Angiotensin converting enzyme 2
| Angiotensin converting enzyme 2 | ||
|---|---|---|
|
||
| Ribbon model of the human angiotensin converting enzyme 2, according to PDB 1R42 | ||
| other names |
|
|
|
Existing structure data : 3SCL , 1R42 , 1R4L , 2AJF , 3D0G , 3D0H , 3D0I , 3KBH , 3SCL , 3SCJ , 3SCK |
||
| Properties of human protein | ||
| Mass / length primary structure | 805 amino acids | |
| Secondary to quaternary structure | Heterodimer | |
| Cofactor | Zn 2+ , Cl - | |
| Isoforms | 2 | |
| Identifier | ||
| Gene names | ACE2 ; ACEH | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 3.4.17.23 , hydrolase | |
| MEROPS | M02.006 | |
| Response type | hydrolysis | |
| Substrate | Angiotensin II , angiotensin I. | |
| Products | Angiotensin (1-7), angiotensin (1-9) | |
| Occurrence | ||
| Homology family | CLU_014364_3_0 | |
| Parent taxon | Eukaryotes , bacteria | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 59272 | 70008 |
| Ensemble | ENSG00000130234 | ENSMUSG00000015405 |
| UniProt | Q9BYF1 | Q8R0I0 |
| Refseq (mRNA) | NM_021804 | NM_001130513 |
| Refseq (protein) | NP_068576 | NP_001123985 |
| Gene locus | Chr X: 15.56 - 15.6 Mb | Chr X: 164.14 - 164.19 Mb |
| PubMed search | 59272 |
70008
|
Angiotensin-converting enzyme 2 ( English Angiotensin-converting enzyme 2 , short ACE2 ) is a metallocarboxypeptidase , and a type 1 transmembrane protein with homology to the angiotensin converting enzyme (ACE), which is mainly in eukaryotes , but also in bacteria occurs. ACE2 plays an important role in the renin-angiotensin-aldosterone system (RAAS), which controls the volume balance of the human body and regulates blood pressure .
PCR analyzes showed that ACE2 in heart , and in the lung , kidney , in the endothelium and in the gastrointestinal tract expressed is. Also, ACE2 is a receptor for various coronaviruses , including SARS-CoV and SARS-CoV-2 , to enter cells .
structure
ACE2 contains 20 α-helical segments and nine 3 10 -helices , which together make up about 62% of the structure. In addition, ACE2 has six short β-sheet segments that make up about 3.5% of the structure. The extracellular region of human ACE2 consists of two domains , on the one hand the zinc metallopeptidase domain (also called peptidase domain (PD), position 19-615) and on the other hand the C -terminal collectrin homology domain (or collectrin-like domain) Domain, CLD), which is disordered. The metallopeptidase domain can also be divided into two subdomains (I and II), between which the active center is located. Subdomain I contains the N terminus and the zinc ion and subdomain II contains the C terminus. Both subdomains are connected with an α-helix.
The zinc ion is coordinated in the active center by the amino acid residues His374, His378, Glu402 and a water molecule (in the native state ) . These amino acid residues together form the "HE XX H + E" motif (H = histidine , E = glutamic acid , X = unknown amino acid; see one -letter code ), which is conserved in clan MA in metalloproteases . The chloride ion is coordinated by the residues Arg169, Trp477 and Lys481 in subdomain II.
Physiological function
Protective effect against cardiovascular diseases
ACE2 is a multifunctional enzyme that enhances the vasoconstrictive effects of angiotensin II through the formation of vasodilatory peptides such as angiotensin- (1-7) (also known as Ang- (1-7)) and through the hydrolysis of vasoactive peptides such as apelin-13 , Neurotensin , kinestinin , dynorphin and bradykinin fragments.
Angiotensin II, the main actor in the renin-angiotensin-aldosterone system, binds mainly to the angiotensin II receptor type 1 (AT 1 receptor) and thus causes cell growth , proliferation and migration . In the event of dysregulation, these processes influence the remodeling of the heart and the blood vessel system, which can lead to various cardiovascular diseases . The so-called counter-regulatory axis of the RAAS ( English counter-regulatory axis ) supported by the enzyme and its products ACE2 angiotensin (1-9) proceeds (also Ang- (1-9) known) and angiotensin (1-7) Angiotensin- (1-7) inhibiting the action of angiotensin II by binding to the Mas receptor , plays a protective role in cardiovascular diseases. The cardioprotective effect is partly based on the formation of nitric oxide (NO). Ang- (1-7) stimulates the phosphorylation of endothelial nitric oxide synthase in Mas-expressing cells, among other things via the PI3K / Akt signaling pathway. This activates the synthase and produces nitric oxide.
The binding of angiotensin- (1-9) to the angiotensin II receptor type 2 (AT 2 receptor) in the heart leads to a reduction in collagen synthesis and thus to a reduction in fibrosis of the heart, as well as a decrease in Rho kinase -Activity to weaken the hypertrophy of the heart. The binding of angiotensin (1-9) to the AT 2 receptor in blood vessels promotes vasodilation through increased NO concentration or through crosstalk with the BK 2 receptor. However, the AT 2 receptor leads to increased vasoconstriction under angiotensin- (1-9). The increased NO concentration is to be regarded as a delayed compensation reaction. The main effect, however, is the clear vasoconstriction under angiotensin II. The protective effect is mainly based on the capillary end flow path. The other angiotensin-II degradation products play a relatively minor role in medicine.
mechanism
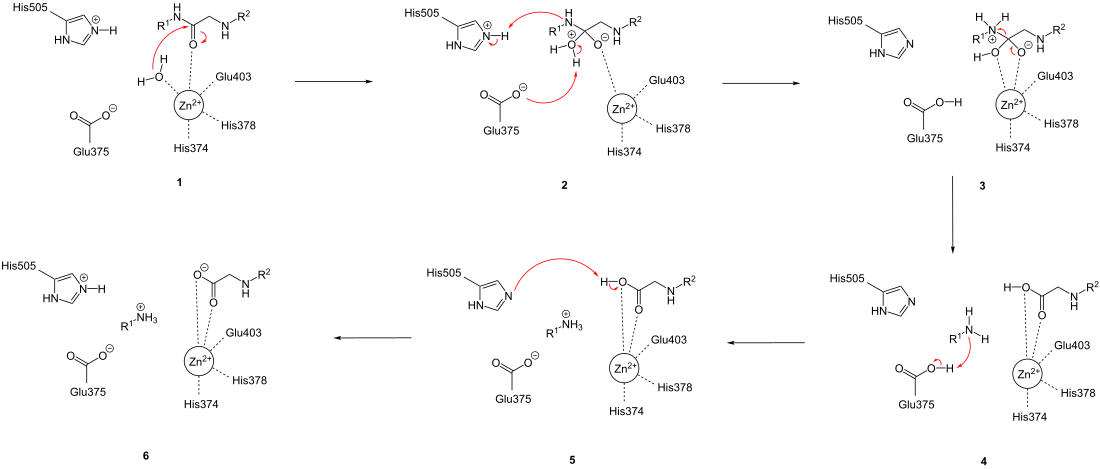
ACE2 catalyzes the hydrolysis of peptide bonds at the C -terminal end with the help of a zinc ion in the active site. With the octapeptide angiotensin II as a substrate , the heptapeptide angiotensin- (1-7) and the amino acid L- phenylalanine are formed . With the decapeptide angiotensin I as substrate, the nonapeptide angiotensin- (1-9) and the amino acid L- leucine are formed . A general peptide is used as a substrate to simplify the illustration of the reaction mechanism .
During the reaction, the enzyme-substrate complex (in 1 ) is first converted to the tetrahedral intermediate (in 2 ). To do this, the zinc-bound water molecule carries out a nucleophilic attack on the carbonyl group of the peptide ( 1 ), which leads to a transfer of protons from the water molecule to the amino acid residue Glu375. At the same time, a proton is transferred from His505 to the nitrogen atom of the amino acid to be split off ( 2 ). This is followed by the disintegration of the tetrahedral intermediate and the cleavage of the peptide bond ( 3 ), which leads to proton transfer from Glu375 to the free amino group of the split-off amino acid ( 4 ). Then a proton is transferred back from the carboxy group of the oligopeptide to His505 directly ( 5 ) or indirectly by proton exchange with the solvent .
SARS-CoV
The SARS coronavirus (SARS-CoV) is able to bind to the human enzyme ACE2 using the spike (S) protein of the virus envelope . When the ACE2 virus complex is transported to the endosomes , the S protein is cleaved by the endopeptidase cathepsin L , so that the virus enters the cell through pH- dependent and receptor-mediated endocytosis (which occurs independently of clathrin and caveolae ). Another possibility for cell entry is the activation of the S protein by TMPRSS2 and the resulting membrane fusion on the cell surface .
The S-protein of SARS-CoV is composed of two subunits. The S1 subunit contains the receptor binding domain ( english receptor-binding domain , RBD), which can bind to ACE2. When the RBD binds to ACE2, this causes conformational changes in the S2 subunit, which facilitate fusion of the virus envelope with the cell membrane . The amino acid residues 424-494 of RBD form the receptor-binding motif ( English receptor-binding motif , RBM). Within the 14 residues of the RBM that are in direct contact with 18 residues of the ACE2, six of them are tyrosine residues that contribute to the specific recognition of ACE2. In addition, several cysteine residues also contribute to recognition through the formation of disulfide bridges . The amino acid residues Asn479 and Thr487 of the RBM influence the course of SARS and the SARS-CoV tropism . Asn479 is present in most of the human SARS-CoV-S protein sequences . Any changes in positions 479 and 487 of the amino acid sequence of the RBD can affect zoonotic or human-to-human transmissions. For a zoonotic transmission (in the case of SARS transmission of SARS-CoV the larvae Rollers on man) has the RBD of larvae Rollers at position 479 a lysine residue , the to steric hindrance and electrostatic interference with residues of the N -terminal helix of ACE2 as His34 leads. With a Lys479 → Asn479 mutation , the obstructive interactions with the N -terminal helix are avoided and the affinity between RBD and ACE2 is increased, so that it could play a role in zoonotic transmission. In addition, the salt bridges formed in a hydrophobic environment between Lys31 and Glu35 of human ACE2 serve to release binding energy and thus to increase virus-receptor interactions. Thr487 also increases the affinity between RBD and ACE2. The γ- methyl group of Thr487 ensures that the side chain of Lys353 on ACE2 is positioned in such a way that a salt bridge is formed with Asp38 on ACE2 and could thus play a role in human-to-human transmission.
SARS-CoV-2
In the event of a SARS-CoV-2 infection, the virion has contact with human cells and is absorbed into the cell interior. In most cells, this process is triggered by the binding of the spike protein in the virus envelope to an ACE2 protein in the cell membrane. The cellular serine protease TMPRSS2 is usually required for penetration. If SARS-CoV-2 penetrates into alveolar epithelial cells ( pneumocytes ), this can lead to respiratory symptoms.
These symptoms are more severe in patients with pre-existing cardiovascular disease, presumably due to an increased ACE2 density on the cell membrane compared to healthy individuals. By increasing the ACE2 level, the equilibrium is shifted in the direction of the counter-regulatory axis. The SARS-CoV-2 infection via ACE2 leads to the inflammation-promoting cytokine release via the angiotensin II-AT 1 R axis; this represents a possible therapeutic target via the IL-6 - STAT3 axis.
Treatment with inhibitors of the renin-angiotensin-aldosterone system ( RAAS inhibitors ) has an influence on the extent of the infection. Different RAAS inhibitors each have different effects on the ACE2 level. In Lewis rats ( laboratory rats developed in the 1950s ), when either ACE inhibitors or angiotensin receptor blockers were administered, the Ace2 mRNA level was increased compared to rats given placebos . In particular in the heart of the rat, the Ace2 mRNA level is increased 4.7-fold when treated with lisinopril and 2.8-fold when treated with losartan . ACE2 activity is increased with lisinopril treatment, but not with losartan treatment, compared to placebo. When treated with captopril , ACE2 expression can be increased significantly in rats with acute lung failure . In rat models of acute lung failure, ACE activity and angiotensin II expression are increased, whereas ACE2 activity and angiotensin (1-7) expression are reduced.
While angiotensin receptor blockers and mineralocorticoid receptor blockers have been shown to increase ACE2 expression and activity in various experimental and clinical models, administration of ACE inhibitors increases the Ace2 mRNA levels of the heart, but did not have any in experimental models Influence on ACE2 activity. In addition, in an animal model of diabetic nephropathy, administration of aliskiren (a direct renin inhibitor ) was associated with a reduction in ACE2 expression. For the treatment of COVID-19 , the YouAn Hospital in Beijing ( Chinese 北京 佑安 医院 ) used intravenous transplantation of ACE2-negative mesenchymal stem cells (MSC), especially for patients in critical condition.
There are clinical concerns about ACE2 regulation with RAAS inhibitors and statins for the treatment of COVID-19.
The German Federal Institute for Drugs and Medical Devices has approved a clinical trial of a recombinant ACE2 on seriously ill COVID-19 patients.
See also
Individual evidence
- ↑ C. Tikellis, MC Thomas: Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. In: International journal of peptides. Volume 2012, 2012, p. 256294, doi : 10.1155 / 2012/256294 , PMID 22536270 , PMC 3321295 (free full text).
- ^ ACE-2: The SARS Receptor Identified. In: R&D Systems. Accessed February 16, 2020 .
- ↑ W. Li, MJ Moore, N. Vasilieva, J. Sui, SK Wong, MA Berne, M. Somasundaran, JL Sullivan, K. Luzuriaga, TC Greenough, H. Choe, M. Farzan: Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. In: Nature . Volume 426, number 6965, November 2003, pp. 450-454, doi : 10.1038 / nature02145 , PMID 14647384 .
- ↑ a b Yushun Wan, Jian Shang, Rachel Graham, Ralph S. Baric, Fang Li: Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS. In: Journal of Virology. , doi : 10.1128 / JVI.00127-20 .
- ↑ H. Hofmann, K. pyrC, L. van der Hoek, M. Geier, B. Berkhout, p Pöhlmann: Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. In: Proceedings of the National Academy of Sciences . Volume 102, number 22, May 2005, pp. 7988-7993, doi : 10.1073 / pnas.0409465102 , PMID 15897467 , PMC 1142358 (free full text).
- ↑ a b R. Yan, Y. Zhang, Y. Li, L. Xia, Y. Guo, Q. Zhou: Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. In: Science . Volume 367, number 6485, 03 2020, pp. 1444–1448, doi : 10.1126 / science.abb2762 , PMID 32132184 , PMC 7164635 (free full text).
- ^ Structure Watch. In: Nature Reviews Molecular Cell Biology . Volume 6, number 823, 2005, p. 823, doi : 10.1038 / nrm1780 .
- ↑ a b c P. Towler, B. Staker, SG Prasad, S. Menon, J. Tang, T. Parsons, D. Ryan, M. Fisher, D. Williams, NA Dales, MA Patane, MW Pantoliano: ACE2 X -ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. In: Journal of Biological Chemistry . Volume 279, Number 17, April 2004, pp. 17996-18007, doi : 10.1074 / jbc.M311191200 , PMID 14754895 .
- ↑ C. Vickers, P. Hales, V. Kaushik, L. Dick, J. Gavin, J. Tang, K. Godbout, T. Parsons, E. Baronas, F. Hsieh, S. Acton, M. Patane, A. Nichols, P. Tummino: Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. In: Journal of Biological Chemistry . Volume 277, Number 17, April 2002, pp. 14838-14843, doi : 10.1074 / jbc.M200581200 , PMID 11815627 .
- ^ MK Raizada, AJ Ferreira: ACE2: a new target for cardiovascular disease therapeutics. In: Journal of cardiovascular pharmacology. Volume 50, Number 2, August 2007, pp. 112-119, doi : 10.1097 / FJC.0b013e3180986219 , PMID 17703127 (review).
- ^ AJ Ferreira, RA Santos, AP Almeida: Angiotensin- (1-7): cardioprotective effect in myocardial ischemia / reperfusion. In: Hypertension. Volume 38, number 3 Pt 2, September 2001, pp. 665-668, doi : 10.1161 / 01.hyp.38.3.665 , PMID 11566952 .
- ↑ KB Brosnihan, P. Li, CM Ferrario: Angiotensin- (1-7) dilates canine coronary arteries through kinins and nitric oxide. In: Hypertension. Volume 27, Number 3, March 1996, pp. 523-528, doi : 10.1161 / 01.hyp.27.3.523 , PMID 8613197 .
- ↑ Walkyria Oliveira Sampaio, Robson Augusto Souza dos Santos, Raphael Faria-Silva, Leonor Tapias da Mata Machado, Ernesto L. Schiffrin, Rhian M. Touyz: Angiotensin- (1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt -dependent pathways. In: Hypertension. Volume 49, Number 1, January 2007, pp. 185-192, doi : 10.1161 / 01.HYP.0000251865.35728.2f , PMID 17116756 .
- ↑ CA McKinney, C. Fattah, CM Loughrey, G. Milligan, SA Nicklin: Angiotensin- (1-7) and angiotensin- (1-9): function in cardiac and vascular remodelling. In: Clinical science. Volume 126, Number 12, June 2014, pp. 815-827, doi : 10.1042 / CS20130436 , PMID 24593683 (review).
- ↑ M. Donoghue, F. Hsieh, E. Baronas, K. Godbout, M. Gosselin, N. Stagliano, M. Donovan, B. Woolf, K. Robison, R. Jeyaseelan, RE Breitbart, S. Acton: A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. In: Circulation research. Volume 87, Number 5, September 2000, pp. E1-E9, doi : 10.1161 / 01.res.87.5.e1 , PMID 10969042 .
- ↑ S. Keidar, M. Kaplan, A. Gamliel-Lazarovich: ACE2 of the heart: From angiotensin I to angiotensin (1-7). In: Cardiovascular research. Volume 73, Number 3, February 2007, pp. 463-469, doi : 10.1016 / j.cardiores.2006.09.006 , PMID 17049503 (review).
- ↑ W. Wang, SM McKinnie, M. Farhan, M. Paul, T. McDonald, B. McLean, C. Llorens-Cortes, S. Hazra, AG Murray, JC Vederas, GY Oudit: angiotensin-converting enzyme 2 and metabolizes Partially Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects in the Cardiovascular System. In: Hypertension. Volume 68, number 2, 08 2016, pp. 365-377, doi : 10.1161 / HYPERTENSIONAHA.115.06892 , PMID 27217402 .
- ↑ Y. Inoue, N. Tanaka, Y. Tanaka, S. Inoue, K. Morita, M. Zhuang, T. Hattori, K. Sugamura: Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. In: Journal of Virology . Volume 81, number 16, August 2007, pp. 8722-8729, doi : 10.1128 / JVI.00253-07 , PMID 17522231 , PMC 1951348 (free full text).
- ↑ G. Simmons, DN Gosalia, AJ Rennekamp, JD Reeves, SL Diamond, P. Bates: Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. In: Proceedings of the National Academy of Sciences . Volume 102, number 33, August 2005, pp. 11876-11881, doi : 10.1073 / pnas.0505577102 , PMID 16081529 , PMC 1188015 (free full text).
- ↑ H. Wang, P. Yang, K. Liu, F. Guo, Y. Zhang, G. Zhang, C. Jiang: SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. In: Cell research. Volume 18, Number 2, February 2008, pp. 290-301, doi : 10.1038 / cr.2008.15 , PMID 18227861 .
- ↑ S. Matsuyama, N. Nagata, K. Shirato, M. Kawase, M. Takeda, F. Taguchi: Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. In: Journal of Virology . Volume 84, number 24, December 2010, pp. 12658-12664, doi : 10.1128 / JVI.01542-10 , PMID 20926566 , PMC 3004351 (free full text).
- ^ SK Wong, W. Li, MJ Moore, H. Choe, M. Farzan: A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. In: Journal of Biological Chemistry . Volume 279, Number 5, January 2004, pp. 3197-3201, doi : 10.1074 / jbc.C300520200 , PMID 14670965 .
- ↑ L. Du, Y. He, Y. Zhou, S. Liu, BJ Zheng, S. Jiang: The spike protein of SARS-CoV - a target for vaccine and therapeutic development. In: Nature reviews. Microbiology. Volume 7, number 3, March 2009, pp. 226-236, doi : 10.1038 / nrmicro2090 , PMID 19198616 , PMC 2750777 (free full text) (review).
- ↑ F. Li, W. Li, M. Farzan, SC Harrison: Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. In: Science . Volume 309, Number 5742, September 2005, pp. 1864-1868, doi : 10.1126 / science.1116480 , PMID 16166518 .
- ^ F. Li: Structural analysis of major species barriers between humans and palm civets for severe acute respiratory syndrome coronavirus infections. In: Journal of Virology . Volume 82, Number 14, July 2008, pp. 6984-6991, doi : 10.1128 / JVI.00442-08 , PMID 18448527 , PMC 2446986 (free full text).
- ↑ a b c F. Li, W. Li, M. Farzan, SC Harrison: Interactions between SARS coronavirus and its receptor. In: Advances in Experimental Medicine and Biology . Volume 581, 2006, pp. 229-234, doi : 10.1007 / 978-0-387-33012-9_38 , PMID 17037534 .
- ↑ K. Wu, G. Peng, M. Wilken, RJ Geraghty, F. Li: Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. In: Journal of Biological Chemistry . Volume 287, number 12, March 2012, pp. 8904-8911, doi : 10.1074 / jbc.M111.325803 , PMID 22291007 , PMC 3308800 (free full text).
- ↑ M. Hoffmann, H. Kleine-Weber, S. Schroeder, N. Krüger, T. Herrler, S. Erichsen, TS Schiergens, G. Herrler, NH Wu, A. Nitsche, MA Müller, C. Drosten, S. Pöhlmann: SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. In: Cell . [electronic publication before printing] March 2020, doi : 10.1016 / j.cell.2020.02.052 , PMID 32142651 , PMC 7102627 (free full text).
- ↑ a b Ying-Ying Zheng, Yi-Tong Ma, Jin-Ying Zhang, Xiang Xie: Reply to: 'Interaction between RAAS inhibitors and ACE2 in the context of COVID-19'. In: Nature Reviews Cardiology. March 30, 2020, doi : 10.1038 / s41569-020-0369-9 .
- ↑ T. Hirano, M. Murakami: COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. In: Immunity. [electronic publication before printing] April 2020, doi : 10.1016 / j.immuni.2020.04.003 , PMID 32325025 , PMC 7175868 (free full text).
- ↑ YY Zheng, YT Ma, JY Zhang, X. Xie: COVID-19 and the cardiovascular system. In: Nature reviews. Cardiology. [Electronic publication before going to press] March 5, 2020, doi : 10.1038 / s41569-020-0360-5 , PMID 32139904 .
- ↑ CM Ferrario, J. Jessup, MC Chappell, DB Averill, KB Brosnihan, EA Tallant, DI Diz, PE Gallagher: Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. In: Circulation . Volume 111, Number 20, May 2005, pp. 2605-2610, doi : 10.1161 / CIRCULATIONAHA.104.510461 , PMID 15897343 .
- ↑ Y. Li, Z. Zeng, Y. Li, W. Huang, M. Zhou, X. Zhang, W. Jiang: Angiotensin-converting enzyme inhibition attenuates lipopolysaccharide-induced lung injury by regulating the balance between angiotensin-converting enzyme and angiotensin-converting enzyme 2 and inhibiting mitogen-activated protein kinase activation. In: Shock. Volume 43, number 4, April 2015, pp. 395-404, doi : 10.1097 / SHK.0000000000000302 , PMID 25768373 .
- ↑ RM Wösten-van Asperen, R. Lutter, PA Specht, GN Moll, JB van Woensel, CM van der Loos, H. van Goor, J. Kamilic, S. Florquin, AP Bos: Acute respiratory distress syndrome leads to reduced ratio of ACE / ACE2 activities and is prevented by angiotensin- (1-7) or an angiotensin II receptor antagonist. In: The Journal of pathology. Volume 225, Number 4, December 2011, pp. 618-627, doi : 10.1002 / path.2987 , PMID 22009550 .
- Jump up ↑ S. Keidar, A. Gamliel-Lazarovich, M. Kaplan, E. Pavlotzky, S. Hamoud, T. Hayek, R. Karry, Z. Abassi: Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients . In: Circulation research. Volume 97, Number 9, October 2005, pp. 946-953, doi : 10.1161 / 01.RES.0000187500.24964.7A , PMID 16179584 .
- ↑ JC Zhong, JY Ye, HY Jin, X. Yu, HM Yu, DL Zhu, PJ Gao, DY Huang, M. Shuster, H. Loibner, JM Guo, XY Yu, BX Xiao, ZH Gong, JM Penninger, GY Oudit: Telmisartan attenuates aortic hypertrophy in hypertensive rats by the modulation of ACE2 and profilin-1 expression. In: Regulatory peptides. Volume 166, number 1-3, January 2011, pp. 90-97, doi : 10.1016 / j.regpep.2010.09.005 , PMID 20854846 .
- ↑ SR Tipnis, NM Hooper, R. Hyde, E. Karran, G. Christie, AJ Turner: A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. In: Journal of Biological Chemistry . Volume 275, Number 43, October 2000, pp. 33238-33243, doi : 10.1074 / jbc.M002615200 , PMID 10924499 .
- ↑ W. Ding, X. Li, W. Wu, H. He, Y. Li, L. Gao, L. Gan, M. Wang, S. Ou, J. Liu: [Aliskiren inhibits angiotensin II / angiotensin 1- 7 (Ang II / Ang1-7) signal pathway in rats with diabetic nephropathy]. In: Xi bao yu fen zi mian yi xue za zhi = Chinese journal of cellular and molecular immunology. Volume 34, Number 10, October 2018, pp. 891-895, PMID 30554582 (Chinese).
- ↑ Zikuan Leng, Rongjia Zhu, Wei Hou, Yingmei Feng, Yanlei Yang, Qin Han, Guangliang Shan, Fanyan Meng, Dongshu Du, Shihua Wang, Junfen Fan, Wenjing Wang, Luchan Deng, Hongbo Shi, Hongjun Li, Zhongjie Hu, Fengchun Zhang, Jinming Gao, Hongjian Liu, Xiaoxia Li, Yangyang Zhao, Kan Yin, Xijing He, Zhengchao Gao, Yibin Wang, B. o. Yang, Ronghua Jin, Ilia Stambler, Lee Wei Lim, Huanxing Su, Alexey Moskalev, Antonio Cano , Sasanka Chakrabarti, Kyung-Jin Min, Georgina Ellison-Hughes, Calogero Caruso, Kunlin Jin, Robert Chunhua Zhao: Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. In: Aging and Disease. Volume 11, March 9, 2020, p. 216, doi : 10.14336 / AD.2020.0228 .
- ↑ AM South, D. Diz, MC Chappell: COVID-19, ACE2 and the Cardiovascular Consequences. In: American Journal of Physiology - Heart and Circulatory Physiology. [Electronic publication before printing] March 31, 2020, doi : 10.1152 / ajpheart.00217.2020 , PMID 32228252 .
- ↑ ESH UPDATE ON COVID-19. In: eshonline.org. European Society of Hypertension, March 19, 2020, accessed April 8, 2020 .
- ↑ B. Williams, G. Mancia, W. Spiering, E. Agabiti Rosei, M. Azizi, M. Burnier, DL Clement, A. Coca, G. de Simone, A. Dominiczak, T. Kahan, F. Mahfoud, J. Redon, L. Ruilope, A. Zanchetti, M. Kerins, SE Kjeldsen, R. Kreutz, S. Laurent, GY Lip, R. McManus, K. Narkiewicz, F. Ruschitzka, RE Schmieder, E. Shlyakhto, C Tsioufis, V. Aboyans, I. Desormais: 2018 ESC / ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. In: Journal of hypertension. Volume 36, number 10, 10 2018, pp. 1953-2041, doi : 10.1097 / HJH.0000000000001940 , PMID 30234752 .
- ↑ Coronavirus SARS-CoV-2. In: bfarm.de. Federal Institute for Drugs and Medical Devices, 2020, accessed on April 10, 2020 (the page is updated regularly, see also versions from March 25 and April 10 in the Web Archive).