Prickly palms
| Prickly palms | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Aiphanes horrida |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Aiphanes | ||||||||||||
| Willd. |
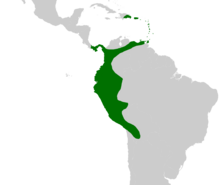
The thorn palms ( Aiphanes ), also stiletto palms , are a genus of 25 species of the palm family (Arecaceae), which are distributed in the Lesser Antilles and from Venezuela to Bolivia along the Andes. Except for one species that still reaches Panama, the holly palms are missing in Central America. They are particularly noticeable because of their spines that are up to 25 centimeters long and almost completely cover the plants.
description
Prickly palms are perennial , woody plants. Due to different types of branching, the palms can have different appearance ( habitus ). For example, there are solitary single trees of Aiphanes erinaceae , but also shrub-like growth forms with over 20 trunks that stand on open plains.
Characteristic are the approximately one millimeter to over 25 centimeter long spines that cover almost the entire plant. The trunk, leaves and flower stalks and bracts of the inflorescences are reinforced. The spines are gray to black, but yellow in Aiphanes erinacea , Aiphanes simplex and Aiphanes tricuspidata . The spines grow out of a group of round, thick-walled cells that initially form a thickening (pulvinus). Small spines are single cells with a strongly thickened (sclerchymatized) cell wall. Large spines consist of external sclerenchymal cells and internal parenchyma .
The number of chromosomes is controversial, in the literature contradicting information can be found, such as for Aiphanes minima n = 15 or n = 18, and for Aiphanes horrida n = 16 or n = 15. Probably the differences within a species are, however around artifacts. Probably, but not verified, n = 15 for the whole genus. For the closely related Acrocomia , Gastrococcus and Astrocaryum , n = 15 applies in each case.
root
Almost all species form adventitious roots above ground , which sometimes give the lower part of the trunk a cone shape. At the same time, they are also support roots and thus compensate for the lack of secondary growth in thickness of the stem axis. The adventitious roots are grayish to reddish brown and often branched. They reach a diameter between five and 15 millimeters. They are covered with whitish, wart-like lenticels that arise endogenously and are called pneumatophores or respiratory roots. They are used for gas exchange on very moist soil.
The exodermis is clearly pronounced. The cortex consists mainly of parenchyma with irregular, air-filled intercellular spaces . The endodermis is lignified. The stele is surrounded by marrow . So far, arbuscular mycorrhizal fungi have been detected in the root cortex of Aiphanes macroloba , Aiphanes ulei and Aiphanes weberbaueri .
Stem axis
The stem axis is branched or unbranched. It can be very short and in Aiphanes acaulis and Aiphanes spicata it can be completely underground. At Aiphanes grandis it grows to over 20 meters high and a diameter of up to 20 centimeters.
The trunk is always clearly marked in a ring shape by leaf scars and reinforced with spines that stand in a ring or spiral around the nodes . The length of the internodes varies and reflects different growth rates. In the small species such as Aiphanes chiribogensis they are usually between one and two centimeters long , in the large species such as Aiphanes eggersii up to 15 centimeters long.
Branches arise from the basal (subterranean or just above the surface of the earth) leaf axils, or rarely distally directly from the stem axis.
The cross-section corresponds to the characteristic structure of the monocot: on the outside lies the epidermis , which surrounds a layer of primary bark . This layer is usually very thin or hardly present, but in Aiphanes macroloba it is very pronounced and about 5 millimeters thick. Further inside there is a cylinder made of parenchyma, in which the vascular bundles run in a distributed manner ( ataktostele ). The outer part of the cylinder consists of black fibers of sclerenchyma.
leaves
Almost all species have a screwy (disperse) leaf position ( phyllotaxis ), only with Aiphanes linearis , Aiphanes verrucosa and Aiphanes lindeniana the leaves are two-lined (distich). The leaves of all species on young plants are two-lined, but this quickly disappears. The leaves are large leaflets ( palm fronds ), the number of which varies from three to more than 20 per crown.
The young leaves are in cylindrical leaf shells, which are densely covered with spines in all species. The leaf envelope then breaks open on both sides, revealing the stalked leaf and a clearly visible ligule . This then recedes, but residues also remain visible on old leaves. The leaf covering falls in all species with the exception of Aiphanes hirsuta subsp. fosteriorum clean. The young leaves are densely covered with multi-branched leaf hairs ( trichomes ).
The length of the petioles varies from a few centimeters to over a meter. Short petioles are notched at the top, while long ones are rounded without a notch. The central main axis of the pinnate leaf ( rachis ) extends distally into a long thread (filament), which then breaks off over time. Leaf stalks and rhachis are often densely covered with spines, but in a few species they are completely unarmed.
The arrangement of the individual leaflets on the leaf spindle varies greatly between species. It ranges from completely irregular to grouped in clusters to pairs of opposite leaves.
As it grows, the leaflet is incised twice from the tip downwards (basipetal) to form three uneven lobes. The central rib of the underside of the leaf is reinforced with one or more spines in all species. In some species, such as Aiphanes ulei , the upper side of the leaf is also covered with rows of spines. In Aiphanes minima , the spines can also grow out of the side ribs.
Leaf anatomy
The leaf blade is dorsiventral . The epidermis is only one layer thick. The cells are rhombohedral or spindle-shaped. The outer cell wall is weakly to strongly cutinized . The anticlinic cell walls, i.e. perpendicular to the leaf surface, are periodically thickened. The thickenings are reminiscent of pearls strung on a string. The stomata (stomata) are preferably abaxial, that is at the leaf base. These are seldom sunk in or lifted out.
The hypodermis, i.e. the layer directly under the epidermis, is also only one cell thick. The cells are twice as wide as those of the epidermis. The air cavities under the stomata are surrounded by nine cells. The chlorenchyme is uniform and one to three cells thick. Rhaphids , bundles of needle-shaped calcium oxalate crystals, are common. Fibers not associated with the vascular bundles are less than five micrometers in diameter and have no sclerenchyme. They lie in strands of two to four layers.
The leaf veins run in the mesophyll , they consist of an outer parenchymal layer, which often does not completely enclose larger veins in particular, and an inner one to seven cell thick layer of sclerenchyma. The main vein phloem consists of two to four strands.
Inflorescences
Prickly palms bloom several times in life, so they are persistent (pollakanth). The flowers are single-sexed ( monoecious ).
The inflorescences are initially upright and can tilt during anthesis , when the flowers open, until they droop. They are often bent as the fruit ripens. Almost all species have one inflorescence per leaf axis, but Aiphanes gelatinosa often has three. The bracts (bracts) are highly variable, they can be thickened and woody or thin and papery. They are often armed with spines, thin bracts often fall off, while woody ones remain.
The inflorescence is piston-like in almost all species , but simply branched. The inflorescence axis consists of a reinforced inflorescence stalk (Pedunculus) with a diameter of three to 50 millimeters and a rhachis, branching off from the axes of the first order (rhachillae), on which the flowers then sit. With Aiphanes acaulis , Aiphanes spicata and Aiphanes macroloba , however, the inflorescence is always unbranched , with Aiphanes simplex mostly. On the flower stalk sit flat, rounded and on the sides winged bracteoles .
The flowers are always in groups of three consisting of two male flowers (with Androeceum ) and one female flower (with Gynoeceum ). At the tip of the inflorescences (distal) there are seldom groups of two in which the female flower is missing. With Aiphanes deltoidea and Aiphanes minima there are seldom groups of four, each consisting of two sex pairs.
Male flowers
The male flowers are stalked or almost sessile. The flower envelope consists of three free, keeled and membrane-like sepals (sepals) and three free or fleshy petals that are fused at the base . Both are pointed. The flower color varies from cream to yellow-orange, or from white to purple or purple. Often the flowers are still green before the anthesis.
The sepals consist of only one or two layers of narrow cells. The petals are significantly thicker, in addition to the epidermis they consist of five or six layers of parenchymal cells, with many rhaphids and tannin-containing cells, and a layer of fibers that are not associated with the vascular bundles. They are by a single vascular bundle with two tracheas from metaxylem supplies. The inner epidermis consists of a layer of large cells with a tough cytoplasm and large cell nuclei .
The six stamens are in two whorls of three each. They are supplied with three to four metaxylem tracheas by a single vascular bundle. The stamens are fused at the base. They stand upright and are never longer than the petals. They have ten to 15 cells in diameter and consist of parenchyma. The anthers open towards the center of the flower (intrors) or to the side (latrors). They are rounded and curved, or upright - in anthesis they often incline almost to the horizontal. The length varies between 0.3 and four millimeters and correlates with the size of the petals. The pollen sacs are rich in raphids. There are small, glandular rudiments of pistils that are transformed into nectaries .
Pollen
The pollen grains are monosulcat, that is, they have only one germinal furrow. This is often in the southern half of the pollen grain (meridionosulcat). Three-armed germinal furrows (trichotomosulcat) are rarely found. They are spherical to ellipsoidal, rarely triangular. The long axis is between 20 and 30 micrometers long. The diameter varies between 20 and 30 micrometers.
The outer layer of the pollen grains (exine) is completely or partially covered with a tectum, a layer that covers the rod-shaped structures called columellae. Often there are short or long thorns, warts or more or less overgrown delicate outgrowths on the tectum. The exine structure and ornamentation is altogether much more diverse than in other Bactridinae genera.
Female flowers
The inflorescence of the female flowers consists of three free, broadly keeled, thin, paper-like sepals and three curved or only slightly keeled, fleshy petals. The latter are fused at half the length basal. The sepals are four to five cells thick and contain a layer of fibers that are not associated with the vascular bundles. The coloring of the female flowers seems to follow that of the male. In the case of Aiphanes deltoidea , however, in contrast to the male orange flowers, they are greenish.
Each flower contains six sterile stamens, staminodes . They have grown together to form a pointed, lobed cup-shaped shell, which in turn has grown together with the lower half of the sepals. The three spherical carpels are fused (synkarp) and the stylus whose length corresponds approximately to the stamens, wears a tripartite scar . Small amounts of nectar are secreted just below the scars and at the opening of the central stylus canal, between the scars .
Fruits and seeds
The fruits of the prickly palms are in most species round, red, single-seeded stone fruits , with a thick, hard, woody core surrounding the seed ( endocarp ). The endocarp is between 0.5 and two millimeters thick and brown or black in color. It has three distinct germ pores, each of which is surrounded by round, overlying fibers in a star-shaped pattern. The middle pericarp (mesocarp) is fleshy and juicy. The diameter of the fruits varies between five and 25 millimeters and is quite small compared to other Cocoeae.
Variations arise, for example, with Aiphanes macroloba with ellipsoidal fruits, some species, such as Aiphanes grandis , produce fruits with beak-shaped outgrowths. The color of some species also differs from red , for example Aiphanes grandis has pale green fruits , and other species, such as Aiphanes verrucosa , white fruits. For example, purple fruits can be found in Aiphanes hirsuta .
The seed coat is thin. The nutrient tissue ( endosperm ) is white and homogeneous, often with an irregular cavity inside. The taste of the endosperm is sweet and reminiscent of coconut , the oil content fluctuates greatly within the genus and is, for example, 37% in Aiphanes horrida , but 65% in Aiphanes minima . The essential component of the oil is lauric acid , in Aiphanes horrida almost 63%. The embryo is light brown in color, has an inverted conical shape and is 0.5 to one millimeter long. Its tip points to one of the three germ pores.
In addition to iso-rhapontigenin, piceatannol and luteolin , aiphenol, a new stilbene compound that functions as an inhibitor of cyclooxygenase reactions was isolated from the fruits of Aiphanes horrida .
distribution
Prickly palms are common in the Lesser Antilles , as well as from Venezuela to Bolivia along the Andes . Aiphanes hirsuta subsp. hirsuta reaches Panama, otherwise the genus is absent in Central America. The eastern border of the distribution area is marked by the western border of the Amazon basin and reaches Brazil in a very thin strip on the border with Peru. Alleged finds in Guyana and from the south of Venezuela could not be confirmed.
The diversity center of the genus, i.e. the area with the greatest biodiversity, is located in western Colombia and Ecuador, a sub-center is found in northeastern Peru. The most widespread species is Aiphanes horrida , which is found from Trinidad to Bolivia, with a gap from central Colombia to central Peru. Many other species have only a small or very small range.
In Tanzania in Africa there are neophytic occurrences of Aiphanes horrida , which spread wildly but are not classified as invasive .
Prickly palms can inhabit altitudes of up to 2800 meters, although other species are common in the lowlands than in the highlands. The Aiphanes species have also adapted to very different locations and there are species that specialize in both very humid and very dry conditions. The plants also behave differently in open terrain than in densely overgrown forest areas. While Aiphanes ulei , Aiphanes weberbaueri , Aiphanes parvifolia or Aiphanes tricuspidata cannot survive in open terrain, Aiphanes erinacea or Aiphanes hirsuta , for example, adapt to the changed conditions and form many short stems and many more inflorescences.
Danger
As with many genera with highly specialized or endemic species, many species are endangered. The International Union for Conservation of Nature and Natural Resources (IUCN) lists six of the 25 species in a hazard category on its Red List .
Are three types as "high risk" ( endangered ) ( Aiphanes grandis , Aiphanes leiostachys and Aiphanes verrucosa ) and another three as "endangered" ( vulnerable ) ( Aiphanes chiribogensis , Aiphanes duquei , Aiphanes lindeniana ). In three species ( Aiphanes chiribogensis , Aiphanes grandis , Aiphanes verrucosa ) the situation is constantly deteriorating and further deterioration is to be feared. Aiphanes grandis in particular is endangered, as none of the remaining populations in Ecuador are located within a protected area. At least Aiphanes erinacea is also endangered, even if the species is not listed on the red list.
Habitat destruction is the main reason for endangerment for all species. Locations are mainly destroyed by clearing and reclamation of the land. For Aiphanes erinacea it could be shown that the species only germinates in untouched forests, but dies in forests that are only slightly thinned; this probably also applies to other species of spiny palm.
ecology
Most species bloom year-round and in a single population individuals can be found in all stages of budding, flowering, and fructation at the same time, and often multiple stages can be found on a single individual at the same time. This is mainly due to the relatively uniform climatic conditions in large parts of the distribution area. However, there are also individual populations that develop seasonally in parallel, such as a population of Aiphanes horrida near Canavi in Bolivia or populations at very high altitudes.
The anthesis lasts at least 80 days, measured in Aiphanes chiribogensis, and a new flower appears within about 25 days after the end of the anthesis of the old flower, measured in Aiphanes eggersii .
pollination
The inflorescences are non-overlapping proterandric , which means that the male flowers release the pollen at a time when the stigmas of the female flowers are not yet capable of being covered. An individual rarely has more than one inflorescence at the same time - this prevents self-pollination .
The sting palm species are pollinated by various insects ( entomophilia ), with some species wind pollination ( anemophilia ) also plays a role. Finn Borchsenius investigated the pollination of Aiphanes chiribogensis , Aiphanes eggersi and Aiphanes erinaceae in western Ecuador . He found many larvae of small butterflies (Microlepidoptera) in the male flowers of Aiphanes eggersii , but concluded that they were pollinated by bees (Apiformes) and wind. The flowers of Aiphanes erinacea are visited by hundreds of flies, especially fruit flies (Drosophilidae), hover flies (Syrphidae), midges (Ceratopogonidae) and leaf beetles (Chrysomelidae), which are probably also responsible for pollination. He did not observe bees here. The flowers of Aiphanes chiribogensis are visited by significantly fewer insects. Here you can find fruit flies, fungus gnats (Mycetophilidae), sciarid gnats ( Sciaridae), gall gnats (Cecidomyiidae), midges and small butterflies. There are no bees or hoverflies.
The flowers of Aiphanes grandis and Aiphanes minima smell sweet to attract insects. An analysis of the scent of the male flower of Aiphanes minima revealed 15 ingredients. The main components are pentadecane (75.5%), tetradecane (3.9%), 1,3,7-nonatriene linalool (1.2%) and dihydro-β-ionone (1.2%).
Aiphanes horrida is visited by stingless bees (Meliponini) and Schnabelkerfen (Hemiptera), pollination takes place by bees. Gloss beetles (Nitidulidae) and weevils (Curculionidae) are found exclusively in male flowers.
Overall, the species with large, white or yellow flowers and linear anthers are most likely pollinated by bees, and the species with small, mostly white to purple flowers with small, oval anthers, mainly by flies.
Seed spread
The ripe fruits of Aiphanes horrida are eaten by squirrels ( Sciurus ), which can climb the trunk despite the many spines. The fruits are high in energy, contain many vitamins and are probably also eaten by many other animals. The spines on the whole plant serve to protect against herbivores and animals that want to climb the trunks to get to the fruits. The bright red fruits of Aiphanes horrida are also eaten by the fat swallow ( Steatornis caripensis ), which swallows them whole and spreads the seeds ( endochory ).
use
The fruits of Aiphanes horrida are sold under the name Corozo or Mararay in many markets in Colombia and can even be found in supermarkets in Medellín . They are eaten raw. Candied the fruits are very popular as sweets in the Andes. Aiphanes horrida is the only species of the genus that is cultivated for its fruits; the fruits of the other species are only collected in nature.
The fruits of Aiphanes linearis are also tasty and are eaten in Colombia. The kernels of Aiphanes minima are edible and are traded as nuts.
Despite the spines, Aiphanes horrida and Aiphanes minima are occasionally used as a specimen plant in gardens. The two species are widespread in botanical gardens . The wood of prickly palms has no economic importance.
Botanical history and etymology
Specimens of the genus Aiphanes were first collected by Charles Plumier , a French missionary and botanist who made three trips to the Caribbean between 1689 and 1695. He made drawings and described two species, which he named Palma dactylifera, aculeata, fructu corallino, major and Palma dactylifera, aculeata, fructu corallino, minor . Both were specimens of today's Aiphanes minima species . The same species was then described again in 1763 by Nikolaus Joseph von Jacquin as Palma grigri martinicensibus .
In 1779, José Mutis made a very detailed description of the species now known as Aiphanes lindeniana . In 1791 the German botanist Joseph Gärtner described the seeds of Aiphanes minima in his book De fructibus et seminibus plantarum under the name Bactris minima - minima is thus the oldest recognized species epithet of an Aiphanes species.
The generic name Aiphanes was first used in 1801 by Karl Ludwig Willdenow in a lecture at the Royal Academy of Sciences in Berlin . The name is made up of the ancient Greek αεί , ai (= always) and φανερός , phaneros (= obvious, visible, conspicuous). Ironically, prickly palms are usually difficult to spot in dense vegetation and, for this reason, have rarely been collected in herbaria. The name probably refers more to the striking appearance of the plants.
Willdenow only described one species of the genus Aiphanes aculeata , which is now a synonym for Aiphanes horrida . The holotypical specimen was collected by Franz Bredemeyer in Caucagua , Venezuela. Bredemeyer then went to Schönbrunn and took either the holotype or the seeds of this specimen with him. In 1809 Joseph Franz von Jacquin described the same holotype or a redrawn specimen under the name Caryota horrida - today the epipheton horrida is recognized as the valid one.
Between 1794 and 1816 several palm trees were described under the generic name Martinezia - but the genus was inconsistent and was synonymous with Aiphanes by Carl Friedrich Philipp von Martius in 1847 . Since Martinezia was the older name, the generic name Aiphanes was no longer used until 1932. However, the genus Martinezia contained many palms that are not to be equated with the thorn palms. Carl Burret therefore reintroduced the name Aiphanes in 1932 . The majority of the species from Martinezia was placed in the genus Euterpe , which is why Martinezia is now a synonym for Euterpe . Most of the species described by Burret were collected by Wilhelm Kalbreyer , who had traveled to northern Colombia between 1877 and 1881 and brought with him an extensive collection of palm trees from which 69 new palm species could be described.
Between 1932 and 1996, 15 other species of spiny palm were described, bringing the number of species to 47. In 1996, Finn Borchsenius and Rodrigo Bernal published an extensive monograph on the genus in which the number of species was reduced to 22. Since then, however, other new species have been described.
Systematics
External system
John Dransfield and colleagues assign the genus Aiphanes within the family Arecaceae to the subfamily Arecoideae , Tribus Cocoseae , Subtribus Bactridinae . This includes the genera Acrocomia , Astrocaryum , Desmoncus and Bactris . The relationships within the subtribes have not been clearly clarified, and different studies have come to different results. Common characteristics of the genera are thorns on at least parts of the plants and the neotropical distribution area.
In addition to the prickly palm trees of this tribe, there are also other genera of prickly palm trees not only in the Neotropics, but these are not closely related to the genus Aiphanes .
A molecular genetic investigation from 2002 resulted in the following cladogram , which represents one of several suggested relationships within the subtribe Bactridinae.
|
|
|
||||||||||||||||||||||||
|
|
Internal system
Today, 25 recognized species belong to the genus (as of 2007). The position of Aiphanes leiospatha Burret is unclear , the species is listed as an " Unplaced Name " . The number has now grown to 32 (as of 2018).
The genus Aiphanes is a morphologically clearly defined unit. Their position as a monophyletic group is therefore not in doubt.
Carl Burret divided the genus in 1992 into two subgenera Macroanthera and Brachyanthera . He distinguished these mainly on the basis of the morphological characteristics of the flowers, especially the length of the anthers and the position of the inflorescences. However, these features are so vague that a clear assignment is not possible. Nevertheless, the three species in the subgenus Macroanthera seem to be closely related, although it is not clear whether this group is monophyletic. Today, the classification is usually no longer followed - in the absence of a newer breakdown it is still used here.
The species of the genus are:
| Sections and species of the genus Aiphanes |
|---|
|
Subgenus Macroanthera Burret
Subgenus Brachyanthera Burret
Not assigned
|
literature
Much of the information in this article has been obtained from the following sources:
- Finn Borchsenius, Rodrigo Bernal: Aiphanes (Palmae) . In: Finn Borchsenius, Rodrigo Bernal (eds.): Flora Neotropica . tape 70/73 . The New York Botanical Garden, 1996, ISBN 0-89327-408-9 , ISSN 0071-5794 .
- Carmen Ulloa Ulloa, Peter Møller Jørgensen: Aiphanes . In: Arboles y arbustos de los Andes del Ecuador . 2004 ( full text ).
Web links
- Aiphanes on the IUCN Red List of Threatened Species2007.
- The genus in Ecuador on palmbase.org ( Memento from June 15, 2009 in the Internet Archive )
- Pictures of many species at fieldmuseum.org ( Memento from March 30, 2008 in the Internet Archive )
Individual evidence
- ↑ MA Sowunmi: Pollen morphology of the palmae and its bearing on taxonomy . In: Review of Palaeobotany and Palynology . tape 13 , no. 1 , February 1972, p. 1-80 , doi : 10.1016 / 0034-6667 (72) 90044-9 .
- ↑ Dongho Lee, Muriel Cuendet, Jose Schunke Vigo, James G. Graham, Fernando Cabieses, Harry HS Fong, John M. Pezzuto, A. Douglas Kinghorn: A Novel Cyclooxygenase-Inhibitory Stilbenolignan from the Seeds of Aiphanes aculeata . In: Organic Letters . tape 3 , no. June 14 , 2001, p. 2169-2171 , doi : 10.1021 / ol015985j .
- ↑ Aiphanes horrida . In: Darwin Initiative (ed.): Usambara Invasive Plants . ( tropical-biology.org ( memento of October 3, 2008 on the Internet Archive ) [accessed December 14, 2007]). Aiphanes horrida ( Memento of the original from October 3, 2008 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ A b Jens-Christian Svenning: The effect of land-use on the local distribution of palm species in an Andean rain forest fragment in northwestern Ecuador . In: Biodiversity and Conservation . tape 7 , 1998, pp. 1529-1537 , doi : 10.1023 / A: 1008831600795 .
- ↑ Finn Borchsenius: Flowering biology and insect visitation of three Ecuadorean Aiphanes species . In: Principes . tape 37 , 1993, pp. 139-150 .
- ↑ JT Knudsen, L. Tollsten. F. Ervik: Flower Scent and Pollination in Selected Neotropical Palms . In: Plant Biology . No. 3 , 2001, p. 642-653 , doi : 10.1055 / s-2001-19366 .
- ^ Mark J. Plotkin, Lisa Famolare: Sustainable Harvest and Marketing of Rain Forest Products . Island Press , Washington, DC 1992, ISBN 978-1-55963-169-3 , pp. 159 .
- ↑ Geo Coppens d'Eeckenbrugge, Dimary Libreros Ferla: Aiphanes . In: Fruits from America, An ethnobotanical inventory . ( ciat.cgiar.org ( July 17, 2006 memento on the Internet Archive ) [accessed January 11, 2008]). Aiphanes ( Memento of the original from July 17, 2006 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Food and Agriculture Organization of the United Nations [FAO] (ed.): Tropical Palms . 1998, ISBN 92-5104213-6 ( full text ).
- ↑ GE Wickens: Edible Nuts . Ed .: Food and Agriculture Organization of the United Nations [FAO] . 1995, ISBN 92-5103748-5 .
- ^ Karl Ludwig Willdenow: About some new South American palm trees . In: Collection of the German treatises which were read out in the Royal Academy of Sciences in Berlin . 1803, p. 250 f . ( full text ).
- ↑ Helmut Genaust: Etymological dictionary of botanical plant names. 3rd, completely revised and expanded edition. Nikol, Hamburg 2005, ISBN 3-937872-16-7 , p. 46 (reprint from 1996).
- ↑ a b Rafaël Govaerts, J. Henderson; SF Zona; DR Hodel; A. Henderson: World Checklist of Arecaceae . Ed .: The Board of Trustees of the Royal Botanic Gardens, Kew . 2006 ( kew.org [accessed December 14, 2007]).
- ↑ a b M. Burret: The palm genera Martinezia and Aiphanes . In: Notblatt des Königl. botanical garden and museum in Berlin . tape 11 , no. 107 , December 15, 1932, p. 557-577 , doi : 10.2307 / 3995129 .
- ↑ Rodrigo G. Bernal, Gloria Galeano-Garces, Andrew Henderson: Neotypification of Colombian Palms Collected by W. Kalbreyer . In: Taxon . tape 38 , no. 1 , February 1989, p. 98-107 , doi : 10.2307 / 1220905 .
- ^ Carlos E. Cerón, Rodrigo Bernal: Una Nueva Especie de Aiphanes (Palmae) del Occidente de Ecuador . In: Caldasia . tape 26 , no. 2 , December 2004, p. 433–438 ( icn.unal.edu.co ( Memento from June 20, 2013 in the Internet Archive ) [PDF]). Una Nueva Especie de Aiphanes (Palmae) del Occidente de Ecuador ( Memento of the original from June 20, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Gloria Galeano, Rodrigo Bernal: New Species and New Records of Columbian Palms . In: Caldasia . tape 24 , no. 2 , December 2002, p. 277-292 ( icn.unal.edu.co [PDF]).
- ↑ Santos Miguel Niño, LJ Dorr, FW Stauffer: Una nueva especie de Aiphanes (Arecaceae) de la Cordillera de Mérida, Venezuela. . In: Sida, Contributions to Botany . tape 21 , no. 3 , 2005, p. 1599-1606 ( online [PDF]).
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq Rafaël Govaerts (Ed.) : Aiphanes. In: World Checklist of Selected Plant Families (WCSP) - The Board of Trustees of the Royal Botanic Gardens, Kew . Retrieved April 16, 2020.
- ^ John Dransfield, Natalie W. Uhl, Conny B. Asmussen, William J. Baker, Madeline M. Harley, Carl E. Lewis: Genera Palmarum. The Evolution and Classification of Palms. 2nd edition, Royal Botanic Gardens, Kew 2008, ISBN 978-1-84246-182-2 , p. 430 ff.
- ↑ John Dowe: Spiny Palms . In: Palms & Cycads . No. 12 , 1986 ( pacsoa.org.au ( memento of March 22, 2012 in the Internet Archive ) [accessed January 11, 2008]). Spiny Palms ( Memento of the original from March 22, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ William J. Hahn: A phylogenetic analysis of the Arecoid Line of palms based on plastid DNA sequence data . In: Molecular Phylogenetics and Evolution . tape 23 , no. 2 , June 2002, p. 189-204 , doi : 10.1016 / S1055-7903 (02) 00022-2 .
- ↑ R. Govaerts: Aiphanes leiospatha . In: The Board of Trustees of the Royal Botanic Gardens, Kew (ed.): World Checklist of Arecaceae . 2006 ( apps.kew.org [accessed December 14, 2007]).
- ↑ Borchsenius, Bernal: Aiphanes (Palmae). P. 33.