Friday the 13th and Ethanol: Difference between pages
m Reverted edits by 63.228.7.58 (talk) to last version by Soliloquial |
|||
| Line 1: | Line 1: | ||
{{Redirect|Grain alcohol|the distilled high-proof liquor of similar name|neutral grain spirit}} |
|||
{{about|the superstition}} |
|||
{{otheruses3|Ethanol (disambiguation)}} |
|||
{{chembox |
|||
| ImageFileL1 = Ethanol-2D-skeletal.svg |
|||
| ImageFileL2 = Ethanol_flat_structure.png |
|||
| ImageFileR1 = Ethanol-3D-vdW.png |
|||
| ImageFileR2 = Ethanol-3D-balls.png |
|||
| IUPACName = Ethanol |
|||
| OtherNames = Ethyl alcohol; grain alcohol; pure alcohol; hydroxyethane; drinking alcohol; ethyl hydrate |
|||
| Section1 = {{Chembox Identifiers |
|||
| SMILES = CCO |
|||
| CASNo = 64-17-5 |
|||
| ChemSpiderID = 682 |
|||
| RTECS = KQ6300000 |
|||
}} |
|||
| Section2 = {{Chembox Properties |
|||
| Formula = C<sub>2</sub>H<sub>6</sub>O |
|||
| MolarMass = 46.06844(232) g/mol |
|||
| Appearance = colorless clear liquid |
|||
| Density = 0.789 g/cm³, liquid |
|||
| Solubility = Fully [[miscible]] |
|||
| MeltingPt = −114.3 °C (158.8 K) |
|||
| BoilingPt = 78.4 °C, 173.1 F (351.6 K) |
|||
| pKa = 15.9 |
|||
| Viscosity = 1.200 mPa·s ([[Poise|cP]]) at 20.0 °C |
|||
| Dipole = 5.64 fC·fm (1.69 [[Debye|D]]) (gas) |
|||
}} |
|||
| Section7 = {{Chembox Hazards |
|||
| FlashPt = 286.15 K (13 °C or 55.4 °F) |
|||
| EUClass = Highly Flammable ('''F''') |
|||
| NFPA-H = 2 |
|||
| NFPA-F = 3 |
|||
| NFPA-R = 0 |
|||
| RPhrases = {{R11}} {{R20}} {{R21}} {{R22}} {{R36}} |
|||
| SPhrases = {{S2}}, {{S7}}, {{S16}} |
|||
}} |
|||
| Section8 = {{Chembox Related |
|||
| Function = [[alcohol]]s }} |
|||
}} |
|||
'''Ethanol''', also called '''ethyl alcohol''', '''pure alcohol''', '''grain alcohol''', or '''drinking alcohol''', is a [[volatility (chemistry)|volatile]], [[flammable]], colorless liquid. It is a [[psychoactive drug]], best known as the type of [[alcohol]] found in [[alcoholic beverages]] and in [[thermometers]]. In common usage, it is often referred to simply as ''alcohol''. |
|||
'''Friday the 13th''' is superstitiously considered a day of bad [[luck]] in [[English language|English]]-, [[French language|French]]- and [[Portuguese language|Portuguese]]-speaking countries around the world, as well as in [[Austria]], [[Germany]], [[Estonia]], [[Finland]], [[The Netherlands]], [[Belgium]], [[Republic of Ireland]], [[Poland]], [[Bulgaria]], [[Denmark]], [[Iceland]], [[Lithuania]], [[Republic of Macedonia]], [[Sweden]], [[Norway]], [[Czech Republic]], [[Slovakia]], [[Slovenia]], [[Hungary]], and the [[Philippines]]. |
|||
Ethanol is abbreviated as '''EtOH''', using the common organic chemistry notation of representing the ethyl group (C<sub>2</sub>H<sub>5</sub>) with '''Et'''. This designation is used both by EMS and Hospital ER staff when describing alcohol intoxication, and is found in most [[chemistry]] textbooks as well. |
|||
Similar [[superstition]]s exist in some other traditions. In [[Greece]], [[Romania]] and [[Spanish language|Spanish]]-speaking countries, for example, it is Tuesday the 13th that is considered unlucky. In [[Italy]], it is [[Friday]] the 17th. |
|||
The fear of Friday the 13th is called ''paraskavedekatriaphobia'',<ref>Alternative spellings include paskevodekatriaphobia or paraskevidekatriaphobia.</ref> a word derived from the concatenation of the Greek words ''Paraskeví'' (Παρασκευή) (meaning ''Friday''), and ''dekatreís'' (δεκατρείς) (meaning ''thirteen''), attached to ''phobía'' (φοβία) (meaning ''fear''). The term is a specialized form of [[triskaidekaphobia]], a simple ''[[phobia]]'' (fear) of the number thirteen appearing in any case. |
|||
Ethanol is a straight-chain alcohol, and its [[chemical formula|molecular formula]] is C<sub>2</sub>H<sub>5</sub>OH. An alternative notation is CH<sub>3</sub>-CH<sub>2</sub>-OH, which indicates that the carbon of a methyl group (CH<sub>3</sub>-) is attached to the carbon of a methylene group (-CH<sub>2</sub>-), which is attached to the oxygen of a [[Hydroxyl|hydroxyl group (-OH)]]. |
|||
Its [[empirical formula]] is [[Carbon|C]]<sub>2</sub>[[Hydrogen|H]]<sub>6</sub>[[Oxygen|O]], making it an [[isomer]] of [[dimethyl ether]]. |
|||
Except for use of fire, the [[ethanol fermentation|fermentation]] of sugar into ethanol is the earliest [[organic reaction]] known to humanity. The intoxicating effects of ethanol consumption have been known since ancient times. In modern times, ethanol intended for industrial use is also produced from by-products of petroleum refining. |
|||
Ethanol has widespread use as a solvent of substances intended for human contact or consumption, including scents, flavorings, colorings, and medicines. In chemistry, it is both an essential solvent and a feedstock for the synthesis of other products. It has a long history as a fuel for heat and light and also as a fuel for [[internal combustion engine]]s. |
|||
==History== |
==History== |
||
[[Image:Alcohol flame.jpg|thumb|left|Ethanol being used as fuel for a burner.]] |
|||
Both the [[13 (number)|number thirteen]] and Friday have been considered unlucky: |
|||
Ethanol has been used by humans since prehistory as the intoxicating ingredient of [[alcoholic beverage]]s. Dried residues on 9000-year-old pottery found in China imply that alcoholic beverages were used even among [[Neolithic]] people.<ref name="Roach">{{cite journal|author=Roach, J.|date=July 18, 2005|url=http://news.nationalgeographic.com/news/2005/07/0718_050718_ancientbeer.html|title= 9,000-Year-Old Beer Re-Created From Chinese Recipe|journal=National Geographic News|accessdate = 2007-09-03}}</ref> Its isolation as a relatively pure compound was first achieved by Persian [[alchemist]], [[Muhammad ibn Zakarīya Rāzi|Zakarīya Rāzi]] (Rhazes), who was renowned for his perfected methods of [[distillation]] and [[extraction]]. |
|||
Other chemists who contributed to the development of [[distillation]] techniques during the [[Abbasid]] [[caliphate]], other than Razi, include [[Geber|Jabir ibn Hayyan]] (Geber) and [[Al-Kindi]] (Alkindus). |
|||
Writings attributed to Jabir ibn Hayyan (721–815) mention the flammable vapors of boiled wine. Al-Kindi (801–873) unambiguously described the distillation of wine.<ref name="al-Hassan">{{cite web |url=http://www.history-science-technology.com/Notes/Notes%207.htm |title=Alcohol and the Distillation of Wine in Arabic Sources |accessdate = 2008-03-29 |last=Hassan |first=Ahmad Y |authorlink=Ahmad Y Hassan |work=History of Science and Technology in Islam}}</ref> |
|||
*In [[numerology]], the number twelve is considered the number of completeness, as reflected in the twelve months of the year, twelve recognized signs of the zodiac, the twelve tribes of Israel, the twelve [[Apostle]]s of Jesus, etc., whereas the number thirteen was considered irregular, transgressing this completeness.<ref name="NatGeo"/> There is also a [[The Thirteen Club|superstition]], thought by some to derive from the [[Last Supper]], that having thirteen people seated at a table will result in the death of one of the diners. |
|||
*[[Good Friday|Friday]], as the day on which [[Jesus Christ]] was [[Crucifixion|crucified]], has been viewed both positively and negatively among Christians. |
|||
In 1796, Johann Tobias Lowitz obtained pure ethanol by filtering distilled ethanol through [[Activated carbon|activated charcoal]]. |
|||
Despite the reputation of the two separated elements, there is no evidence for a link between the two before the 19th century. The earliest known reference in English occurs in a 1869 biography of [[Gioachino Rossini]]: |
|||
[[Antoine Lavoisier]] described ethanol as a compound of carbon, hydrogen, and oxygen, and in 1808 [[Nicolas-Théodore de Saussure]] determined ethanol's chemical formula.<ref>''[http://www.1911encyclopedia.org/Alcohol Alcohol]'' in the [[Encyclopædia Britannica Eleventh Edition]]</ref> Fifty years later, [[Archibald Scott Couper]] published the structural formula of ethanol, which placed ethanol among the first chemical compounds to have their chemical structure determined.<ref name="Couper">{{cite journal|author=Couper, A.S.|year=1858|title=On a new chemical theory|journal=Philosophical magazine|format=online reprint|volume=16|issue=104–116|url=http://web.lemoyne.edu/~giunta/couper/couper.html|accessdate = 2007-09-03}}</ref> |
|||
:''[Rossini] was surrounded to the last by admiring and affectionate friends; and if it be true that, like so many other Italians, he regarded Friday as an unlucky day, and thirteen as an unlucky number, it is remarkable that on Friday, the 13th of November, he died.''<ref>Henry Sutherland Edwards, ''The Life of Rossini'', 1869, p. 340.</ref> |
|||
Ethanol was first prepared synthetically in 1826 through the independent efforts of Henry Hennel in Great Britain and S.G. Sérullas in |
|||
However, only in the 20th century did the superstition receive greater audience, as |
|||
[[France]]. In 1828, [[Michael Faraday]] prepared ethanol by [[Acid catalysis|acid-catalyzed]] hydration of [[ethylene]], a process similar to that which is used today for industrial ethanol synthesis.<ref name=Hennell>{{cite journal|author=Hennell, H. |year=1828|title=On the mutual action of sulfuric acid and alcohol, and on the nature of the process by which ether is formed|journal=Philosophical Transactions|volume=118|issue=365–371}}</ref> |
|||
Ethanol was used as lamp fuel in the United States as early as 1840, but a tax levied on industrial alcohol during the [[American Civil War|Civil War]] made this use uneconomical. This tax was repealed in 1906,<ref name=siegel>{{cite news|url=http://www.npr.org/templates/story/story.php?storyId=7426827|title=Ethanol, Once Bypassed, Now Surging Ahead|author=Robert Siegel|publisher=NPR|date=2007-02-15|accessdate = 2007-09-22}}</ref> and from 1908 onward [[Ford Model T]] automobiles could be adapted to run on ethanol.<ref name=dipardo>{{cite web|url=http://tonto.eia.doe.gov/FTPROOT/features/biomass.pdf|title=Outlook for Biomass Ethanol Production and Demand|publisher=United States Department of Energy|author=Joseph DiPardo|accessdate =2007-09-22|format=PDF}}</ref> With the advent of [[Prohibition]] in 1920 though, sellers of ethanol fuel were accused of being allied with [[moonshine]]rs,<ref name="siegel"/> and ethanol fuel again fell into disuse until late in the 20th century. |
|||
:''Friday the 13th doesn't even merit a mention in E. Cobham Brewer's voluminous 1898 edition of the Dictionary of Phrase and Fable, though one does find entries for "Friday, an Unlucky Day" and "Thirteen Unlucky." When the date of ill fate finally does make an appearance in later editions of the text, it is without extravagant claims as to the superstition's historicity or longevity.''<ref name="why">[http://urbanlegends.about.com/cs/historical/a/friday_the_13th_4.htm Why Friday the 13th Is Unlucky]</ref> |
|||
==Physical properties== |
|||
Though the superstition developed relatively recently, much older origins are often claimed for it, most notably in the [[novel]] ''[[The Da Vinci Code]]'' (and later the film), which traced the belief to the arrest of the [[Knights Templar]] on Friday [[October 13]], [[1307]].<ref name="why"/> |
|||
[[Image:Spiritusflamme mit spektrum.png|thumb|right|Ethanol burning with its spectrum depicted.]] |
|||
[[Image:Chemistry, Combustion of Ethanol 002.jpg|thumb|right|Ethanol burning in a shallow dish.]] |
|||
Ethanol is a volatile, [[flammable]], colorless liquid that has a strong characteristic odor. It burns with a smokeless blue flame that is not always visible in normal light. |
|||
==Effects in people and cultures== |
|||
According to the ''Stress Management Center and Phobia Institute'' in [[Asheville, North Carolina]], an estimated 17 to 21 million people in the [[United States]] are affected by a fear of this day. Some people are so paralyzed by fear that they avoid their normal routines in doing business, taking flights or even getting out of bed. "It's been estimated that <nowiki>[</nowiki>[[United_States_dollar|US]]<nowiki>]</nowiki>$800 or $900 million is lost in business on this day".<ref name="NatGeo"> |
|||
John Roach, "[http://news.nationalgeographic.com/news/2004/02/0212_040212_friday13.html Friday the 13th Phobia Rooted in Ancient History]", ''National Geographic News'', August 12, 2004.</ref> Despite this, representatives for both Delta and Continental Airlines say that their airlines don't suffer from any noticeable drop in travel on those Fridays.<ref>Josh Sens, "[http://www.viamagazine.com/top_stories/articles/lucky_1304.asp Some Don't Count on lucky]", ''Via Magazine'', January 2004.</ref> |
|||
The physical properties of ethanol stem primarily from the presence of its [[hydroxyl]] group and the shortness of its carbon chain. Ethanol’s [[hydroxyl]] group is able to participate in hydrogen bonding, rendering it more viscous and less volatile than less polar organic compounds of similar molecular weight. |
|||
A [[British Medical Journal]] study has shown that there is a significant increase in traffic-related accidents on Fridays the 13th.<ref>T. J. Scanlon, R. N. Luben, F. X. Scanlon, N. Singleton, "Is Friday the 13th bad for your health?", ''[[British Medical Journal]]'', issue 307 (1993), p. 1584–1586.</ref> |
|||
Ethanol is a versatile solvent, [[miscible]] with water and with many organic solvents, including [[acetic acid]], [[acetone]], [[benzene]], [[carbon tetrachloride]], [[chloroform]], [[diethyl ether]], [[ethylene glycol]], [[glycerol]], [[nitromethane]], [[pyridine]], and [[toluene]].<ref name="crc"/><ref name="merck"/> It is also miscible with light aliphatic hydrocarbons, such as [[pentane]] and [[hexane]], and with aliphatic chlorides such as [[1,1,1-Trichloroethane|trichloroethane]] and [[tetrachloroethylene]].<ref name="merck">''Merck Index of Chemicals and Drugs'', 9th ed.</ref> |
|||
===Fewer accidents=== |
|||
The Dutch Centre for Insurance Statistics (CVS) on [[June 12]], 2008, stated that "fewer accidents and reports of fire and theft occur when the 13th of the month falls on a Friday than on other Fridays, because people are preventatively more careful or just stay home; but statistically speaking, driving is a little bit safer on Friday 13th; in the last two years, Dutch insurers received reports of an average 7,800 traffic accidents each Friday; but the average figure when the 13th fell on a Friday was just 7,500.<ref>[http://www.mirror.co.uk/news/topstories/2008/06/13/friday-13th-is-no-longer-unlucky-89520-20605817/ www.mirror.co.uk, Friday 13th is no longer unlucky]</ref><ref>[http://uk.reuters.com/article/oddlyEnoughNews/idUKL1268660720080613 uk.reuters.com, Dutch study shows Friday 13th not more unlucky]</ref> |
|||
Ethanol’s miscibility with water contrasts with that of longer-chain alcohols (five or more carbon atoms), whose water miscibility decreases sharply as the number of carbons increases.<ref name="m_and_b"/> |
|||
==Occurrence== |
|||
{{Cleanup|date=June 2007}} |
|||
{{-}} |
|||
<table border=0><tr><td valign=top> |
|||
The following months have a Friday the 13th: |
|||
{| class="wikitable" |
|||
|- |
|||
! Month !! Years !! [[Dominical Letter|Dominical</br>Letter]] |
|||
|- |
|||
| January || 2006, 2012, 2017, 2023 || style="text-align:center" |A, AG |
|||
|- |
|||
| February || 2004, 2009, 2015, 2026 || style="text-align:center" |D, DC |
|||
|- |
|||
| March || 2009, 2015, 2020, 2026 || style="text-align:center" |D, ED |
|||
|- |
|||
| April || 2001, 2007, 2012, 2018 || style="text-align:center" |G, AG |
|||
|- |
|||
| May || 2005, 2011, 2016, 2022 || style="text-align:center" |B, CB |
|||
|- |
|||
| June || 2003, [[2008]], 2014, 2025 || style="text-align:center" |E, FE |
|||
|- |
|||
| July || 2001, 2007, 2012, 2018 || style="text-align:center" |G, AG |
|||
|- |
|||
| August || 2004, 2010, 2021, 2027 || style="text-align:center" |C, DC |
|||
|- |
|||
| September || 2002, 2013, 2019, 2024 || style="text-align:center" |F, GF |
|||
|- |
|||
| October || 2006, 2017, 2023, 2028 || style="text-align:center" |A, BA |
|||
|- |
|||
| November || 2009, 2015, 2020, 2026 || style="text-align:center" |D, ED |
|||
|- |
|||
| December || 2002, 2013, 2019, 2024 || style="text-align:center" |F, GF |
|||
|} |
|||
Hydrogen bonding causes pure ethanol to be [[hygroscopic]] to the extent that it readily absorbs water from the air. The polar nature of the hydroxyl group causes ethanol to dissolve many ionic compounds, notably [[sodium hydroxide|sodium]] and [[potassium hydroxide]]s, [[magnesium chloride]], [[calcium chloride]], [[ammonium chloride]], [[ammonium bromide]], and [[sodium bromide]].<ref name="merck"/> [[Sodium chloride|Sodium]] and [[potassium chloride]]s are slightly soluble in ethanol.<ref name="merck"/> Because the ethanol molecule also has a nonpolar end, it will also dissolve nonpolar substances, including most [[essential oil]]s<ref name="merckoils">''Merck Index of Chemicals and Drugs'', 9th ed.; monographs 6575 through 6669</ref> and numerous flavoring, coloring, and medicinal agents. |
|||
</td><td valign=top> |
|||
The following years have Fridays the 13th in these months: |
|||
Two unusual phenomena are associated with mixtures of ethanol and water. Ethanol-water mixtures have less volume than the sum of their individual components. Mixing equal volumes of ethanol and water results in only 1.92 volumes of mixture.<ref name = "ChemTech"> Kroschwitz and Howe-Grant, editors, ''Encyclopedia of Chemical Technology'', 4th ed., (New York: John Wiley & Sons), vol. 9, 813.</ref><ref name="crc">''CRC Handbook of Chemistry'', 44th ed.</ref> The addition of even a few percent of ethanol to water sharply reduces the [[surface tension]] of water. This property partially explains the “[[tears of wine]]” phenomenon. When wine is swirled in a glass, ethanol evaporates quickly from the thin film of wine on the wall of the glass. As the wine’s ethanol content decreases, its surface tension increases and the thin film “beads up” and runs down the glass in channels rather than as a smooth sheet. |
|||
{| class="wikitable" |
|||
Mixtures of ethanol and water that contain more than about 50% ethanol are [[flammable]] and easily ignited. An alcohol stove has been developed in India which runs on 50% ethanol/water mixture.<ref>{{cite journal | author = Anil K. Rajvanshi, S.M. Patil, and B. Mendonca | volume = 11 | issue = 1 | year = 2007 | pages = 94–99 | journal = [[Energy for Sustainable Development]]}}</ref> [[Alcoholic proof]] is a widely used measure of how much ethanol (i.e., alcohol) such a mixture contains. In the 18th century, proof was determined by adding a liquor (such as [[rum]]) to gunpowder. If the gunpowder still just exploded, that was considered to be “100 degrees proof” that it was “good” liquor — hence it was called “100 degrees proof.” |
|||
Ethanol-water solutions that contain less than 50% ethanol may also be flammable if the solution is first heated. Some cooking methods call for [[wine]] to be added to a hot pan, causing it to flash boil into a vapor, which is then ignited to burn off excess alcohol. |
|||
Ethanol is slightly more refractive than water, having a [[refractive index]] of 1.36242 (at ''λ''=589.3 nm and 18.35 °C).<ref name="crc"/> |
|||
==Chemical properties== |
|||
{{detail|Alcohol}} |
|||
Ethanol is classified as a primary alcohol, meaning that the carbon to which its hydroxyl group is attached has at least two hydrogen atoms attached to it as well. |
|||
The chemistry of ethanol is largely that of its [[hydroxyl]] group. |
|||
===Acid-base chemistry=== |
|||
Ethanol's hydroxyl causes the molecule to be slightly basic. It is however,so very slightly basic it is almost neutral, like pure water. The [[pH]] of 100% ethanol is 7.33, compared to 7.00 for pure water. Ethanol can be quantitatively converted to its [[conjugate base]], the [[Alkoxide|ethoxide]] ion (CH<sub>3</sub>CH<sub>2</sub>O<sup>−</sup>), by reaction with an [[alkali metal]] such as [[sodium]]:<ref name=m_and_b/> |
|||
:2CH<sub>3</sub>CH<sub>2</sub>OH + 2[[sodium|Na]] → 2CH<sub>3</sub>CH<sub>2</sub>ONa + [[hydrogen|H<sub>2</sub>]], |
|||
or a very strong base such as sodium hydride: |
|||
:CH<sub>3</sub>CH<sub>2</sub>OH + NaH → CH<sub>3</sub>CH<sub>2</sub>ONa + [[hydrogen|H<sub>2</sub>]]. |
|||
This reaction is not possible in an aqueous solution, as water is more acidic, so that hydroxide is preferred over ethoxide formation. |
|||
===Halogenation=== |
|||
Ethanol reacts with [[hydrogen halide]]s to produce [[haloalkane|ethyl halides]] such as [[ethyl chloride]] and [[ethyl bromide]]: |
|||
:CH<sub>3</sub>CH<sub>2</sub>OH + [[hydrochloric acid|HCl]] → [[ethyl chloride|CH<sub>3</sub>CH<sub>2</sub>Cl]] + [[water|H<sub>2</sub>O]] |
|||
HCl reaction requires a catalyst such as [[zinc chloride]].<ref name="s_and_h">{{cite book|author=Streitweiser, Andrew Jr.; Heathcock, Clayton H.|title=Introduction to Organic Chemistry|year=1976|publisher=MacMillan|isbn=0-02-418010-6}}</ref> Hydrogen chloride in the presence of their respective zinc chloride is known as [[Lucas reagent]].<ref name=m_and_b/><ref name=s_and_h/> |
|||
:CH<sub>3</sub>CH<sub>2</sub>OH + [[Hydrobromic acid|HBr]] → [[Ethyl bromide|CH<sub>3</sub>CH<sub>2</sub>Br]] + [[water|H<sub>2</sub>O]] |
|||
HBr requires [[refluxing]] with a [[sulfuric acid]] catalyst.<ref name=s_and_h/> |
|||
Ethyl halides can also be produced by reacting ethanol with more specialized [[Halogenation|halogenating agents]], such as [[thionyl chloride]] for preparing ethyl chloride, or [[phosphorus tribromide]] for preparing ethyl bromide.<ref name=m_and_b/><ref name=s_and_h/> |
|||
:CH<sub>3</sub>CH<sub>2</sub>OH + SOCl<sub>2</sub> → CH<sub>3</sub>CH<sub>2</sub>Cl + SO<sub>2</sub> + HCl |
|||
===Ester formation=== |
|||
Under acid-catalyzed conditions, ethanol reacts with [[carboxylic acid]]s to produce ethyl [[ester]]s and water: |
|||
:[[carboxylic acid|RCOOH]] + HOCH<sub>2</sub>CH<sub>3</sub> → [[ester|RCOOCH<sub>2</sub>CH<sub>3</sub>]] + [[water|H<sub>2</sub>O]]. |
|||
For this reaction to produce useful yields it is necessary to remove water from the reaction mixture as it is formed. |
|||
Ethanol can also form esters with inorganic acids. [[Diethyl sulfate]] and [[triethyl phosphate]], prepared by reacting ethanol with [[sulfuric acid|sulfuric]] and [[phosphoric acid]] respectively, are both useful ethylating agents in [[organic synthesis]]. [[Ethyl nitrite]], prepared from the reaction of ethanol with [[sodium nitrite]] and [[sulfuric acid]], was formerly a widely-used [[diuretic]]. |
|||
===Dehydration=== |
|||
Strong acid desiccants, such as sulfuric acid, cause ethanol's dehydration to form either [[diethyl ether]] or [[ethylene]]: |
|||
:2 CH<sub>3</sub>CH<sub>2</sub>OH → [[diethyl ether|CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub>]] + [[water|H<sub>2</sub>O]] |
|||
:CH<sub>3</sub>CH<sub>2</sub>OH → [[ethylene|H<sub>2</sub>C=CH<sub>2</sub>]] + [[water|H<sub>2</sub>O]] |
|||
Which product, diethyl ether or ethylene, predominates depends on the precise reaction conditions. |
|||
===Oxidation=== |
|||
Ethanol can be oxidized to [[acetaldehyde]], and further oxidized to [[acetic acid]]. In the human body, these oxidation reactions are catalyzed by [[enzyme]]s. In the laboratory, aqueous solutions of strong oxidizing agents, such as [[chromic acid]] or [[potassium permanganate]], oxidize ethanol to acetic acid, and it is difficult to stop the reaction at acetaldehyde at high yield. Ethanol can be oxidized to acetaldehyde, without over oxidation to acetic acid, by reacting it with [[pyridinium chromic chloride]].<ref name="s_and_h"/> |
|||
The direct oxidation of ethanol to acetic acid using chromic acid is given below. |
|||
:C<sub>2</sub>H<sub>5</sub>OH + 2[O] → CH<sub>3</sub>COOH + H<sub>2</sub>O |
|||
The oxidation product of ethanol, acetic acid, is spent as nutrient by the human body as [[acetyl CoA]], where the acetyl group can be spent as energy or used for biosynthesis. |
|||
===Chlorination=== |
|||
When exposed to [[chlorine]], ethanol is both oxidized and its [[alpha carbon]] chlorinated to form the compound, [[chloral]]. |
|||
:4Cl<sub>2</sub> + C<sub>2</sub>H<sub>5</sub>OH → CCl<sub>3</sub>CHO + 5HCl |
|||
===Combustion=== |
|||
[[Combustion]] of ethanol forms [[carbon dioxide]] and [[water]]: |
|||
:C<sub>2</sub>H<sub>5</sub>OH(g) + 3 O<sub>2</sub>(g) → 2 CO<sub>2</sub>(g) + 3 H<sub>2</sub>O(l); (ΔH<sub>r</sub> = −1409 kJ/mol<ref>{{cite journal|title=Heats of Formation of Simple Organic Molecules|author=Frederick D. Rossini|journal=[[Ind. Eng. Chem.]]|year=1937|volume=29|issue=12|pages=1424–1430|doi=10.1021/ie50336a024}}</ref>) |
|||
==Production== |
|||
[[Image:Ethanol Flasche.jpg|thumb|left|94% denatured ethanol sold in a bottle for household use.]] |
|||
Ethanol is produced both as a [[petrochemical]], through the hydration of [[ethylene]], and biologically, by [[fermentation (biochemistry)|fermenting]] sugars with [[yeast]].<ref name="Mills-Ecklund">Mills, G.A.; Ecklund, E.E. "[http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.eg.12.110187.000403?journalCode=energy Alcohols as Components of Transportation Fuels]." ''Annual Review of Energy.'' November 1987. Vol. 12, 47–80. Retrieved on September 2, 2007.</ref> Which process is more economical is dependent upon the prevailing prices of petroleum and of grain feed stocks. |
|||
===Ethylene hydration=== |
|||
Ethanol for use as industrial feedstock is most often made from [[petrochemical]] feed stocks, typically by the [[acid]]-[[catalysis|catalyzed]] hydration of [[ethylene]], represented by the [[chemical equation]] |
|||
:[[ethylene|C<sub>2</sub>H<sub>4</sub>]]<sub>(g)</sub> + [[water|H<sub>2</sub>O]]<sub>(g)</sub> → CH<sub>3</sub>CH<sub>2</sub>OH<sub>(l)</sub>. |
|||
The catalyst is most commonly [[phosphoric acid]],<ref name=r_and_c>{{cite book|author=Roberts, John D.; Caserio, Marjorie C.|year=1977|publisher=W. A. Benjamin, Inc|title=Basic Principles of Organic Chemistry|isbn=0-8053-8329-8}}</ref> [[adsorption|adsorbed]] onto a porous support such as [[diatomaceous earth]] or [[charcoal]]. This catalyst was first used for large-scale ethanol production by the [[Shell Oil Company]] in 1947.<ref name="ECT4 820">Lodgsdon, J.E. (1994). "Ethanol." In J.I. Kroschwitz (Ed.) ''Encyclopedia of Chemical Technology, 4th ed.'' vol. 9, p. 820. New York: John Wiley & Sons.</ref> The reaction is carried out with an excess of high pressure steam at 300°C. |
|||
In an older process, first practiced on the industrial scale in 1930 by [[Union Carbide]],<ref name="ECT4 817">Lodgsdon, J.E. (1994). p. 817</ref> but now almost entirely obsolete, ethylene was hydrated indirectly by reacting it with concentrated [[sulfuric acid]] to produce [[ethyl sulfate]], which was then [[hydrolysis|hydrolyzed]] to yield ethanol and regenerate the sulfuric acid:<ref name=s_and_h/> |
|||
:[[ethylene|C<sub>2</sub>H<sub>4</sub>]] + [[sulfuric acid|H<sub>2</sub>SO<sub>4</sub>]] → [[ethyl sulfate|CH<sub>3</sub>CH<sub>2</sub>SO<sub>4</sub>H]] |
|||
:[[ethyl sulfate|CH<sub>3</sub>CH<sub>2</sub>SO<sub>4</sub>H]] + [[water|H<sub>2</sub>O]] → CH<sub>3</sub>CH<sub>2</sub>OH + [[sulfuric acid|H<sub>2</sub>SO<sub>4</sub>]] |
|||
===Fermentation=== |
|||
{{details|Ethanol fermentation}} |
|||
Ethanol for use in [[alcoholic beverage]]s, and the vast majority of ethanol for use as fuel, is produced by fermentation. When certain species of [[yeast]], most importantly, ''[[Saccharomyces cerevisiae]]'', [[metabolism|metabolize]] [[polysaccharide|sugar]] in the absence of [[oxygen]], they produce ethanol and [[carbon dioxide]]. The chemical equation below summarizes the conversion: |
|||
:[[glucose|C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>]] → 2 CH<sub>3</sub>CH<sub>2</sub>OH + 2 [[carbon dioxide|CO<sub>2</sub>]]. |
|||
The process of culturing yeast under conditions to produce alcohol is called [[fermentation]]. Ethanol's toxicity to yeast limits the ethanol concentration obtainable by brewing. The most ethanol-tolerant strains of yeast can survive up to approximately 15% ethanol by volume.<ref name="mosttolerant">Morais, P.B.; Rosa, C.A.; Linardi, V.R.; Carazza, F.; Nonato, E.A. "[http://www.springerlink.com/content/h32k825756g41318/ Production of fuel alcohol by Saccharomyces strains from tropical habitats]." ''Biotechnology Letters.'' November 1996. Vol. 18, No. 11, 1351–1356. Retrieved on September 2, 2007.</ref> |
|||
The fermentation process must exclude oxygen. If oxygen is present, yeast undergo [[aerobic respiration]] which produces [[carbon dioxide]] and water rather than ethanol. |
|||
In order to produce ethanol from starchy materials such as [[cereal grain]]s, the [[starch]] must first be converted into sugars. In brewing [[beer]], this has traditionally been accomplished by allowing the grain to germinate, or [[malt]], which produces the [[enzyme]], [[amylase]]. When the malted grain is [[mashing|mashed]], the amylase converts the remaining starches into sugars. For fuel ethanol, the hydrolysis of starch into glucose can be accomplished more rapidly by treatment with dilute sulfuric acid, [[fungi|fungally]] produced amylase, or some combination of the two.<ref name=hydrolysis>Badger, P.C. "[http://www.hort.purdue.edu/newcrop/ncnu02/v5-017.html Ethanol From Cellulose: A General Review]." p. 17–21. In: J. Janick and A. Whipkey (eds.), Trends in new crops and new uses. ASHS Press, 2002, Alexandria, VA. Retrieved on September 2, 2007.</ref> |
|||
===Cellulosic ethanol=== |
|||
{{main|Cellulosic ethanol}} |
|||
Sugars for [[ethanol fermentation]] can be obtained from [[cellulose]].<ref>Taherzadeh M.J., Karimi K. (2007): Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review, BioResources, 2(3): 472-499</ref><ref>Taherzadeh M.J., Karimi K. (2007): Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: A review, BioResources, 2(4): 707-738</ref> Until recently, however, the cost of the [[cellulase]] enzymes capable of hydrolyzing cellulose has been prohibitive. The Canadian firm [[Iogen Corp.|Iogen]] brought the first cellulose-based ethanol plant on-stream in 2004.<ref name=Ritter>Ritter, S.K. (May 31, 2004). "Biomass or Bust." ''Chemical & Engineering News'' '''82'''(22), 31–34.</ref> Its primary consumer so far has been the Canadian government, which, along with the [[United States Department of Energy]], has invested heavily in the commercialization of cellulosic ethanol. Deployment of this technology could turn a number of cellulose-containing agricultural by-products, such as [[corncob]]s, [[straw]], and [[sawdust]], into renewable energy resources. Other enzyme companies are developing genetically engineered fungi that produce large volumes of cellulase, xylanase, and hemicellulase enzymes. These would convert agricultural residues such as [[corn stover]], wheat straw, and sugar cane bagasse and energy crops such as [[switchgrass]] into fermentable sugars.<ref>{{cite web|author=Clines, Tom|title=Brew Better Ethanol|publisher=Popular Science Online|month=July|year=2006|url=http://www.popsci.com/popsci/energy/6756226d360ab010vgnvcm1000004eecbccdrcrd.html}}</ref> |
|||
Cellulose-bearing materials typically also contain other [[polysaccharide]]s, including [[hemicellulose]]. When [[hydrolysis|hydrolyzed]], hemicellulose decomposes into mostly five-carbon sugars such as [[xylose]]. ''S. cerevisiae'', the yeast most commonly used for ethanol production, cannot metabolize xylose. Other yeasts and bacteria are under investigation to ferment xylose and other [[pentose]]s into ethanol.<!--<ref>{{cite web|url=http://www.lub.lu.se/cgi-bin/show_diss.pl?db=global&fname=tec_748.html|title=www.lub.lu.se/cgi-bin/show_diss.pl?db=global&fname=tec_748.html}}</ref> --><ref>{{cite web|url=http://www.metabolicengineering.gov/me2001/2001Kompala.pdf|title=Maximizing Ethanol Production by Engineered Pentose-Fermenting ''Zymononas mobilis''|author=Dhinakar S. Kompala|publisher=Department of Chemical Engineering, University of Colorado at Boulder|accessdaymonth=May 21|accessyear=2007|format=PDF}}</ref> |
|||
On January 14, 2008, [[General Motors]] announced a partnership with Coskata, Inc. The goal is to produce cellulosic ethanol cheaply, with an eventual goal of US$1 per U.S. gallon ($0.30/L) for the fuel. The partnership plans to begin producing the fuel in large quantity by the end of 2008. By 2011 a full-scale plant will come on line, capable of producing 50 to 100 million gallons of ethanol a year (200–400 [[megalitre|ML]]/[[year|a]]).<ref>{{cite web|author=Mick, Jason|title=Cellulosic Ethanol Promises $1 per Gallon Fuel From Waste|date=[[2008-01-14]]|work=DailyTech.com |url=http://www.dailytech.com/Cellulosic+Ethanol+Promises+1+per+Gallon+Fuel+From+Waste/article10320.htm|accessdate=2008-01-15}}</ref> |
|||
===Prospective technologies=== |
|||
[[Image:SDethnl1.jpg|thumb|left|Ethanol plant in [[Turner County, South Dakota|Turner County]], [[South Dakota]]]] |
|||
The [[anaerobic bacteria|anaerobic bacterium]] ''[[Clostridium]] ljungdahlii'', recently discovered in commercial chicken wastes, can produce ethanol from single-carbon sources including [[synthesis gas]], a mixture of [[carbon monoxide]] and [[hydrogen]] that can be generated from the partial [[combustion]] of either [[fossil fuel]]s or [[biomass]]. Use of these bacteria to produce ethanol from synthesis gas has progressed to the pilot plant stage at the BRI Energy facility in [[Fayetteville, Arkansas|Fayetteville]], [[Arkansas]].<ref>{{cite web|url=http://www.brienergy.com/|title=Providing for a Sustainable Energy Future|publisher=Bioengineering Resources, inc|accessdaymonth=May 21|accessyear=2007}}</ref> |
|||
Another prospective technology is the closed-loop ethanol plant.<ref name="clAlc">{{cite journal|url=http://www.renewableenergyaccess.com/rea/news/story?id=46414|title= Closed-Loop Ethanol Plant to Start Production|date=November 2, 2006|publisher=www.renewableenergyaccess.com|accessdate = 2007-09-03}}</ref> Ethanol produced from corn has a number of critics who suggest that it is primarily just recycled fossil fuels because of the energy required to grow the grain and convert it into ethanol. There is also the issue of competition with use of corn for food production. However, the closed-loop ethanol plant attempts to address this criticism. In a closed-loop plant, the energy for the distillation comes from fermented manure, produced from cattle that have been fed the by-products from the distillation. The leftover manure is then used to fertilize the soil used to grow the grain. Such a process is expected to lower the fossil fuel consumption used during conversion to ethanol by 75%.<ref name="Rapier-1">Rapier, R. (June 26, 2006) [http://i-r-squared.blogspot.com/2006/06/e3-biofuels-responsible-ethanol.html "E3 Biofuels: Responsible Ethanol"] R-Squared Energy Blog</ref> Although [[Anaerobic digestion|energy]] can be created from the collection of [[methane]] from livestock manure, this can be mutually exclusive to the production of ethanol and should not be tagged on to it to make ethanol production seem more efficient or enviromentally friendly. |
|||
Though in an early stage of research, there is some development of alternative production methods that use feed stocks such as municipal waste or recycled products, rice hulls, sugarcane bagasse, small diameter trees, wood chips, and switchgrass.<ref>{{cite web|url=http://www.truthout.org/issues_06/041607ED.shtml|title=Air Pollution Rules Relaxed for US Ethanol Producers|publisher=Truthout|date=2007-04-12|accessdaymonth=May 21|accessyear=2007}}</ref> |
|||
===Testing=== |
|||
[[Image:Ethanol near IR spectrum.png|thumb|right|240px|[[Near infrared spectrum]] of liquid ethanol.]] |
|||
Breweries and [[biofuel]] plants employ two methods for measuring ethanol concentration. Infrared ethanol sensors measure the vibrational frequency of dissolved ethanol using the CH band at 2900 cm<sup>−1</sup>. This method uses a relatively inexpensive solid state sensor that compares the CH band with a reference band to calculate the ethanol content. The calculation makes use of the [[Beer-Lambert law]]. Alternatively, by measuring the density of the starting material and the density of the product, using a [[hydrometer]], the change in specific gravity during fermentation indicates the alcohol content. This inexpensive and indirect method has a long history in the beer brewing industry. |
|||
===Purification=== |
|||
{{main|Ethanol purification}} |
|||
Ethylene hydration or brewing produces an ethanol–water mixture. For most industrial and fuel uses, the ethanol must be purified. [[Fractional distillation]] can concentrate ethanol to 95.6% by weight (89.5 mole%). This mixture is an [[azeotrope]] with a boiling point of 78.1 °C, and cannot be further purified by distillation. |
|||
In one common industrial method to obtain absolute alcohol, a small quantity of [[benzene]] is added to [[rectified spirit]] and the mixture is then distilled. Absolute alcohol is obtained in the third fraction, which distills over at 78.3 °C (351.4 K).<ref name="m_and_b">{{cite book|author=Morrison, Robert Thornton; Boyd, Robert Neilson|title=Organic Chemistry, 2nd ed.|year=1972|publisher=Allyn and Bacon, inc.}}</ref> Because a small amount of the benzene used remains in the solution, absolute alcohol produced by this method is not suitable for consumption, as benzene is [[carcinogenic]].<ref>{{cite journal |author=Snyder R, Kalf GF |title=A perspective on benzene leukemogenesis |journal=Crit. Rev. Toxicol. |volume=24 |issue=3 |pages=177–209 |year=1994 |pmid=7945890 |doi=10.3109/10408449409021605}}</ref> |
|||
There is also an absolute alcohol production process by [[desiccation]] using [[glycerol]]. Alcohol produced by this method is known as spectroscopic alcohol—so called because the absence of benzene makes it suitable as a solvent in [[spectroscopy]]. |
|||
Other methods for obtaining absolute ethanol include desiccation using adsorbents such as starch or [[zeolite]]s, which adsorb water preferentially, as well as [[azeotropic distillation]] and [[extractive distillation]].<br clear=all> |
|||
==Grades of ethanol== |
|||
===Denatured alcohol=== |
|||
{{Main|Denatured alcohol}} |
|||
Pure ethanol and alcoholic beverages are heavily taxed, but ethanol has many uses that do not involve consumption by humans. To relieve the tax burden on these uses, most jurisdictions waive the tax when an agent has been added to the ethanol to render it unfit to drink. These include bittering agents such as [[denatonium benzoate]] and toxins such as [[methanol]], [[naphtha]], and [[pyridine]]. Products of this kind are called ''denatured alcohol.''<ref>{{cite web|url=http://www.procurement.umich.edu/Contracts/Denatured_Alchohol.pdf|title=U-M Program to Reduce the Consumption of Tax-free Alcohol; Denatured Alcohol a Safer, Less Expensive Alternative|publisher=University of Michigan|accessdate=2007-09-29|format=PDF}}</ref><ref>Great Britain (2005). ''[http://www.opsi.gov.uk/si/si2005/20051524.htm The Denatured Alcohol Regulations 2005].'' Statutory Instrument 2005 No. 1524.</ref> |
|||
===Absolute ethanol=== |
|||
Absolute or anhydrous alcohol generally refers to purified ethanol, containing no more than one percent [[water]]. Absolute alcohol not intended for human consumption often contains trace amounts of toxic benzene (used to remove water by [[azeotropic distillation]]). Consumption of this form of ethanol can be fatal over a short time period. Generally this kind of ethanol is used as solvents for lab and industrial settings where water will disrupt a desired reaction. |
|||
Pure ethanol is classed as 200 [[proof (alcohol)|proof]] in the USA, equivalent to 175 degrees proof in the UK system. |
|||
==Use== |
|||
===As a fuel=== |
|||
{|class=wikitable style="float:right; margin-left:1em; text-align:right" |
|||
!colspan=4|[[Energy density|Energy content]] of some fuels compared with ethanol:<ref name=TEDB>[http://www-cta.ornl.gov/data/Appendix_B.html Appendix B, Transportation Energy Data Book] from the [[Center for Transportation Analysis]] of the [[Oak Ridge National Laboratory]]</ref> |
|||
|- |
|- |
||
! align=left|Fuel type || MJ/L || MJ/kg || [[octane rating|Research<br>octane<br>number]] |
|||
! Year !! Months !! [[Dominical Letter|Dominical</br>Letter]] |
|||
|- |
|- |
||
|align=left|[[Methanol]]||17.9||19.9||123 |
|||
| 2001 || April, July || [[Common year starting on Monday|G]] |
|||
|- |
|||
| 2002 || September, December || [[Common year starting on Tuesday|F]] |
|||
|- |
|||
| 2003 || June || [[Common year starting on Wednesday|E]] |
|||
|- |
|||
| 2004 || February, August || [[Leap year starting on Thursday|DC]] |
|||
|- |
|||
| 2005 || May || [[Common year starting on Saturday|B]] |
|||
|- |
|||
| 2006 || January, October || [[Common year starting on Sunday|A]] |
|||
|- |
|||
| 2007 || April, July || [[Common year starting on Monday|G]] |
|||
|- |
|||
| 2008 || June || [[Leap year starting on Tuesday|FE]] |
|||
|- |
|||
| 2009 || February, March, November || [[Common year starting on Thursday|D]] |
|||
|- |
|||
| 2010 || August || [[Common year starting on Friday|C]] |
|||
|- |
|- |
||
|align=left|[[ethanol fuel|Ethanol]]||23.5||<ref>Calculated from heats of formation. Does not correspond exactly to the figure for MJ/l divided by density.</ref>31.1||129 |
|||
| 2011 || May || [[Common year starting on Saturday|B]] |
|||
|- |
|- |
||
|align=left|[[Liquefied natural gas]]||25.3||~55|| |
|||
| 2012 || January, April, July || [[Leap year starting on Sunday|AG]] |
|||
|- |
|- |
||
|align=left|[[Autogas]] ([[Liquified petroleum gas|LPG]])<br>(60% [[Propane]] + 40% [[Butane]])||26.8||50.|| |
|||
| 2013 || September, December || [[Common year starting on Tuesday|F]] |
|||
|- |
|- |
||
|align=left|[[Aviation gasoline]]<br>(high-octane gasoline, not jet fuel)||33.5||46.8|| |
|||
| 2014 || June || [[Common year starting on Wednesday|E]] |
|||
|- |
|- |
||
|align=left|[[Alcohol fuel|Gasohol]]<br>(90% gasoline + 10% ethanol)||33.7||47.1||93/94 |
|||
| 2015 || February, March, November || [[Common year starting on Thursday|D]] |
|||
|- |
|- |
||
|align=left|Regular Gasoline||34.8||<ref>[http://www.eere.energy.gov/hydrogenandfuelcells/pdfs/storage.pdf|Thomas, George. Overview of Storage Development DOE Hydrogen Program [pdf]. Livermore, CA. Sandia National Laboratories. 2000.]</ref>44.4||min. 91 |
|||
| 2016 || May || [[Leap year starting on Friday|CB]] |
|||
|- |
|- |
||
|align=left|Premium Gasoline||||||max. 95 |
|||
| 2017 || January, October || [[Common year starting on Sunday|A]] |
|||
|- |
|- |
||
|align=left|[[Diesel]]||38.6||45.4||25 |
|||
| 2018 || April, July || [[Common year starting on Monday|G]] |
|||
|- |
|- |
||
|align=left|[[Charcoal]], extruded||50||23|| |
|||
| 2019 || September, December || [[Common year starting on Tuesday|F]] |
|||
|- |
|||
| 2020 || March, November || [[Leap year starting on Wednesday|ED]] |
|||
|- |
|||
| 2021 || August || [[Common year starting on Friday|C]] |
|||
|- |
|||
| 2022 || May || [[Common year starting on Saturday|B]] |
|||
|- |
|||
| 2023 || January, October || [[Common year starting on Sunday|A]] |
|||
|- |
|||
| 2024 || September, December || [[Leap year starting on Monday|GF]] |
|||
|- |
|||
| 2025 || June || [[Common year starting on Wednesday|E]] |
|||
|- |
|||
| 2026 || February, March, November || [[Common year starting on Thursday|D]] |
|||
|- |
|||
| 2027 || August || [[Common year starting on Friday|C]] |
|||
|- |
|||
| 2028 || October || [[Leap year starting on Saturday|BA]] |
|||
|} |
|} |
||
{{main|Ethanol fuel}} |
|||
</td></tr></table> |
|||
The largest single use of ethanol is as a motor [[fuel]] and [[fuel additive]]. The largest national fuel ethanol industries exist in [[Brazil]] (gasoline sold in Brazil contains at least 25% ethanol and anhydrous ethanol is also used as fuel in more than 90% of new cars sold in the country). The Brazilian production of ethanol is praised for the high [[carbon sequestration]] capabilities of the [[sugar cane]] [[plantations]], thus making it a real option to combat [[climate change]].<ref name="WaPo-Brazil">Reel, M. (August 19, 2006) [http://www.washingtonpost.com/wp-dyn/content/article/2006/08/19/AR2006081900842.html "Brazil's Road to Energy Independence"], ''[[Washington Post]]''.</ref> |
|||
This sequence, here given for 2001–2028, repeats every 28 years from 1901 to 2099. The months with a Friday the 13th are determined by the [[Dominical letter]] (G, F, GF, etc.) of the year. Any month that begins on a Sunday will contain a Friday the 13th. |
|||
[[Henry Ford]] designed the first mass-produced automobile, the famed Model T Ford, to run on pure anhydrous (ethanol) alcohol—he said it was "the fuel of the future". Today, however, 100% pure ethanol is not approved as a motor vehicle fuel in the U.S. '''Added to gasoline, ethanol reduces ground-level ozone formation''' by lowering volatile organic compound and hydrocarbon emissions, decreasing carcinogenic benzene, and butadiene, emissions, and particulate matter emissions from gasoline combustion.<ref>{{cite web|url=http://journeytoforever.org/ethanol.html#E|title=Etnanol Fuel}}</ref> |
|||
Every year has at least one and at most three Fridays the 13th, with 688 occurrences during each 400-year [[Gregorian calendar|Gregorian]] cycle (146,097 days).{{Fact|date=May 2008}} |
|||
Combustion of ethanol in an internal combustion engine yields many of the products of incomplete combustion that are produced by gasoline and significantly larger amounts of [[formaldehyde]] and related species such as [[formalin]], [[acetaldehyde]], etc..<ref>California Air Resources Board,Definition of a Low Emission Motor Vehicle in Compliance with the Mandates of Health and Safety Code Section 39037.05,second release, October 1989</ref> This leads to a significantly larger photochemical reactivity that '''generates much more ground level [[ozone]].'''<ref>A.Lowi& W.P.L.Carter; A Method for Evaluating the Atmospheric Ozone Impact of Actual Vehicle emissions, S.A.E. Technical Paper, Warrendale,PA; march 1990</ref> This data has been assembled into The Clean Fuels Report comparison of fuel emissions<ref>T.T.M.Jones,The Clean Fuels Report: A Quantitative Comparison Of Motor Fuels, Related Pollution and Technologies: 2008.[http://www.cleanfuelsreport.com]</ref> and shows that ethanol exhaust generates 2.14 times as much ozone as does gasoline exhaust. When this is added into the custom "Localised Pollution Index (LPI)" of The Clean Fuels Report the local pollution, i.e. that which contributes to smog, is 1.7 on a scale where gasoline is 1.0 and higher numbers signify greater pollution. This issue has been formalised by the [[California Air Resouces Board]] in 2008<ref>citation needed</ref> by recognising control standards for formaldehydes et al as an emissions control group much like the conventional [[NOx]] and Reactive Organic Gases (ROGs). |
|||
The longest period that can occur without a Friday the 13th is fourteen months, either from July to September the following year (e.g. in 2001/2002 and 2012/13), or from August to October in a leap year (e.g. in 2027/28). |
|||
Prior to the development of [[electronic fuel injection]] (EFI) and computerized engine management, the lower energy content of ethanol required that the engine [[carburetor]] be ''rejetted'' to permit a larger volume of fuel to mix with the intake air. EFI is able to actively compensate for varying fuel energy densities by [[oxygen sensor| monitoring the oxygen content]] of exhaust gases. However, a standard EFI gasoline engine can typically only tolerate up to 10% ethanol and 90% gasoline. Higher ethanol ratios require either larger-volume [[fuel injector]]s or an increase in [[fuel rail]] pressure to deliver the greater liquid volume needed to equal the energy content of pure gasoline. |
|||
<table border=0><tr><td valign=top> |
|||
Patterns for non leap-years: |
|||
[[Image:Sao Paulo ethanol pump 04 2008 74 zoom.jpg|thumb|left|Ethanol pump station in [[Sao Paulo, Brazil]] where the fuel is available commercially.]] |
|||
{| class=wikitable |
|||
World production of ethanol in 2006 was {{convert|51|GL|usgal}}, with 69% of the world supply coming from Brazil and the United States.<ref>{{cite web|url=http://www.ethanolrfa.org/industry/statistics/#E|title=Renewable Fuels Association Industry Statistics}}</ref> More than 20% of the Brazilian fleet of cars on the streets are able to use 100% ethanol as fuel, which includes ethanol-only engines and [[Flexible-fuel vehicle|flex-fuel]] engines.<ref>{{cite web|url=http://www.estadao.com.br/economia/not_eco178105,0.htm|title=Tecnologia flex atrai estrangeiros|Publisher=Agência Estado}}</ref> Flex-fuel engines in Brazil are able to work with all ethanol, all gasoline or any mixture of both. In the US flex-fuel vehicles can run on 0% to 85% ethanol (15% gasoline) since higher ethanol blends are not yet allowed. Brazil supports this population of ethanol-burning automobiles with large national infrastructure that produces ethanol from domestically grown [[sugar cane]]. [[Sugar cane]] not only has a greater concentration of sucrose than corn (by about 30%), but is also much easier to extract. The [[bagasse]] generated by the process is not wasted, but is utilized in power plants as a surprisingly efficient fuel to produce electricity. |
|||
|- |
|||
! First month occurring !! Second month !! Third month |
|||
|- |
|||
|January||October|| |
|||
|- |
|||
|February||March||November |
|||
|- |
|||
|April||July|| |
|||
|- |
|||
|May|||| |
|||
|- |
|||
|June|||| |
|||
|- |
|||
|August|||| |
|||
|- |
|||
|September||December|| |
|||
|- |
|||
|} |
|||
[[Image:Ethanol Car.jpg|thumb|right|A [[Ford Taurus]] "fueled by clean burning ethanol" owned by [[New York City]].]] |
|||
</td><td valign=top> |
|||
[[Image:USPS-E85 fuel-St Paul-20070127.jpg|thumb|right|[[United States Postal Service]] vehicle running on [[E85]], a "flex-fuel" blend in [[Saint Paul, Minnesota|Saint Paul]], [[Minnesota]].]] |
|||
Patterns for leap years: |
|||
The United States fuel ethanol industry is based largely on [[Maize|corn]]. According to the Renewable Fuels Association, as of October 30, 2007, 131 grain ethanol bio-refineries in the United States have the capacity to produce 7.0 billion US gallons (26 GL) of ethanol per year. An additional 72 construction projects underway (in the U.S.) can add 6.4 billion gallons of new capacity in the next 18 months. Over time, it is believed that a material portion of the ~150 billion gallon per year market for gasoline will begin to be replaced with fuel ethanol.<ref name="rfa1">{{cite web|url=http://www.ethanolrfa.org/media/press/rfa/view.php?id=909|title=First Commercial U.S. Cellulosic Ethanol Biorefinery Announced|date=2006-11-20|publisher=Renewable Fuels Association|accessdaymonth=May 21|accessyear=2006}}</ref> |
|||
{| class=wikitable |
|||
|- |
|||
! First month occurring !! Second month !! Third month |
|||
|- |
|||
|January||April||July |
|||
|- |
|||
|February||August|| |
|||
|- |
|||
|March||November|| |
|||
|- |
|||
|May|||| |
|||
|- |
|||
|June|||| |
|||
|- |
|||
|September||December|| |
|||
|- |
|||
|October|||| |
|||
|} |
|||
</td></tr></table> |
|||
The [[Energy Policy Act of 2005]] requires that 4 billion gallons of "renewable fuel" be used in 2006 and this requirement will grow to a yearly production of 7.5 billion gallons by 2012.<ref name="epa1">{{cite web|url=http://www.epa.gov/otaq/renewablefuels/|title= Renewable Fuel Standard Program|publisher=United States Environmental Protection Agency|date=2007-04-10|accessdaymonth=May 21|accessyear=2007}}</ref> In the United States, ethanol is most commonly blended with gasoline as a 10% ethanol blend nicknamed "gasohol". This blend is widely sold throughout the U.S. [[Midwest]], and in cities required by the [[1990 Clean Air Act]] to oxygenate their gasoline during the winter.{{citation}} Ethanol and [[isobutene]] are also the feedstocks for [[ethyl tert-butyl ether]] (ETBE), an oxygenate antiknock additive. The use of ethanol makes ETBE partially a biofuel, but also more expensive than the similar additive [[methyl tert-butyl ether]] (MTBE), made from [[methanol]] and isobutene.{{citation}} |
|||
Each [[Gregorian calendar|Gregorian]] 400-year cycle contains 146,097 days (365 * 400 = 146,000 normal days plus 97 [[leap year|leap days]]), 146,097 / 7 = 20,871 weeks, and 400 * 12 = 4,800 months. Thus, each cycle contains the same pattern of days of the week (and thus the same pattern of Fridays the 13th), but no day of the month up to the 28th can occur the same number of times on each day of the week (because 4,800 is not divisible by 7). The 13th day of the month is slightly more likely to be a Friday than any other day of the week.<ref>B. H. Brown, "Solution to Problem E36", ''American Mathematical Monthly'', vol. 40, issue 10 (1933), p. 607; [[Jean Meeus]], ''Mathematical Astronomy Morsels IV'', 2007, p. 367.</ref> On average, there is a Friday the 13th once every 212.35 (212 and 241/688) days. |
|||
====Food versus fuel debate==== |
|||
The distribution of the 13th day over the 4,800 months is as follows: |
|||
{{further|[[Food vs fuel]]}} |
|||
{| class=wikitable |
|||
It is disputed whether [[corn ethanol]] as an automotive fuel results in a net energy gain or loss. As reported in "The Energy Balance of Corn Ethanol: an Update,"<ref>{{cite web|url=http://www.transportation.anl.gov/pdfs/AF/265.pdf|title=The Energy Balance of Corn Ethanol: an Update|publisher=United States Department of Agriculture|accessdaymonth=May 21|accessyear=2007|author=Hosein Shapouri, James A. Duffield, and Michael Wang|format=PDF}}</ref> the energy returned on energy invested ([[EROEI]]) for ethanol made from corn in the U.S. is 1.34 (it yields 34% more energy than it takes to produce it). Input energy includes natural gas based fertilizers, farm equipment, transformation from corn or other materials, and transportation. However, other researchers report that the production of ethanol consumes more energy than it yields.<ref name=Pimentel2005>{{cite journal |author = Pimentel D, Patzek TW |year = 2005 |title = Ethanol Production Using Corn, Switchgrass, and Wood; Biodiesel Production Using Soybean and Sunflower |journal = Natural Resources Research |volume = 14 |issue = 1 |pages = 65–76 |doi = 10.1007/s11053-005-4679-8}}</ref><ref>{{cite web|url=http://www.news.cornell.edu/stories/July05/ethanol.toocostly.ssl.html|title=Cornell ecologist's study finds that producing ethanol and biodiesel from corn and other crops is not worth the energy|publisher=Cornell University|accessdaymonth=July 5|accessyear=2005|author=Lang, Susan S.}}</ref> In comparison, sugar cane ethanol EROEI is at around 8 (it yields 8 joules for each joule used to produce it).{{Fact|date=April 2008}} Recent research suggests that cellulosic crops such as [[switchgrass]] provide a much better net energy production than corn, producing over five times as much energy as the total used to produce the crop and convert it to fuel.<ref>{{cite web|url=http://www.pnas.org/cgi/reprint/0704767105v1 |title=Net energy of cellulosic ethanol from switchgrass|author=M.R. Schmer, K.P. Vogel, R.B. Mitchell, R.K. Perrin|publisher=U.S. Dept. of Agrigulture|date=2007-11-21|accessdate = 2008-01-13}}</ref> If this research is confirmed, cellulosic crops will most likely displace corn as the main fuel crop for producing bioethanol. |
|||
|- |
|||
Michael Grunwald reports that one person could be fed 365 days "on the corn needed to fill an ethanol-fueled SUV".<ref>''The Clean Energy Scam'', '''TIME''', April 7, 2008, pages 40–41. </ref> He further reports that though "hyped as an eco-friendly fuel, ethanol increases global warming, destroys forests and inflates food prices." Environmentalists, livestock farmers, and opponents of subsidies say that increased ethanol production won't meet energy goals and may damage the environment, while at the same time causing worldwide food prices to soar. Some of the controversial subsidies in the past have included more than $10 billion to [[Archer-Daniels-Midland]] since 1980.<ref>{{cite web|url=http://www.cato.org/pub_display.php?pub_id=6079|title=Ethanol Keeps ADM Drunk On Tax Dollars.|publisher=CATO Institute|author=Doug Bandow|date=1997-10-02|accessdate = 2007-09-03}}</ref>{{POV-statement|date=April 2008}}<!-- Why is this subsidy controversial? Don't a lot of farming-related operations get such subsidies? --> Critics also speculate that as ethanol is more widely used, changing irrigation practices could greatly increase pressure on water resources. In October 2007, 28 environmental groups decried the Renewable Fuels Standard (RFS), a legislative effort intended to increase ethanol production, and said that the measure will "lead to substantial environmental damage and a system of biofuels production that will not benefit family farmers...will not promote sustainable agriculture and will not mitigate global climate change."<ref name=csm>[http://www.csmonitor.com/2007/1115/p02s02-uspo.html The Politics of Ethanol Outshine its Costs]</ref><ref name="bw0307">{{cite journal|journal=Business Week|title=Ethanol's Growing List of Enemies|url=http://www.businessweek.com/bwdaily/dnflash/content/mar2007/db20070316_016207.htm?campaign_id=rss_topStories|date=March 19, 2007|accessdate = 2007-09-03|author=Moira Herbst}}</ref> |
|||
! Day of the week !! Number of occurrences |
|||
|- |
|||
Recent articles have also blamed subsidized ethanol production for the nearly 200% increase in milk prices since 2004,<ref name="cnn0607">{{cite journal|journal=CNNMoney.com|title=Corn and milk: A 1-2 inflation combo|url=http://money.cnn.com/2007/06/19/news/economy/commodity_prices/index.htm|date=June 19, 2007|accessdate = 2007-09-03|author=Jeff Cox}}</ref> although that is disputed by some{{Fact|date=March 2008}}. Especially since the price of fuel has driven up the costs to cultivate, grow, harvest, ship, refine, bring to market, etc, all commodities including; but not limited to, milk. Not to mention the presence of speculators, and the recent growing interest in the commodities market by investors who have been scared away from a falling stock market. |
|||
|Sunday||687 |
|||
|- |
|||
Ethanol production uses the starch portion of corn, but the leftover protein can be used to create a high-nutrient, low-cost animal feed.<ref>[http://www.npr.org/templates/story/story.php?storyId=89598524 Fuel, Food Demand Raise Corn, Soybean Prices]</ref> |
|||
|Monday||685 |
|||
|- |
|||
In 2007 the United Nations' independent expert on the right to food, called for a five-year moratorium on biofuel production from food crops, to allow time for development of non-food sources. He called recent increases in food costs because of fuel production, such as the quadrupling of world corn price in one year, a growing "catastrophe" for the poor.<ref>{{cite news | url = http://www.livescience.com/environment/071027-ap-biofuel-crime.html | title=UN Expert Calls Biofuel 'Crime Against Humanity' | author = Edith M. Lederer, Associated Press | date = 2007-10-27 }}</ref> In February 2007, [[2007–2008 world food price crisis|riots]] occurred in Mexico because of the skyrocketing price of tortillas. Ethanol has been credited as the reason for this increase in food prices<ref>http://news.bbc.co.uk/2/hi/americas/6319093.stm</ref>. The demand for corn has had a rippling effect on many corn-based products, like tortillas. The effects of ethanol and the increasing cost of food have also been felt in Pakistan, Indonesia, and Egypt.<ref>{{cite web|url=http://www.openmarket.org/2008/04/08/ethanol-subsidies-cause-food-riots-in-mexico-pakistan-indonesia-yemen-and-egypt|title=Ethanol Subsidies Cause Food Riots in Mexico, Pakistan, Indonesia, Yemen, and Egypt|author=Posted by Hans Bader|publisher=Open Market blog|date=2008-04-08}}</ref> |
|||
|Tuesday||685 |
|||
|- |
|||
Oil has historically had a much higher [[EROEI]] than corn produced ethanol, according to some{{Fact|date=March 2008}}. However, oil must be refined into gasoline before it can be used for automobile fuel. Refining, as well as exploration and drilling, consumes energy. The difference between the energy in the fuel (output energy) and the energy needed to produce it (input energy) is often expressed as a percent of the input energy and called net energy gain (or loss). Several studies released in 2002 estimated that the net energy gain for [[corn ethanol]] is between 21 and 34 percent. The net energy loss for [[MTBE]] is about 33 percent. When added to gasoline, ethanol can replace MTBE as an anti-knock agent without poisoning drinking water as MTBE does. In Brazil, where the broadest and longest ethanol producing experiment took place, improvements in agricultural practices and ethanol production improvements led to an increase in ethanol net energy gain from 300% to over 800% in recent years.{{Fact|date=May 2008}} It must be noted that Brazil produces ethanol more efficiently because its primary input is the sugar from sugar cane rather than starches from corn. Consuming known oil reserves is increasing oil exploration and drilling energy consumption which is reducing [[EROEI|oil EROEI]] (and [[energy balance]]) further.<ref>{{cite web|url=http://oregon.gov/ENERGY/RENEW/Biomass/forum.shtml|title=Ethanol Energy Balances|author=David Andress & Associates|month=November | year=2002}}</ref> |
|||
|Wednesday||687 |
|||
|- |
|||
Opponents claim that corn ethanol production does not result in a net energy gain or that the consequences of large scale ethanol production to the food industry and environment offset any potential gains from ethanol. It has been estimated that "if every bushel of U.S. [[maize|corn]], [[wheat]], [[rice]] and [[soybean]] were used to produce ethanol, it would only cover about 4% of [[Energy policy of the United States|U.S. energy needs]] on a net basis."<ref>{{cite news|url=http://www.bloomberg.com/apps/news?pid=20601039&refer=columnist_wasik&sid=aOS8e5kvDESE|title=Forget the Ethanol Myth -- Avoid Biofuel Bubble: John F. Wasik|date=2007-07-23|publisher=Bloomberg.com|accessdate = 2007-07-25}}</ref> Many of the issues raised could likely be fixed by techniques now in development that produce ethanol from agricultural waste, such as paper waste, switchgrass, and [[Energy crop|other materials]], but EIA Forecasts Significant Shortfall in Cellulosic Biofuel Production Compared to Target Set by Renewable Fuel Standard.<ref>{{cite web|url=http://www.greencarcongress.com/cellulosic_ethanol/index.html|title=Study Finds Net Energy of Cellulosic Ethanol from Switchgrass Much Higher Than Expected|date=2008-01-07|publisher=Green Car Congress|accessdate = 2008-01-13}}</ref> |
|||
|Thursday||684 |
|||
|- |
|||
Proponents cite the potential gains to the U.S. economy both from domestic fuel production and increased demand for corn. Optimistic calculations project that the United States is capable of producing enough ethanol to completely replace gasoline consumption.{{Fact|date=April 2008}} In comparison, Brazil's ethanol consumption today covers more than 50% of all energy used by vehicles in that country. |
|||
|'''Friday'''||'''688''' |
|||
|- |
|||
In the United States, preferential regulatory and tax treatment of ethanol automotive fuels introduces complexities beyond its energy economics alone. North American automakers have in 2006 and 2007 promoted a blend of 85% ethanol and 15% gasoline, marketed as [[E85]], and their [[Flexible-fuel vehicle|flex-fuel vehicles]], ''e.g.'' [[General Motors|GM's]] "[http://www.livegreengoyellow.com/ Live Green, Go Yellow]" campaign.<ref name=autochannel>{{cite web|title=GM Announces E85 Fuel Card Promotion On FlexFuel Vehicles|url=http://theautochannel.com/news/2006/05/02/005502.html|accessdate = 2007-09-04|publisher=The Auto Channel}}</ref> The apparent motivation is the nature of U.S. [[CAFE|Corporate Average Fuel Economy (CAFE)]] standards, which give an effective 54% fuel efficiency bonus to vehicles capable of running on 85% alcohol blends over vehicles not adapted to run on 85% alcohol blends.<ref>{{cite web|title=CAFE Credits for Flex Fuel Vehicles Undermine Improvements in Fuel Economy|publisher=Public Citizen|date=[[2006-09-27]]|url =http://www.citizen.org/autosafety/vehicles/enviro/articles.cfm?ID=15763| accessdate=2007-09-03}}</ref> In addition to this auto manufacturer-driven impetus for 85% alcohol blends, the [[United States Environmental Protection Agency]] had authority to mandate that minimum proportions of oxygenates be added to automotive gasoline on regional and seasonal bases from 1992 until 2006 in an attempt to reduce air pollution, in particular [[ground-level ozone]] and [[smog]].<ref "epars">{{cite web|url=http://www.epa.gov/otaq/rfg_regs.htm|title=Regulations & Standards|publisher=United States Environmental Protection Agency|accessdate=2007-09-04}}</ref> In the United States, incidents of methyl tert(iary)-butyl ether ([[MTBE]]) groundwater contamination have been recorded in the majority of the 50 states,<ref>{{cite web|url=http://gao.gov/new.items/d02753t.pdf|title=MTBE Contamination from Underground Storage Tanks|publisher=United States General Accounting Office|date=2002-05-21|accessdate =2007-10-09|format=PDF}}</ref> and the State of [[California]]'s ban on the use of MTBE as a gasoline additive has further driven the more widespread use of ethanol as the most common fuel oxygenate.<ref name="epamtbe">{{cite web|url=http://www.epa.gov/mtbe/faq.htm|title=Methyl Tertiary Butyl Ether (MTBE)|publisher=United State Environmental Protection Agency|accessdate = 2007-09-04}}</ref> |
|||
|Saturday||684 |
|||
|} |
|||
A February 7, 2008 [[Associated Press]] article stated, "The widespread use of ethanol from corn could result in nearly twice the greenhouse gas emissions as the gasoline it would replace because of expected land-use changes, researchers concluded Thursday. The study challenges the rush to biofuels as a response to global warming."<ref>[http://www.guardian.co.uk/worldlatest/story/0,,-7291645,00.html Study: Ethanol May Add to Global Warming] Associated Press, February 7, 2008</ref> |
|||
One acre of land can yield about 7,110 pounds (3,225 kg) of corn, which can be processed into 328 gallons (1240.61 liters) of ethanol. That is about 26.1 pounds (11.84 kg) of corn per gallon. |
|||
Much overlooked in most discussions about ethanol from corn are the by-products from the production of ethanol. Depending on the way it is processed, the processing yields several beneficial products, some of which are used for food production and feedstocks. |
|||
==Planned events on Fridays the 13th== |
|||
Some events are intentionally scheduled for Friday the 13th for dramatic effect. They include: |
|||
===Ethanol fuel cells=== |
|||
* The 2008 [[Tamil language|Tamil]] block-buster film ''[[Dasavathaaram]]'' was released on Friday, [[June 13]], [[2008]] in India, Malaysia, Singapore, Australia, USA and UK. |
|||
{{main|Direct-ethanol fuel cell}} |
|||
*[[Black Sabbath]]'s eponymous [[Black Sabbath (album)|debut album]] was released in the UK on Friday, [[February 13]], [[1970]]. |
|||
Ethanol may be used as a fuel to power [[Direct-ethanol fuel cell]]s ([[Direct-ethanol fuel cell|DEFC]]) in order to produce electricity and the by-products of [[water]] ([[H2O|H<sub>2</sub>O]]) and [[carbon dioxide]] ([[carbon dioxide|CO<sub>2</sub>]]).<ref name="DEFC-chem">[http://www.fctec.com/fctec_types_dmfc.asp Direct Methanol Fuel Cells (DMFC)] FCTec.</ref> [[Platinum]] is commonly used as an [[anode]] in such fuel cells in order to achieve a [[power density]] that is comparable to competing technologies. Until recently the high price of platinum has been cost prohibitive. A company called [http://www.acta-nanotech.com Acta Nanotech] has created platinum free [[nanostructure]]d [[anode]]s using more common and therefore less expensive metals.<ref name="Acta-car">[http://www.acta-nanotech.com/index.php?option=com_content&task=view&id=131&Itemid=66 Offenburg students test world's first ethanol powered fuel cell vehicle] Acta.</ref> A vehicle using a [[Direct-ethanol fuel cell|DEFC]] and non-platinum nanostructured anodes was used in the [[Royal Dutch Shell|Shell]] [[Eco-Marathon]] 2007 by a team from [[Offenburg]] Germany which achieved an efficiency of 2716 [[kilometers per liter]] (6388 [[miles per gallon]]).<ref name="Offenburg-team">[http://schluckspecht.net Willkommen beim Projekt "Schluckspecht" der Hochschule Offenburg] .</ref> |
|||
*The [[The End (A Series of Unfortunate Events)|13th book]] in ''[[A Series of Unfortunate Events]]'' was released on Friday, [[October 13]], [[2006]] by [[Lemony Snicket]], also known as novelist [[Daniel Handler]]. |
|||
*[[Port Dover#Tourism and commerce |Friday the Thirteenth]], a gathering for motorcycle enthusiasts held in the [[Canadian]] [[beach]] town of [[Port Dover, Ontario]] every Friday the 13th. |
|||
* The films ''[[The Happening (2008 film)|The Happening]]'' and ''[[The Incredible Hulk (2008 film)|The Incredible Hulk]]'' were released on Friday, [[June 13]], [[2008]]. |
|||
* The first TV special on [[iCarly]], "iCarly Saves TV" was aired on [[June 13]], [[2008]]. |
|||
* The Canadian release of the [[Microsoft]]'s [[Zune]] music player was on [[June 13]], [[2008]]. |
|||
* The remake of the original ''[[Friday the 13th (franchise)#Future|Friday the 13th]]'' is planned to release on Friday, [[February 13]], [[2009]]. |
|||
* Birthday of Thomas Gramberg |
|||
===Rocket fuel=== |
|||
==Natural events on Fridays the 13th== |
|||
Ethanol was commonly used as fuel in early [[bipropellant]] [[rocket]] vehicles, in conjunction with an [[oxidizer]] such as liquid oxygen. The German [[V-2]] rocket of [[World War II]], credited with beginning the space age, used ethanol, mixed with water to reduce the combustion chamber temperature.<ref>{{cite web|url=http://daviddarling.info/encyclopedia/V/V-2.html|title=The Internet Encyclopedia of Science: V-2|author=David Darling}}</ref><ref name=braeunig>Braeunig, Robert A. [http://braeunig.us/space/propel.htm "Rocket Propellants."] (Website). Rocket & Space Technology, 2006. Retrieved on [[2007]]-[[08-23]].</ref> The V-2's design team helped develop U.S. rockets following World War II, including the ethanol-fueled [[Redstone (rocket)|Redstone rocket]], which launched the first U.S. satellite.<ref>[http://science.ksc.nasa.gov/history/rocket-history.txt "A Brief History of Rocketry."] NASA Historical Archive, via science.ksc.nasa.gov.</ref> Alcohols fell into general disuse as more efficient rocket fuels were developed.<ref name="braeunig" /> |
|||
Due to the large number of events that happen in the world, a similar list could be compiled for any combination of day of the month and day of the week. |
|||
*The [[Black Friday (1939)|Black Friday bushfires]] in [[Victoria (Australia)|Victoria]], [[Australia]] occurred on Friday, [[January 13]], [[1939]]. |
|||
*The Uruguayan Rugby team [[Uruguayan Air Force Flight 571|infamously crashed in the Andes mountain range]] on Friday, [[13 October]] [[1972]] |
|||
*[[Hurricane Charley]] made landfall near Port Charlotte, Florida on Friday, [[August 13]], [[2004]]. |
|||
*The "[[Lake Storm "Aphid"|Friday the 13th Storm]]" struck [[Buffalo, New York|Buffalo]], [[New York]] on [[October 13]], [[2006]]. |
|||
*[[UNIX time]] will reach 1,234,567,890 decimal seconds on [[February 13]], [[2009]] at 23:31:30 [[GMT]]. |
|||
*The asteroid [[99942 Apophis]] will make its close encounter on Friday, [[April 13]], [[2029]].<!--as per article on 99942 Apophis--> |
|||
=== |
===Alcoholic beverages=== |
||
{{main|Alcoholic beverage}} |
|||
<!--If a person doesn't have an article on Wikipedia, please do not add them to this list.--> |
|||
Ethanol is the principal psychoactive constituent in [[alcoholic beverage]]s, with [[depressant]] effects on the [[central nervous system]]. It has a complex mode of action and affects multiple systems in the brain, most notably ethanol acts as an agonist to the [[GABA receptors]].<ref>{{cite journal|author=Chastain G|title=Alcohol, neurotransmitter systems, and behavior|journal=The Journal of general psychology|volume=133|issue=4|pages=329–35|year=2006|pmid=17128954 |doi=10.3200/GENP.133.4.329-335}}</ref> Similar psychoactives include those which also interact with [[GABA receptors]], such as [[gamma-hydroxybutyric acid]] (GHB).<ref name="boggan2"/> Ethanol is metabolized by the body as an energy-providing carbohydrate nutrient, as it metabolizes into [[acetyl CoA]], an intermediate common with [[glucose]] metabolism, that can be used for energy in the [[citric acid cycle]] or for biosynthesis. |
|||
Alcoholic beverages vary considerably in their ethanol content and in the foodstuffs from which they are produced. Most alcoholic beverages can be broadly classified as [[fermented beverage]]s, beverages made by the action of yeast on sugary foodstuffs, or as [[distilled beverage]]s, beverages whose preparation involves concentrating the ethanol in fermented beverages by [[distillation]]. The ethanol content of a beverage is usually measured in terms of the volume fraction of ethanol in the beverage, expressed either as a percentage or in [[alcoholic proof]] units. |
|||
<table border=0><tr><td valign=top> |
|||
Fermented beverages can be broadly classified by the foodstuff from which they are fermented. [[Beer]]s are made from [[cereal grain]]s or other [[starch]]y materials, [[wine]]s and [[cider]]s from [[fruit juice]]s, and [[mead]]s from [[honey]]. Cultures around the world have made fermented beverages from numerous other foodstuffs, and local and national names for various fermented beverages abound. |
|||
{| border="0" cellpadding="2" class="wikitable" width="100%" |
|||
! Born on Friday the 13th !! Date of Birth |
|||
Distilled beverages are made by distilling fermented beverages. Broad categories of distilled beverages include [[whiskey]]s, distilled from fermented cereal grains; [[brandy|brandies]], distilled from fermented fruit juices, and [[rum]], distilled from fermented [[molasses]] or [[sugarcane]] juice. [[Vodka]] and similar [[neutral grain spirits]] can be distilled from any fermented material (grain or [[potatoes]] are most common); these spirits are so thoroughly distilled that no tastes from the particular starting material remain. Numerous other spirits and liqueurs are prepared by infusing flavors from [[fruit]]s, [[herb]]s, and [[spice]]s into distilled spirits. A traditional example is [[gin]], which is created by infusing [[juniper]] berries into a neutral grain alcohol. |
|||
|- |
|||
| [[Oliviero de Fabritiis]] || [[13 June]], [[1902]] |
|||
In a few beverages, ethanol is concentrated by means other than distillation. [[Applejack (beverage)|Applejack]] is traditionally made by [[freeze distillation]], by which water is frozen out of fermented [[apple cider]], leaving a more ethanol-rich liquid behind. [[Eisbier]] (more commonly, ''[[eisbock]]'') is also freeze-distilled, with [[beer]] as the base beverage. [[Fortified wine]]s are prepared by adding brandy or some other distilled spirit to partially-fermented wine. This kills the yeast and conserves some of the [[sugar]] in grape juice; such beverages are not only more ethanol-rich, but are often sweeter than other wines. |
|||
|- |
|||
| [[Georges Simenon]] || [[13 February]], [[1903]] |
|||
Alcoholic beverages are sometimes used in cooking, not only for their inherent flavors, but also because the alcohol dissolves hydrophobic flavor compounds which water cannot. |
|||
|- |
|||
| [[Samuel Beckett]] || [[13 April]], [[1906]] |
|||
===Feedstock=== |
|||
|- |
|||
{{main|Chemical derivatives of ethanol}} |
|||
| [[Bud Freeman]] || [[13 April]], [[1906]] |
|||
|- |
|||
Ethanol is an important industrial ingredient and has widespread use as a base chemical for other organic compounds. These include ethyl [[halide]]s, ethyl [[ester]]s, [[diethyl ether]], [[acetic acid]], [[butadiene]], and ethyl [[amine]]s. |
|||
| [[Johannes Bjelke-Petersen|Sir Joh Bjelke-Petersen]] || [[13 January]], [[1911]] |
|||
*[http://www.petroupdates.com Ethanol Feedstock Market] |
|||
|- |
|||
| [[Fred Daly]] || [[13 June]], [[1913]] |
|||
===Antiseptic use=== |
|||
|- |
|||
Ethanol is used in medical wipes and in most common antibacterial [[hand sanitizer]] gels at a concentration of about 62% ([[percentage]] by volume, not weight) as an [[antiseptic]]. Ethanol kills organisms by denaturing their [[protein]]s and dissolving their [[lipid]]s and is effective against most [[bacterium|bacteria]] and [[fungus|fungi]], and many [[virus]]es (including [[SARS]] [http://query.nytimes.com/gst/fullpage.html?res=9D0CE3D9133CF936A35756C0A9659C8B63]), but is ineffective against bacterial [[spore]]s.<ref>{{cite journal |author=McDonnell G, Russell AD |title=Antiseptics and disinfectants: activity, action, and resistance |journal=Clin. Microbiol. Rev. |volume=12 |issue=1 |pages=147–79 |year=1999 |pmid=9880479 |url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=9880479}}</ref> |
|||
| [[Eileen Farrell]] || [[13 February]], [[1920]] |
|||
|- |
|||
===Antidote use=== |
|||
| [[Stuart Wagstaff]] || [[13 February]], [[1925]] |
|||
Ethanol can be used as an antidote for poisoning by other toxic alcohols, in particular [[methanol]]<ref name=cambridge>{{cite web|url=http://www-clinpharm.medschl.cam.ac.uk/pages/teaching/topics/poison/poison9.html| title=Methanol Poisoning|publisher=Cambridge University School of Clinical Medicine|accessdate = 2007-09-04}}</ref> and [[ethylene glycol]]. Ethanol [[competitive inhibition|competes]] with other alcohols for the [[alcohol dehydrogenase]] enzyme, preventing metabolism into toxic [[aldehyde]] and [[carboxylic acid]] derivatives.<ref>{{cite journal |author=Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA |title=American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning |journal=J. Toxicol. Clin. Toxicol. |volume=40 |issue=4 |pages=415–46 |year=2002 |pmid=12216995 |doi=10.1081/CLT-120006745}}</ref> |
|||
|- |
|||
| [[Jennifer Vyvyan]] || [[13 March]], [[1925]] |
|||
===Other uses=== |
|||
|- |
|||
*Ethanol is easily [[miscible]] in [[water (molecule)|water]] and is a good [[solvent]]. Ethanol is less polar than water and is used in [[perfume]]s, [[paint]]s and [[tincture]]s. |
|||
| [[Margaret Thatcher]] || [[13 October]], [[1925]] |
|||
*Ethanol is also used in design and sketch art markers, such as [[Copic]], and [[Tria]]. |
|||
|- |
|||
| [[Fidel Castro]] || [[13 August]], [[1926]] |
|||
==Effects on humans== |
|||
|- |
|||
{{main|Short-term effects of alcohol}} |
|||
| [[Herbert Ross]] || [[13 May]], [[1927]] |
|||
The [[National Institute on Alcohol Abuse and Alcoholism]] maintains a database of alcohol-related health effects. <ref>{{cite web|url=http://etoh.niaaa.nih.gov/Archive.htm|title=ETOH Archival Database (1972–2003)|accessdate=2008-03-31|work=Alcohol and Alcohol Problems Science Database|publisher=[[National Institute on Alcohol Abuse and Alcoholism|NIAAA]]}}</ref> |
|||
|- |
|||
| [[Rosalind Elias]] || [[13 March]], [[1931]] |
|||
{|class=wikitable style="float:right; width:25em; margin-left: 1em" |
|||
|- |
|||
! BAC (mg/dL) || Symptoms<ref name="Pohorecky & Brick">Pohorecky, L.A., and J. Brick. (1988). "Pharmacology of ethanol." ''Pharmacology & Therapeutics'' '''36'''(3), 335–427.</ref> |
|||
| [[T. J. Cloutier]] || [[13 October]], [[1939]] |
|||
|- |
|||
| [[Carol Lynley]] || [[13 February]], [[1942]] |
|||
|- |
|||
| [[Zoë Wanamaker]] || [[13 May]], [[1949]] |
|||
|- |
|||
| [[Yuri Ahronovich]] || [[13 May]], [[1949]] |
|||
|- |
|||
| [[Peter Davison]] || [[13 April]], [[1951]] |
|||
|- |
|||
| [[Max Weinberg]] || [[13 April]], [[1951]] |
|||
|- |
|||
| [[Deborah Raffin]] || [[13 March]], [[1953]] |
|||
|- |
|||
| [[Steve Buscemi]] || [[13 December]], [[1957]] |
|||
|- |
|||
| [[Julia Louis-Dreyfus]] || [[13 January]], [[1961]] |
|||
|- |
|||
| [[Will Clark]] || [[13 March]], [[1964]] |
|||
|- |
|- |
||
| 50 || Euphoria, talkativeness, relaxation |
|||
| [[Kate Walsh (actress)|Kate Walsh]] || [[13 October]], [[1967]] |
|||
|- |
|- |
||
| 100 || Central nervous system depression, impaired motor and sensory function, impaired cognition |
|||
| [[Tim Story (film director)|Tim Story]] || [[13 March]], [[1970]] |
|||
|- |
|- |
||
| >140 || Decreased blood flow to brain |
|||
| [[Sara Cox|Sara Cox]] || [[13 December]], [[1974]] |
|||
|- |
|- |
||
| 300 || Stupefaction, possible unconsciousness |
|||
| [[Sarah Connor (singer)|Sarah Connor]] || [[13 June]], [[1980]] |
|||
|- |
|- |
||
| 400 || Possible death |
|||
| [[Mary-Kate and Ashley Olsen]] || [[13 June]], [[1986]] |
|||
|- |
|- |
||
| >550 || Death |
|||
| [[Marco Andretti]] || [[13 March]], [[1987]] |
|||
}- |
|||
| [[Thomas Gramberg]] || [[13 July]], [[1990]] |
|||
|} |
|} |
||
</td><td valign=top> |
|||
===Effects on the central nervous system=== |
|||
{| border="0" cellpadding="2" class="wikitable" width="100%" |
|||
Ethanol is a central nervous system depressant and has significant psychoactive effects in sublethal doses; for specifics, see [[Effects of alcohol on the body#Effects by dose|effects of alcohol on the body by dose]]. Based on its abilities to change the [[human consciousness]], ethanol is considered a [[drug]].<ref>[http://www.nlm.nih.gov/medlineplus/ency/article/001944.htm "Alcohol Use"] ''MedlinePlus Medical Encyclopedia'', U.S. National Library of Medicine and National Institutes of Health. Retrieved on [[2007]]-[[09-27]]</ref> Death from ethyl alcohol consumption is possible when blood alcohol level reaches 0.4%. A blood level of 0.5% or more is commonly fatal. Levels of even less than 0.1% can cause [[intoxication]], with unconsciousness often occurring at 0.3–0.4%.<ref name=yost/> |
|||
! Died on Friday the 13th!! Date of Death |
|||
|- |
|||
The amount of ethanol in the body is typically quantified by [[blood alcohol content]] (BAC), the [[milligram]]s of ethanol per 100 [[milliliter]]s of blood. The table at right summarizes the symptoms of ethanol consumption. Small doses of ethanol generally produce euphoria and relaxation; people experiencing these symptoms tend to become talkative and less inhibited, and may exhibit poor judgment. At higher dosages (BAC > 100 mg/dl), ethanol acts as a [[central nervous system]] [[depressant]], producing at progressively higher dosages, impaired sensory and motor function, slowed cognition, stupefaction, unconsciousness, and possible death. |
|||
| [[Sam Patch]] || [[13 November]], [[1829]]<ref>{{cite journal |last=Thomas |first=W. Stehpen |coauthors= Ruth Rosenberg-Naparsteck |year=1988 |month=October |title=Sleep City The Sesquicentenneial History of Mt. Hope Cemetery|journal=Rochester History |volume=L |issue=4 | pages=4 | publisher=Rochester Public Library | issn =0035-7413 |url=http://www.rochester.lib.ny.us/~rochhist/v50_1988/v50i4.pdf |accessdate=2007-12-31}} </ref> |
|||
|- |
|||
In America, about half of the deaths in car accidents occur in alcohol-related crashes.<ref>{{cite journal|author=Hingson R, Winter M|title=Epidemiology and consequences of drinking and driving |journal=Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism|volume=27|issue=1|pages=63–78|year=2003|pmid=15301401|url=http://pubs.niaaa.nih.gov/publications/arh27-1/63-78.pdf}}</ref> There is no completely-safe level of alcohol for driving; the risk of a fatal [[car accident]] rises with the level of alcohol in the driver's blood.<ref>{{cite journal|author=Naranjo CA, Bremner KE|title=Behavioural correlates of alcohol intoxication|journal=[[Addiction (journal)|Addiction]]|volume=88|issue=1|pages=25–35|year=1993|pmid=8448514|doi=10.1111/j.1360-0443.1993.tb02761.x}}</ref> However, most [[drunk driving]] laws governing the acceptable levels in the blood while driving or operating heavy machinery set typical upper limits of blood alcohol content (BAC) between 0.05% to 0.08%. |
|||
| [[Gioachino Rossini]] || [[13 November]], [[1868]] |
|||
|- |
|||
===Effects on metabolism=== |
|||
| [[Diamond Jim Brady]] || [[13 April]], [[1917]] |
|||
{{Main|Alcohol metabolism}} |
|||
|- |
|||
Ethanol within the human body is converted into [[acetaldehyde]] by [[alcohol dehydrogenase]] and then into [[acetic acid]] by [[acetaldehyde dehydrogenase]]. The product of the first step of this breakdown, acetaldehyde,<ref name=boggan1>{{cite web|url=http://chemcases.com/alcohol/alc-06.htm|accessdate=2007-09-29|author=Dr. Bill Boggan|title=Metabolism of Ethyl Alcohol in the Body|publisher=Chemases.com}}</ref> is more toxic than ethanol. Acetaldehyde is linked to most of the clinical effects of alcohol. It has been shown to increase the risk of developing cirrhosis of the liver,<ref name=boggan2>{{cite web|url=http://chemcases.com/alcohol/alc-07.htm|accessdate=2007-09-29|author=Dr. Bill Boggan|title=Effects of Ethyl Alcohol on Organ Function|publisher=Chemases.com}}</ref> multiple forms of cancer, and alcoholism. |
|||
| [[Henry Segrave|Sir Henry Segrave]] || [[13 June]], [[1930]] |
|||
|- |
|||
===Drug interactions=== |
|||
| [[Arnold Schoenberg]] || [[13 July]], [[1951]] |
|||
Ethanol can intesify the sedation caused by other [[central nervous system]] [[depressant]] drugs such as [[barbiturate]]s, [[benzodiazepine]]s, [[opioid]]s, and [[phenothiazine]]s<ref name=yost>{{cite journal|url=http://postgradmed.com/issues/2002/12_02/yost1.htm|title=Acute care for alcohol intoxication|publisher=Postgraduate Medicine Online|author=David A. Yost, MD|volume=112|number=6|month=December|year=2002|accessdate=2007-09-29|format={{dead link|date=August 2008}} – <sup>[http://scholar.google.co.uk/scholar?hl=en&lr=&q=intitle%3AAcute+care+for+alcohol+intoxication&as_publication=&as_ylo=&as_yhi=&btnG=Search Scholar search]</sup>}}</ref> |
|||
|- |
|||
| [[Martita Hunt]] || [[13 June]], [[1969]] |
|||
===Magnitude of effects=== |
|||
|- |
|||
Some individuals have less-effective forms of one or both of the metabolizing enzymes, and can experience more-severe symptoms from ethanol consumption than others. Conversely, those who have acquired [[alcohol tolerance]] have a greater quantity of these enzymes, and metabolize ethanol more rapidly.<ref>{{cite journal|author=Agarwal DP, Goedde HW|title=Pharmacogenetics of alcohol metabolism and alcoholism|journal=Pharmacogenetics|volume=2|issue=2|pages=48–62|year=1992|pmid=1302043|doi=10.1097/00008571-199204000-00002}}</ref> |
|||
| [[Hubert Humphrey]] || [[13 January]], [[1978]] |
|||
|- |
|||
===Birth defects=== |
|||
| [[Ralph Kirkpatrick]] || [[13 April]], [[1984]] |
|||
Ethanol is classified as a [[teratogen]]. See [[fetal alcohol syndrome]]. |
|||
|- |
|||
| [[Benny Goodman]] || [[13 June]], [[1986]] |
|||
===Other effects=== |
|||
|- |
|||
Frequent drinking of alcoholic beverages has been shown to be a major contributing factor in cases of elevated blood levels of [[triglycerides]].<ref>{{cite web|url=http://americanheart.org/presenter.jhtml?identifier=4778|title=Triglycerides |
|||
| [[Gerald Moore]] || [[13 March]], [[1987]] |
|||
|accessdate =2007-09-04|publisher=American Heart Association}}</ref> |
|||
|- |
|||
| [[Stuart Challender]] || [[13 December]], [[1991]] |
|||
Ethanol is not a [[carcinogen]].<ref name=msdsbj>{{cite web|url=http://www.cise.columbia.edu/clean/msds/ethanol.pdf|title=Material Data Safety Sheet|publisher=Burdick and Jackson|accessdate = 2007-10-25|format=PDF}}</ref><ref name=aacr>{{cite web|url=http://www.aacr.org/PDF_files/2004Prevention/Program/2004_Prevention_Abstracts.pdf|title=Animal Models for Carcinogenesis and Chemoprevention, abstract #C42: Effects of Chronic Ethanol Intake on Cyclin D1 Levels and Altered Foci in Diethylnitrosamine-initiated Rats|author=Pollyanna R. Chavez, Xiang-Dong Wang, Jean Meyer|publisher=USDA|accessdate=2007-10-24|format=PDF}}</ref> However, the first metabolic product of ethanol, [[acetaldehyde]], is toxic, [[mutagen]]ic, and carcinogenic. Also, ethanol's effect on the [[liver]] can contribute to [[immune suppression]]. Consequently, consumption of alcoholic beverages can be an aggravating factor in [[carcinogenesis]]. |
|||
|- |
|||
| [[Tupac Shakur]] || [[13 September]], [[1996]] |
|||
==See also== |
|||
|- |
|||
{{portal|Pharmacy and Pharmacology|Tabletten.JPG}} |
|||
|[[Tony Roper]] || [[13 October]], [[2000]] |
|||
{{EnergyPortal}} |
|||
|- |
|||
{{col-begin}} |
|||
|[[Tim Russert]] || [[13 June]], [[2008]] |
|||
{{col-break}} |
|||
|- |
|||
*[[1-propanol]] |
|||
|} |
|||
*[[2,2,2-trichloroethanol]] |
|||
</td></tr></table> |
|||
*[[Alcoholic beverage]] |
|||
*[[Biobutanol]] |
|||
*[[Biodiesel]] |
|||
*[[Breathalyzer]] |
|||
*[[Cellulosic ethanol]] |
|||
*[[Cellulosic ethanol commercialization]] |
|||
*[[Corn ethanol]] |
|||
*[[Corn liquor]] |
|||
{{col-break}} |
|||
*[[Denatured alcohol]] |
|||
*[[Ethanol (data page)]] |
|||
*[[Ethanol fuel]] |
|||
*[[Ethanol fuel in Brazil]] |
|||
*[[Isopropyl alcohol]] |
|||
*[[List of energy topics]] |
|||
*[[Rubbing alcohol]] |
|||
*[[Timeline of alcohol fuel]] |
|||
{{col-end}} |
|||
==References== |
==References== |
||
{{reflist}} |
{{reflist|2}} |
||
==Further reading== |
|||
{{refbegin}} |
|||
*"Alcohol." (1911). In Hugh Chisholm (Ed.) ''[[Encyclopædia Britannica Eleventh Edition]].'' [http://91.1911encyclopedia.org/A/AL/ALCOHOL.htm Online reprint] |
|||
*Lodgsdon, J.E. (1994). "Ethanol." In J.I. Kroschwitz (Ed.) ''Encyclopedia of Chemical Technology, 4th ed.'' vol. 9, pp. 812–860. New York: John Wiley & Sons. |
|||
*Smith, M.G., and M. Snyder. (2005). "Ethanol-induced virulence of ''Acinetobacter baumannii''". ''American Society for Microbiology meeting''. June 5 – June 9. Atlanta. |
|||
*[http://sci-toys.com/ingredients/alcohol.html Sci-toys website explanation of US denatured alcohol designations] |
|||
*Boyce, John M., and Pittet Didier. (2003). [http://cdc.gov/handhygiene/ “Hand Hygiene in Healthcare Settings.”] [[Centers for Disease Control]], [[Atlanta, Georgia|Atlanta]], [[Georgia (U.S. state)|Georgia]], United States. |
|||
*{{cite web|title=VS1000A Series In-Line Ethanol Sensors for the Beverage and BioFuel Industry|author=Rene Martinez VitalSensors Technologies LLC|url=http://vitalsensorstech.com/VitalSensors%20VS-1000A%20Ethanol%20Alcohol%20Sensor%20Data%20Sheet.pdf}} Martinez describes the theory and practice of measuring brix on-line in beverages. |
|||
{{refend}} |
|||
==External links== |
==External links== |
||
*[http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc00/icsc0044.htm International Labour Organization] ethanol safety information |
|||
{{wiktionarypar| paraskavedekatriaphobia}} |
|||
*[http://www.npi.gov.au/database/substance-info/profiles/35.html National Pollutant Inventory – Ethanol Fact Sheet] |
|||
{{Illustrated Wikipedia|Friday wikiworld comic.jpg}} |
|||
*[http://ethanol-information.com/ Ethanol Information] |
|||
* [http://news.nationalgeographic.com/news/2004/02/0212_040212_friday13.html Friday the 13th Phobia Rooted in Ancient History] |
|||
*[http://theethanolsource.com/ Ethanol Facts] |
|||
* [http://urbanlegends.about.com/cs/historical/a/friday_the_13th.htm Why Friday the 13th Is Unlucky] (About.com) |
|||
*[http://compchemwiki.org/index.php?title=Ethanol Coordinates of the ethanol molecule] on Computational Chemistry Wiki. Accessed on September 8, 2005. |
|||
* [http://www.howstuffworks.com/friday-thirteenth.htm How Friday the 13th works] (HowStuffWorks.com) |
|||
*[http://www.bluerhinos.co.uk/molview/indv.php?id=4 Molview from bluerhinos.co.uk] See Ethanol in 3D |
|||
* [[National Geographic]]: [http://news.nationalgeographic.com/news/2004/02/0212_040212_friday13.html ''Friday the 13th Phobia Rooted in Ancient History''] |
|||
*[http://webbook.nist.gov/cgi/cbook.cgi?Name=ethanol&Units=SI National Institute of Standards and Technology] chemical data on ethanol |
|||
* [http://altreligion.about.com/library/weekly/aa101306a.htm Friday the 13th and the Templars] - About.com article |
|||
*[http://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:16236 ChEBI – biology related] |
|||
* [http://www.opinionjournal.com/taste/?id=110009086 Your Unlucky Day: The religious roots of triskaidekaphobia.] |
|||
*[http://cbot.com/ Chicago Board of Trade] news and market data on ethanol futures |
|||
* [http://www.viamagazine.com/top_stories/articles/lucky_1304.asp Some don't count on Lucky 13] - Via Magazine. |
|||
* [http://skepdic.com/paraskevidekatriaphobia.html Paraskevidekatriaphobia] - skepdic.com article on Friday the 13th |
|||
{{Alcohols}} |
|||
* http://www.infoplease.com/spot/friday13th.html |
|||
{{Antiseptics and disinfectants}} |
|||
* [http://www.snopes.com/luck/friday13.asp A world of Luck: Friday the 13th] - Snopes.com article |
|||
{{Antidotes}} |
|||
* [http://mathworld.wolfram.com/Triskaidekaphobia.html Triskaidekaphobia on MathWorld] |
|||
[[Category: |
[[Category:Anatomical preservation]] |
||
[[Category: |
[[Category:Alcohol]] |
||
[[Category: |
[[Category:Alcohols]] |
||
[[Category:Anxiolytics]] |
|||
[[Category:Drugs]] |
|||
[[Category:Rocket fuels]] |
|||
[[Category:Household chemicals]] |
|||
[[Category:Alcohol solvents]] |
|||
[[Category:Teratogens]] |
|||
[[Category:Disinfectants]] |
|||
[[Category:Oxygenates]] |
|||
[[Category:Straight-chain alcohols]] |
|||
[[af:Etanol]] |
|||
[[be-x-old:Пятніца 13]] |
|||
[[ |
[[ar:إثانول]] |
||
[[bn:ইথানল]] |
|||
[[cs:Pátek třináctého]] |
|||
[[ |
[[bs:Etanol]] |
||
[[ |
[[bg:Етанол]] |
||
[[ca:Etanol]] |
|||
[[el:Παρασκευή και 13]] |
|||
[[ |
[[cs:Ethanol]] |
||
[[ |
[[da:Ætanol]] |
||
[[ |
[[de:Ethanol]] |
||
[[ |
[[et:Etanool]] |
||
[[el:Αιθανόλη]] |
|||
[[he:יום שישי ה-13]] |
|||
[[ |
[[es:Etanol]] |
||
[[eo:Etanolo]] |
|||
[[nl:Vrijdag de dertiende]] |
|||
[[ |
[[eu:Etanol]] |
||
[[ |
[[fa:اتانول]] |
||
[[ |
[[fr:Éthanol]] |
||
[[ |
[[gl:Etanol]] |
||
[[ |
[[ko:에탄올]] |
||
[[ |
[[hr:Etanol]] |
||
[[id:Etanol]] |
|||
[[sk:Piatok trinásteho]] |
|||
[[ |
[[is:Etanól]] |
||
[[ |
[[it:Etanolo]] |
||
[[ |
[[he:אתנול]] |
||
[[la:Ethanol]] |
|||
[[vi:Thứ sáu ngày 13]] |
|||
[[lv:Etanols]] |
|||
[[uk:П'ятниця 13-го]] |
|||
[[ |
[[lb:Ethanol]] |
||
[[lt:Etanolis]] |
|||
[[hu:Etanol]] |
|||
[[ml:എഥനോള്]] |
|||
[[ms:Etanol]] |
|||
[[nl:Ethanol]] |
|||
[[ja:エタノール]] |
|||
[[no:Etanol]] |
|||
[[nn:Etanol]] |
|||
[[pl:Alkohol etylowy]] |
|||
[[pt:Etanol]] |
|||
[[ro:Etanol]] |
|||
[[qu:Ethanul]] |
|||
[[ru:Этанол]] |
|||
[[simple:Ethanol]] |
|||
[[sk:Etanol]] |
|||
[[sl:Etanol]] |
|||
[[sh:Alkohol]] |
|||
[[fi:Etanoli]] |
|||
[[sv:Etanol]] |
|||
[[ta:எத்தனால்]] |
|||
[[th:เอทานอล]] |
|||
[[vi:Êtanol]] |
|||
[[tg:Итонул]] |
|||
[[tr:Etanol]] |
|||
[[uk:Етанол]] |
|||
[[ur:ایتھنول]] |
|||
[[zh-yue:乙醇]] |
|||
[[zh:乙醇]] |
|||
Revision as of 16:24, 12 October 2008
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ethanol
| |||
| Other names
Ethyl alcohol; grain alcohol; pure alcohol; hydroxyethane; drinking alcohol; ethyl hydrate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.000.526 | ||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C2H6O | |||
| Molar mass | 46.06844(232) g/mol | ||
| Appearance | colorless clear liquid | ||
| Density | 0.789 g/cm³, liquid | ||
| Melting point | −114.3 °C (158.8 K) | ||
| Boiling point | 78.4 °C, 173.1 F (351.6 K) | ||
| Fully miscible | |||
| Acidity (pKa) | 15.9 | ||
| Viscosity | 1.200 mPa·s (cP) at 20.0 °C | ||
| 5.64 fC·fm (1.69 D) (gas) | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 286.15 K (13 °C or 55.4 °F) | ||
| Related compounds | |||
| Supplementary data page | |||
| Ethanol (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug, best known as the type of alcohol found in alcoholic beverages and in thermometers. In common usage, it is often referred to simply as alcohol.
Ethanol is abbreviated as EtOH, using the common organic chemistry notation of representing the ethyl group (C2H5) with Et. This designation is used both by EMS and Hospital ER staff when describing alcohol intoxication, and is found in most chemistry textbooks as well.
Ethanol is a straight-chain alcohol, and its molecular formula is C2H5OH. An alternative notation is CH3-CH2-OH, which indicates that the carbon of a methyl group (CH3-) is attached to the carbon of a methylene group (-CH2-), which is attached to the oxygen of a hydroxyl group (-OH).
Its empirical formula is C2H6O, making it an isomer of dimethyl ether.
Except for use of fire, the fermentation of sugar into ethanol is the earliest organic reaction known to humanity. The intoxicating effects of ethanol consumption have been known since ancient times. In modern times, ethanol intended for industrial use is also produced from by-products of petroleum refining.
Ethanol has widespread use as a solvent of substances intended for human contact or consumption, including scents, flavorings, colorings, and medicines. In chemistry, it is both an essential solvent and a feedstock for the synthesis of other products. It has a long history as a fuel for heat and light and also as a fuel for internal combustion engines.
History

Ethanol has been used by humans since prehistory as the intoxicating ingredient of alcoholic beverages. Dried residues on 9000-year-old pottery found in China imply that alcoholic beverages were used even among Neolithic people.[1] Its isolation as a relatively pure compound was first achieved by Persian alchemist, Zakarīya Rāzi (Rhazes), who was renowned for his perfected methods of distillation and extraction. Other chemists who contributed to the development of distillation techniques during the Abbasid caliphate, other than Razi, include Jabir ibn Hayyan (Geber) and Al-Kindi (Alkindus).
Writings attributed to Jabir ibn Hayyan (721–815) mention the flammable vapors of boiled wine. Al-Kindi (801–873) unambiguously described the distillation of wine.[2]
In 1796, Johann Tobias Lowitz obtained pure ethanol by filtering distilled ethanol through activated charcoal.
Antoine Lavoisier described ethanol as a compound of carbon, hydrogen, and oxygen, and in 1808 Nicolas-Théodore de Saussure determined ethanol's chemical formula.[3] Fifty years later, Archibald Scott Couper published the structural formula of ethanol, which placed ethanol among the first chemical compounds to have their chemical structure determined.[4]
Ethanol was first prepared synthetically in 1826 through the independent efforts of Henry Hennel in Great Britain and S.G. Sérullas in France. In 1828, Michael Faraday prepared ethanol by acid-catalyzed hydration of ethylene, a process similar to that which is used today for industrial ethanol synthesis.[5]
Ethanol was used as lamp fuel in the United States as early as 1840, but a tax levied on industrial alcohol during the Civil War made this use uneconomical. This tax was repealed in 1906,[6] and from 1908 onward Ford Model T automobiles could be adapted to run on ethanol.[7] With the advent of Prohibition in 1920 though, sellers of ethanol fuel were accused of being allied with moonshiners,[6] and ethanol fuel again fell into disuse until late in the 20th century.
Physical properties


Ethanol is a volatile, flammable, colorless liquid that has a strong characteristic odor. It burns with a smokeless blue flame that is not always visible in normal light.
The physical properties of ethanol stem primarily from the presence of its hydroxyl group and the shortness of its carbon chain. Ethanol’s hydroxyl group is able to participate in hydrogen bonding, rendering it more viscous and less volatile than less polar organic compounds of similar molecular weight.
Ethanol is a versatile solvent, miscible with water and with many organic solvents, including acetic acid, acetone, benzene, carbon tetrachloride, chloroform, diethyl ether, ethylene glycol, glycerol, nitromethane, pyridine, and toluene.[8][9] It is also miscible with light aliphatic hydrocarbons, such as pentane and hexane, and with aliphatic chlorides such as trichloroethane and tetrachloroethylene.[9]
Ethanol’s miscibility with water contrasts with that of longer-chain alcohols (five or more carbon atoms), whose water miscibility decreases sharply as the number of carbons increases.[10]
Hydrogen bonding causes pure ethanol to be hygroscopic to the extent that it readily absorbs water from the air. The polar nature of the hydroxyl group causes ethanol to dissolve many ionic compounds, notably sodium and potassium hydroxides, magnesium chloride, calcium chloride, ammonium chloride, ammonium bromide, and sodium bromide.[9] Sodium and potassium chlorides are slightly soluble in ethanol.[9] Because the ethanol molecule also has a nonpolar end, it will also dissolve nonpolar substances, including most essential oils[11] and numerous flavoring, coloring, and medicinal agents.
Two unusual phenomena are associated with mixtures of ethanol and water. Ethanol-water mixtures have less volume than the sum of their individual components. Mixing equal volumes of ethanol and water results in only 1.92 volumes of mixture.[12][8] The addition of even a few percent of ethanol to water sharply reduces the surface tension of water. This property partially explains the “tears of wine” phenomenon. When wine is swirled in a glass, ethanol evaporates quickly from the thin film of wine on the wall of the glass. As the wine’s ethanol content decreases, its surface tension increases and the thin film “beads up” and runs down the glass in channels rather than as a smooth sheet.
Mixtures of ethanol and water that contain more than about 50% ethanol are flammable and easily ignited. An alcohol stove has been developed in India which runs on 50% ethanol/water mixture.[13] Alcoholic proof is a widely used measure of how much ethanol (i.e., alcohol) such a mixture contains. In the 18th century, proof was determined by adding a liquor (such as rum) to gunpowder. If the gunpowder still just exploded, that was considered to be “100 degrees proof” that it was “good” liquor — hence it was called “100 degrees proof.”
Ethanol-water solutions that contain less than 50% ethanol may also be flammable if the solution is first heated. Some cooking methods call for wine to be added to a hot pan, causing it to flash boil into a vapor, which is then ignited to burn off excess alcohol.
Ethanol is slightly more refractive than water, having a refractive index of 1.36242 (at λ=589.3 nm and 18.35 °C).[8]
Chemical properties
Ethanol is classified as a primary alcohol, meaning that the carbon to which its hydroxyl group is attached has at least two hydrogen atoms attached to it as well.
The chemistry of ethanol is largely that of its hydroxyl group.
Acid-base chemistry
Ethanol's hydroxyl causes the molecule to be slightly basic. It is however,so very slightly basic it is almost neutral, like pure water. The pH of 100% ethanol is 7.33, compared to 7.00 for pure water. Ethanol can be quantitatively converted to its conjugate base, the ethoxide ion (CH3CH2O−), by reaction with an alkali metal such as sodium:[10]
or a very strong base such as sodium hydride:
- CH3CH2OH + NaH → CH3CH2ONa + H2.
This reaction is not possible in an aqueous solution, as water is more acidic, so that hydroxide is preferred over ethoxide formation.
Halogenation
Ethanol reacts with hydrogen halides to produce ethyl halides such as ethyl chloride and ethyl bromide:
HCl reaction requires a catalyst such as zinc chloride.[14] Hydrogen chloride in the presence of their respective zinc chloride is known as Lucas reagent.[10][14]
HBr requires refluxing with a sulfuric acid catalyst.[14]
Ethyl halides can also be produced by reacting ethanol with more specialized halogenating agents, such as thionyl chloride for preparing ethyl chloride, or phosphorus tribromide for preparing ethyl bromide.[10][14]
- CH3CH2OH + SOCl2 → CH3CH2Cl + SO2 + HCl
Ester formation
Under acid-catalyzed conditions, ethanol reacts with carboxylic acids to produce ethyl esters and water:
- RCOOH + HOCH2CH3 → RCOOCH2CH3 + H2O.
For this reaction to produce useful yields it is necessary to remove water from the reaction mixture as it is formed.
Ethanol can also form esters with inorganic acids. Diethyl sulfate and triethyl phosphate, prepared by reacting ethanol with sulfuric and phosphoric acid respectively, are both useful ethylating agents in organic synthesis. Ethyl nitrite, prepared from the reaction of ethanol with sodium nitrite and sulfuric acid, was formerly a widely-used diuretic.
Dehydration
Strong acid desiccants, such as sulfuric acid, cause ethanol's dehydration to form either diethyl ether or ethylene:
- 2 CH3CH2OH → CH3CH2OCH2CH3 + H2O
- CH3CH2OH → H2C=CH2 + H2O
Which product, diethyl ether or ethylene, predominates depends on the precise reaction conditions.
Oxidation
Ethanol can be oxidized to acetaldehyde, and further oxidized to acetic acid. In the human body, these oxidation reactions are catalyzed by enzymes. In the laboratory, aqueous solutions of strong oxidizing agents, such as chromic acid or potassium permanganate, oxidize ethanol to acetic acid, and it is difficult to stop the reaction at acetaldehyde at high yield. Ethanol can be oxidized to acetaldehyde, without over oxidation to acetic acid, by reacting it with pyridinium chromic chloride.[14]
The direct oxidation of ethanol to acetic acid using chromic acid is given below.
- C2H5OH + 2[O] → CH3COOH + H2O
The oxidation product of ethanol, acetic acid, is spent as nutrient by the human body as acetyl CoA, where the acetyl group can be spent as energy or used for biosynthesis.
Chlorination
When exposed to chlorine, ethanol is both oxidized and its alpha carbon chlorinated to form the compound, chloral.
- 4Cl2 + C2H5OH → CCl3CHO + 5HCl
Combustion
Combustion of ethanol forms carbon dioxide and water:
- C2H5OH(g) + 3 O2(g) → 2 CO2(g) + 3 H2O(l); (ΔHr = −1409 kJ/mol[15])
Production

Ethanol is produced both as a petrochemical, through the hydration of ethylene, and biologically, by fermenting sugars with yeast.[16] Which process is more economical is dependent upon the prevailing prices of petroleum and of grain feed stocks.
Ethylene hydration
Ethanol for use as industrial feedstock is most often made from petrochemical feed stocks, typically by the acid-catalyzed hydration of ethylene, represented by the chemical equation
The catalyst is most commonly phosphoric acid,[17] adsorbed onto a porous support such as diatomaceous earth or charcoal. This catalyst was first used for large-scale ethanol production by the Shell Oil Company in 1947.[18] The reaction is carried out with an excess of high pressure steam at 300°C.
In an older process, first practiced on the industrial scale in 1930 by Union Carbide,[19] but now almost entirely obsolete, ethylene was hydrated indirectly by reacting it with concentrated sulfuric acid to produce ethyl sulfate, which was then hydrolyzed to yield ethanol and regenerate the sulfuric acid:[14]
- C2H4 + H2SO4 → CH3CH2SO4H
- CH3CH2SO4H + H2O → CH3CH2OH + H2SO4
Fermentation
Ethanol for use in alcoholic beverages, and the vast majority of ethanol for use as fuel, is produced by fermentation. When certain species of yeast, most importantly, Saccharomyces cerevisiae, metabolize sugar in the absence of oxygen, they produce ethanol and carbon dioxide. The chemical equation below summarizes the conversion:
The process of culturing yeast under conditions to produce alcohol is called fermentation. Ethanol's toxicity to yeast limits the ethanol concentration obtainable by brewing. The most ethanol-tolerant strains of yeast can survive up to approximately 15% ethanol by volume.[20]
The fermentation process must exclude oxygen. If oxygen is present, yeast undergo aerobic respiration which produces carbon dioxide and water rather than ethanol.
In order to produce ethanol from starchy materials such as cereal grains, the starch must first be converted into sugars. In brewing beer, this has traditionally been accomplished by allowing the grain to germinate, or malt, which produces the enzyme, amylase. When the malted grain is mashed, the amylase converts the remaining starches into sugars. For fuel ethanol, the hydrolysis of starch into glucose can be accomplished more rapidly by treatment with dilute sulfuric acid, fungally produced amylase, or some combination of the two.[21]
Cellulosic ethanol
Sugars for ethanol fermentation can be obtained from cellulose.[22][23] Until recently, however, the cost of the cellulase enzymes capable of hydrolyzing cellulose has been prohibitive. The Canadian firm Iogen brought the first cellulose-based ethanol plant on-stream in 2004.[24] Its primary consumer so far has been the Canadian government, which, along with the United States Department of Energy, has invested heavily in the commercialization of cellulosic ethanol. Deployment of this technology could turn a number of cellulose-containing agricultural by-products, such as corncobs, straw, and sawdust, into renewable energy resources. Other enzyme companies are developing genetically engineered fungi that produce large volumes of cellulase, xylanase, and hemicellulase enzymes. These would convert agricultural residues such as corn stover, wheat straw, and sugar cane bagasse and energy crops such as switchgrass into fermentable sugars.[25]
Cellulose-bearing materials typically also contain other polysaccharides, including hemicellulose. When hydrolyzed, hemicellulose decomposes into mostly five-carbon sugars such as xylose. S. cerevisiae, the yeast most commonly used for ethanol production, cannot metabolize xylose. Other yeasts and bacteria are under investigation to ferment xylose and other pentoses into ethanol.[26]
On January 14, 2008, General Motors announced a partnership with Coskata, Inc. The goal is to produce cellulosic ethanol cheaply, with an eventual goal of US$1 per U.S. gallon ($0.30/L) for the fuel. The partnership plans to begin producing the fuel in large quantity by the end of 2008. By 2011 a full-scale plant will come on line, capable of producing 50 to 100 million gallons of ethanol a year (200–400 ML/a).[27]
Prospective technologies

The anaerobic bacterium Clostridium ljungdahlii, recently discovered in commercial chicken wastes, can produce ethanol from single-carbon sources including synthesis gas, a mixture of carbon monoxide and hydrogen that can be generated from the partial combustion of either fossil fuels or biomass. Use of these bacteria to produce ethanol from synthesis gas has progressed to the pilot plant stage at the BRI Energy facility in Fayetteville, Arkansas.[28]
Another prospective technology is the closed-loop ethanol plant.[29] Ethanol produced from corn has a number of critics who suggest that it is primarily just recycled fossil fuels because of the energy required to grow the grain and convert it into ethanol. There is also the issue of competition with use of corn for food production. However, the closed-loop ethanol plant attempts to address this criticism. In a closed-loop plant, the energy for the distillation comes from fermented manure, produced from cattle that have been fed the by-products from the distillation. The leftover manure is then used to fertilize the soil used to grow the grain. Such a process is expected to lower the fossil fuel consumption used during conversion to ethanol by 75%.[30] Although energy can be created from the collection of methane from livestock manure, this can be mutually exclusive to the production of ethanol and should not be tagged on to it to make ethanol production seem more efficient or enviromentally friendly.
Though in an early stage of research, there is some development of alternative production methods that use feed stocks such as municipal waste or recycled products, rice hulls, sugarcane bagasse, small diameter trees, wood chips, and switchgrass.[31]
Testing
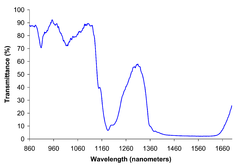
Breweries and biofuel plants employ two methods for measuring ethanol concentration. Infrared ethanol sensors measure the vibrational frequency of dissolved ethanol using the CH band at 2900 cm−1. This method uses a relatively inexpensive solid state sensor that compares the CH band with a reference band to calculate the ethanol content. The calculation makes use of the Beer-Lambert law. Alternatively, by measuring the density of the starting material and the density of the product, using a hydrometer, the change in specific gravity during fermentation indicates the alcohol content. This inexpensive and indirect method has a long history in the beer brewing industry.
Purification
Ethylene hydration or brewing produces an ethanol–water mixture. For most industrial and fuel uses, the ethanol must be purified. Fractional distillation can concentrate ethanol to 95.6% by weight (89.5 mole%). This mixture is an azeotrope with a boiling point of 78.1 °C, and cannot be further purified by distillation.
In one common industrial method to obtain absolute alcohol, a small quantity of benzene is added to rectified spirit and the mixture is then distilled. Absolute alcohol is obtained in the third fraction, which distills over at 78.3 °C (351.4 K).[10] Because a small amount of the benzene used remains in the solution, absolute alcohol produced by this method is not suitable for consumption, as benzene is carcinogenic.[32]
There is also an absolute alcohol production process by desiccation using glycerol. Alcohol produced by this method is known as spectroscopic alcohol—so called because the absence of benzene makes it suitable as a solvent in spectroscopy.
Other methods for obtaining absolute ethanol include desiccation using adsorbents such as starch or zeolites, which adsorb water preferentially, as well as azeotropic distillation and extractive distillation.
Grades of ethanol
Denatured alcohol
Pure ethanol and alcoholic beverages are heavily taxed, but ethanol has many uses that do not involve consumption by humans. To relieve the tax burden on these uses, most jurisdictions waive the tax when an agent has been added to the ethanol to render it unfit to drink. These include bittering agents such as denatonium benzoate and toxins such as methanol, naphtha, and pyridine. Products of this kind are called denatured alcohol.[33][34]
Absolute ethanol
Absolute or anhydrous alcohol generally refers to purified ethanol, containing no more than one percent water. Absolute alcohol not intended for human consumption often contains trace amounts of toxic benzene (used to remove water by azeotropic distillation). Consumption of this form of ethanol can be fatal over a short time period. Generally this kind of ethanol is used as solvents for lab and industrial settings where water will disrupt a desired reaction.
Pure ethanol is classed as 200 proof in the USA, equivalent to 175 degrees proof in the UK system.
Use
As a fuel
| Energy content of some fuels compared with ethanol:[35] | |||
|---|---|---|---|
| Fuel type | MJ/L | MJ/kg | Research octane number |
| Methanol | 17.9 | 19.9 | 123 |
| Ethanol | 23.5 | [36]31.1 | 129 |
| Liquefied natural gas | 25.3 | ~55 | |
| Autogas (LPG) (60% Propane + 40% Butane) |
26.8 | 50. | |
| Aviation gasoline (high-octane gasoline, not jet fuel) |
33.5 | 46.8 | |
| Gasohol (90% gasoline + 10% ethanol) |
33.7 | 47.1 | 93/94 |
| Regular Gasoline | 34.8 | [37]44.4 | min. 91 |
| Premium Gasoline | max. 95 | ||
| Diesel | 38.6 | 45.4 | 25 |
| Charcoal, extruded | 50 | 23 | |
The largest single use of ethanol is as a motor fuel and fuel additive. The largest national fuel ethanol industries exist in Brazil (gasoline sold in Brazil contains at least 25% ethanol and anhydrous ethanol is also used as fuel in more than 90% of new cars sold in the country). The Brazilian production of ethanol is praised for the high carbon sequestration capabilities of the sugar cane plantations, thus making it a real option to combat climate change.[38]
Henry Ford designed the first mass-produced automobile, the famed Model T Ford, to run on pure anhydrous (ethanol) alcohol—he said it was "the fuel of the future". Today, however, 100% pure ethanol is not approved as a motor vehicle fuel in the U.S. Added to gasoline, ethanol reduces ground-level ozone formation by lowering volatile organic compound and hydrocarbon emissions, decreasing carcinogenic benzene, and butadiene, emissions, and particulate matter emissions from gasoline combustion.[39]
Combustion of ethanol in an internal combustion engine yields many of the products of incomplete combustion that are produced by gasoline and significantly larger amounts of formaldehyde and related species such as formalin, acetaldehyde, etc..[40] This leads to a significantly larger photochemical reactivity that generates much more ground level ozone.[41] This data has been assembled into The Clean Fuels Report comparison of fuel emissions[42] and shows that ethanol exhaust generates 2.14 times as much ozone as does gasoline exhaust. When this is added into the custom "Localised Pollution Index (LPI)" of The Clean Fuels Report the local pollution, i.e. that which contributes to smog, is 1.7 on a scale where gasoline is 1.0 and higher numbers signify greater pollution. This issue has been formalised by the California Air Resouces Board in 2008[43] by recognising control standards for formaldehydes et al as an emissions control group much like the conventional NOx and Reactive Organic Gases (ROGs).
Prior to the development of electronic fuel injection (EFI) and computerized engine management, the lower energy content of ethanol required that the engine carburetor be rejetted to permit a larger volume of fuel to mix with the intake air. EFI is able to actively compensate for varying fuel energy densities by monitoring the oxygen content of exhaust gases. However, a standard EFI gasoline engine can typically only tolerate up to 10% ethanol and 90% gasoline. Higher ethanol ratios require either larger-volume fuel injectors or an increase in fuel rail pressure to deliver the greater liquid volume needed to equal the energy content of pure gasoline.

World production of ethanol in 2006 was 51 gigalitres (1.3×1010 US gal), with 69% of the world supply coming from Brazil and the United States.[44] More than 20% of the Brazilian fleet of cars on the streets are able to use 100% ethanol as fuel, which includes ethanol-only engines and flex-fuel engines.[45] Flex-fuel engines in Brazil are able to work with all ethanol, all gasoline or any mixture of both. In the US flex-fuel vehicles can run on 0% to 85% ethanol (15% gasoline) since higher ethanol blends are not yet allowed. Brazil supports this population of ethanol-burning automobiles with large national infrastructure that produces ethanol from domestically grown sugar cane. Sugar cane not only has a greater concentration of sucrose than corn (by about 30%), but is also much easier to extract. The bagasse generated by the process is not wasted, but is utilized in power plants as a surprisingly efficient fuel to produce electricity.


The United States fuel ethanol industry is based largely on corn. According to the Renewable Fuels Association, as of October 30, 2007, 131 grain ethanol bio-refineries in the United States have the capacity to produce 7.0 billion US gallons (26 GL) of ethanol per year. An additional 72 construction projects underway (in the U.S.) can add 6.4 billion gallons of new capacity in the next 18 months. Over time, it is believed that a material portion of the ~150 billion gallon per year market for gasoline will begin to be replaced with fuel ethanol.[46]
The Energy Policy Act of 2005 requires that 4 billion gallons of "renewable fuel" be used in 2006 and this requirement will grow to a yearly production of 7.5 billion gallons by 2012.[47] In the United States, ethanol is most commonly blended with gasoline as a 10% ethanol blend nicknamed "gasohol". This blend is widely sold throughout the U.S. Midwest, and in cities required by the 1990 Clean Air Act to oxygenate their gasoline during the winter. {{citation}}: Empty citation (help) Ethanol and isobutene are also the feedstocks for ethyl tert-butyl ether (ETBE), an oxygenate antiknock additive. The use of ethanol makes ETBE partially a biofuel, but also more expensive than the similar additive methyl tert-butyl ether (MTBE), made from methanol and isobutene. {{citation}}: Empty citation (help)
Food versus fuel debate
It is disputed whether corn ethanol as an automotive fuel results in a net energy gain or loss. As reported in "The Energy Balance of Corn Ethanol: an Update,"[48] the energy returned on energy invested (EROEI) for ethanol made from corn in the U.S. is 1.34 (it yields 34% more energy than it takes to produce it). Input energy includes natural gas based fertilizers, farm equipment, transformation from corn or other materials, and transportation. However, other researchers report that the production of ethanol consumes more energy than it yields.[49][50] In comparison, sugar cane ethanol EROEI is at around 8 (it yields 8 joules for each joule used to produce it).[citation needed] Recent research suggests that cellulosic crops such as switchgrass provide a much better net energy production than corn, producing over five times as much energy as the total used to produce the crop and convert it to fuel.[51] If this research is confirmed, cellulosic crops will most likely displace corn as the main fuel crop for producing bioethanol.
Michael Grunwald reports that one person could be fed 365 days "on the corn needed to fill an ethanol-fueled SUV".[52] He further reports that though "hyped as an eco-friendly fuel, ethanol increases global warming, destroys forests and inflates food prices." Environmentalists, livestock farmers, and opponents of subsidies say that increased ethanol production won't meet energy goals and may damage the environment, while at the same time causing worldwide food prices to soar. Some of the controversial subsidies in the past have included more than $10 billion to Archer-Daniels-Midland since 1980.[53][neutrality is disputed] Critics also speculate that as ethanol is more widely used, changing irrigation practices could greatly increase pressure on water resources. In October 2007, 28 environmental groups decried the Renewable Fuels Standard (RFS), a legislative effort intended to increase ethanol production, and said that the measure will "lead to substantial environmental damage and a system of biofuels production that will not benefit family farmers...will not promote sustainable agriculture and will not mitigate global climate change."[54][55]
Recent articles have also blamed subsidized ethanol production for the nearly 200% increase in milk prices since 2004,[56] although that is disputed by some[citation needed]. Especially since the price of fuel has driven up the costs to cultivate, grow, harvest, ship, refine, bring to market, etc, all commodities including; but not limited to, milk. Not to mention the presence of speculators, and the recent growing interest in the commodities market by investors who have been scared away from a falling stock market.
Ethanol production uses the starch portion of corn, but the leftover protein can be used to create a high-nutrient, low-cost animal feed.[57]
In 2007 the United Nations' independent expert on the right to food, called for a five-year moratorium on biofuel production from food crops, to allow time for development of non-food sources. He called recent increases in food costs because of fuel production, such as the quadrupling of world corn price in one year, a growing "catastrophe" for the poor.[58] In February 2007, riots occurred in Mexico because of the skyrocketing price of tortillas. Ethanol has been credited as the reason for this increase in food prices[59]. The demand for corn has had a rippling effect on many corn-based products, like tortillas. The effects of ethanol and the increasing cost of food have also been felt in Pakistan, Indonesia, and Egypt.[60]
Oil has historically had a much higher EROEI than corn produced ethanol, according to some[citation needed]. However, oil must be refined into gasoline before it can be used for automobile fuel. Refining, as well as exploration and drilling, consumes energy. The difference between the energy in the fuel (output energy) and the energy needed to produce it (input energy) is often expressed as a percent of the input energy and called net energy gain (or loss). Several studies released in 2002 estimated that the net energy gain for corn ethanol is between 21 and 34 percent. The net energy loss for MTBE is about 33 percent. When added to gasoline, ethanol can replace MTBE as an anti-knock agent without poisoning drinking water as MTBE does. In Brazil, where the broadest and longest ethanol producing experiment took place, improvements in agricultural practices and ethanol production improvements led to an increase in ethanol net energy gain from 300% to over 800% in recent years.[citation needed] It must be noted that Brazil produces ethanol more efficiently because its primary input is the sugar from sugar cane rather than starches from corn. Consuming known oil reserves is increasing oil exploration and drilling energy consumption which is reducing oil EROEI (and energy balance) further.[61]
Opponents claim that corn ethanol production does not result in a net energy gain or that the consequences of large scale ethanol production to the food industry and environment offset any potential gains from ethanol. It has been estimated that "if every bushel of U.S. corn, wheat, rice and soybean were used to produce ethanol, it would only cover about 4% of U.S. energy needs on a net basis."[62] Many of the issues raised could likely be fixed by techniques now in development that produce ethanol from agricultural waste, such as paper waste, switchgrass, and other materials, but EIA Forecasts Significant Shortfall in Cellulosic Biofuel Production Compared to Target Set by Renewable Fuel Standard.[63]
Proponents cite the potential gains to the U.S. economy both from domestic fuel production and increased demand for corn. Optimistic calculations project that the United States is capable of producing enough ethanol to completely replace gasoline consumption.[citation needed] In comparison, Brazil's ethanol consumption today covers more than 50% of all energy used by vehicles in that country.
In the United States, preferential regulatory and tax treatment of ethanol automotive fuels introduces complexities beyond its energy economics alone. North American automakers have in 2006 and 2007 promoted a blend of 85% ethanol and 15% gasoline, marketed as E85, and their flex-fuel vehicles, e.g. GM's "Live Green, Go Yellow" campaign.[64] The apparent motivation is the nature of U.S. Corporate Average Fuel Economy (CAFE) standards, which give an effective 54% fuel efficiency bonus to vehicles capable of running on 85% alcohol blends over vehicles not adapted to run on 85% alcohol blends.[65] In addition to this auto manufacturer-driven impetus for 85% alcohol blends, the United States Environmental Protection Agency had authority to mandate that minimum proportions of oxygenates be added to automotive gasoline on regional and seasonal bases from 1992 until 2006 in an attempt to reduce air pollution, in particular ground-level ozone and smog.[66] In the United States, incidents of methyl tert(iary)-butyl ether (MTBE) groundwater contamination have been recorded in the majority of the 50 states,[67] and the State of California's ban on the use of MTBE as a gasoline additive has further driven the more widespread use of ethanol as the most common fuel oxygenate.[68]
A February 7, 2008 Associated Press article stated, "The widespread use of ethanol from corn could result in nearly twice the greenhouse gas emissions as the gasoline it would replace because of expected land-use changes, researchers concluded Thursday. The study challenges the rush to biofuels as a response to global warming."[69]
One acre of land can yield about 7,110 pounds (3,225 kg) of corn, which can be processed into 328 gallons (1240.61 liters) of ethanol. That is about 26.1 pounds (11.84 kg) of corn per gallon.
Much overlooked in most discussions about ethanol from corn are the by-products from the production of ethanol. Depending on the way it is processed, the processing yields several beneficial products, some of which are used for food production and feedstocks.
Ethanol fuel cells
Ethanol may be used as a fuel to power Direct-ethanol fuel cells (DEFC) in order to produce electricity and the by-products of water (H2O) and carbon dioxide (CO2).[70] Platinum is commonly used as an anode in such fuel cells in order to achieve a power density that is comparable to competing technologies. Until recently the high price of platinum has been cost prohibitive. A company called Acta Nanotech has created platinum free nanostructured anodes using more common and therefore less expensive metals.[71] A vehicle using a DEFC and non-platinum nanostructured anodes was used in the Shell Eco-Marathon 2007 by a team from Offenburg Germany which achieved an efficiency of 2716 kilometers per liter (6388 miles per gallon).[72]
Rocket fuel
Ethanol was commonly used as fuel in early bipropellant rocket vehicles, in conjunction with an oxidizer such as liquid oxygen. The German V-2 rocket of World War II, credited with beginning the space age, used ethanol, mixed with water to reduce the combustion chamber temperature.[73][74] The V-2's design team helped develop U.S. rockets following World War II, including the ethanol-fueled Redstone rocket, which launched the first U.S. satellite.[75] Alcohols fell into general disuse as more efficient rocket fuels were developed.[74]
Alcoholic beverages
Ethanol is the principal psychoactive constituent in alcoholic beverages, with depressant effects on the central nervous system. It has a complex mode of action and affects multiple systems in the brain, most notably ethanol acts as an agonist to the GABA receptors.[76] Similar psychoactives include those which also interact with GABA receptors, such as gamma-hydroxybutyric acid (GHB).[77] Ethanol is metabolized by the body as an energy-providing carbohydrate nutrient, as it metabolizes into acetyl CoA, an intermediate common with glucose metabolism, that can be used for energy in the citric acid cycle or for biosynthesis.
Alcoholic beverages vary considerably in their ethanol content and in the foodstuffs from which they are produced. Most alcoholic beverages can be broadly classified as fermented beverages, beverages made by the action of yeast on sugary foodstuffs, or as distilled beverages, beverages whose preparation involves concentrating the ethanol in fermented beverages by distillation. The ethanol content of a beverage is usually measured in terms of the volume fraction of ethanol in the beverage, expressed either as a percentage or in alcoholic proof units.
Fermented beverages can be broadly classified by the foodstuff from which they are fermented. Beers are made from cereal grains or other starchy materials, wines and ciders from fruit juices, and meads from honey. Cultures around the world have made fermented beverages from numerous other foodstuffs, and local and national names for various fermented beverages abound.
Distilled beverages are made by distilling fermented beverages. Broad categories of distilled beverages include whiskeys, distilled from fermented cereal grains; brandies, distilled from fermented fruit juices, and rum, distilled from fermented molasses or sugarcane juice. Vodka and similar neutral grain spirits can be distilled from any fermented material (grain or potatoes are most common); these spirits are so thoroughly distilled that no tastes from the particular starting material remain. Numerous other spirits and liqueurs are prepared by infusing flavors from fruits, herbs, and spices into distilled spirits. A traditional example is gin, which is created by infusing juniper berries into a neutral grain alcohol.
In a few beverages, ethanol is concentrated by means other than distillation. Applejack is traditionally made by freeze distillation, by which water is frozen out of fermented apple cider, leaving a more ethanol-rich liquid behind. Eisbier (more commonly, eisbock) is also freeze-distilled, with beer as the base beverage. Fortified wines are prepared by adding brandy or some other distilled spirit to partially-fermented wine. This kills the yeast and conserves some of the sugar in grape juice; such beverages are not only more ethanol-rich, but are often sweeter than other wines.
Alcoholic beverages are sometimes used in cooking, not only for their inherent flavors, but also because the alcohol dissolves hydrophobic flavor compounds which water cannot.
Feedstock
Ethanol is an important industrial ingredient and has widespread use as a base chemical for other organic compounds. These include ethyl halides, ethyl esters, diethyl ether, acetic acid, butadiene, and ethyl amines.
Antiseptic use
Ethanol is used in medical wipes and in most common antibacterial hand sanitizer gels at a concentration of about 62% (percentage by volume, not weight) as an antiseptic. Ethanol kills organisms by denaturing their proteins and dissolving their lipids and is effective against most bacteria and fungi, and many viruses (including SARS [2]), but is ineffective against bacterial spores.[78]
Antidote use
Ethanol can be used as an antidote for poisoning by other toxic alcohols, in particular methanol[79] and ethylene glycol. Ethanol competes with other alcohols for the alcohol dehydrogenase enzyme, preventing metabolism into toxic aldehyde and carboxylic acid derivatives.[80]
Other uses
- Ethanol is easily miscible in water and is a good solvent. Ethanol is less polar than water and is used in perfumes, paints and tinctures.
- Ethanol is also used in design and sketch art markers, such as Copic, and Tria.
Effects on humans
The National Institute on Alcohol Abuse and Alcoholism maintains a database of alcohol-related health effects. [81]
| BAC (mg/dL) | Symptoms[82] |
|---|---|
| 50 | Euphoria, talkativeness, relaxation |
| 100 | Central nervous system depression, impaired motor and sensory function, impaired cognition |
| >140 | Decreased blood flow to brain |
| 300 | Stupefaction, possible unconsciousness |
| 400 | Possible death |
| >550 | Death |
Effects on the central nervous system
Ethanol is a central nervous system depressant and has significant psychoactive effects in sublethal doses; for specifics, see effects of alcohol on the body by dose. Based on its abilities to change the human consciousness, ethanol is considered a drug.[83] Death from ethyl alcohol consumption is possible when blood alcohol level reaches 0.4%. A blood level of 0.5% or more is commonly fatal. Levels of even less than 0.1% can cause intoxication, with unconsciousness often occurring at 0.3–0.4%.[84]
The amount of ethanol in the body is typically quantified by blood alcohol content (BAC), the milligrams of ethanol per 100 milliliters of blood. The table at right summarizes the symptoms of ethanol consumption. Small doses of ethanol generally produce euphoria and relaxation; people experiencing these symptoms tend to become talkative and less inhibited, and may exhibit poor judgment. At higher dosages (BAC > 100 mg/dl), ethanol acts as a central nervous system depressant, producing at progressively higher dosages, impaired sensory and motor function, slowed cognition, stupefaction, unconsciousness, and possible death.
In America, about half of the deaths in car accidents occur in alcohol-related crashes.[85] There is no completely-safe level of alcohol for driving; the risk of a fatal car accident rises with the level of alcohol in the driver's blood.[86] However, most drunk driving laws governing the acceptable levels in the blood while driving or operating heavy machinery set typical upper limits of blood alcohol content (BAC) between 0.05% to 0.08%.
Effects on metabolism
Ethanol within the human body is converted into acetaldehyde by alcohol dehydrogenase and then into acetic acid by acetaldehyde dehydrogenase. The product of the first step of this breakdown, acetaldehyde,[87] is more toxic than ethanol. Acetaldehyde is linked to most of the clinical effects of alcohol. It has been shown to increase the risk of developing cirrhosis of the liver,[77] multiple forms of cancer, and alcoholism.
Drug interactions
Ethanol can intesify the sedation caused by other central nervous system depressant drugs such as barbiturates, benzodiazepines, opioids, and phenothiazines[84]
Magnitude of effects
Some individuals have less-effective forms of one or both of the metabolizing enzymes, and can experience more-severe symptoms from ethanol consumption than others. Conversely, those who have acquired alcohol tolerance have a greater quantity of these enzymes, and metabolize ethanol more rapidly.[88]
Birth defects
Ethanol is classified as a teratogen. See fetal alcohol syndrome.
Other effects
Frequent drinking of alcoholic beverages has been shown to be a major contributing factor in cases of elevated blood levels of triglycerides.[89]
Ethanol is not a carcinogen.[90][91] However, the first metabolic product of ethanol, acetaldehyde, is toxic, mutagenic, and carcinogenic. Also, ethanol's effect on the liver can contribute to immune suppression. Consequently, consumption of alcoholic beverages can be an aggravating factor in carcinogenesis.
See also
|
References
- ^ Roach, J. (July 18, 2005). "9,000-Year-Old Beer Re-Created From Chinese Recipe". National Geographic News. Retrieved 2007-09-03.
- ^ Hassan, Ahmad Y. "Alcohol and the Distillation of Wine in Arabic Sources". History of Science and Technology in Islam. Retrieved 2008-03-29.
- ^ Alcohol in the Encyclopædia Britannica Eleventh Edition
- ^ Couper, A.S. (1858). "On a new chemical theory" (online reprint). Philosophical magazine. 16 (104–116). Retrieved 2007-09-03.
- ^ Hennell, H. (1828). "On the mutual action of sulfuric acid and alcohol, and on the nature of the process by which ether is formed". Philosophical Transactions. 118 (365–371).
- ^ a b Robert Siegel (2007-02-15). "Ethanol, Once Bypassed, Now Surging Ahead". NPR. Retrieved 2007-09-22.
- ^ Joseph DiPardo. "Outlook for Biomass Ethanol Production and Demand" (PDF). United States Department of Energy. Retrieved 2007-09-22.
- ^ a b c CRC Handbook of Chemistry, 44th ed.
- ^ a b c d Merck Index of Chemicals and Drugs, 9th ed.
- ^ a b c d e Morrison, Robert Thornton; Boyd, Robert Neilson (1972). Organic Chemistry, 2nd ed. Allyn and Bacon, inc.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Merck Index of Chemicals and Drugs, 9th ed.; monographs 6575 through 6669
- ^ Kroschwitz and Howe-Grant, editors, Encyclopedia of Chemical Technology, 4th ed., (New York: John Wiley & Sons), vol. 9, 813.
- ^ Anil K. Rajvanshi, S.M. Patil, and B. Mendonca (2007). Energy for Sustainable Development. 11 (1): 94–99.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f Streitweiser, Andrew Jr.; Heathcock, Clayton H. (1976). Introduction to Organic Chemistry. MacMillan. ISBN 0-02-418010-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Frederick D. Rossini (1937). "Heats of Formation of Simple Organic Molecules". Ind. Eng. Chem. 29 (12): 1424–1430. doi:10.1021/ie50336a024.
- ^ Mills, G.A.; Ecklund, E.E. "Alcohols as Components of Transportation Fuels." Annual Review of Energy. November 1987. Vol. 12, 47–80. Retrieved on September 2, 2007.
- ^ Roberts, John D.; Caserio, Marjorie C. (1977). Basic Principles of Organic Chemistry. W. A. Benjamin, Inc. ISBN 0-8053-8329-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Lodgsdon, J.E. (1994). "Ethanol." In J.I. Kroschwitz (Ed.) Encyclopedia of Chemical Technology, 4th ed. vol. 9, p. 820. New York: John Wiley & Sons.
- ^ Lodgsdon, J.E. (1994). p. 817
- ^ Morais, P.B.; Rosa, C.A.; Linardi, V.R.; Carazza, F.; Nonato, E.A. "Production of fuel alcohol by Saccharomyces strains from tropical habitats." Biotechnology Letters. November 1996. Vol. 18, No. 11, 1351–1356. Retrieved on September 2, 2007.
- ^ Badger, P.C. "Ethanol From Cellulose: A General Review." p. 17–21. In: J. Janick and A. Whipkey (eds.), Trends in new crops and new uses. ASHS Press, 2002, Alexandria, VA. Retrieved on September 2, 2007.
- ^ Taherzadeh M.J., Karimi K. (2007): Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review, BioResources, 2(3): 472-499
- ^ Taherzadeh M.J., Karimi K. (2007): Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: A review, BioResources, 2(4): 707-738
- ^ Ritter, S.K. (May 31, 2004). "Biomass or Bust." Chemical & Engineering News 82(22), 31–34.
- ^ Clines, Tom (2006). "Brew Better Ethanol". Popular Science Online.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Dhinakar S. Kompala. "Maximizing Ethanol Production by Engineered Pentose-Fermenting Zymononas mobilis" (PDF). Department of Chemical Engineering, University of Colorado at Boulder.
{{cite web}}: Unknown parameter|accessdaymonth=ignored (help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - ^ Mick, Jason (2008-01-14). "Cellulosic Ethanol Promises $1 per Gallon Fuel From Waste". DailyTech.com. Retrieved 2008-01-15.
{{cite web}}: Check date values in:|date=(help) - ^ "Providing for a Sustainable Energy Future". Bioengineering Resources, inc.
{{cite web}}: Unknown parameter|accessdaymonth=ignored (help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - ^ "Closed-Loop Ethanol Plant to Start Production". www.renewableenergyaccess.com. November 2, 2006. Retrieved 2007-09-03.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Rapier, R. (June 26, 2006) "E3 Biofuels: Responsible Ethanol" R-Squared Energy Blog
- ^ "Air Pollution Rules Relaxed for US Ethanol Producers". Truthout. 2007-04-12.
{{cite web}}: Unknown parameter|accessdaymonth=ignored (help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - ^ Snyder R, Kalf GF (1994). "A perspective on benzene leukemogenesis". Crit. Rev. Toxicol. 24 (3): 177–209. doi:10.3109/10408449409021605. PMID 7945890.
- ^ "U-M Program to Reduce the Consumption of Tax-free Alcohol; Denatured Alcohol a Safer, Less Expensive Alternative" (PDF). University of Michigan. Retrieved 2007-09-29.
- ^ Great Britain (2005). The Denatured Alcohol Regulations 2005. Statutory Instrument 2005 No. 1524.
- ^ Appendix B, Transportation Energy Data Book from the Center for Transportation Analysis of the Oak Ridge National Laboratory
- ^ Calculated from heats of formation. Does not correspond exactly to the figure for MJ/l divided by density.
- ^ George. Overview of Storage Development DOE Hydrogen Program [pdf. Livermore, CA. Sandia National Laboratories. 2000.]
- ^ Reel, M. (August 19, 2006) "Brazil's Road to Energy Independence", Washington Post.
- ^ "Etnanol Fuel".
- ^ California Air Resources Board,Definition of a Low Emission Motor Vehicle in Compliance with the Mandates of Health and Safety Code Section 39037.05,second release, October 1989
- ^ A.Lowi& W.P.L.Carter; A Method for Evaluating the Atmospheric Ozone Impact of Actual Vehicle emissions, S.A.E. Technical Paper, Warrendale,PA; march 1990
- ^ T.T.M.Jones,The Clean Fuels Report: A Quantitative Comparison Of Motor Fuels, Related Pollution and Technologies: 2008.[1]
- ^ citation needed
- ^ "Renewable Fuels Association Industry Statistics".
- ^ "Tecnologia flex atrai estrangeiros".
{{cite web}}: Unknown parameter|Publisher=ignored (|publisher=suggested) (help) - ^ "First Commercial U.S. Cellulosic Ethanol Biorefinery Announced". Renewable Fuels Association. 2006-11-20.
{{cite web}}: Unknown parameter|accessdaymonth=ignored (help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - ^ "Renewable Fuel Standard Program". United States Environmental Protection Agency. 2007-04-10.
{{cite web}}: Unknown parameter|accessdaymonth=ignored (help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - ^ Hosein Shapouri, James A. Duffield, and Michael Wang. "The Energy Balance of Corn Ethanol: an Update" (PDF). United States Department of Agriculture.
{{cite web}}: Unknown parameter|accessdaymonth=ignored (help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Pimentel D, Patzek TW (2005). "Ethanol Production Using Corn, Switchgrass, and Wood; Biodiesel Production Using Soybean and Sunflower". Natural Resources Research. 14 (1): 65–76. doi:10.1007/s11053-005-4679-8.
- ^ Lang, Susan S. "Cornell ecologist's study finds that producing ethanol and biodiesel from corn and other crops is not worth the energy". Cornell University.
{{cite web}}: Unknown parameter|accessdaymonth=ignored (help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - ^ M.R. Schmer, K.P. Vogel, R.B. Mitchell, R.K. Perrin (2007-11-21). "Net energy of cellulosic ethanol from switchgrass". U.S. Dept. of Agrigulture. Retrieved 2008-01-13.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ The Clean Energy Scam, TIME, April 7, 2008, pages 40–41.
- ^ Doug Bandow (1997-10-02). "Ethanol Keeps ADM Drunk On Tax Dollars". CATO Institute. Retrieved 2007-09-03.
- ^ The Politics of Ethanol Outshine its Costs
- ^ Moira Herbst (March 19, 2007). "Ethanol's Growing List of Enemies". Business Week. Retrieved 2007-09-03.
- ^ Jeff Cox (June 19, 2007). "Corn and milk: A 1-2 inflation combo". CNNMoney.com. Retrieved 2007-09-03.
- ^ Fuel, Food Demand Raise Corn, Soybean Prices
- ^ Edith M. Lederer, Associated Press (2007-10-27). "UN Expert Calls Biofuel 'Crime Against Humanity'".
- ^ http://news.bbc.co.uk/2/hi/americas/6319093.stm
- ^ Posted by Hans Bader (2008-04-08). "Ethanol Subsidies Cause Food Riots in Mexico, Pakistan, Indonesia, Yemen, and Egypt". Open Market blog.
- ^ David Andress & Associates (2002). "Ethanol Energy Balances".
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "Forget the Ethanol Myth -- Avoid Biofuel Bubble: John F. Wasik". Bloomberg.com. 2007-07-23. Retrieved 2007-07-25.
- ^ "Study Finds Net Energy of Cellulosic Ethanol from Switchgrass Much Higher Than Expected". Green Car Congress. 2008-01-07. Retrieved 2008-01-13.
- ^ "GM Announces E85 Fuel Card Promotion On FlexFuel Vehicles". The Auto Channel. Retrieved 2007-09-04.
- ^ "CAFE Credits for Flex Fuel Vehicles Undermine Improvements in Fuel Economy". Public Citizen. 2006-09-27. Retrieved 2007-09-03.
{{cite web}}: Check date values in:|date=(help) - ^ "Regulations & Standards". United States Environmental Protection Agency. Retrieved 2007-09-04.
- ^ "MTBE Contamination from Underground Storage Tanks" (PDF). United States General Accounting Office. 2002-05-21. Retrieved 2007-10-09.
- ^ "Methyl Tertiary Butyl Ether (MTBE)". United State Environmental Protection Agency. Retrieved 2007-09-04.
- ^ Study: Ethanol May Add to Global Warming Associated Press, February 7, 2008
- ^ Direct Methanol Fuel Cells (DMFC) FCTec.
- ^ Offenburg students test world's first ethanol powered fuel cell vehicle Acta.
- ^ Willkommen beim Projekt "Schluckspecht" der Hochschule Offenburg .
- ^ David Darling. "The Internet Encyclopedia of Science: V-2".
- ^ a b Braeunig, Robert A. "Rocket Propellants." (Website). Rocket & Space Technology, 2006. Retrieved on 2007-08-23.
- ^ "A Brief History of Rocketry." NASA Historical Archive, via science.ksc.nasa.gov.
- ^ Chastain G (2006). "Alcohol, neurotransmitter systems, and behavior". The Journal of general psychology. 133 (4): 329–35. doi:10.3200/GENP.133.4.329-335. PMID 17128954.
- ^ a b Dr. Bill Boggan. "Effects of Ethyl Alcohol on Organ Function". Chemases.com. Retrieved 2007-09-29.
- ^ McDonnell G, Russell AD (1999). "Antiseptics and disinfectants: activity, action, and resistance". Clin. Microbiol. Rev. 12 (1): 147–79. PMID 9880479.
- ^ "Methanol Poisoning". Cambridge University School of Clinical Medicine. Retrieved 2007-09-04.
- ^ Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA (2002). "American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning". J. Toxicol. Clin. Toxicol. 40 (4): 415–46. doi:10.1081/CLT-120006745. PMID 12216995.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "ETOH Archival Database (1972–2003)". Alcohol and Alcohol Problems Science Database. NIAAA. Retrieved 2008-03-31.
- ^ Pohorecky, L.A., and J. Brick. (1988). "Pharmacology of ethanol." Pharmacology & Therapeutics 36(3), 335–427.
- ^ "Alcohol Use" MedlinePlus Medical Encyclopedia, U.S. National Library of Medicine and National Institutes of Health. Retrieved on 2007-09-27
- ^ a b David A. Yost, MD (2002). "Acute care for alcohol intoxication" ([dead link] – Scholar search). 112 (6). Postgraduate Medicine Online. Retrieved 2007-09-29.
{{cite journal}}: Cite journal requires|journal=(help); External link in|format=|month=ignored (help) - ^ Hingson R, Winter M (2003). "Epidemiology and consequences of drinking and driving" (PDF). Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 27 (1): 63–78. PMID 15301401.
- ^ Naranjo CA, Bremner KE (1993). "Behavioural correlates of alcohol intoxication". Addiction. 88 (1): 25–35. doi:10.1111/j.1360-0443.1993.tb02761.x. PMID 8448514.
- ^ Dr. Bill Boggan. "Metabolism of Ethyl Alcohol in the Body". Chemases.com. Retrieved 2007-09-29.
- ^ Agarwal DP, Goedde HW (1992). "Pharmacogenetics of alcohol metabolism and alcoholism". Pharmacogenetics. 2 (2): 48–62. doi:10.1097/00008571-199204000-00002. PMID 1302043.
- ^ "Triglycerides". American Heart Association. Retrieved 2007-09-04.
- ^ "Material Data Safety Sheet" (PDF). Burdick and Jackson. Retrieved 2007-10-25.
- ^ Pollyanna R. Chavez, Xiang-Dong Wang, Jean Meyer. "Animal Models for Carcinogenesis and Chemoprevention, abstract #C42: Effects of Chronic Ethanol Intake on Cyclin D1 Levels and Altered Foci in Diethylnitrosamine-initiated Rats" (PDF). USDA. Retrieved 2007-10-24.
{{cite web}}: CS1 maint: multiple names: authors list (link)
Further reading
- "Alcohol." (1911). In Hugh Chisholm (Ed.) Encyclopædia Britannica Eleventh Edition. Online reprint
- Lodgsdon, J.E. (1994). "Ethanol." In J.I. Kroschwitz (Ed.) Encyclopedia of Chemical Technology, 4th ed. vol. 9, pp. 812–860. New York: John Wiley & Sons.
- Smith, M.G., and M. Snyder. (2005). "Ethanol-induced virulence of Acinetobacter baumannii". American Society for Microbiology meeting. June 5 – June 9. Atlanta.
- Sci-toys website explanation of US denatured alcohol designations
- Boyce, John M., and Pittet Didier. (2003). “Hand Hygiene in Healthcare Settings.” Centers for Disease Control, Atlanta, Georgia, United States.
- Rene Martinez VitalSensors Technologies LLC. "VS1000A Series In-Line Ethanol Sensors for the Beverage and BioFuel Industry" (PDF). Martinez describes the theory and practice of measuring brix on-line in beverages.
External links
- International Labour Organization ethanol safety information
- National Pollutant Inventory – Ethanol Fact Sheet
- Ethanol Information
- Ethanol Facts
- Coordinates of the ethanol molecule on Computational Chemistry Wiki. Accessed on September 8, 2005.
- Molview from bluerhinos.co.uk See Ethanol in 3D
- National Institute of Standards and Technology chemical data on ethanol
- ChEBI – biology related
- Chicago Board of Trade news and market data on ethanol futures