Lithium-ion accumulator
A lithium-ion battery (including the lithium ion battery , lithium ion battery , lithium ion secondary battery or short rechargeable lithium battery ; fachsprachlich [ liːtiʊm ]) is the generic term for batteries based on lithium compounds in all three phases of the electrochemical cell. The reactive materials in both the negative and positive electrodes and the electrolyte contain lithium ions.
Lithium-ion accumulators have a high specific energy compared to other types of accumulators, but in most applications they require electronic protective circuits because they react disadvantageously to both deep discharge and overcharging.
General
In terms of their chemical structure, lithium-ion accumulators are divided into a large number of different types such as the lithium-cobalt dioxide accumulator , lithium-manganese dioxide accumulator , lithium-iron-phosphate accumulator and less common variants such as the lithium-titanate accumulator and tin-sulfur -Lithium-ion accumulator . The majority of the accumulators on the market are lithium-cobalt dioxide accumulators, often in the form of a lithium-polymer accumulator . The characteristics such as cell voltage, temperature sensitivity, charge and discharge end voltage and the maximum permitted charge or discharge current vary depending on the design and are essentially dependent on the electrode material and electrolyte used. For this reason, the specification of the exact type, for example lithium iron phosphate battery, is more informative than the unspecific specification of the generic term lithium-ion battery .
What all lithium-ion accumulators have in common is that the cells must be sealed gas-tight and can be operated in any position. The specific energy is in the order of 150 Wh / kg and the energy density in the order of 400 Wh / l, which means that lithium-ion batteries are of particular interest in the field of mobile applications as electrical energy storage devices and allow the construction of smaller and lighter batteries. The temperature - dependent self-discharge rate is in the range of almost 0% to 8% per month, the typical temperature range for use is approx. −30 ° C to +60 ° C.
Another essential feature of all lithium-ion accumulators is that the cells are chemically unable to cope with overcharging. When several cells are connected in series in order to achieve a higher electrical voltage, additional measures in the form of a battery management system (BMS) and balancer must usually be provided to compensate for the tolerances in the capacity between the cells .
Most types of lithium-ion accumulators are damaged by overtemperature, because some of the commonly used oxides such as cobalt (II) oxide and mixed oxides such as lithium-nickel-manganese-cobalt-oxides lead to a thermal effect from temperatures of approx. 180 ° C Run through comes. Oxides such as nickel dioxide , which allow the construction of lithium-ion accumulators with a comparatively high specific capacity, have a strong tendency towards thermal runaway and are therefore practically not used in commercial applications. During thermal runaway, the chemical decomposition of the oxide in the accumulator releases oxygen, which reacts chemically with the cell components such as the electrolyte and thus leads to a self-increasing exothermic reaction that can no longer be sustained from the outside and thermal destruction of the accumulator. This applies to all known cathode materials, in particular also to lithium iron phosphate , but these differ both in the so-called onset temperature at which the exothermic reaction is triggered and in the energy released in the process.
In contrast to the non-rechargeable lithium batteries and the group of lithium accumulators such as the lithium-air accumulator , which use metallic lithium in their construction, lithium-ion accumulators have no metallic lithium inside - lithium is in all lithium-ion accumulator types bound in the host lattice of a carrier material in the form of an intercalation. Depending on the type, around 80 g to 130 g of chemically pure lithium are required for the production of batteries with a storage capacity of one kilowatt hour . The lithium ions occurring in lithium-ion accumulators are monovalent , which leads to higher diffusion compared to multivalent ions.
Related to the lithium-ion accumulators in structure and process are the sodium-ion accumulators , which use the alkali metal sodium as an ion source and have a similar type variation, but basically have a lower energy density for physical reasons.
history
As early as the 1970s, the basic functional principle of reversible alkali metal ion intercalation in carbon electrodes and oxidic electrodes and their application in lithium batteries were researched and published at the Technical University of Munich ( Jürgen Otto Besenhard and others), even if at that time the practical applicability as Lithium battery electrodes was not recognized. In the 1970s, Stanley Whittingham found a promising cathode material for lithium batteries in the form of titanium disulfide, which could accommodate lithium ions in its atomic spaces. The anode was still made of metallic lithium and was too explosive for practical purposes; one cell delivered a little over 2 volts.
The lithium cobalt dioxide accumulator , also known as LiCoO 2 accumulator, was the first available electrode chemistry system for a lithium ion accumulator. Its usefulness as an electrode material was discovered in 1980 by a research group led by John B. Goodenough at the University of Oxford . The cathode consists of the eponymous substance lithium cobalt (III) oxide . Goodenough found the material after a systematic search, based on the fact that he considered a metal oxide with incorporated lithium ions to be more promising than the metal sulfide from Whittingham. It reached cell voltages of 4 volts. The successful industrial implementation was first achieved in 1985 in Japan by Akira Yoshino and colleagues at A&T Battery, a subsidiary of Toshiba and Asahi Kasei . It built on Goodenough's anode concept and used petroleum coke for the cathode instead of the reactive metallic lithium , which like the anode could absorb lithium ions. An advantage in addition to the relatively low weight was the durability, as they were not based on the chemical reactions that break up the electrode material, but on the flow of lithium ions between anode and cathode.
The first commercially available lithium-ion battery was taken as a lithium secondary battery Cobaltdioxid by Sony in 1991 to the market and in the Hi8 - Video camera CCD TR 1 used. The battery, made up of two cells connected in series, has a voltage of 7.2 V and a capacity of around 1200 mAh. To date (2016), batteries of this type are offered with capacities of up to 6900 mAh and are used in a variety of devices.
Whittingham, Goodenough and Yoshino received the Nobel Prize in Chemistry in 2019 for developing the lithium-ion battery .
Areas of application
Initially, lithium-ion batteries mainly supplied portable devices with a high energy requirement, for which conventional nickel-cadmium or nickel-metal hydride batteries were too heavy or too large, e.g. mobile phones , tablets , digital cameras , camcorders , notebooks , handheld consoles, airsoft weapons or flashlights . They can now be found in almost all areas. In electromobility, they serve as energy storage for pedelecs , electric cars , modern electric wheelchairs and hybrid vehicles . They also established themselves early on in RC model making . Due to their low weight, in connection with brushless DC motors and the corresponding controllers, they are well suited as drive units in model aircraft construction. Lithium-ion batteries have been used in power tools such as cordless screwdrivers and in gardening tools since 2003 . The Boeing 787 uses lithium cobalt oxide batteries (LiCoO 2 ), which, however, were given a steel casing after several fires. Other aircraft are equipped with lithium iron phosphate batteries. Lithium-ion battery systems are also used in battery storage power plants and solar batteries . A list of such battery storage systems can be found in the article List of Battery Storage Power Plants .
principle
In the charged lithium-ion battery, the electrical potential difference between the electrodes is used in an electrochemical process with a change in the substance of the electrodes to generate electricity. In the accumulator, lithium ions (Li + ) can migrate freely through the electrolyte between the two electrodes, from which the name of the accumulator is derived. In contrast to the lithium ions, the transition metal and graphite structures of the electrodes are stationary and protected from direct contact by a separator. The mobility of the lithium ions is necessary to balance the external current flow during charging and discharging so that the electrodes themselves remain (largely) electrically neutral.
The negative electrode is a graphite intercalation compound with the general composition Li x C n , with lithium being present as a cation . When discharging, the intercalation compound releases electrons that flow to the positive electrode via the external circuit. At the same time, the same number of Li + ions migrate from the intercalation compound through the electrolyte to the positive electrode. At the positive electrode, it is not the lithium ions that take up the electrons from the external circuit, but the structures of the transition metal compounds that are present there. Depending on the type of battery, these can be cobalt, nickel, manganese or iron ions that change their charge. When the battery is discharged, the lithium is still present in the positive electrode in ion form.
Since the affinity of the lithium ions for the material of the positive electrode is greater than their affinity for the negative (graphite) electrode, energy is released when the lithium ions flow from the negative to the positive electrode.
Electrons can move freely as electron gas within both electrodes and migrate to the external conductors or enter the electrode from the conductors, but cannot migrate between the electrodes within the accumulator. The partition is electron impermeable, which prevents a short circuit.
construction
The materials used include:
Negative electrode (when discharging: anode; when charging: cathode)
- Graphite and related carbons, where lithium intercalation occurs, are still the most important materials.
- nanocrystalline , amorphous silicon (alloying with lithium)
- Lithium titanates such as Li 4 Ti 5 O 12 (used with lithium titanate batteries )
- Tin dioxide (SnO 2 )
electrolyte
- Salts such as lithium hexafluorophosphate (LiPF 6 ), lithium tetrafluoroborate (LiBF 4 ) or lithium bis (oxalato) borate (LiBOB) dissolved in anhydrous aprotic solvents such as. B. ethylene carbonate , propylene carbonate , dimethyl carbonate , diethyl carbonate or 1,2-dimethoxyethane .
- Polymers made from polyvinylidene fluoride (PVDF) or polyvinylidene fluoride hexafluoropropene (PVDF-HFP) in a lithium polymer battery
- Lithium phosphate nitride (Li 3 PO 4 N)
- Lithium titanium thiophosphate (LiTi 2 (PS 4 ) 3 )
- LISICON (Li 2 + 2x Zn 1 − x GeO 4 )
Positive electrode (when discharging: cathode; when charging: anode)
- Lithium cobalt (III) oxide (LiCoO 2 , "LCO") and related layer compounds . In 2008, LiCoO 2 was used in around 90% of all lithium-ion batteries sold .
- Lithium-nickel-manganese-cobalt oxides such as. B. LiNi 0.33 Co 0.33 Mn 0.33 O 2 , the mixed oxides of the mentioned LiCoO 2 , of LiNiO 2 and LiMnO 2 . A manganese-free variant is the oxide LiNi 1 − x Co x O 2 .
- Lithium-Nickel-Cobalt-Aluminum-Oxide ("NCA", LiNi 1 − x − y Co x Al y O 2 ) z. B. LiNi 0.85 Co 0.1 Al 0.05 O 2
- Spinels like the lithium manganese oxide LiMn 2 O 4 ("LMO")
- Lithium iron phosphate (LiFePO 4 , "LFP") in the lithium iron phosphate accumulator
separator
- Polyolefin - membrane with and without nanokeramischer layer
- High purity alumina (HPA) separator
Current arrester
- Copper foil on the negative side. Aluminum is not used here because aluminum would react with lithium on the negative electrode.
- Aluminum foil on the positive side. The inexpensive and lightweight material is used here, which is protected from corrosion by passivation in suitable electrolytes .
Reaction equations
The following are examples of the chemical reaction equations that apply to the lithium-manganese battery for discharging and charging.
Negative electrode (discharging):
Positive electrode (discharging):
Redox equation:
Designs
Commercially available single cells of lithium-ion accumulators are usually designed in a cylindrical shape, as single cells (prismatic cells) assembled with a special housing or as pouch cells . The cylindrical design is marked with a five-digit number and enables easy replacement. The first two digits indicate the cell diameter in millimeters, the third and fourth digits the length of the cell in millimeters. The following table shows some common cell sizes with typical capacity values. The specific values for the capacity are rough guide values and depend on the specific cell type and manufacturer.
| Zellbe- drawing |
Typical capacity (in Ah) |
Dimensions (ø × l in mm) |
Note |
|---|---|---|---|
| 10180 | 0.3-0.4 | 10 × 18 | Design like 2 ⁄ 5 AAA cell |
| 10280 | 0.3-0.4 | 10 × 28 | Construction like 2 ⁄ 3 AAA cell |
| 10440 | 0.3-0.4 | 10 × 44 | Construction like AAA cell |
| 13450 | 0.5-0.7 | 13 × 45 | Use with e-cigarettes |
| 14250 | 0.25-0.3 | 14 × 25 | Design like 1 ⁄ 2 AA cell |
| 14430 | 0.6-0.7 | 14 × 43 | Design like 4 ⁄ 5 AA cell |
| 14500 | 0.7-0.8 | 14 × 53 | Construction like AA cell |
| 14650 | 0.9-1.6 | 14 × 65 | |
| 16340 | 0.6-1.0 | 16 × 34 | |
| 16500 | 0.8-1.2 | 16 × 50 | |
| 16650 | 2-3 | 16 × 65 | Narrow design of the 18650 |
| 17500 | 0.7-1.2 | 17.3 x 50 | Construction like A-cell |
| 17650 | 1.2-2.5 | 17 × 65 | |
| 18350 | 0.7-1.2 | 18 × 35 | |
| 18500 | 1.1-2.2 | 18.3 x 49.8 | |
| 18650 | 0.8-3.5 | 18.6 x 65.2 | Widespread design, u. a. in electric cars, e-cigarettes and flashlights |
| 21700 | 3-5 | 21 × 70 | Application in traction batteries of electric cars |
| 23430 | 3.3-5.2 | 23 × 43 | Like sub-C cell |
| 25500 | 3.7-5 | 24.3 x 49.2 | |
| 26500 | 2-4 | 26 × 50 | Construction like C-cell |
| 26650 | 3.3-5.2 | 26.5 x 65.4 | Widespread design, u. a. in electric cars |
| 32600 | 5.5-6 | 32 x 61.9 | Design like D-cell |
properties
Since lithium-ion accumulator is the generic term for a large number of possible combinations of materials for anode, cathode and separator, it is difficult to make general statements. Depending on the material combination, the properties sometimes differ significantly. Added to this is the continuous improvement by the battery manufacturers, who in recent years have been able to achieve considerable improvements, particularly in the known problem areas such as durability and safety, while the specific energy has only been increased to a comparatively small extent.
No memory effect
In lithium iron phosphate cells, an anomaly was discovered in the course of the discharge voltage curve , which the discoverers call the "memory effect". However, this effect cannot be compared with the well-known memory effect in NiCd and NiMH batteries and has no direct negative effects on the user.
lifespan
Lithium-ion batteries deteriorate both with use, with a full charge and discharge being referred to as a cycle, and without use simply over time (calendar life). In particular, the majority of the first-generation lithium-ion batteries installed in consumer devices only had a short lifespan. In some cases, users were able to notice considerable capacity losses after just one year; after two to three years, many a lithium ion battery had already become unusable. It turned out that the gradual loss of capacity depended less on the number of charge-discharge cycles and more on the storage conditions: the higher the temperature and the fuller the battery, the more likely it was to fail. As a rule, parasitic irreversible chemical reactions are cited as the reason for this.
With current lithium-ion batteries, the calendar service life is significantly longer, so that now the cycle life usually determines how long the battery can be used.
The cycle life depends on the type and quality of the battery, the temperature and the type of use of the battery, in particular the (discharge) charging stroke, end-of-charge voltage and the strength of the charging and discharging currents. The cycle life is drastically reduced at high temperatures, which is why the battery is best used at room temperature. Low temperatures during operation, but not during storage, are also harmful. Shallow charging and discharging improves the shelf life significantly, which means that a lithium-ion battery, of which only 50% of the maximum capacity is discharged instead of 100% and then recharged, can last more than double the number of cycles. The reason for this is that when the battery is fully discharged and fully charged, the electrodes are subjected to high loads. Ideally, with such shallowly cyclized batteries, both the end-of-charge voltage is reduced and the end-of-discharge voltage is increased. Strong charging and discharging currents also increase the mechanical and thermal loads and thus have a negative effect on the number of cycles.
Increasingly, however, better lithium-ion batteries with a longer shelf life are also being sold in the end consumer sector.
Apple stated in 2009 that the batteries installed in the new models of the MacBook Pro family can be recharged up to 1,000 times before they only reached 80% of their original capacity. This should correspond to a tripling of the service life compared to conventional batteries. If the application rules learned from the bad batteries of the first generations (mostly LiCoO 2 batteries) are observed - operation and storage at the lowest possible temperature; Storage only when partially charged; generally neither fully charged nor fully discharged - the number of cycles that can be achieved with the newer batteries could also be higher. A community portal on the battery life of laptop batteries gives an average number of cycles of 424 with a remaining capacity of 82% with a number of cases of 1,644, whereby cases of 60% loss after only 120 cycles are not uncommon.
Efficiency
- Coulomb efficiency
- The Coulomb efficiency or the Coulomb efficiency is typically approximately 100%, that is, almost the entire charge that has flowed into the accumulator can also be removed from it again. The Coulomb efficiency is only lower during the first few cycles, since some of the lithium ions react irreversibly with the electrolyte solution at the anode and cathode, forming cover layers.
- Storage capacity depending on the discharge current
- The storage capacity as a function of the discharge current can be described approximately by the Peukert equation . The higher the discharge current, the less electrical energy can be drawn from the battery. For lithium-ion batteries, the Peukert number is around 1.05.
- Energy efficiency
- As with every accumulator, there is a loss of energy due to the internal resistance during both charging and discharging. Typical overall efficiency levels of early lithium-cobalt dioxide accumulators (before 2006) were around 90%. If small charging and discharging currents are used in relation to the maximum current carrying capacity of the accumulator, more than 98% can be achieved.
Loading time
Like the service life, the charging time also depends on a number of factors, in the case of higher charging capacities primarily on the temperature. Short charging times have a negative effect on the electrode material, so that the service life and number of cycles are shortened.
| material | tension |
|---|---|
| LiCoO 2 | 3.6V |
| LiMnO 2 | 3.7-3.8V |
| LiFePO 4 | 3.3V |
| Li 2 FePO 4 F | 3.6V |
tension
A conventional LiCoO 2 battery delivers a nominal voltage of 3.6 volts , which is around three times as high as that of a nickel-metal hydride battery (NiMH battery). The end-of-charge voltage is up to 4.3 volts. The final discharge voltage is 2.5 volts; deep discharge leads to irreversible damage and loss of capacity. The cell voltage, however, depends on the cathode material used and is therefore slightly different from battery type to battery type.
Gravimetric power density
The power density is the power that an accumulator delivers in relation to its mass and is typically 300–1500 W / kg, but several thousand W / kg are also possible with newer accumulators.
Specific energy and energy density
The mass-related specific energy is more than twice as high as that of the nickel-cadmium accumulator, for example, and is 90–250 Wh / kg, the volume-related energy density is 200–500 Wh / l, depending on the materials used. Applications that require a particularly long service life, for example for use in electric cars, often only partially charge and discharge the lithium-ion battery (e.g. from 30 to 80% instead of from 0 to 100%), which is the number of possible charge-discharge cycles increased disproportionately, but the usable energy density reduced accordingly.
price
The cost of lithium-ion batteries is falling steadily. As of 2019, the costs have fallen by more than 80% within 8 years. Further cost reductions are expected due to both technical advances and an increase in production capacities.
The price development of lithium-ion batteries plays a major role in the switch to electromobility , as their costs account for a greater proportion of the sales price and follow-up costs of electric vehicles than the drive technology of combustion vehicles. Predictions about future developments and price reductions have not always been fulfilled in the past. In 2015, however, there was a significant price drop of 35% compared to the previous year. The price is usually given in US dollars per kilowatt hour. According to a manufacturer survey by Bloomberg , the price fell by 73%, from USD 1,000 in 2010 to USD 273 in 2016. It fell to around USD 300 in 2015, sometimes also USD 200. However, the price development of electric vehicles did not reflect the reduction in cell prices from 2015. A February 2017 study by the U.S. Department of Energy indicates that the cost of the traction battery - which includes encapsulation, housing, electronics, and temperature management - has a lag behind the price of the cells.
Practical use
Because of the large number of possible materials for the negative and positive electrodes and the separator, it is difficult to make general statements for lithium-ion batteries. The various types are optimized by the manufacturers for a wide variety of uses and sometimes differ greatly in how they are handled.
charge
The end-of-charge voltage is typically 4.0-4.2 V, sometimes 4.35 V, which enables slightly higher capacities, but at the expense of a reduced number of cycles. Since lithium-ion batteries do not have a memory effect and do not have to be formed, they are always charged in the same way: with most commercially available batteries, the high-quality charging process only activates the charging function when the cell voltage is at least 2.5 V. up to approx. 2.9 V only with a constant current of approx. 0.1 C , above that with a constant current of approx. 0.3 C (cell-friendly charging) up to max. 1 C charged to the end-of-charge voltage. Depending on the type, fast-charging cells can also tolerate 2 C, 4 C or even 8 C. The abbreviation C stands for the relative charging current based on the capacity (i.e. A / Ah) and should not be confused with the unit Coulomb (i.e. As) ; a charging current of 0.75 C means that a battery with a capacity of 1 Ah of 0.75 A is loaded. In general, it is possible to charge lithium-ion batteries with a lower charging current than the nominal current; this usually also increases the number of cycles that can be achieved.
If the accumulator reaches the end-of-charge voltage of z. B. 4.2 V, this voltage is maintained. The charging current then continues to decrease over time, the fuller the battery becomes. As soon as the current falls below a certain value (e.g. C / 10 or even only 3 percent of the initial current) or it no longer drops over a longer period of time, charging is ended. The end-of-charge voltage of 4.1 V to 4.3 V, depending on the product, may be exceeded with a small tolerance (e.g. 50 mV). The use of a slightly lower end-of-charge voltage, however, is not critical. A certain reduction in capacity is usually offset by a significant increase in the number of usable charge-discharge cycles.
discharge
The voltage of the lithium-ion battery initially drops quite quickly from the final charge voltage reached to the nominal voltage (approx. 3.6 to 3.7 V) during discharge, but then hardly drops further over a long period of time. The cell voltage only begins to drop sharply again shortly before complete discharge. The final discharge voltage is around 2.5 V depending on the cell type; this must not be exceeded, otherwise the cell will be destroyed by irreversible chemical processes. However, many electronic devices switch at significantly higher voltages, e.g. B. 3.0V.
We recommend charging (discharging) lithium-ion batteries “flat”, as this extends their service life. If a lithium-ion battery is always discharged from 100% charge level to 0% before it is charged again, it will only achieve the minimum number of cycles. It is better, depending on the type, e.g. B. to apply 70% depth of discharge. This means that the battery has 30% remaining capacity when it is recharged. Some manufacturers specify the cycle life depending on the degree of discharge .
As a general rule, high discharge currents both reduce the nominal capacity of a battery, as the higher voltage drop across the internal resistance means that the end-of-discharge voltage is reached earlier and also reduce the number of cycles due to the higher mechanical and thermal load. In earlier publications, an optimal discharge current of 0.2 C (that is, a discharge current of one fifth of the nominal value of the nominal capacity in Ah) is indicated. For a battery with a capacity of 5 Ah, this would be 1 A.
Storage / self-discharge
Lithium-ion batteries age faster with a higher state of charge and higher temperatures. It would be advantageous, but unrealistic in practice, to have an only slightly charged, coolly stored, regularly checked battery that would be charged before use and, if necessary, partially discharged again after use. With a lot of effort and at the crucial moment, the battery might be empty, you could extend the service life a little. However, it is important to check the charge level and recharge batteries that are rarely used every 18 to 24 months in order to prevent deep discharge.
Older sources state a self-discharge at 5 ° C of about 1 to 2% / month, at 20 ° C about 30% / month. Current data indicate a self-discharge of 3% / month even at room temperature. A battery should be recharged to 55 to 75% approximately every six months. Lithium-ion accumulators must not discharge below 2.7 V per cell, even during storage. Any liquid or gel electrolytes in the cell must not freeze, which corresponds to a minimum temperature of −25 ° C.
If local conditions permit, it is advantageous to store the lithium batteries outdoors for safety reasons. A fire protection concept should also be in place, as lithium batteries based on lithium cobalt (III) oxide can run out thermally. Resistant steel containers or fire protection cabinets can prevent a chain reaction by providing protection against sparks, flames, heat and projectiles (splinters from a battery explosion). In addition, the steel containers or fire protection cabinets should have a gas management system. In this way, gases that develop in the event of a battery fire can be safely removed from the container and an explosion of the container can be prevented.
Transportation / transportation
Every transport of a lithium-ion battery is a transport of dangerous goods, regardless of whether the battery is damaged or not. Regulations of the "European Agreement on the International Carriage of Dangerous Goods by Road" (ADR) regulate the transport of new and used lithium batteries.
Due to the high risk of fire in the event of a short circuit or exposure to water, special safety regulations apply to the transport of lithium accumulators / batteries:
- All lithium batteries have been classified as class 9 dangerous goods since January 1, 2009. In order to be able to ship them, the cell or battery manufacturer must first pass the UN transport test UN / DOT 38.3 ( English UN Transportation Testing ) can be carried out by an accredited test laboratory. There are eight tests in total. This test must be documented, from 2019 (mandatory from 2020) this will be done in a test report that is specified in terms of content / form.
- for road transport: the "European Agreement concerning the International Carriage of Dangerous Goods by Road" (ADR). The transitional provision according to 1.6.1.20 ADR applies.
- for sea transport: the IMDG code
- for air transport: the ICAO Technical Instructions (ICAO TI) or the IATA Dangerous Goods Regulations (IATA DGR)
- the law on the transport of dangerous goods (GGBefG, Germany)
- the "Hazardous Goods Ordinance on Road, Rail and Inland Shipping" (GGVSEB, Germany)
- Many parcel services, airlines and airports also have their own transport regulations for lithium-ion batteries, although smaller batteries (approx. <100 Wh) are usually unproblematic. For the DHL shipping of items that contain lithium-ion accumulators or batteries, z. B. a regulation for the transport of dangerous substances and objects .
Basically, the dangerous goods regulations differentiate between "small" and "large" lithium batteries. For lithium-ion batteries, small means a max. Nominal energy of 20 Wh per cell or 100 Wh per battery. For lithium metal batteries, cells with a lithium content of up to 1 g or batteries with a lithium content of up to 2 g are considered small. Small cells / batteries, whether transported individually, in or with devices (consumers), are subject to simplified conditions, while large cells / batteries are subject to more extensive transport conditions under dangerous goods law. The relief for small cells / batteries is largely the same in road, sea and air transport, although there are stricter requirements for air transport with regard to the amount of batteries permitted in the package. The basis is the special provision SV 188 ADR / IMDG code or the corresponding packing instructions PI 965 - PI 970, each Section II.
The shipping of packages with small cells / batteries is usually carried out with a clearly visible label, which indicates the content. If the button cells are built-in, this marking is not required. Parcels with a max. Contains 2 small lithium batteries used in devices, provided the shipment does not consist of more than 2 such packages.
The sticker contains information about / about it
- the presence of lithium-ion cells or batteries, recognizable by the listed UN number
- that there is a risk of ignition if damaged,
- a phone number where additional information can be obtained.
There are also detailed regulations for lithium batteries in the luggage of air travelers, for example individual lithium batteries without devices / consumers such as power banks or replacement batteries may not be transported in checked baggage, but must be carried in hand baggage. Airlines are obliged to inform their passengers of the relevant regulations on dangerous goods in their luggage through notices, questions during check-in or clear instructions during check-in on the Internet. The national aviation authorities such as the German LBA have also published the relevant provisions.
disposal
Proper disposal enables the batteries to be recycled and thus certain substances such as cobalt, manganese, nickel, zinc or copper to be recovered. Basically, the following applies to disposal in Germany: The manufacturers are obliged to take back old batteries free of charge (§ 5 BattG) and to treat them according to the state of the art and to recycle them (§ 14 BattG). In the Battery Act, notification and return obligations as well as recycling requirements are specified.
(Integrated) electronics
Lithium-ion batteries are very sensitive to incorrect treatment, which is why this type of battery was not used at first, although it had been known since the 1980s. Integrated circuits have become very inexpensive; This is why lithium-ion batteries can now be operated in conjunction with electronics (BMS = battery management system), which has significantly increased safety when handling this type of battery. In small and medium-sized battery packs, this electronics is usually integrated; it serves to protect against deep discharge, overcharging and thermal overload. A self-resetting fuse prevents overcurrent or short circuit. The processor control used is matched to the properties of the respective battery type. Battery packs in which several cells are connected in series to increase the voltage often also have electronics that use so-called " cell balancing " to adjust the charge status of all cells in a pack to one another. In particular, the charging must be ended or at least the charging current must be reduced when the first cell exceeds the maximum voltage, and the discharge when the first cell falls below the minimum voltage.
Overload
If you attempt to overcharge a battery with integrated monitoring electronics, the cell is separated from the external contacts until the excessive voltage is no longer present. After that, it can usually be used again without any problems. Not all batteries available on the market contain such monitoring electronics. If various lithium-ion batteries are overcharged, metallic lithium can be deposited on the anode and / or oxygen is released from the cathode. At best, the latter outgasses through a safety valve or reacts with the electrolyte or anode. This causes the accumulator to heat up and can even catch fire. Other lithium-ion batteries such as the LiFePO 4 battery are thermally stable, but are also irreversibly damaged if overcharged.
Deep discharge
If the cells are deeply discharged , an internal fuse or a BMS that may be present switches off the battery, usually only temporarily. There is then no longer any voltage at all at the external contacts of the battery pack, i.e. it cannot be discharged any further. Some chargers refuse to recharge such a defective accumulator because in this case no voltage can be measured at the external contacts. However, the battery is usually switched back to the contacts by its protective electronics as soon as an external voltage is applied. In such cases it can help to use a different charger.
In general, deep discharge usually leads to irreversible damage and a loss of capacity. If a cell has been discharged below 1.5 V, it should no longer be used, as there is a high probability that bridges have formed which will lead to a short circuit. The cell becomes unstable and becomes very hot. There may be a risk of fire.
Charger
Conventional lithium-ion batteries may only be charged with a special charging circuit . The electronics control the charge-dependent charging current and, in particular, monitor the exact end-of- charge voltage to be maintained . Even if there is an internal protective circuit, charging should only be carried out with suitable devices. Fast chargers should always be used under supervision and, if possible, away from flammable materials.
Operating and ambient temperature
Since the chemical processes (including the decomposition of the accumulator during aging) run more slowly in the cold and the viscosity of the electrolytes used in Li cells increases significantly, the internal resistance of the lithium-ion accumulator also increases when it is cold , which reduces the power that can be output . In addition, the electrolytes used may freeze at temperatures around −25 ° C. Some manufacturers specify the working range as 0–40 ° C. For many cells, however, 18-25 ° C is optimal. Below 10 ° C, the increased internal resistance of some species can reduce the performance so much that it is not long enough to operate a camcorder or digital camera . However, there are lithium-ion batteries with special electrolytes that can be used down to −54 ° C. Charging at low temperatures usually results in very severe aging , which is accompanied by an irreversible loss of capacity. For this reason, 0 ° C is specified as the lower permissible temperature for most batteries during the charging process. If the operating temperatures are too high, in many systems a layer forms on the anode due to the decomposition of the electrolyte, which greatly increases the internal cell resistance. Most manufacturers therefore limit the temperature during the discharge process to 60 ° C. Lithium-ion accumulators heat up during the discharge process, especially with high currents. In many cases, the maximum temperature depends linearly on the discharge rate.
Dangers when handling lithium-ion batteries
Mechanical stress
Mechanical damage such as objects penetrating the battery cell can lead to internal electrical short circuits . The high currents flowing lead to the heating of the accumulator. Housing made of plastic can melt and catch fire. Under certain circumstances, a mechanical defect cannot be immediately recognized from the outside. An internal short circuit can still occur a long time after the mechanical defect. External damage can also cause air and, in particular, humidity to penetrate into the cell and cause undesirable chemical reactions.
Chemical reaction

With a charged Li-Ion battery, overheating (also locally due to overload) or external damage can lead to thermal runaway , in which the energy stored in the battery is released in a very short time through direct chemical reaction in the form of heat. This can lead to a fire, primarily due to the organic electrolyte and its decomposition products.
This is not a classic metal fire, as the total amount of “metallic” (intercalated in graphite) lithium is not very large, even when charged, and due to the compact design it reacts internally with the metal oxide. Ordinary extinguishing agents (foam, carbon dioxide, especially water due to the cooling effect) are therefore effective and can be used safely. The possible fire hazard can lead to costly product recalls.
remedy
Ceramic, more temperature-resistant separators guarantee increased safety. Cell chemicals can also be used which are thermally more stable or whose decomposition does not take place exothermically. For example, reliable lithium iron phosphate batteries can be used instead of inexpensive lithium cobalt dioxide batteries : In addition to being more expensive , they also have a lower energy density and do not allow such compact designs as lithium cobalt dioxide batteries.
Further protective measures integrated directly into the cell concern the electrical connection between the electrode material and the external cell connection. The connection can be designed so that it acts like a fuse and is also torn off when any burst openings are opened. However, these internal cell protection mechanisms are usually irreversible. In addition to the cell-internal protective devices, there are usually other electronic protective circuits in modern batteries. Their functions range from complex battery management systems (BMS) with temperature sensors, charging electronics, battery status monitoring and external communication connections (smart batteries) to simple, mostly reversible safety circuits that are only intended to prevent overcharging or overloading of the battery.
In ergonomic studies it was found that the handling of high-performance lithium-ion batteries, such as their manufacture, installation, storage, disposal and certain operating conditions, has only minor effects on occupational safety. The dangers associated with working with higher electrical voltages and the handling of hazardous substances from the cell chemistry used can be minimized by adapting and consistently implementing existing safety requirements.
Examples of incidents
In automotive construction, particularly high safety requirements due to the high amounts of energy installed sometimes lead to delays in use. Opel postponed the delivery of the Ampera when, three weeks after a crash test of an identical Chevrolet Volt, the battery, which had not been removed, overheated and led to a vehicle fire. As a result, the safety concept of the traction battery was revised.
Dealing with burning electric cars poses new challenges for breakdown services and fire brigades. B. much more water is required for extinguishing. In addition, a special refrigerated container is required for removal.
CO 2 balance
Carbon dioxide is produced during the manufacture of the accumulators . While earlier studies came to the conclusion that around 70 kg of carbon dioxide are released per installed kilowatt hour of battery energy storage capacity, an overview study published in 2017 on the current state of research came to an average of around 110 kg per installed kWh. A review published in 2019 put the CO 2 emissions in the manufacture of the most frequently used NMC type at around 61 to 106 kg CO 2 equivalents .
Depending on various factors such as B. the electricity mix for battery production is between 38 and 356 kg CO 2 -eq / kWh. There are various ways of reducing these CO 2 emissions; for example, by reducing the overall energy requirement or using recycling materials. The use of electricity from renewable energies in battery production is seen as probably the most promising measure for this.
recycling
The lithium in the batteries is currently (2019) not being recycled because it is not profitable. That could change as the number of battery-operated devices increases. The valuable materials cobalt, nickel, copper and aluminum are particularly attractive for process economy and process ecology. The recycling should be implemented in the medium to long term to avoid bottlenecks.
In order to recycle lithium-ion accumulators and primary lithium-ion batteries, various basic operations are combined into complex process chains:
- Deactivate / discharge (especially for traction batteries)
- Dismantling the battery systems (especially for traction batteries)
- mechanical processes (shredding, sorting, sieving, etc.)
- hydrometallurgical processes
- pyrometallurgical processes
The world's first commercial recycling plant (PosLX) was commissioned by POSCO in Gwangyang, South Korea , in 2017 . In this plant, lithium phosphate from old lithium-ion batteries is converted into lithium carbonate , a preliminary product for lithium, using the process patented by POSCO . The new factory has an annual production capacity of 2,500 tons of lithium carbonate. Europe's largest recycling plant is operated by Umicore in Antwerp . In Germany, around 20,000 tonnes can be recycled in 2020, but this is not sufficient with the increasing number of electric vehicles.
So far, all processes for recycling are not efficient in terms of energy consumption .
Embodiments
Cell chemistry
Materials on the positive side
| designation | (+) - electrode materials | Abbreviations | Cell tension | typical operating range | Charging (end-of-charge voltage) | Discharge (cut-off voltage) | Specific energy | Charging cycles |
|---|---|---|---|---|---|---|---|---|
| Lithium cobalt dioxide accumulator | LiCoO 2 | ICR, LCO | 3.6V | 3.0-4.2V | 0.7-1C (4.2V) | ≤ 1C (2.5 V) | 150-200 Wh / kg | 500-1000 |
| Lithium manganese accumulator | LiMnO 2 / LiMn 2 O 4 | IMR, LMO, LMS | 3.7-3.8V | 3.0-4.2V | 0.7-1C (4.2V) | 1C, some cells 10C (2.5 V) | 100-150 Wh / kg | 300-700 |
| Lithium-nickel-manganese-cobalt accumulator | LiNi x Mn y Co z O 2 | INR, NMC, NCM | 3.6-3.7V | 3.0-4.2V | 0.7-1C (4.2V) | 1C, some cells 2C (2.5 V) | 150-220 Wh / kg | 1000-2000 |
| Lithium-nickel-cobalt-aluminum accumulator | LiNi x Co y Al z O 2 | NCA | 3.6V | 3.0-4.2V | 0.7C (4.2V) | 1C (3.0 V) | 200-260 Wh / kg | 500 |
| Lithium iron phosphate accumulator | LiFePO 4 | IFR, LFP | 3.2-3.3V | 2.5-3.65V | 1C (3.65 V) | 1C, some cells 25C (2.5 V) | 90-120 Wh / kg | 2000 and more |
Materials on the negative side
| designation | (-) - electrode material | Abbreviations | Cell tension | typical operating range | Charging (end-of-charge voltage) | Discharge (cut-off voltage) | Specific energy | Charging cycles |
|---|---|---|---|---|---|---|---|---|
| Lithium graphite battery (standard lithium ion cell) | C. | C. | 3.2V-4.0V | 3.4-3.8V | 1C | up to 10C | ||
| Lithium titanate accumulator | Li 4 Ti 5 O 12 | LTO | 2.4V | 1.8-2.85V | 1C (2.85 V) | up to 10C (2.5 V) | 50-80 Wh / kg | 3000-7000 |
Electrolytes
All of the mentioned electrode materials can be combined with a polymer electrolyte, so that a lithium polymer accumulator is created. Liquid electrolytes in a porous separator are common.
Lithium cobalt dioxide accumulator
The positive electrode of the lithium cobalt dioxide accumulator consists of the eponymous lithium cobalt (III) oxide (LiCoO 2 ). Almost all commercially available mobile electronics use lithium cobalt dioxide accumulators.
Lithium-nickel-manganese-cobalt accumulator
Lithium-Nickel-Manganese-Cobalt Oxide , NMC for short, or NCM for short, have become the most important material used in traction batteries . Most electric cars, including those from Daimler or BMW , but apart from products from Tesla and some Chinese manufacturers, use NMC batteries as of 2019.
Lithium-nickel-cobalt-aluminum accumulator
The lithium-nickel-cobalt-aluminum battery is a version of the lithium-ion battery with lithium-nickel-cobalt-aluminum oxide (LiNi x Co y Al z O 2 , NCA) as the cathode material. This battery is characterized by its high energy density (240–270 Wh / kg for cyl. Cells in 18650 format) and a long service life. NCA cells are mainly produced as traction batteries by Panasonic and Tesla .
Lithium manganese accumulator
When lithium manganese battery is lithium manganese oxide used as an active material in the positive electrode. The negative electrode, which is the anode when the battery is discharged, consists either of conventional graphite (high-energy cells) or of an amorphous carbon structure ( amorphous carbon , in high-performance cells). The larger anode surface results in improved resistance to high currents. As of 2012, the cells are used in pedelecs and e-bikes from various manufacturers (including the Swiss pedelec manufacturer Flyer ), as well as in hybrid electric vehicles (e.g. Nissan Fuga Hybrid, Infinity Mh ) and electric cars (e.g. Nissan Leaf ) . AESC, for example, produces large-format cells for traction batteries for Nissan .
Lithium iron phosphate accumulator
The lithium iron phosphate battery (LiFePO 4 battery) is a version of the lithium-ion battery in which the conventional lithium cobalt oxide cathode has been replaced by a lithium iron phosphate cathode. This battery is characterized by high charging and discharging currents, very good temperature stability and a long service life. The nominal voltage is 3.2 V or 3.3 V, the energy density is 100–120 Wh / kg
Further developments to improve the technical properties are doping with yttrium (LiFeYPO 4 ) and sulfur atoms .
Lithium titanate accumulator
The lithium titanate battery is a sub-category of the lithium-ion battery in which the conventional graphite electrode (negative pole) is replaced by a sintered electrode made of lithium titanium spinel (Li 4 Ti 5 O 12 ). The much stronger chemical bond of lithium in the titanate prevents the formation of a surface layer, which is one of the main reasons for the rapid aging of many conventional Li-ion batteries. This increases the number of possible charging cycles. Since the titanate can no longer react with oxides from the cathode, the thermal runaway of the battery is also prevented, even in the event of mechanical damage. In addition, thanks to the lithium titanate anode, the battery can also be operated at low temperatures in a temperature range of −40 to +55 ° C, in contrast to conventional lithium-ion batteries.
Other types of accumulators
With the dual carbon accumulator, both electrodes, both the cathode and the anode, are made of porous graphite. This type of accumulator is in the research stage and as of 2019 has no economic significance. It does not belong to the lithium ion cells in the narrower sense, because Li + ions are not stored on the positive pole side as usual when discharging .
Special construction methods
Lithium polymer accumulator
The lithium-polymer accumulator does not represent an independent cell chemistry, although the majority of all lithium-polymer accumulators on the market are of the lithium-cobalt dioxide accumulator type and are often equated with colloquially. The essential property of the polymer accumulator is the type of design of the normally liquid electrolyte, which is present as a solid to gel-like film on a polymer basis and thus allows various designs such as flat cells in the mechanical structure of the cell. There are practically no restrictions on the external shape of lithium polymer batteries.
Different material combinations
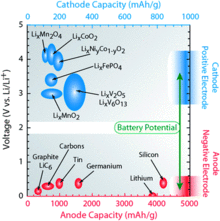
There are numerous material combinations available for storing lithium ions. The chemical storage materials change the properties of the accumulator decisively, so that they can be used to adjust to special requirements. The illustration shows a comparison of numerous cathode and anode materials and shows the potential difference between the materials.
Through the additional use of different special separators (e.g. ceramic separators ), electrolytes (e.g. ionic liquids ) and packaging materials, further properties of the accumulator can be set so that they can also meet extreme requirements.
The following are special requirements for lithium-ion batteries:
- High and low temperature resistance
- Radiation tolerance (e.g. gamma radiation in the aerospace industry)
- High and low pressure resistance (up to rough vacuum )
- Special form factors for foil bodies or connection poles
- Shock resistance
- Amagnetism
- Maximizing energy density or power density
- Fast charge capability
- Intrinsic safety
- Bending flexibility
Although these possibilities exist, industrial mass production relies on the use of established storage materials, such as e.g. B. lithium cobalt (III) oxide and graphite. Only a few specialist manufacturers, such as B. the German company Custom Cells Itzehoe GmbH and the American company Yardney Technical Products Inc., offer special solutions. The figure on the right shows a battery developed by the Fraunhofer Institute for Silicon Technology (ISIT), which has been optimized in terms of its energy density, pressure resistance and unusual shape (hexagonal) for use in an autonomous underwater vehicle (AUV) .
literature
- Lucien F. Trueb, Paul Rüetschi: Batteries and accumulators. Mobile energy sources for today and tomorrow . Springer, Berlin 1998, ISBN 3-540-62997-1 .
- Jeff Dahn, Grant M. Ehrlich: Lithium-Ion Batteries. In: Thomas B. Reddy (Ed.): Linden's Handbook of Batteries. 4th edition. McGraw-Hill, New York 2011, ISBN 978-0-07-162421-3 , chapter 26
- Claus Daniel, Jürgen O. Besenhard: Handbook of Battery Materials. Wiley-VCH, Weinheim 2011, ISBN 3-527-32695-2 .
- Masaki Yoshio, Ralph J. Brodd, Akiya Kozawa (Eds.): Lithium-Ion Batteries. Science and Technologies. Springer, New York 2009, ISBN 978-0-387-34444-7 .
- Reiner Korthauer (Ed.): Handbook lithium-ion batteries. Heidelberg: Springer Verlag, 2013, ISBN 978-3-642-30653-2 .
- Peter Kurzweil, Otto K. Dietlmeier: Electrochemical storage. Springer Vieweg, Wiesbaden 2015, ISBN 978-3-658-10900-4 .
- P. Birke, M. Schiemann: Accumulators: past, present and future of electrochemical energy storage. H. Utz Verlag, Munich 2013, ISBN 978-3-8316-0958-1 , Chapter 3.1 .: Lithium-ion accumulators. Pp. 192-235.
- Dell, Rand: Understanding Batteries. Royal Society of Chemistry, 2001, ISBN 0-85404-605-4 , pp. 147-153, Chapter 10.4: The Lithium-ion Battery.
Web links
- Compendium: Li ‐ ion batteries (July 2015, PDF; 1.2 MB) BMWi ICT funding program for electric mobility II
- Script about various types of lithium batteries (as of Feb. 2005, PDF; 3.4 MB) Graz University of Technology, Institute for Chemical Technology of Inorganic Materials
- Battery forum Germany, information portal with links to the general battery compendium and lexicon
- Compendium: Lithium-ion batteries from the German Battery Forum
- Comprehensive information on lithium-ion batteries in notebooks
- Thomas Kretschmann: Ten times better batteries in prospect. In: Tom's Hardware. December 21, 2007
- Matthias Gräbner: Wiry silicon. In: Telepolis. December 22, 2007
- IATA Lithium Battery Guidance (IATA regulations for the transport of lithium batteries by air)
- Investigation of the fire behavior of lithium-ion and lithium-metal batteries in various applications and derivation of tactical recommendations , research report by the Research Center for Fire Protection Technology, Karlsruhe Institute of Technology - KIT (PDF)
Individual evidence
- ↑ Grant M. Ehrlich: Handbook Of Batteries . Ed .: David Linden, Thomas B. Reddy. 3. Edition. McGraw-Hill, 2002, ISBN 0-07-135978-8 , Chapter 35: Lithium-Ion Batteries, pp. 35.1-35.94 .
- ↑ Grant M. Ehrlich: Handbook Of Batteries . Ed .: David Linden, Thomas B. Reddy. 3. Edition. McGraw-Hill, 2002, ISBN 0-07-135978-8 , Chapter 35: Lithium-Ion Batteries, pp. 35.2 .
- ↑ Grant M. Ehrlich: Handbook Of Batteries . Ed .: David Linden, Thomas B. Reddy. 3. Edition. McGraw-Hill, 2002, ISBN 0-07-135978-8 , Chapter 35: Lithium-Ion Batteries, pp. 35.3 .
- ↑ Grant M. Ehrlich: Handbook Of Batteries . Ed .: David Linden, Thomas B. Reddy. 3. Edition. McGraw-Hill, 2002, ISBN 0-07-135978-8 , Chapter 35: Lithium-Ion Batteries, pp. 35.8-35.9 .
- ↑ Peter J. Bugryniec, Jonathan N. Davidson, Solomon F. Brown: Assessment of thermal runaway in commercial lithium iron phosphate cells due to overheating in an oven test . In: Energy Procedia . tape 151 , October 2018, ISSN 1876-6102 , p. 74–78 , doi : 10.1016 / j.egypro.2018.09.030 .
- ↑ Thomas B. Reddy, Sohrab Hossain: Handbook Of Batteries . Ed .: David Linden, Thomas B. Reddy. 3. Edition. McGraw-Hill, 2002, ISBN 0-07-135978-8 , Chapter 34: Rechargeable Lithium Batteries (Ambient Temperature), pp. 34.1-34.4 .
- ↑ Naoki Nitta, Feixiang Wu, Jung Tae Lee, Gleb Yushin: Li-ion battery materials: present and future . In: Materials Today . tape 18 , no. 5 , June 1, 2015, p. 252–264 , doi : 10.1016 / j.mattod.2014.10.040 ( sciencedirect.com [accessed March 25, 2017]).
- ↑ Jang-Yeon Hwang, Seung-Taek Myung, Yang-Kook Sun: Sodium-ion batteries: present and future . In: Chemical Society Reviews . tape 46 , no. June 12 , 2017, p. 3529 - 3614 , doi : 10.1039 / C6CS00776G .
- ^ JO Besenhard, HP Fritz: Cathodic reduction of graphite in organic solutions of alkali and NR 4 + salts . In: Journal of Electroanalytical Chemistry and Interfacial Electrochemistry . tape 53 , no. 2 , June 25, 1974, p. 329-333 , doi : 10.1016 / S0022-0728 (74) 80146-4 .
- ^ JO Besenhard: The Electrochemical Preparation and Properties of Ionic Alkali Metal and NR 4 Graphite Intercalation Compounds in Organic Electrolytes . In: Carbon . tape 14 , no. 2 , 1976, p. 111-115 , doi : 10.1016 / 0008-6223 (76) 90119-6 .
- ^ R. Schöllhorn, R. Kuhlmann, JO Besenhard: Topotactic redox reactions and ion exchange of layered MoO 3 bronzes . In: Materials Research Bulletin . tape 11 , no. 1 , January 1976, p. 83-90 , doi : 10.1016 / 0025-5408 (76) 90218-X .
- ^ JO Besenhard, R. Schöllhorn: The discharge reaction mechanism of the MoO 3 electrode in organic electrolytes . In: Journal of Power Sources . tape 1 , no. 3 , 1976, p. 267-276 , doi : 10.1016 / 0378-7753 (76) 81004-X .
- ^ JO Besenhard, G. Eichinger: High energy density lithium cells: Part I. Electrolytes and anodes . In: Journal of Electroanalytical Chemistry and Interfacial Electrochemistry . tape 68 , no. 1 , February 25, 1976, p. 1-18 , doi : 10.1016 / S0022-0728 (76) 80298-7 .
- ^ G. Eichinger, JO Besenhard: High energy density lithium cells: Part II. Cathodes and complete cells . In: Journal of Electroanalytical Chemistry and Interfacial Electrochemistry . tape 72 , no. 1 , August 25, 1976, p. 1-31 , doi : 10.1016 / S0022-0728 (76) 80072-1 .
- ↑ a b c Recognition for the Nobel Prize in Chemistry 2019 . nobelprize.org
- ↑ K. Mizushima, PC Jones, PJ Wiseman, JB Goodenough: Li x CoO 2 (0 <x <l): A New Cathode Material For Batteries Of High Energy Density . In: Materials Research Bulletin . tape 15 , 1980, pp. 783-789 , doi : 10.1016 / 0025-5408 (80) 90012-4 .
- ↑ Battery for Sony CCD Tr1. In: akku-gebiet.de. Retrieved March 4, 2012 .
- ↑ Memory effect with lithium-ion batteries. heise online , April 15, 2013, accessed on January 17, 2017.
- ↑ M. Wohlfahrt-Mehrens, C. Vogler, J. Garche: Aging mechanisms of lithium cathode materials. In: Journal of Power Sources. 127, No. 1-2, 2004, pp. 58-64, doi: 10.1016 / j.jpowsour.2003.09.034 .
- ↑ Sébastien Patoux, Lucas Sannier, Hélène Lignier, Yvan Reynier, Carole Bourbon, Séverine Jouanneau, Frédéric Le Cras, Sébastien Martinet ,: High voltage nickel manganese spinel oxides for Li-ion batteries . In: Electrochimica Acta . tape 53 , no. May 12 , 2008, pp. 4137-4145 , doi : 10.1016 / j.electacta.2007.12.054 .
- ↑ Gaurav Sharma, Yi Jin, YS Lin: Lithium Ion Batteries with Alumina Separator for Improved Safety . In: Journal of The Electrochemical Society . tape 164 , no. 6 , January 1, 2017, ISSN 0013-4651 , p. A1184 – A1191 , doi : 10.1149 / 2.1091706jes ( ecsdl.org [accessed June 18, 2018]).
- ↑ thunderheartreviews.com
- ↑ Isidor Buchmann: Is lithium-ion the ideal battery? (No longer available online.) In: Platform for the promotion and dissemination of electric vehicles. Archived from the original on November 22, 2015 ; Retrieved December 17, 2011 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Tsuyoshi Sasaki, Yoshio Ukyo, Petr Novák: Memory effect in a lithium-ion battery . In: nature materials . April 14, 2013, doi : 10.1038 / NMAT3623 .
- ↑ Isidor Buchmann: Will lithium-ion batteries power the new millennium? ( Memento of October 17, 2015 in the Internet Archive ) September 2008.
- ^ Richard L. Hartmann II: An aging model for lithium-ion cells. Pp. 75–79 ( PDF file; 3.4 MB ).
- ↑ https://www.apple.com/de/newsroom/2009/06/08Apple-Updates-MacBook-Pro-Family-with-New-Models-Innovative-Built-in-Battery-for-Up-to-40 -Percent-Longer-Battery-Life / In: apple.com . June 8, 2009, accessed July 31, 2017.
- ↑ https://macbookbatterydatabase.com/ , accessed August 1, 2017.
- ↑ a b Life-Cycle Testing of Mars Surveyor Program Lander Lithium Ion Battery Achieved Over 10,000 Low-Earth-Orbit Cycles. ( Memento from September 9, 2013 in the Internet Archive ) In: NASA.gov , October 16, 2006.
- ↑ PDF at www.et-inf.fho-emden.de ( Memento of the original from April 12, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b c d Charging lithium-ion batteries. In: BORDER RADIO CLUB newsletter. March 2006.
- ^ E-One Moli Energy: Announcement of a new cell ( Memento of March 11, 2007 in the Internet Archive ); Lithium-ion battery: ITWissen.info (September 4, 2016)
- ↑ Kurzweil, Dietlmeier: Elektrochemische Speicher p. 223 Tab. 318.
- ↑ Nancy Haegel et al: terawatt-scale photovoltaics: Transform global energy . In: Science . tape 364 , no. 6443 , 2019, p. 836-838 , doi : 10.1126 / science.aaw1845 .
- ↑ faz.net: The Battle for the Battery , February 20, 2015, accessed on August 1, 2017.
- ↑ Will electric cars soon be cheaper than petrol? In: wiwo.de. June 1, 2015, accessed August 1, 2017 .
- ^ Clair Curry: Lithium-ion Battery Costs and Market. (PDF) BNEF, July 5, 2017, accessed on May 7, 2018 (English).
- ^ Battery Cost Plunge Seen Changing Automakers Most in 100 Years. In: Bloomberg.com. October 11, 2016, accessed August 1, 2017 .
- ↑ Are falling battery prices lowering the price of electric cars? In: Energyload.eu. July 25, 2016, accessed August 1, 2017 .
- ^ Cost and Price Metrics for Automotive Lithium-Ion Batteries. (PDF) In: energy.gov. February 2017, accessed August 1, 2017 .
- ↑ LiIon: many cells have an end-of-charge voltage of 4.35 V! ALC update planned? , elv.de, 2013.
- ↑ Product description SCiB from Toshiba ( Memento of the original from August 27, 2010 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Batteries: short-lived ex works. ( Memento of the original from May 24, 2009 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. In: TecChannel.de. P. 4 of 15
- ↑ Lithium-ion battery. In: qsl.net.
- ↑ a b Winston Battery: WB-LYP100AHA. Data sheet LiFePO 4 cell 3.2 V 100 Ah, added February 11, 2012.
- ↑ Bernhard Haluschak: Batteries: Short-lived ex works. ( Memento of the original from June 24, 2008 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. In: Tecchannel. September 23, 2005.
- ↑ M. Frehner, 2007: Everything about batteries, section: Self-discharge. In: funkcom.ch , accessed on February 15, 2012.
- ↑ Increased fire risk in hospitals: storage problems with lithium-ion batteries. Accessed June 27, 2019 (German).
- ↑ UN transport regulation for lithium batteries. Retrieved March 8, 2017 .
- ↑ Lithium Battery Testing Under UN / DOT 38.3. (PDF) TÜV Süd America, accessed on March 11, 2017 (English).
- ↑ Regulations for the transport of dangerous substances and objects. ( Memento of the original from May 9, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. DHL Vertriebs GmbH & Co OHG Market Communication (PDF; 132 kB)
- ^ SV 188. Retrieved on February 10, 2019 .
- ↑ ADR 2019 - subsection 5.2.1.9
- ^ Federal Aviation Office - Passenger Luggage. Retrieved February 10, 2019 .
- ↑ Increased fire risk in hospitals: storage problems with lithium-ion batteries. Accessed June 27, 2019 (German).
- ↑ BattG - Law on the placing on the market, taking back and environmentally friendly disposal of batteries and accumulators. Retrieved June 27, 2019 .
- ↑ Isidor Buchmann: When was the battery invented? ( Memento from January 22, 2012 in the Internet Archive ) In: Batteries-Montage-Zentrum GmbH.
- ↑ Battery Management Systems (BMS). In: Electropaedia.
- ↑ Multi-Cell Li-ion Battery Pack OCP / Analog Front End at intersil ( Memento of the original from April 1, 2010 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Thomas Waldmann, Marcel Wilka, Michael Kasper, Meike Fleischhammer, Margret Wohlfahrt-Mehrens: Temperature dependent aging mechanisms in Lithium-ion batteries - A Post-Mortem study. In: Journal of Power Sources. Volume 262, 2014, pp. 129–135, doi: 10.1016 / j.jpowsour.2014.03.112 .
- ↑ Thomas Waldmann, Margret Wohlfahrt-Mehrens: In-Operando Measurement of Temperature Gradients in Cylindrical Lithium-Ion Cells during High-Current Discharge. In: ECS Electrochemistry Letters. Volume 4, 2015, pp. A1-A3, doi: 10.1149 / 2.0031501eel .
- ↑ Thomas Waldmann, Gunther Bisle, Björn-Ingo Hogg, Stefan Stumpp, Michael A. Danzer, Michael Kasper, Peter Axmann, and Margret Wohlfahrt-Mehrens: Influence of Cell Design on Temperatures and Temperature Gradients in Lithium-Ion Cells: An In Operando Study. In: Journal of The Electrochemical Society. Volume 162, 2015, pp. A921-A927, doi: 10.1149 / 2.0561506jes .
- ↑ DGUV: FAQ list of the working group “Framework for Electromobility”. (PDF) August 1, 2016, accessed on March 14, 2019 .
- ↑ Gigaset recalls phone batteries due to overheating. In: heise online . December 15, 2011, accessed January 17, 2017 .
- ↑ Electromobility - assessment of changes relevant to ergonomics In: baua.de , Federal Institute for Occupational Safety and Health , 2012, accessed on March 28, 2012.
- ↑ Opel does not sell an Ampera for the time being. In: heise.de
- ↑ Opel parent company gets the risk of flames under control. In: Spiegel Online . January 6, 2012, accessed February 13, 2012 .
- ↑ Electric car at the gas station in Ratingen burns out completely. In: nrz.de . September 19, 2019, accessed October 21, 2019 .
- ↑ Jörn Kerckhoff: Mobility Turnaround: When Electric Cars Burn. In: moz.de . September 25, 2019, accessed October 21, 2019 .
- ↑ Breakdown services have to upgrade because of electric cars. In: 20min.ch . October 18, 2019, accessed October 19, 2019 .
- ↑ Electric car caught fire on the A4 - the route between Goldau and Küssnacht was blocked. In: luzernerzeitung.ch . October 21, 2019, accessed October 21, 2019 .
- ↑ M. Armand, J.-M. Tarascon: Building better batteries . In: Nature . tape 451 , 2008, p. 652-657 , doi : 10.1038 / 451652a .
- ↑ Boucar Diouf, Ramchandra Pode: Potential of lithium-ion batteries in renewable energy . In: Renewable Energy . tape 76 , 2015, p. 375-380 , doi : 10.1016 / j.renene.2014.11.058 .
- ↑ D. Larcher, JM. Tarascon: Towards greener and more sustainable batteries for electrical energy storage . In: Nature Chemistry . tape 7 , 2015, p. 19-29 , doi : 10.1038 / NCHEM.2085 .
- ^ Jens F. Peters et al .: The environmental impact of Li-Ion batteries and the role of key parameters - A review . In: Renewable and Sustainable Energy Reviews . tape 67 , p. 491–506 , doi : 10.1016 / j.rser.2016.08.039 .
- ↑ Erik Emilsson, Lisbeth Dahllöf: Lithium-Ion Battery Vehicle Production . IVL. Retrieved December 2, 2019.
- ↑ Linda Ager-Wick Ellingsen: Identifying key assumptions and differences in life cycle assessment studies of lithium-ion traction batteries with focus on greenhouse gas emissions . In: Transportation Research Part D: Transport and Environment . tape 55 , 2017, p. 82–90 , doi : 10.1016 / j.trd.2017.06.028 .
- ↑ SZ of March 13, 2019: Why lithium from batteries still ends up in the trash
- ↑ a b c Christian Hanisch, Jan Diekmann, Alexander Stieger, Wolfgang Haselrieder & Arno Kwade: Handbook of Clean Energy Systems - Recycling of Lithium-Ion Batteries . Ed .: Jinyue Yan, Luisa F. Cabeza, Ramteen Sioshansi. 5 Energy Storage edition. John Wiley & Sons, Ltd, 2015, ISBN 978-1-118-99197-8 , chap. 27 , p. 2865-2888 , doi : 10.1002 / 9781118991978.hces221 .
- ↑ Jung Min-hee: Urban Lithium Mine POSCO Starts Commercial Lithium Production for First Time in the World. Business Korea, February 8, 2017, accessed February 10, 2017 .
- ^ Moon Ji-woong: Posco begins commercial lithium carbonate production in Korea. Pulse News, February 7, 2017, accessed February 10, 2017 .
- ↑ a b Hellmuth Nordwig: The laborious recycling of lithium-ion batteries. In: Current research (broadcast on DLF ). January 23, 2019, accessed October 10, 2019 .
- ↑ Spektrum.de of June 9, 2020, The Old Burden of Electromobility , accessed on June 13, 2020.
- ↑ Lithium-ion batteries Battery Forum Germany (Competence Network Lithium-Ion Batteries e.V.)
- ↑ Flyer: Product overview , accessed May 10, 2012.
- ↑ Flyer (NL): Section: Actieradius (reference to the use of lithium-manganese technology) , accessed May 10, 2012.
- ↑ More range for electric cars. Schott AG
- ↑ tietEK: technology concept for inspectation and exploration of the deep sea , Fraunhofer IOSB
- ↑ Altana and CCI cooperate in the development of energy storage systems. In: chemanager-online.com , January 23, 2014.