4-pentenoic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4-pentenoic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 8 O 2 | |||||||||||||||
| Brief description |
colorless to light yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 100.12 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.98 g cm −3 (20 ° C ) |
|||||||||||||||
| Melting point |
<−22 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
0.267 mmHg (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4283 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
4-pentenoic acid is a linear, unsaturated carboxylic acid with a terminal double bond , which smells strongly of cheese and is used as a flavoring substance .
In the future, 4-pentenoic acid (in addition to the isomeric 2-pentenoic acid and 3-pentenoic acid) could become important as a starting material for cellulose-based biofuels.
In current considerations for the production of adipic acid , the intermediate product for polyamide 6.6 , from lignocellulose-containing biomass , 4-pentenoic acid and its methyl ester, methyl 4-pentenoate, play an important role.
Occurrence and representation
Classic laboratory processes for the preparation of 4-pentenoic acid are the malonic ester synthesis and the acetoacetic ester synthesis with allyl bromide or from 1,2,3-tribromopropane (practically quantitatively from allyl bromide and bromine ) as modified malonic ester synthesis.
The alkaline hydrolysis of the substituted malonic ester yields the substituted malonic ester, the second terminal bromine atom being split off as hydrogen bromide by the malonic ester anion. Hydrolysis and decarboxylation lead to the sodium salt of 4-bromo-4-pentenoic acid, which is reduced to 4-pentenoic acid by the action of ethanol and sodium.
Oxidation of 4-pentenal (from cyclopentene or acetaldehyde diallylacetal ) with oxygen also produces 4-pentenoic acid in relatively modest yields (up to 38%). 4-pentenoic acid is also obtained in the reaction of propiolactone with vinyl magnesium bromide in the presence of copper (I) chloride as a catalyst in a yield of 59%.
Allyl alcohol reacts with the Orthoester trimethyl under acid catalysis with propionic acid in the Johnson variant of the Claisen rearrangement to the 4-pentenoate, which gives 4-pentenoic acid in 70% yield after alkaline hydrolysis and acidification.
The continuous isomerization, in particular of methyl 3-pentenoate to methyl 4-pentenoate, is of technical interest. and hydrolysis to 4-pentenoic acid. The required methyl 3-pentenoate precipitates in yields of> 90% (as approx. 70% 3- ( E ) - trans and 30% 3- ( Z ) - cis mixture) in the carbonylation of 1,3-butadiene with CO and methanol in a pyridine / 3-picoline mixture with dicobalt octacarbonyl as a catalyst. Isomerization with palladium on acidic ion exchangers or zeolites yields isomer mixtures with up to 10 percent by weight of 4-pentenoic acid ester, which is removed from the mixture by distillation. The 3-esters in the distillation bottom are returned to the isomerization reaction.
Despite recycling of the unchanged 3-pentenoic acid ester, this route to 4-pentenoic acid is hardly economical.
4-pentenoic acid is a component of in the ring-opening acid hydrolysis of γ-valerolactone resulting Pentensäuregemisches a total of five isomers: 4-pentenoic acid, 3-pentenoic acid (in cis - and trans - configuration) and the thermally stable 2-pentenoic acid (cis and trans ). Under somewhat milder conditions and complete conversion of the starting materials, the reaction of a γ-valerolactone / methanol mixture takes place to form the isomeric pentenoic esters, from which 4-pentenoic acid can be isolated after hydrolysis.
properties
4-pentenoic acid is corrosive and gives off a strong cheese odor.
Applications
Bromine compounds such as B. N-bromosuccinimide or iodine or iodine chloride convert 4-pentenoic acid almost quantitatively into the corresponding halomethyl-butyrolactones.
The 5-methylenebutyrolactone is obtained from the iodomethyl butyrolactone by dehydrohalogenation using diazabicycloundecene DBU.
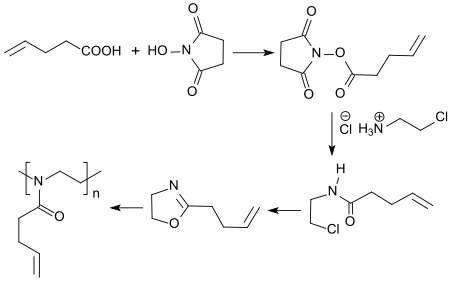
4-pentenoic acid is used to synthesize the monomer 2- (3-butenyl) -2-oxazoline,
at its terminal double bond in homo- and copolymers in so-called thiol-ene- click addition reactions , thiol- functionalized molecules can be added very gently and efficiently.
By incorporating 4-pentenoic acid into the neutral thermoresponsive polymer N-isopropylacrylamide , copolymeric spherical microgels are obtained, the diameter of which changes drastically with a shift in pH.
4-pentenoic acid reacts with sulfuric acid or iron triflate with intramolecular cyclization to form γ-valerolactone.
The reaction is reversible and gives mixtures of the isomeric pentenoic acids.
As a secondary product of the alkaline hydrolysis of γ-valerolactone, a platform chemical made from renewable raw materials , 4-pentenoic acid has recently attracted more attention. By decarboxylation of acidic zeolites n- arise butenes , the acidic ion exchanger (Amberlyst 70) in an overall yield of 77% at the C 8+ alkenes di- or can be oligomerized. After hydrogenation, the alkenes obtained can be used as biogenic gasoline or diesel fuel.
The isomeric pentenoic acids obtained during the acid hydrolysis of γ-valerolactone can be hydrogenated to valeric acid and reacted with alcohols to give the corresponding esters. The ethyl valerate has gasoline-like properties, the higher esters can be used as a diesel substitute.
The implementation of isomeric pentenoic acid or pentenoic ester mixtures from the hydrolysis of γ-valerolactone for the production of the polyamide 6 monomer ε-caprolactam (after hydroformylation to 5-formylvaleric acid and reductive amination ) or the polyamide 6.6 building block adipic acid by carbonylation could have future potential in the presence of water with palladium acetate and the phosphine ligand 1,2-bis (di-tert-butylphosphinomethyl) benzene with a shift of the double bond from the 2- and 3- to the 5-position or dimethyl adipate by methoxycarbonylation in the presence of methanol and the hydroformylation catalyst system dicarbonylacetylacetonato -rhodium (I) [Rh (acac) CO) 2 ] / tri- (sodium-meta-sulfonatophenyl) -phosphine . The diol component 1,6-hexanediol for polyester or from hexamethylenediamine , the diamine building block for polyamide 6.6, is accessible from the adipic acid ester .
In experiments on animals and cell organelles, the inhibition of fatty acid oxidation and the blood sugar-lowering effect of 4-pentenoic acid could be demonstrated.
Individual evidence
- ↑ a b Entry on 4-Pentenoic Acid at TCI Europe, accessed on May 15, 2017.
- ↑ a b c d e f g h Entry on 4-pentenoic acid in the GESTIS substance database of the IFA , accessed on May 15, 2017(JavaScript required) .
- ↑ Data sheet 4-Pentenoic Acid from Sigma-Aldrich , accessed on May 15, 2017 ( PDF ).
- ↑ a b c 4-pentenoic acid. In: thegoodscentcompany.com. The Good Scent Co., accessed May 15, 2017 .
- ^ R. Madsen, B. Fraser-Reid: 4-Pentenoic Acid . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2002, doi : 10.1002 / 047084289X.ru00128 .
- ↑ a b P. Palkovits: Pentenic acid as a trailblazer for cellulose-based biofuels . In: Angew. Chem. Band 122 , no. 26 , 2010, p. 4434–4436 , doi : 10.1002 / anie.201002061 .
- ↑ a b Patent WO2012134397A1 : Synthesis of diacids. Filed on March 28, 2012 , published on October 4, 2012 , Applicant: Agency for Science, Technology and Research, Singapore, Inventor: PK Wong et al
- ↑ PJ Deuss, K. Barta, JG de Vries: Homogeneous catalysis for the conversion of biomass and biomass-derived platform chemicals . In: Catal. Sci. Technol. tape 4 , 2014, p. 1174-1196 , doi : 10.1039 / C3CY01058A ( rsc.org ).
- ↑ a b Patent WO2012131027A1 : Process for the preparation of alkanoic acid esters in a carbonylation process using palladium bidentate bisphosphonate ligands. Registered on March 30, 2012 , published on October 4, 2012 , applicant: DSM IP Assets BV, inventor: JG de Vries, N. Sereinig, EWM van de Vordervoort, MCC Janssen.
- ↑ A. Messerschmidt: Investigations on the unsaturated acids. About allylacetic acid and valerolactone . In: Justus Liebigs Ann. Chem. Band 208 , no. 1–2 , 1881, pp. 92-104 , doi : 10.1002 / jlac.18812080107 .
- ^ JR Johnson, WL McEwen: 1,2,3-Tribromopropane In: Organic Syntheses . 5, 1925, p. 99, doi : 10.15227 / orgsyn.005.0099 ; Coll. Vol. 1, 1941, p. 209 ( PDF ).
- ↑ a b F.C. Whitmore: Organic Chemistry, Volume 1, reissued 2012 . Van Nostrand, New York 1951, ISBN 978-0-486-31115-9 , pp. 12 .
- ↑ Patent US8362296B2 : Process for preparing 4-pentenoic acid. Registered on August 19, 2010 , published on January 29, 2013 , applicant: BASF SE, inventors: JH Teles, M. Schelper, K. Gumlich, M. Chabanas, C. Müller, A. Meier.
- ^ S. Günther: Synthesis of polymers based on the renewable raw material glycerine, dissertation . University of Hamburg 2012 ( uni-hamburg.de [PDF]).
- ↑ T. Sato, T. Kawara, M. Kawashima, T. Fujisawa: Copper-catalyzed reaction of Grignard reagents with β-propiolactones: A convenient method for the synthesis of β-substituted propionic acids . In: Chem. Lett. tape 9 , no. 5 , 1980, pp. 571-574 , doi : 10.1246 / cl.1980.571 .
- ↑ a b V. Jäger, HJ Günther: Synthesis of γ-methylene butyrolactones (4-penten-4-olides) . In: Tetrahedron Lett. tape 18 , no. 29 , 1977, pp. 2543-2546 , doi : 10.1016 / S0040-4039 (01) 83815-6 .
- ↑ Patent EP0266689B1 : Process for the production of 4-pentenoic acid esters. Registered on October 30, 1987 , published on January 2, 1992 , applicant: BASF AG, inventor: W. Hoelderich, H. Aichinger, F. Naeumann, R. Fischer.
- ↑ Patent DE3040432A1 : Process for the production of 3-pentenoic acid esters. Applied on October 27, 1980 , published on May 19, 1981 , Applicants: Mitsubishi Gas Chemical Co., Inc., Inventors: N. Isogai, M. Hosokawa, T. Okawa, N. Wakui, T. Watanabe.
- ↑ Patent EP0157311A2 : Process for the separation of methyl 4-pentenoate from such mixtures and mixtures containing methyl 3-pentenoate. Registered on March 22, 1985 , published on October 9, 1985 , applicant: BASF AG, inventor: H.-W. Schneider, R. Kummer, D. Zimmerling.
- ↑ Patent WO2012134397A1 : Synthesis of diacids. Filed on March 28, 2012 , published on October 4, 2012 , Applicant: Agency for Science, Technology and Research, Inventor: PK Wong et al
- ↑ YA Cheng et al .: Efficient medium ring size bromolactonization using a sulfur-based zwitterionic catalyst . In: J. Am. Chem. Soc. tape 134 , no. 40 , 2012, p. 16492-16495 , doi : 10.1021 / ja307210n .
- ^ RD Crouch, A. Tucker-Schwartz, K. Barker: Iodolactonization of 4-pentenoic acid . In: J. Chem. Educ. tape 83 , no. 6 , 2006, p. 921 , doi : 10.1021 / ed083p921 .
- ↑ N. Windmon, V. Dragojlovic: Phase vanishing halolactonization of neat substances . In: Beilstein J. Org. Chem. Volume 4 , 2008, p. 29 , doi : 10.3762 / bjoc.4.29 .
- ↑ A. Gress, A. Völkel, H. Schlaad: Thio-click modification of poly [2- (3-butenyl) -2-oxazoline] . In: Macromolecules . tape 40 , no. 22 , 2007, p. 7928-7933 , doi : 10.1021 / ma71357r .
- ↑ M. Karg, I. Pastoriza-Santos, B. Rodriguez-Gonzá, R. von Klitzing, S. Wellert, T. Hellweg: Temperature, pH, and ionic strength induced changes in the swelling behavior of PNIPAM-Poly (allyl acetic acid) copolymer microgels . In: Langmuir . tape 24 , no. 12 , 2008, p. 6300-6306 , doi : 10.1021 / la702996p .
- ↑ K. Komeyama, Y. Mieno, S. Yukawa, T. Morimoto, K. Takaki: Cationic iron-catalyzed addition of carboxylic acids to olefins . In: Chem. Lett. tape 36 , no. 6 , 2007, p. 752-753 , doi : 10.1246 / cl.2007.752 .
- ↑ JQ Bond, D. Wang, DM Alonso, JA Dumesic: Interconversion between γ-valerolactone and pentenoic acid combined with decarboxylation to form butene over silica / alumina . In: J. Catal. tape 281 , no. 2 , 2011, p. 290–299 , doi : 10.1016 / j.jcat.2011.05.011 .
- ↑ JQ Bond, DM Alonso, D. Wang, RM West, JA Dumesic: Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels . In: Science . tape 327 , no. 5969 , 2010, p. 1110–1114 , doi : 10.1126 / science.1184362 .
- ↑ J.-P. Lange, R. Price, PM Ayoub, J. Louis, L. Petrus, L. Clark, H. Gosselink: Valeric biofuels: A platform of cellulosic transportation fuels . In: Angew. Chem. Band 122 , no. 26 , 2010, p. 4581–4585 , doi : 10.1002 / anie.201000655 .
- ↑ a b Patent WO2014111446A1 : Process for the preparation of formylvaleric acid and adipic acid. Registered on January 16, 2014 , published on July 24, 2014 , applicant: DSM IP Assets BV, inventors: RFMJ Parton, MCC Janssen, B. Engendahl, JG de Vries.
- ↑ J.-P. Lange, JZ Vestering, RJ Haan: Towards 'bio-based' nylon: Conversion of γ-valerolactone to methyl pentenoate under catalytic distillation conditions . In: Chem. Commun. tape 33 , 2007, p. 3488-3490 , doi : 10.1039 / B705782B .
- ^ C. Corredor, K. Brendel, R. Bressler: Studies on the mechanism of the hypoglycemic action of 4-pentenoic acid . In: Proc. Natl. Acad. Sci. USA . tape 58 , no. 6 , 1967, p. 2299-2306 , PMC 223835 (free full text).
- ↑ H. Schulz: Metabolism of 4-pentenoic acid and inhibition of thiolase by metabolites of 4-pentenoic acid . In: Biochemistry . tape 22 , no. 8 , 1983, p. 1827-1832 , doi : 10.1021 / bi00277a013 .