Cycads
| Cycads | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Cycas circinalis , young (standing in the center) and old (hanging to the side) male cones |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name of the class | ||||||||||||
| Cycadopsida | ||||||||||||
| Brongn. | ||||||||||||
| Scientific name of the order | ||||||||||||
| Cycadales | ||||||||||||
| Dumb. |
The cycads (Cycadales) are one of the five groups of seed plants living today, of very different sizes, and comprise around 320 species . Its German name refers to its external appearance, which is characterized by a palm-like or underground trunk and fern-like leaves. Because their ovules are not enclosed in carpels, they belong to the naked samers . The female and male reproductive organs are in most species in cone-like organs and are always on different individuals ( diocyte ).
Cycads are found in the tropics around the world , but are largely absent from tropical rainforests. The use of the stems as a source of starch was particularly important in the past; today some species are popular ornamental plants .
features
Shoot axes
The cycads form two types of shoot axes , tree-shaped trunks and subterranean rhizomes .
Tree-shaped trunks have a tuft of leaves at their upper end and thus have a palm-like appearance. The trunks are usually compact, straight and unbranched. They often reach heights of growth of five to ten meters, some species such as Lepidozamia hopei even reach 15 meters. In old age some species are prostrate, rock-dwelling species even drooping. The formation of root shoots can lead to the formation of groups.


Underground trunks can be bulbous and then have no leaf scars (in Bowenia, Chigua, Stangeria and Zamia ). In other species, the leaf scars are preserved ( Cycas , Encephalartos and many Macrozamia species).
In many species, the leaf bases are heavily involved in the structure of the trunk. The trunks of some genera and most of the leaf stalks have the fern- typical stairs tracheids , often with echoes of pits (araucariaid type). There are only tracheids, there are no tracheas . The marrow is very pronounced. It is partially rich in starch and serves as a place to store reserve substances.
The general structure of the trunk is that of a Eustele . The secondary wood is not very pronounced. It is rich in mucus ducts and has very broad radial medullary rays (manoxylated wood). On the other hand, there is water-bearing fabric on the outside.
Few species, such as the representatives of Dioon , have a single cambium . They form a uniform wooden cylinder (monoxylic type). Other representatives, such as Cycas, Macrozamia and Encephalartos, have a different form of secondary growth in thickness: the first cambium stops working. Outside the normal, first wooden cylinder , a cambium or several consecutively forms in the bark , outside the primary phloem . This cambium forms the xylem and phloem. This creates several xylem rings (polyxyle wood). In some cases, phloem is also released inward - a very rare case with secondary growth in thickness.
A characteristic feature that only the cycads have within the Nacktsamer are the leaf tracks: The vascular bundles of at least some leaves of a plant arise on the side of the stem facing away from the leaf and encircle the stem in a belt-like manner.
root

The primary root of the cycads is a taproot . In cycads with an underground trunk, the taproot is a contractile root: it can pull the trunk into the ground. It compensates for the growth in height of the trunk. In these species, the roots are mostly succulent and bulbous and serve to store water and reserve materials .
In the tree-shaped cycads, the tap root is soon replaced by a secondary, extensive root system. This also serves to anchor and support the trunk. The secondary roots are slender, lignified and have little storage capacity.
A third form of roots are the coralloid roots. They occur in all cycads, but not out of order, so they are a synapomorphism . These are side roots that grow sideways or upwards and sit just below or even above the soil surface. At their end they form a nodular structure. This contains nitrogen-fixing cyanobacteria from the genera Nostoc , Calothrix and Anabaena , which enable the cycads to grow on very sterile soils.
The occurrence of mycorrhiza has also been proven in some genera .
leaves


The leaves are simply pinnate. Exceptions are the genus Bowenia and the two species Cycas debaoensis and Cycas multipinnata , which have bipinnate leaves. The leaves have a distinct petiole that merges into the rachis on which the leaflets sit. The leaf length ranges from 20 centimeters for Zamia pygmaea to around seven meters for Encephalartos laurentianus . Most species form the new leaves of a vintage mostly all at once. Few species, such as Macrozamia sect. Macrozamia , Stangeria eriopus and some Zamia species each form one leaf after the other. The cycads are generally evergreen, but some species shed the old leaves just before the new ones form.
The leaflets are extremely diverse. Their length ranges from three centimeters for Zamia pygmaea to 50 centimeters long and 30 centimeters wide for Zamia wallisii , their shape from lanceolate to obovate, from straight to sickle-shaped. In some species such as Cyca micholitzii and some species of Macrozamia sect. Parazamia , the leaflets are branched one to three times. The consistency ranges from paper to leathery to stiff. The surface ranges from smooth and shiny to rough and uneven and furrowed. The furrows are created by the fact that the larger leaf veins are sunk. The color of the leaflets ranges from yellow-green and blue-green to various tones from light and dark green to purple-green, bronze-green, to silver and blue. The last two colors are created by layers of wax on the leaf surface. The leaf veins usually run parallel to the longitudinal axis of the leaflet. Chigua , Cycas and Stangeria form distinct midribs.
There are two ways in which the leaflets attach to the rachis: articulated feathers break off easily from the rachis and also fall off after the rachis has died. The second leaflet shape runs down the rachis and therefore does not fall off separately. In most species, the leaflets are flat and at right angles to the rachis. They are seldom keeled, inclined upwards or downwards so that the leaflets overlap.
The leaf margin of the leaflets can be whole, more often it is serrated, toothed, provided with thorns or forms thorny lobes. It is flat, wavy, rolled back, or thickened.
The young, growing leaves are covered with hairs ( trichomes ), sometimes densely to woolly hairy. Usually the leaves soon become bare, in some species such as Encephalartos hirsutus the leaves remain hairy for a few months. However, adult leaves are usually smooth and shiny.
In addition to the leaves just described, cycads also form lower leaves (cataphylls) and sporophylls , the latter usually in the form of cones . These three leaf shapes are usually formed in a fixed order in the annual cycle. At the beginning of the growing season, which usually coincides with the rainy season, cataphylls are first formed, which is immediately followed by a new crown of leaves and then the cones. Some species either produce new leaves or cones each year. The cataphylls are scale-shaped, usually a few centimeters long and fleshy when fresh, later dry and paper.
Cones
Cycads are dioeciously separated, there are male and female individuals ( diocyte ). The formation of female and male cones on one plant at the same time was never observed. After traumatic events such as transplanting, severe damage from cold, heat or drought, a change of sex was observed. The sex determination should be done through the interaction of several plant hormones .
The cones are the reproductive organs and consist of strongly derived leaves, the sporophylls . These are arranged in a spiral, the cone has limited growth. The cones can be up to 75 centimeters long and 40 kilograms. The male cones are smaller and have more sporophylls. The cone morphology is important for the delimitation and determination of the genera.
The cones arise at the end of the shoot. Carried the further growth of the plant through a side bud that the unused through the journal Education Spitzenmeristem replaces. The trunk is thus a sympodium . Exceptions are the female representatives of Cycas , in which no cones are formed and the point meristem forms leaves again after the sporophylls.
- Male cones
The individual sporophylls are wedge-shaped and carry the sporangia on the underside . At Zamia they are also on the leaf margin and on the top. During the ripening period, the cone axis stretches and the sporophylls, which had been dense up to that point, move apart, the sporangia become visible. The sporangia are located on the underside, the number per sporophyll ranges from a few in Zamia to over 1000 in Cycas . The wall of the sporangia is several layers of cells thick. The sporangia tear open and release the pollen (the micro-meiospores). This falls on the smooth surface of the underlying sporophylls. At the same time, the temperature of the cone also increases and fragrances are produced. The heat development ( thermogenesis ) takes place via the alternative, cyanide-resistant path of the respiratory chain and is much more pronounced in male cones than in female. The scent is described as musty, fruity or sweet. Inside the cone, the lower sporophylls release the pollen first, then the upper ones. The pollen discharge takes from a few days to several weeks.
- Female cones

The female cones are generally larger than the male of the same species, at the same time they have fewer, but significantly larger, sporophylls. Each sporophyll sits with a stalk on the axis of the cone. The bulla, the outward-facing side of the sporophyll, sits on the stem. On the inside of the bulla, pointing to the cone axis, there are two ovules , and more in Cycas , one on each side of the sporophyll stalk. When the ovules are ripe for pollination, the sporophylls move apart a little. The female cones also heat up and produce fragrances.
In the genus Cycas , the female sporophylls do not form a cone. They are screwy at the end of the stem axis and are clearly leaf-like, albeit brownish. The sporophylls are either open, long and loose, or they are closed and form a hilly structure.
Seeds
The seed coat consists of three layers: the outer layer is fleshy ( sarcotesta ), the middle layer is lignified ( sclerotesta ), and the innermost layer, the endotesta , is a thin membrane. The sarcotesta is often conspicuously colored and edible for animals, so it serves the directional spread of seeds by animals ( zoochory ). The species from the Cycas rumphii complex have another, spongy tissue inside, which allows the seeds to swim. The species grow on the seashore, the floating seeds are used for spreading. Inside the semen is the female gametophyte with the embryo .
The seeds are usually egg-shaped, but can also be spherical or cylindrical. Their size ranges from 15 millimeters for Zamia pygmaea to 60 millimeters for Macrozamia macdonellii and Cycas micronesia . The scar at the point of attachment of the seed to the sporophyll is called Chalaza and often has a species-specific shape. The surface of the sclerotesta can be smooth, rough, fibrous, or grooved. The color of the sarcotesta is very variable and ranges from green to yellow to various shades of red.
There are two types of seeds: the platyspermic seeds are flattened and only occur in the genus Cycas . They tear open lengthways and divide the sclerotesta into two equal halves. The gametophyte becomes visible in the crack, and shortly afterwards the radicle of the seedling appears. The other genera have radiospermic seeds: At the micropylene end of the seed there is a round pore that is burst by the radicula of the seedling directly below.

ingredients
The cycads contain poisonous substances, pseudocyanogenic compounds, from which hydrocyanic acid is split off when exposed to alkaline solutions . These are azoxy compounds in a glycosidic bond . Cycasin , the glucoside of methylazoxymethanol , is represented in all the cycads examined . It does not occur outside of cycads.
Many cycads contain the non-proteinogenic amino acid β-methyl-amino-L-alanine (BMAA). These and other compounds have been linked to neurological damage. The connection with the "Zamia staggers" ataxia of the hind legs of cattle and sheep that graze on cycads is considered to be certain. The accumulation of Alzheimer's and Parkinson's disease in humans, known as “Guam dementia”, has been linked to chronic poisoning due to the consumption of starch obtained from cycads and / or the bats that feed on the seeds, but it has not been finally clarified.
The toxins are located in the cones in specialized cells ( idioblasts ), the so-called gold cells because of their color.
Reproduction

Numerous sporogenic cells surrounded by a tapetum are formed in the young sporangia . The tapetum dissolves, forms a syncytium and nourishes the sporogenic cells. The spore mother cells develop from the sporogenic cells. These undergo a meiosis and each form a tetrad of haploid pollen grains.
The ovules have an integument from which the seed coat is created in the course of seed maturation. In the nucellus a megaspore mother cell is formed by a functional meiosis from the megaspore is apparent, while the remaining three cells die. The female gametophyte is formed by initially free nuclear divisions, followed by cell wall formation. In the end, the gametophyte consists of a few thousand cells and usually forms two to six archegonia . Only Microcycas forms up to 100 archegonia. The egg cell is up to six millimeters in size.
The ovules on the female sporophylls form a nectar-like drop on the micropyle in the receptive stage . It is created by dissolving the cells under the micropyle, which also creates a pollen chamber. At the end of its readiness to receive, the drop dries up and retreats into the pollen chamber together with the pollen grains it contains. The micropyle is also closed by drying.
The pollination occurs by insects, primarily beetle ( Cantharophilie ). Most of the flower visitors are weevils (Curculionidae), which only act as pollinators in cycads. The second group of pollinators are the fringed winged birds (Thysanoptera). In addition, there is pollination by the wind ( anemophilia ).
The development of the male gametophyte in the pollen grain begins in the sporangium . The first cell division produces the prothallium cell and the antheridia initial. From this, the tube cell and the generative cell arise through renewed division. At this stage the pollen grain is released from the sporangium.
The pollen grain germinates in the pollen chamber and forms a short pollen tube that penetrates the female tissue and also feeds on the tissue of the ovule. It usually takes several months for the pollen tube to reach the egg cell and fertilization to take place. During this time, the sperm are formed in the pollen tube: the generative cell divides into a sterile cell and the spermatogenic cell. The latter divides and forms two sperm cells. With Microcycas , additional sperm cells are created from the sterile cell. The sperm have a lash line, so they are spermatozoids . Besides ginkgo, cycads are the only recent seed plants with spermatozoids. With a size of up to 300 micrometers, the spermatozoids of the cycads are among the largest known. They are released when the pollen tube is torn open and swim into the neck of the egg sac. The high osmotic potential of the cell fluid released when the pollen tube is torn open leads to the shrinking of the cervical cells of the archegonium and the exposure of the egg cell. When entering the egg, the spermatozoids lose their lash line. It can take up to seven months from pollination to fertilization, in some species the seed has already fallen from the mother plant by this time.
After fertilization, the nucleus of the zygote divides rapidly and forms 256 to 1024 free cell nuclei without cell formation and without changing the total volume of the nuclei; the individual core is therefore getting smaller and smaller. Then the cell walls are formed and the cells form three regions: at one end cells develop that take up nutrients from the surrounding gametophyte tissue. The middle region forms the suspensor, which pushes the embryo deeper into the gametophyte. The actual embryo is created at the other end. It forms two cotyledons , which are, however, greatly reduced. However, at least one further (subsequent) sheet is formed, at least one or two more are created.
The mature seed consists of three genetically different parts: the seed coat is diploid tissue from the sporophyte mother plant, the endosperm-like tissue is the haploid gametophyte and the embryo is the young, diploid daughter plant.
development
During germination, the primary root (radicula) appears first and grows downwards. The primary taproot forms when the ground touches the ground. Only then does the leaf already laid in the seed appear. At the tip of the shoot, leaf leaves and cataphylls are formed alternately. Young plants initially only form one or a few leaves per growth period, later the storage organs are large enough to allow the formation of a larger number of leaves. During this time, the trunk also reaches its final diameter. At this time, the plant also begins the regular alternation of leaves and cataphylls, as well as the formation of cones.
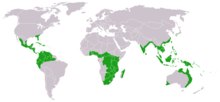
Distribution and locations
The cycads are a group of tropical plants. Their distribution area extends from the 30th north to the 35th south latitude. However, only a few species occur directly at the equator , and then more at higher altitudes, in areas with lower temperatures and humidity than in the tropical rainforest . In the latter, only the representatives of the genera Zamia and Chigua can be found. The majority of cycads are found just outside this area. Only a few species can survive frost or long periods of cold, examples are Encephalartos ghellinckii , Encephalartos cycadifolius and Macrozamia occidua . They grow at greater heights of up to 2500 meters, where frosts and snow regularly occur.
Even though cycads are widespread around the world, the individual genera are limited to individual continents: Ceratozamia , Dioon and Microcycas occur north of the equator in America , with Cycas the area also extends south of the equator. Chigua is only known from Colombia and Microcycas only from Cuba . The genera Bowenia, Lepidozamia and Macrozamia occur only in Australia , Stangeria only in southern Africa . The ancient genera Cycas and Encephalartos have their focus like the other ancient genera south of the equator, but their areas also extend beyond it to the north.
The locations of the cycads are mostly frost-free, the rain falls in summer, the winters are cool and dry. They are therefore not found in areas with a Mediterranean climate . Precipitation must be at least 350 millimeters per year, but can reach 5500 millimeters. The soils are mostly sand or sandy gravel, with a small amount of clay and humus . The soil must be well drained and well ventilated. Salt is poorly tolerated; only a few species grow on salt-influenced coasts and marshes .
Herbivories and Diseases
The cycads are eaten by relatively few herbivores . Some species of weevils (Curculionidae), which attack various organs, are significant . The larvae of Tranes internatus (Australia), Phacecorynus funerarius and Calandra sommeri (both Africa), as well as Phacecorynus zamiae eat passages in the trunks of cycads and can completely destroy the tip of the shoot. Weevils of the genus Antliarhinus , especially A. zamiae and A. signatus , lay their eggs in seeds of Encephalartos , their larvae devour the seeds. Some butterflies (Lepidoptera) have specialized in cycads , including the genus Eumaeus in America and Zeronopsis leopardina in South Africa. The caterpillars mainly eat the young leaves and store the toxins (cycasin, etc.) of the cycads in their bodies and use them for their own defense. Mealybugs (Pseudococcidae), scale insects (Coccoidea), aphids (Aphidoidea) and spider mites (Tetranychidae) are mostly only in Palm Farm crops are more important. Pathogens ( fungi and bacteria ) are only important in the seedling stage.
Hazard and protection
Many species of cycads only inhabit a small area, and their populations are correspondingly small. Many species are endangered by foraging, but to a greater extent by the loss of their locations ( deforestation , agriculture, settlement development, etc.). The genera Ceratozamia , Chigua , Encephalartos , Microcycas and Stangeria , as well as Cycas beddomei are listed in Appendix I of the Washington Convention on Endangered Species (CITES), the other genera and species in Appendix II. Most of the species are also on the Red List as classified endangered, a number of species have already died out, at least in the wild.
Systematics









External system
The relationships within the seed plants have still not been clarified, despite intensive research. The main reason is the high proportion of extinct seed plant groups, while only five groups are still alive today. Both morphological and molecular genetic studies show the cycads mostly close to the base of the seed plants. A recent, very detailed study showed that only the extinct groups Elkinsia , Lyginopteris and the Medullosales are more basal than the cycads within the seed plants. The medullosales are very often discussed as possible precursors of the cycads, but this is contradicted by the fact that both groups occurred at the same time.
In the past, cycads were often grouped together with the fossil Bennettitales known only from the Mesozoic era . In terms of their morphology, leaves or cataphylls can often not be assigned to one of the two groups, only the cuticle allows an exact assignment. Cladistic analyzes, however, never revealed a closer relationship between the cycads and the Bennettitales.
Internal system
The cycads have already been the subject of phylogenetic studies several times, with different genes and species being analyzed. The cladograms created from it were essentially similar, even if they differed in the position of some genera. The cladogram according to Hill et al. shown:
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
The work by Treutlein and Wink 2002, as well as Chaw et al. 2005 showed similar results. Only the position of the genera Bowenia , Stangeria and Dioon are different. Cycas is always the most basic genus; there is a clade from Lepidozamia , Encephalartos, and Macrozamia , and a clade from New World genera. In Chaw et al. however, after Cycas Dioon was the next basal genus, followed by Bowenia . Stangeria lies with them within the clade with the New World genera ( Zamia , Microcycas and Ceratozamia ). However, the Stangeria and Bowenia, placed in one family, were always relatively far apart. Chigua was always within Zamia , which is why the genus status is more than questionable. Another study by Zgurski et al. 2008 could not clarify the situation of the three genera in question, while the other results were confirmed.
The cycads comprise around 300 recent species in three families and eleven genera. The following system follows Whitelock 2002, which in turn follows DW Stevenson 1992:
- Suborder Cycadineae
- Suborder Zamiineae
- Stangeriaceae family
- family Zamiaceae
- Subfamily Zamioideae
-
Zamieae
tribe
- Sub tribus Zamiinae
- Subertribus Microcycadinae
- Genus Microcycas
- Tribe Ceratozamieae
- Genus Ceratozamia
-
Zamieae
tribe
- Subfamily Encephalartoideae
- Tribe Encephalarteae
- Subtribe Encephalartinae
- Genus Encephalartos
- Macrozamiinae subtribe
- Genus Macrozamia
- Genus Lepidozamia
- Subtribe Encephalartinae
- Tribe Diooeae
- Genus Dioon
- Tribe Encephalarteae
- Subfamily Zamioideae
A World List of Cycads appears at irregular intervals, listing all accepted taxa. The current one appeared in 2007.
Paleobotany
The oldest fossil representatives of the cycads come from the Pennsylvania , so are around 300 million years old. Morphologically, they are largely similar to today's representatives. Characteristic features of the recent representatives such as the omega-shaped petiole vascular bundles and the leaf traces surrounding the trunk emerged in the Permian at the latest . They reached their greatest geographical distribution and number of species in the Mesozoic, especially in the Jurassic and Cretaceous (around 200 to 65 million years ago). In addition to the tall-stemmed forms, there were already short-stemmed forms (such as Charmorgia dijolii ) and those with underground stems ( Antarcticycas schopfii ).
According to a study published in 2011, the 300 or so species of cycads that still exist today have only diversified from a small group of fossil species over the past 12 million years.
Research history
Cycads are not mentioned by the ancient Greek and Roman authors. The plant referred to by Theophrastus as χὑχας was a palm, possibly Hyphaene thebaica . The first reports came to the western world in the 9th century by Arab naturalists with descriptions of Indian cycads, then in the 16th century by Antonio Pigafetta , Fernão Lopes de Castanheda and by Francis Drake , who reported on Cycas from the Moluccas . The first reference to American cycads comes from Giovanni Lerio in 1576.
The first scientific description comes from the pre-Linnean Rheede tot Draakenstein , who described a cycad named Todda panna in 1682 , to which Carl von Linné gave the ancient name Cycas in 1753 , on the erroneous assumption that Theophrastus and Rheede had described the same plant. Linnaeus described the next genus, Zamia , in 1763 . Only in 1834 was followed by Lehmann with Encephalartos . Most recent genera were known by 1868, only Chigua was first described in 1990. Important monographs and revisions of cycads come from Miquel (1861, 1868, 1870), Regel (1876), de Candolle (1868), and Thiselton-Dyer (1884). In the 20th century, CJ Chamberlain presented The living cycads in 1919, a work that is still important today, while the German Julius Schuster caused confusion in 1932 with his contribution to Engler's Das Pflanzenreich . An important monograph by Chamberlain from the 1940s was never published because of his death. The classification of recent cycads, which was applicable at the beginning of 2009, is that given above by Stevenson 1992, the two most important monographs are those by Norstog and Nicholls, The Biology of the Cycads from 1997, and by Whitelock, The Cycads , from 1992.
use
In almost all areas where cycads occur naturally, they have been or are used as starch suppliers. The oldest finds come from Australia and are 6,000 to 7,000 years old. Both the seeds and the pulp of the stems or tubers are used.
The Calusa , Seminoles and Tequesta of the southeastern United States made a pudding from Zamia integrifolia . The Wasanya in Kenya use the seeds of Encephalartos hildebrandtii to produce flour that has a long shelf life. On Ryūkyū , miso was made from the seeds and stems of Cycas revoluta in years when the soy harvest failed . 100 kilograms of seeds provide 27 kilograms of starch. In New Guinea, the Philippines, Fiji, Nicobar Islands and Sri Lanka, in India, Southeast Asia, from Mexico to Peru, the cycads are still occasionally used.
In Florida between 1845 and 1920 starch was also obtained industrially from Zamia integrifolia , the process being based on that of the Seminoles. The production of a single mill was 136 tons per year in 1850, around 1900 a mill produced around eleven tons per week. In 1920 the last mill was closed, probably because the stocks of cycads had dwindled due to exploitation. During the same period there were also mills in the Dominican Republic . From 1921 there was a plant in New South Wales that produced starch from the marrow of Macrozamia communis , but was closed again after several years.
On Ryūkyū, the starch from Cycas revoluta was fermented into a sake- like drink. In Africa, a beer-like drink was made from the pulp of various types of Encephalartos . In Sonora , a tequila- like drink was distilled from Dioon sonorense . When the species became too rare, its use ended.
Due to their content of toxic substances, acute and chronic poisoning occurred again and again. They can cause liver damage and cause cancer.
Around eight to ten species are widely grown as ornamental plants , mainly in subtropical areas, as the plants are not hardy. In addition, the leaves are used for decorative purposes. It is widely used as a "palm frond" on Palm Sunday . In Mexico, whole crowns are chopped off and the leaves are bent down and tied together so that they look like a crown. If the remaining strains survive, they will take 10 to 15 years to regenerate; during this time they do not form a cone.
supporting documents
- Loran M. Whitelock: The Cycads . Timber Press, Portland 2002. ISBN 0-88192-522-5 (Characteristics, reproduction, development)
Individual evidence
- ↑ a b c Walter Zimmermann : Phylogeny of the plants . 2nd edition, G. Fischer, Stuttgart 1959, pp. 375-384. (without ISBN)
- ^ A b Peter R. Crane: Major Clades and Relationships in the "Higher" Gymnosperms . in: Charles B. Beck (Ed.): Origin and Evolution of Gymnosperms . Columbia University Press, New York 1988, ISBN 0-231-06358-X , pp. 218-272, especially 228-241.
- ↑ a b c A. Bresinsky, Ch. Körner, JW Kadereit, G. Neuhaus, U. Sonnewald: Strasburger - Textbook of Botany . 36th edition, Spektrum Akademischer Verlag, Heidelberg 2008, pp. 833-835. ISBN 978-3-8274-1455-7
- ↑ Jack B. Fisher, Andrew P. Vovides: Mycorrhizae Are Present in Cycad Roots The Botanical Review, Volume 70, pp. 16-23.
- ↑ a b c d e f g K. R. Sporne: The Morphology of Gymnosperms . Hutchinson University Library, London 1965. (without ISBN), pp. 103-118.
- ↑ a b Robert Roemer, Irene Terry, Christina Chockley, Jennifer Jacobsen: Experimental evaluation and thermo-physical analysis of thermogenesis in male and female cycad cones . Oecologia, Vol. 144, 2005, pp. 88-97. doi : 10.1007 / s00442-005-0045-0
- ↑ a b c d Eric D. Brenner, Dennis W. Stevenson, Richard W. Twigg: Cycads: evolutionary innovations and the role of plant-derived neurotoxins . Trends in Plant Sciences, Volume 8, 2003, pp. 446-452.
- ↑ Dietrich Frohne, Uwe Jensen: Systematics of the plant kingdom with special consideration of chemical characteristics and plant drugs . 4th revised edition. Gustav Fischer, Stuttgart / Jena / New York 1992, ISBN 3-437-20486-6 , pp. 95 .
- ^ EJ Hermsen, TN Taylor, EL Taylor, D. Wm. Stevenson: Cataphylls of the Middle Triassic cycad Antarcticycas schopfii and new insights into cycad evolution . American Journal of Botany, Volume 93, 2006, pp. 724-738.
- ^ RJ Poort, H. Visscher, DL Dilcher: Zoidogamy in fossil gymnosperms: The centenary of a concept, with special reference to prepollen of late Paleozoic conifers . Proceedings of the National Academy of Science USA, Volume 93, 1996, pp. 11713-11717.
- ↑ The section distribution and locations is based on the chapter Cycads Distribution Past and Present , in: Loran M. Whitelock: The Cycads . Timber Press, Portland 2002, p. 11 f. ISBN 0-88192-522-5
- ↑ Loran M. Whitelock: The Cycads . Timber Press, Portland 2002, pp. 29-32. ISBN 0-88192-522-5
- ↑ Loran M. Whitelock: The Cycads . Timber Press, Portland 2002, pp. 44 f. ISBN 0-88192-522-5
- ^ JS Donaldson, JS (Ed.): Cycads. Status Survey and Conservation Action Plan . IUCN / SSC Cycad Specialist Group. IUCN, Gland, Switzerland and Cambridge, UK 2003; see. also species of Cycadales on IUCN : 2007 IUCN Red List of Threatened Species. , accessed May 3, 2008.
- ^ Sarah Matthews: Phylogenetic relationships among seed plants: Persistent questions and the limits of molecular data . American Journal of Botany, Volume 96, 2009, pp. 228-236. doi : 10.3732 / ajb.0800178
- ↑ James A. Doyle: Seed ferns and the origin of angiosperms . Journal of the Torrey Botanical Society, Volume 133, 2006, pp. 169-209.
- ↑ a b c d Thomas N. Taylor, Edith L. Taylor, Michael Krings: Paleobotany. The Biology and Evolution of Fossil Plants . Second Edition, Academic Press 2009, ISBN 978-0-12-373972-8 , pp. 703-722.
- ↑ KD Hill, MW Chase, DW Stevenson, HG Hills, B. Schutzman: The Families and Genera of Cycads: A Molecular Phylogenetic Analysis of Cycadophyta Based on Nuclear and Plastid DNA Sequences . International Journal of Plant Sciences, Volume 164, 2003, pp. 933-948. doi : 10.1086 / 378538
- ↑ Jens Treutlein, Michael Wink: Molecular phylogeny of cycads inferred from rbcL sequences . Natural Sciences, Volume 89, 2002, pp. 221-225. doi : 10.1007 / s00114-002-0308-0
- ↑ Shu-Miaw Chaw, Terrence W. Walters, Chien-Chang Chang, Shu-Hsuan Hu, Shin-Hsiao Chen: A phylogeny of cycads (Cycadales) inferred from chloroplast matK gene, trnK intron, and nuclear rDNA ITS region . Molecular Phylogenetics and Evolution, Volume 37, 2005, pp. 214-234. doi : 10.1016 / j.ympev.2005.01.006
- ↑ a b Jessie M. Zgurski, Hardeep S. Rai, Quentin M. Fai, David J. Bogler, Javier Francisco-Ortega, Sean W. Graham: How well do we understand the overall backbone of cycad phylogeny? New insights from a large, multi-own plastid data set . Molecular Phylogenetics and Evolution, Volume 47, 2008, pp. 1232-1237. doi : 10.1016 / j.ympev.2008.03.002
- ^ DW Stevenson: A formal classification of the extant cycads . Brittonia, Vol. 44, 1992, pp. 220-223.
- ^ KD Hill, DW Stevenson, R. Osborne: The world list of cycads . In: Proceedings of the 7th International Conference on Cycad Biology (CYCAD 2005), Xalapa, Mexico, January 2005 . Memoirs of the New York Botanical Garden, Volume 97, 2007, pp. 454-483. (pdf; 119 kB)
- ↑ a b N. S. Nagalingum et al .: Recent Synchronous Radiation of a Living Fossil. In: Science , Volume 334, No. 6057, 2011, pp. 796-799, DOI: 10.1126 / science.1209926
- ↑ The section on the history of research is based on: Paolo De Luca: A Historical Perspective on Cycads from Antiquity to the Present . Memoirs of the New York Botanical Garden, Vol. 57, 1990, pp. 1-7.
- ↑ The usage section is based on the chapter Cycads in Human Activities , in: Loran M. Whitelock: The Cycads . Timber Press, Portland 2002, pp. 46-51. ISBN 0-88192-522-5
Web links
- Virtual Cycad Encyclopedia .
- The Cycad Pages of the Royal Botanic Gardens Sydney.
- Christopher J. Earle, last update 2011 Cycadales on www.conifers.org - The Gymsperm Database .
- Cycad Specialist Group of the IUCN
- Cycads in the Solnhofen Fossil Atlas.