amino acids
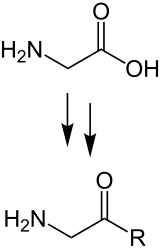
Amino acids ( AS ), uncommonly also aminocarboxylic acids , out of date called amido acids , are chemical compounds with an amino group containing nitrogen (N) and a carboxylic acid group containing carbon (C) and oxygen (O) . Amino acids are found in all living things . They are the building blocks of proteins (protein) and are released when proteins are broken down ( proteolysis ). Essential amino acids an organism cannot produce it itself, so they must be taken in with food.
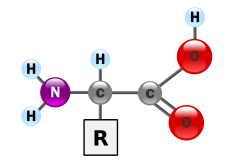
The class of amino acids includes organic compounds that contain at least one amino group (-NH 2 or substituted -NR 2 ) and one carboxy group (-COOH) as functional groups , that is, have structural features of amines and carboxylic acids . Chemically, they can be differentiated according to the position of their amino group to the carboxy group - if the amino group on the C α -atom is immediately adjacent to the terminal carboxy group, this is called α-position and speaks of α-amino acids .
Selected α-amino acids are the natural building blocks of proteins. They are linked together to form chains by the carboxy group of one amino acid forming a peptide bond with the amino group of the next . The amino acids linked in this way to form a polymer differ in their side chains and together determine the shape with which the polypeptide then unfolds in an aqueous environment to form the native protein . This biosynthesis of proteins takes place in all cells on the ribosomes according to genetic information that is available in the form of mRNA .
The base sequence of the mRNA encodes the amino acid sequence in triplets , each base triplet representing a codon that stands for a specific proteinogenic amino acid . The amino acids specified as building blocks for the formation of proteins in a certain order form the proteins. In humans there are 21 different proteinogenic amino acids. After translation , the side chains of some of the amino acids built into the protein can still be modified .
The spectrum of amino acids goes well beyond these twenty proteinogenic ones . So far, over 400 non-proteinogenic naturally occurring amino acids are known that have biological functions. The comparatively rare D amino acids represent a special group. The variety of synthetically produced and that of theoretically possible amino acids is considerably greater.
Some amino acids play a special role as neurotransmitters , as do various breakdown products of amino acids; Biogenic amines not only act as messenger substances in the nervous system, but also develop diverse physiological effects in the organism as hormones and tissue mediators .
The simplest amino acid, glycine , could not only be detected on earth , but also on comets , meteorites and in gas clouds in interstellar space .
history
The first amino acid was isolated from the juice of asparagus ( Asparagus officinalis ) in the Paris laboratory of Louis-Nicolas Vauquelin and his student Pierre Jean Robiquet in 1805 and was then named asparagine . As the last of the usual protein-building amino acids, threonine was discovered in fibrin in 1931 and its structure was clarified by William Rose in 1935 . Through experiments with various feeds, Rose had found that the 19 amino acids that had been discovered so far were not sufficient as an additive. He also determined the essentiality of other amino acids and determined the minimum daily dose required for optimal growth .
In the period between 1805 and 1935, many of the chemists and pharmacists known at the time were involved in isolating amino acids for the first time and clarifying their structure. Emil Fischer , to whom the Fischer projection also goes back, succeeded in finally elucidating the structure of serine (1901), lysine (1902), valine (1906) and cysteine (1908). Even Albrecht Kossel (1896 histidine from Störsperma), Richard Willstätter (1900 proline via synthesis) and Frederick Hopkins (1901 tryptophan from casein ) were Nobel laureates later. The German chemist Ernst Schulze isolated three amino acids for the first time - glutamine from beets in 1877 , phenylalanine in 1881 and arginine from lupins in 1886 - and was involved in the structural analysis of other amino acids. Heinrich Ritthausen had previously obtained crystalline glutamic acid from grain protein , gluten , in 1866 . In 1872 Wilhelm Dittmar clarified the structure of glutamine and glutamic acid, the salts of which are glutamates .

Already in 1810 William Hyde Wollaston discovered the sulfur-containing cystine as "cystic oxide" in bladder stones , but it was not until 1884 that Eugen Baumann discovered the monomeric cysteine . In 1819 Henri Braconnot separated the glycine from glue and Joseph Louis Proust separated the leucine from grain. Eugen von Gorup-Besánez isolated the valine from pancreatic juice in 1856 . Justus von Liebig was able to separate the tyrosine from casein for the first time in 1846 , the structure of which Ludwig von Barth clarified in 1869 . In the hydrolyzate of casein, Edmund Drechsel discovered lysine in 1889 and later John Howard Mueller discovered sulphurous methionine in 1922 as the 19th amino acid, the structural formula of which George Barger and Philip Coine stated in 1928. In molasses had Felix Ehrlich in 1903 as 18, the isoleucine found, a structural isomer of leucine.
Friedrich Wöhler , whose syntheses opened up the field of biochemistry in the 1820s , did not discover any amino acids, but three of his students were involved, along with the aforementioned Gorup-Besánez and Schulze, Georg Städeler (1863 serine from raw silk). 18 of the 20 discovered amino acids were isolated from plant or animal material, only the two amino acids alanine (1850 Adolph Strecker ) and proline (Willstätter) were obtained through organic synthesis. While the analysis of the material composition up to the molecular formula was easy to accomplish with the methods of the time, the structural formula of many amino acids could often only be finally clarified through partial steps of the synthesis, which sometimes only succeeded years later. The structure of asparagine and of aspartic cleared Hermann Kolbe until 1862, 57 years after the first description.
Amino acids owe their generic names to two functional groups, their individual names sometimes due to a light appearance (e.g. arginine , leucine ), a sweet taste (e.g. glycine ) or the material in which they were found (e.g. asparagine , Cysteine , serine , tyrosine ), characteristics of the chemical structure (e.g. proline , valine , isoleucine ) or both (e.g. glutamine , glutamic acid ) and sometimes the starting materials of their synthesis (e.g. alanine ).
That proteins are built up as chains of amino acids, linked by peptide bonds, was first proposed in 1902 at the meeting of German naturalists and doctors in Karlsbad, simultaneously and independently from both Emil Fischer and Franz Hofmeister (Hofmeister-Fischer theory).
structure
| Carbamic acid |
Amino acids consist of at least two carbon atoms. The unstable carbamic acid has only one carbon atom and is therefore not an amino acid, but a carbonic acid amide. Amino acids can be divided into classes depending on the carbon atom on which the amino group is located relative to the carboxy group. If several amino groups are represented in the molecule , the carbon atom whose amino group is closest to the carboxy carbon determines which class of amino acids it is.
| General structure of amino acids (R: side chain) |
| α-amino acid |
| β-amino acid |
| γ-amino acid |
- α-Amino acids: The amino group of the α-amino acids is on the second carbon atom, including the carboxy carbon atom. The count always starts with the carboxy carbon. The IUPAC name is therefore 2-aminocarboxylic acids. The simplest representative of the α-amino acids is the proteinogenic amino acid glycine . All proteinogenic amino acids are α-amino acids.
- The term amino acids often refers to a certain group of α-amino acids, which mainly consists of L- α-amino acids: the proteinogenic amino acids . These are the building blocks of all proteins of all life on earth and, in addition to the nucleic acids, basic building blocks of life .
- β-amino acids: The amino group of the β-amino acids is located on the third carbon atom (including the carboxy carbon atom). The IUPAC name is 3-aminocarboxylic acids. The simplest representative is β-alanine .
- γ-amino acids: The amino group of the γ-amino acids is located on the fourth carbon atom (including the carboxy carbon atom). The IUPAC name is 4-aminocarboxylic acids. The simplest representative is γ-aminobutyric acid (GABA).
The designation of further classes of amino acids follows the same scheme.
The amino acids of a class are distinguished by their side chain R . If the side chain R is different from the other substituents located on the carbon with the amino group, then there is a stereocenter here and there are two enantiomers of the corresponding amino acid . If the side chain R itself contains further stereocenters, diastereomers also result and the number of possible stereoisomers increases correspondingly to the number of further stereocenters. There are four stereoisomers of amino acids with two differently substituted stereocenters .
Aminoacyl group
 Aminoacyl group formed from the amino acid glycine . R here denotes a radical to which the aminoacyl group is attached; for example, a transfer RNA ( tRNA ) is loaded into aminoacyl-tRNA . |
 Aminoacyl group formed from the amino acid L - glutamine . R here denotes a radical to which the aminoacyl group is attached; for example, a transfer RNA ( tRNA ) is loaded into aminoacyl-tRNA . |
Aminoacyl group refers to the monovalent group that is created from an amino acid by removing the hydroxyl (-OH) from the carboxyl group (-COOH), i.e. the univalent radical. An α-aminoacyl group is thus formed from an α-amino acid; from the amino acid tyrosine, for example, the tyrosyl group is created as a special α-aminoacyl group.
Proteinogenic amino acids
Amino acids are referred to as proteinogenic which are used in living organisms as building blocks of proteins during translation according to genetic information. In the biosynthesis of proteins, which takes place on the ribosomes of a cell, selected amino acids are linked by peptide bonds in a certain order to form the polypeptide chain of a protein in the course of protein biosynthesis . The amino acid sequence of the ribosomally formed peptide is predetermined by the genetic information contained in the base sequence of a nucleic acid , with an amino acid being encoded by a base triplet according to the genetic code .
 L- proline (proteinogenic amino acid) |
 D- proline (non-proteinogenic amino acid) |
The proteinogenic amino acids are always α-amino acids. Except for the smallest, glycine , they are chiral and occur with a special spatial arrangement. The amino acid proline has a special feature, the amino group of which has a secondary amine and which therefore does not fit into a protein fold as flexibly as other proteinogenic amino acids - proline, for example, is regarded as a helix breaker in α-helical structures in proteins. Due to the secondary amino group, proline is also referred to as a secondary amino acid - often incorrectly or out of date also as an imino acid .
Of the mirror- inverted enantiomers , only the L amino acids are proteinogenic (for D / L nomenclature see Fischer projection ; in cases such as hydroxyproline there are further stereoisomers ). The molecular components of the cellular apparatus necessary to build proteins - in addition to ribosomes, tRNAs and aminoacyl-tRNA synthetases , which are loaded with amino acids - are themselves also chiral and only recognize the L variant.
Nevertheless, D- amino acids also occur occasionally in living things . However, these are synthesized independently of proteinogenic metabolic pathways and are not used for the ribosomal structure of proteins. For example, D - alanine is incorporated into peptidoglycans of the bacterial cell wall or D - valine is incorporated into bacterial cyclo- depsipeptides such as valinomycin . Different types of archaea , bacteria , fungi and nudibranchs have multi - enzyme complexes called non- ribosomal peptide synthetases with which such (non-proteinogenic) amino acids can be incorporated into a non- ribosomal peptide .
Canonical amino acids
For 20 of the proteinogenic amino acids are found codons in the (most frequently used) Standard Version of the genetic code. These are therefore called standard amino acids or canonical amino acids .
In amino acid sequences , the amino acids are usually given with a name abbreviation in a three-letter code or represented in the one -letter code by a symbol.
| amino acid | Acyl group |
eat- tially? |
Ø in proteins |
||
|---|---|---|---|---|---|
| Surname | Abbr. | symbol | |||
| Alanine | Ala | A. | Alanyl | No | 9.0% |
| Arginine | Arg | R. | Arginyl | semi | 4.7% |
| Asparagine | Asn | N | Asparaginyl | No | 4.4% |
| Aspartic acid | Asp | D. | α-aspartyl | No | 5.5% |
| Cysteine | Cys | C. | Cysteinyl | no * | 2.8% |
| Glutamine | Gln | Q | Glutaminyl | No | 3.9% |
| Glutamic acid | Glu | E. | α-glutamyl | No | 6.2% |
| Glycine | Gly | G | Glycylic | No | 7.5% |
| Histidine | His | H | Histidyl | semi | 2.1% |
| Isoleucine | Ile | I. | Isoleucylic | Yes | 4.6% |
| Leucine | Leu | L. | Leucylic | Yes | 7.5% |
| Lysine | Lys | K | Lysyl | Yes | 7.0% |
| Methionine | Mead | M. | Methionyl | Yes | 1.7% |
| Phenylalanine | Phe | F. | Phenylalanyl | Yes | 3.5% |
| Proline | Per | P | Prolyl | No | 4.6% |
| Serine | Ser | S. | Seryl | No | 7.1% |
| Threonine | Thr | T | Threonyl | Yes | 6.0% |
| Tryptophan | Trp | W. | Tryptophyl | Yes | 1.1% |
| Tyrosine | Tyr | Y | Tyrosyl | no * | 3.5% |
| Valine | Val | V | Valyl | Yes | 6.9% |
| * Essential for children and pregnant women. | |||||
In addition to the codes given above, additional characters are used as placeholders if the precise amino acid cannot be inferred from protein sequencing or X-ray structure analysis.
| Possible amino acids | Abbr. | symbol |
|---|---|---|
| Asparagine or aspartic acid | Asx | B. |
| Glutamine or glutamic acid | Glx | Z |
| Leucine or isoleucine | Xle | J |
| unknown amino acid | Xaa (rarely Unk) | X |
Non-canonical amino acids
In addition to the canonical amino acids, the naturally occurring amino acids include the other amino acids called non-canonical amino acids, including proteinogenic and non-proteinogenic amino acids. Several groups can be distinguished:
- The first group includes those proteinogenic amino acids that are incorporated into proteins through recoding of the genetic material. The 21st and 22nd proteinogenic amino acids belong to this: selenocysteine (in eukaryotes and some bacteria and archaea ) and pyrrolysine (in some bacteria and archaea). Specific tRNAs - tRNA Sec and tRNA Pyl - were found for both amino acids, which enable incorporation into the ribosome during translation. Their anticodon pairs, depending on structural elements in the context of the mRNA (see Secis ), with the codon UGA or UAG ; in the standard code these represent a stop codon . However, not all organisms use the non-canonical proteinogenic amino acids of this group.
amino acid Abbr. symbol Pyrrolysine Pyl O Selenocysteine Sec U
- The usual start codon AUG codes for the amino acid methionine . In addition to the tRNA Met, bacteria have a special tRNA fMet , which is also loaded with methionine and serves as an initiator tRNA . However , the amino acid bound to tRNA i fMet is formylated in bacteria at the N terminus to N -formylmethionine (fMet) before it can become the first amino acid of a peptide chain during initiation on the ribosome. This amino acid derivative formylmethionine is therefore occasionally also counted as (23rd) proteinogenic amino acid. Also, mitochondria and chloroplasts use fMet initial. In contrast, it is not used in translation in the cytosol of eukaryotic cells and in archaea .
- A second group is formed by amino acids that are not proteinogenic in the narrow sense of the word and that arise from canonical amino acids when the amino acid residue R is changed after incorporation into proteins, i.e. H. through one of the many post-translational modifications . Proline can be converted to hydroxyproline , serine to O -phosphoserine, tyrosine to O - phosphotyrosine and glutamate to γ-carboxyglutamate . Glycosylation also represents an important change in the amino acid residue : here, carbohydrate residues are transferred to the amino acid residues, creating glycoproteins .
- The third group, strictly speaking, is non-proteinogenic amino acids, which the organism cannot distinguish from the canonical amino acids and which it incorporates into proteins non-specifically instead. This includes selenomethionine , which can be incorporated in place of methionine , or canavanine , which the organism cannot distinguish from arginine , or azetidine-2-carboxylic acid , which acts as a poisonous proline analogue. Many of the amino acids in this group are toxic as they often lead to misfolding of the protein, which can impair the shape and thus the functionality of the protein. Azetidine-2-carboxylic acid is a toxic component of the lily of the valley , whereby the lily of the valley protects itself against the uncontrolled incorporation of this amino acid into its proteins with a highly specific prolyl tRNA synthetase .
In addition to the canonical 20, humans also use selenocysteine as a proteinogenic amino acid. Of the 20 canonical amino acids, 12 are synthesized by the human organism or by microorganisms living in the human digestive tract. The remaining 8 amino acids are essential for humans, which means that they have to be ingested through food.
The incorporation of artificial, almost arbitrarily constructed amino acids in the course of a protein design is possible, among other things, by replacing the ligand in the corresponding aminoacyl-tRNA synthetase . These methods are partially advanced so far that this target specific proteins, a marker can be obtained, for example, the protein by treatment with specific reagents for fluorescence stimulate (for example, incorporation of norbornene-amino acid via pyrrolysyl-tRNA synthetase / codon CUA). This enables the protein to be precisely localized even without production and reaction with antibodies .
Biochemical significance
Amino acids as building blocks of proteins
L- amino acids are of great importance in biochemistry because they are the building blocks of peptides and proteins . So far, more than twenty so-called proteinogenic amino acids are known. These are first of all those 20 L -α-amino acids which are coded as standard amino acids by codons of three nucleobases each in the DNA according to the standard code . In addition to these canonically named amino acids, two more have been added, selenocysteine and pyrrolysine . Both non-canonical are also α-amino acids, based on the terminal carboxy group , the amino group is bonded to the immediately adjacent carbon atom (C α ). In addition, there are other amino acids that occur as components of proteins or peptides, but are not coded.
Amino acid chains with a chain length of less than 100 amino acids are usually referred to as peptides, the larger ribosomally formed ones are called proteins. The individual amino acids are linked within the chain via peptide bonds (acid amide). An automated method for the synthesis of peptides provides the Merrifield synthesis .
Proteins ingested in the form of food are broken down into L- amino acids during digestion . They are then used in the liver . They are either used for protein synthesis or broken down ( see also: amino acid index ). The most important mechanisms of amino acid breakdown are:
Essential amino acids
Amino acids that an organism needs but cannot produce itself are called essential amino acids and must be taken in with food. All of these essential amino acids are L -α amino acids. For humans, valine , methionine , leucine , isoleucine , phenylalanine , tryptophan , threonine and lysine are essential amino acids. The WHO has also classified the amino acid histidine as an essential amino acid since 1985. There are therefore nine essential amino acids. Conditionally essential or semi-essential amino acids only need to be taken in with food in certain situations, for example during growth or after severe injuries. The remaining amino acids are either synthesized directly or obtained from other amino acids by modification. Cysteine can be synthesized from the essential amino acid methionine. As long as the ability to produce the amino acid tyrosine from phenylalanine is not yet fully developed, this is one of the other essential amino acids in childhood. For a similar reason, tyrosine must also be taken in the case of phenylketonuria . There are also other diseases that impair the amino acid metabolism and may require the intake of an actually non-essential amino acid. All the essential or semi-essential amino acids that the human body needs are contained in chicken eggs.
Plants and microorganisms can synthesize all the amino acids they need by themselves. Therefore, there are no essential amino acids for them .

Chemical-physical properties
The proteinogenic amino acids can be divided into groups according to their residues (see table overview of properties ). An amino acid can appear in different groups at the same time. The overlapping of the groups can be shown graphically in a quantity diagram .
The authors have different views on the properties of the side chain of cysteine: Löffler considers it polar, while Alberts considers it non-polar. Correctly, sulfur is a heteroatom , so the following applies: The side chain of cysteine has weakly polar properties.
Acid and base behavior

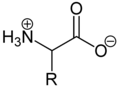
Due to the basic amino group and the acidic carboxylic acid group, amino acids are both bases and acids . As solids and in neutral aqueous solutions, amino acids are present as zwitterions , that is, the amino group is protonated and the carboxy group is deprotonated. The zwitterion can be generalized as follows:
As a zwitterion, the protonated amino group can react as an acid ( proton donor ) and the carboxylate group can react as a base ( proton acceptor ). In acidic solutions, amino acids are present as cations and in basic solutions as anions :
The charge of an amino acid molecule depends on the pH of the solution. In the case of a zwitterion with an acidic and a basic group, the total charge of the molecule is zero at neutral pH. In addition, the side chains of the amino acids have partially acidic or basic charged groups. The pH with a net charge of zero is the isoelectric point (pH I , pI) of an amino acid. The water solubility of an amino acid is lowest at the isoelectric point.
| amino acid | property | free | in protein |
|---|---|---|---|
| Asp | angry | 3.68 | 3.7-4.0 |
| Glu | angry | 4.25 | 4.2-4.5 |
| His | basic | 6.00 | 6.7-7.1 |
| Cys | semi-sour | 8.33 | 8.8-9.1 |
| Tyr | semi-sour | 10.07 | 9.7-10.1 |
| Lys | basic | 10.53 | 9.3-9.5 |
| Arg | basic | 12.48 | - |
For the acid-base behavior of proteinogenic amino acids, the behavior of their side chain (hereinafter referred to as R ) is of particular interest. In proteins, the NH 2 and COOH groups cannot be protonated at a physiological pH value (around pH 7) because of the peptide bond, and therefore cannot be titrated . Exceptions are the amino and carboxy terminus of the protein. The side chain residue R is therefore decisive for the acid-base behavior of proteins and peptides .
The behavior of the side chain R depends on its constitution, that is, whether the side chain itself can act as a proton acceptor or as a proton donor . The proteinogenic amino acids are divided according to the functional groups into those with non-polar or polar amino acid side chains and further subdivided into subgroups sorted by polarity : aliphatic , aromatic , amidated , sulfur-containing , hydroxylated , basic and acidic amino acids.
Although the side chains of tyrosine and cysteine are relatively acidic compared to the other non-polar side chains, they only tend to deprotonate at unphysiologically high pH values. Proline is a secondary amino acid because the N-terminus forms a five-atom ring with the side chain. Within a protein, the carboxy terminus of a preceding amino acid binds to the nitrogen of the proline, which cannot be protonated due to the peptide bond already mentioned. Histidine, tyrosine and methionine each come in two subgroups.
| amino acid | pK 2 COOH |
pK 1 COOH |
Isoelectric point |
pK 1 NH 2 |
pK 2 NH 2 |
|---|---|---|---|---|---|
| Alanine | - | 2.3 | 6.1 | 9.9 | - |
| Arginine | - | 2.81 | 10.76 | 9.09 | 12.5 |
| Asparagine | - | 2.02 | 5.41 | 8.80 | - |
| Aspartic acid | 3.65 | 1.88 | 2.85 | 9.60 | - |
| Cysteine | 8.33 * | 1.71 | 5.05 | 10.78 | - |
| Glutamine | - | 2.17 | 5.65 | 9.13 | - |
| Glutamic acid | 4.25 | 2.19 | 3.22 | 9.67 | - |
| Glycine | - | 2.21 | 5.97 | 9.15 | - |
| Histidine | - | 1.78 | 7.47 | 8.97 | 5.97 |
| Isoleucine | - | 2.32 | 5.94 | 9.76 | - |
| Leucine | - | 2.4 | 5.98 | 9.6 | - |
| Lysine | - | 2.20 | 9.59 | 8.90 | 10.28 |
| Methionine | - | 2.28 | 5.74 | 9.21 | - |
| Phenylalanine | - | 2.58 | 5.84 | 9.24 | - |
| Proline | - | 1.99 | 6.3 | 10.60 | - |
| Serine | - | 2.21 | 5.68 | 9.15 | - |
| Threonine | - | 2.10 | 5.60 | 9.12 | - |
| Tryptophan | - | 2.15 | 5.64 | 9.12 | - |
| Tyrosine | 10.07 ** | 2.20 | 5.66 | 9.11 | - |
| Valine | - | 2.30 | 5.96 | 9.60 | - |
|
* Thiol group ** phenolic hydroxyl group |
|||||
- Aromatic amino acid side chains
- Amidated amino acid side chains
- Sulfur-containing amino acid side chains
- Hydroxylated amino acid side chains
- Basic amino acid side chains
- Acid Amino Acid Side Chains
- Aspartic acid (dissociates to aspartate)
- Glutamic acid (dissociates to glutamate)
The pK value is the pH value at which the titratable groups are protonated and deprotonated in equal parts; the titratable group is then present in equal parts in its basic as in its acidic form (see also: Henderson-Hasselbalch equation ).
It is usually customary to speak of the pK instead of the pK S , i.e. the pK of the acid . In this sense, however, the pK of the lysine would have to be referred to as pK B and the pK of the base . For the sake of simplicity, this notation is generally omitted, since the context also shows whether the group acts as a base or an acid.
The pK is not a constant, but depends on the temperature, the activity , the ionic strength and the immediate environment of the titratable group and can therefore fluctuate widely.
If the pH is higher than the pK of a titratable group, the titratable group is in its basic (deprotonated) form. If the pH is lower than the pK of the titratable group, the titratable group is in its acidic (protonated) form:
- For Asp (pK = 3.86) at pH 7: the side chain is almost completely deprotonated.
- For Lys (pK = 10.53) at pH 7: the side chain is almost completely protonated.
The side chains of basic amino acids are simply positively charged in their protonated (acidic) form and uncharged in their deprotonated (basic) form. The side chains of the acidic amino acids (including cysteine and tyrosine) are uncharged in their protonated (acidic) form and simply negatively charged in their deprotonated (basic) form. Since the behavior of the side chain is completely different when it is charged or uncharged, the pH value plays such an important role in the properties of the side chain.
The titratable side chains influence, for example, the solubility behavior of the corresponding amino acid. In polar solvents, the following applies: Charged side chains make the amino acid more soluble, uncharged side chains make the amino acid more insoluble.
In proteins this can lead to certain sections becoming more hydrophilic or hydrophobic , which means that the folding and thus also the activity of enzymes depends on the pH value. Proteins can therefore be denatured by strongly acidic or basic solutions .
Table overview of the properties
| amino acid | Side chain R | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surname | Abbr. | symbol | Structural formula | Constitutional formula | relative molecular weight |
van der Waals volume |
pola- rity |
Hydro phobi- capacity |
Acidity or basicity |
Acid constant (pK S ) |
| Alanine | Ala | A. | -CH 3 | 15th | 67 | non-polar | +1.8 | neutral | - | |
| Arginine | Arg | R. | -CH 2 CH 2 CH 2 NH-C (NH) NH 2 | 100 | 148 | polar | −4.5 | basic (strong) |
12.48 | |
| Asparagine | Asn | N | -CH 2 CONH 2 | 58 | 96 | polar | −3.5 | neutral | - | |
|
Aspartic acid |
Asp | D. | -CH 2 COOH | 59 | 91 | polar | −3.5 | angry | 3.90 | |
| Cysteine | Cys | C. | -CH 2 SH | 47 | 86 | polar | +2.5 | neutral | 8.18 | |
| Glutamine | Gln | Q | -CH 2 CH 2 CONH 2 | 72 | 114 | polar | −3.5 | neutral | - | |
|
Glutamic acid |
Glu | E. | -CH 2 CH 2 COOH | 73 | 109 | polar | −3.5 | angry | 4.07 | |
| Glycine | Gly | G | -H | 1 | 48 | non-polar | −0.4 | neutral | - | |
| Histidine | His | H | -CH 2 ( C 3 H 3 N 2 ) | 81 | 118 | polar | −3.2 | basic (weak) |
6.04 | |
| Isoleucine | Ile | I. | -CH (CH 3 ) -CH 2 CH 3 | 57 | 124 | non-polar | +4.5 | neutral | - | |
| Leucine | Leu | L. | -CH 2 CH (CH 3 ) 2 | 57 | 124 | non-polar | +3.8 | neutral | - | |
| Lysine | Lys | K | -CH 2 CH 2 CH 2 -CH 2 NH 2 | 72 | 135 | polar | −3.9 | basic | 10.54 | |
| Methionine | Mead | M. | -CH 2 CH 2 SCH 3 | 75 | 124 | non-polar | +1.9 | neutral | - | |
| Phenylalanine | Phe | F. | -CH 2 ( C 6 H 5 ) | 91 | 135 | non-polar | +2.8 | neutral | - | |
| Proline | Per | P | One H is missing from the NH 2 | 42 | 90 | non-polar | −1.6 | neutral | - | |
| Serine | Ser | S. | -CH 2 OH | 31 | 73 | polar | −0.8 | neutral | - | |
| Threonine | Thr | T | -CH (OH) CH 3 | 45 | 93 | polar | −0.7 | neutral | - | |
| Tryptophan | Trp | W. | -CH 2 ( C 8 H 6 N ) | 130 | 163 | non-polar | −0.9 | neutral | - | |
| Tyrosine | Tyr | Y | -CH 2 ( C 6 H 4 ) OH | 107 | 141 | polar | −1.3 | neutral | 10.46 | |
| Valine | Val | V | -CH (CH 3 ) 2 | 43 | 105 | non-polar | +4.2 | neutral | - | |
Stereochemistry
According to the Cahn-Ingold-Prelog convention, 18 of the 20 proteinogenic amino acids have the ( S ) configuration on the α-carbon atom , only cysteine has the ( R ) configuration, since the carbon with the thiol group has a higher priority than the Has carboxylic acid group. Glycine is achiral , so no absolute configuration can be determined.
In addition to the stereocenter on the α-C atom, isoleucine and threonine each have a further stereogenic center in their R group . Proteinogenic isoleucine [ R = -C * H (CH 3 ) CH 2 CH 3 ] is ( S ) -configured there, threonine [ R = -C * H (OH) CH 3 ] ( R ) -configured.
Non-proteinogenic amino acids

To date, over 400 non-proteinogenic (i.e., not incorporated into proteins during translation ) amino acids found in organisms are known. Among them is the L - thyroxine , a hormone produced by the thyroid , L -DOPA , L - ornithine or in almost all types of cyanobacteria proven neurotoxin β-Methylaminoalanin (BMAA).
Most of the non-proteinogenic amino acids are derived from the proteinogenic, which are L -α-amino acids. Nevertheless, β-amino acids ( β-alanine ) or γ-amino acids ( GABA ) can also be formed.
Among the non-proteinogenic amino acids are all D - enantiomers of the proteinogenic L -amino acids. D -serine, in the brain by the serine - racemase from L generated -serine (its enantiomer). It serves both as a neurotransmitter and as a glio transmitter by activating the NMDA receptor , which together with glutamate allows the channel to open. To open the ion channel, glutamate and either glycine or D- serine must bind. D -Serine is a stronger agonist than glycine itself at the glycine binding site of the glutamate receptor of the NMDA type , but was still unknown at the time the glycine binding site was first described. After D -aspartate, D -serine is the second D -amino acid that has been found in humans.
The synthetic amino acids include 2-amino-5-phosphonovaleric acid (APV), an antagonist of the NMDA receptor and the economically important D- phenylglycine [synonym: ( R ) -phenylglycine], which is found in the side chain of many semisynthetic β-lactam antibiotics as Partial structure is included. ( S ) - and ( R ) - tert -leucine [synonym: ( S ) - and ( R ) -β-methylvaline] are synthetic structural isomers of the proteinogenic amino acid ( S ) -leucine and are used as starting material in stereoselective syntheses.
There are also aminosulfonic acids [example: 2-aminoethanesulfonic acid (synonym: taurine )], α-aminophosphonic acids and α-aminophosphinic acids. These are also α-amino acids, but no α-amino carboxylic acids. Instead of a carboxy group (–COOH), these α-amino acids contain a sulfonic acid, phosphonic acid or phosphinic acid group.
| amino acid | Biological importance |
|---|---|
| Thyroxine | Thyroid - hormone |
| GABA | inhibitory neurotransmitter |
| L - homoser | Metabolic intermediate in arginine synthesis |
| Ornithine | Metabolic intermediate in the urea cycle |
| Citrulline | Metabolic intermediate in the urea cycle |
| Argininosuccinate | Metabolic intermediate in the urea cycle |
| L -DOPA | Metabolic intermediate in the synthesis of catecholamines |
| 5-hydroxytryptophan | Metabolic intermediate in serotonin synthesis |
| β-alanine | Building block of coenzyme A |
| β-methylamino-alanine | Neurotoxin of cyanobacteria |
| Ibotenic acid | Mushroom poison |
| D - valine | Part of the antibiotic valinomycin |
| D - alanine | Part of bacterial cell walls |
| D - glutamate | Part of bacterial cell walls |
| 2,6-diaminopimelic acid | Part of bacterial cell walls |
proof
Quantitative photometric detection of amino acids can be carried out, among other things, by the Kaiser test with ninhydrin or with the Folin reagent , whereby primary amines can be detected. For secondary amines, the Isatin test or the Chloranil test are used. Separation and detection of amino acids can also be carried out by capillary electrophoresis or by HPLC , sometimes as liquid chromatography with mass spectrometry coupling . While most amino acids do not absorb UV light with wavelengths above 220 nm, the amino acids phenylalanine, tyrosine, histidine and tryptophan are aromatic and absorb UV light with a maximum between 260 nm and 280 nm. The amino acid composition of a protein can be determined by hydrolysis of the protein to be examined. The slowly occurring racemization of the amino acids in the proteins originally composed exclusively of L-amino acids is investigated during amino acid dating .
Extraction and production
Amino acids are obtained either from natural substances by separating a hydrolyzed protein or by synthetic means. Originally, the development of a synthesis for the various amino acids mainly served to clarify the structure. In the meantime, these structural questions have been resolved and the desired amino acids are specifically represented with the various syntheses, as far as they are still relevant. The syntheses initially give rise to racemic mixtures which can be separated. One method for this is selective enzymatic hydrolysis, which is used to resolve racemates .
The following is an overview of various syntheses that were developed by chemists as early as the mid-19th century. Some of these older syntheses are only of historical interest because of low yields or other problems. However, some of these old methods have been further developed and some are still relevant today for the representation of amino acids. Further details on these syntheses, including the equations for the syntheses, are given under the links to the syntheses and the amino acids given .
- With the cyanohydrin synthesis by the chemist Adolph Strecker in 1850, alanine was first synthesized from acetaldehyde (see Strecker synthesis ).
- A synthesis for the preparation of glycine via the α-fatty acids, which are produced by the reaction of bromine or chlorine fatty acids with ammonia, was by William H. Perkin sen. and Baldwin F. Duppa developed as early as 1859.
- Josef Pöchl discovered the synthesis of azlactone for the preparation of amino acids in 1883 . The exact procedure was only published in 1893 by Emil Erlenmeyer jun. enlightened. This method is therefore also called the Erlenmeyer synthesis . This process was used to produce histidine as well as phenylalanine and thyroxine in 1911 .
- Aspartic acid was first synthesized in 1887 by reducing an α-oximino acid . In 1906 Louis Bouveault used the same method to prepare isoleucine from the oxime of methylethylpyruvic acid ester .
- According to the Gabriel synthesis developed by Siegmund Gabriel , glycine hydrochloride was synthesized in 1889 using phthalimide potassium as the starting chemical. Although this synthesis is outdated for the preparation of glycine , its high yields make it suitable for the production of other amino acids.
- With the cyanohydrin synthesis , Emil Fischer produced serine for the first time using glycolaldehyde in 1902 . In 1906, leucine was synthesized using the malonic ester synthesis he developed . Isoleucine , norleucine , methionine and phenylalanine are further amino acids that can be easily represented with this synthesis.
- Theodor Curtius used the Curtius degradation developed by him for the preparation of α-amino acids through the use of malonic ester derivatives for the synthesis of glycine , alanine , valine and phenylalanine.
- In 1911, tyrosine , phenylalanine and tryptophan were obtained through a condensation of aromatic aldehydes with hydantoin .
- In 1931, George Barger synthesized methionine using a combined phthalimide malonic ester synthesis. Phenylalanine, proline , tyrosine, aspartic acid and serine can also be produced using the same method . Vincent du Vigneaud produced DL - cystine using this method in 1939 .
Amino acids are industrially produced today using the following processes:
- Extraction method : For this, proteins are first hydrolyzed with acids . After precipitation of the amino acid mixture of the hydrolyzate a done chromatographic separation by ion exchange chromatography . In the elution different are polarities of the amino acids used.
- Chemical Synthesis : There are a variety of synthetic methods. Examples are the Strecker synthesis of D , L -valine, Degussa-Synthesis of D , L - cysteine and the synthesis of D , L - methionine from methyl mercaptan , acrolein and hydrogen cyanide . Since the amino acids produced are obtained as a racemate , processes for the separation of enantiomers must then be carried out if pure L or D amino acids are required.
- Enzymatic processes : This process has the advantage of delivering enantiomerically pure L or D amino acids with suitable enzymes as biocatalysts . Examples are the production of L - aspartic acid from fumaric acid with L - aspartase and the production of L - tryptophan from indole and pyruvic acid with tryptopharase.
- Fermentation process : During fermentation , the amino acids are produced with the help of suitable microorganisms . The synthesis process runs through very complex intermediate steps within the cells. One example is the production of L - glutamic acid from glucose . 1 gram of glutamic acid can be obtained from 2 grams of glucose. Most amino acids today are made by fermentation. Every year 6 million tons of glutamic acid and lysine are produced worldwide, partly from hydrolyzed starch or molasses using the bacteria Escherichia coli or Corynebacterium glutamicum .
use
Amino acids are of fundamental importance for human nutrition , especially because the essential amino acids cannot be produced by themselves. As a rule, in the course of a balanced diet, the requirement for essential amino acids is completely covered by animal proteins or a suitable combination of different vegetable proteins (e.g. from cereals and legumes). Vegetable proteins usually have a lower biological value . Animal feeds are also enriched with amino acids, e.g. B. DL - methionine and L - lysine , but also branched amino acids (leucine, isoleucine, valine), which increases their nutritional value. Various amino acids are sold as dietary supplements .
Amino acids and their derivatives are used as additives for food . The human tongue has a glutamate receptor , the activation of which is generally associated with increased taste. Sodium glutamate is therefore used as a flavor enhancer . The sweetener aspartame contains an amino acid. Amino acids are precursors for certain flavoring substances that are created by the Maillard reaction during dry cooking of food .
Amino acids are used in cell biology and microbiology as components of cell culture media. In biochemistry , derivatives of amino acids such as photo-leucine or photo-methionine are used to determine the structure of proteins and others for marking molecules. In addition, amino acids are also used as auxiliaries, e.g. B. as a salt former , buffer . In pharmacy and medicine , L- amino acids are used as infusion solutions for parenteral nutrition and as stabilizers for certain liver diseases . L- Dopa is used for diseases with a deficiency of neurotransmitters . Amino acids are necessary starting materials for synthetic peptide hormones and for the biosynthesis of antibiotics . Magnesium and potassium aspartates play a role in the treatment of heart and circulatory diseases.
Cysteine , or the derivatives acetylcysteine and carbocysteine , are also used for infectious bronchial diseases with increased bronchial secretions . In addition, L cysteine as a reducing agent in the perm used. Amino acids are added to skin care products and shampoos in cosmetics .
metabolism
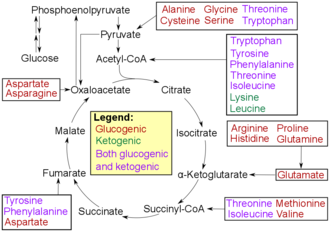
Amino acids can be divided into ketogenic , glucogenic and mixed keto and glucogenic amino acids according to their breakdown pathways . Ketogenic amino acids are added to the citrate cycle during breakdown , glucogenic amino acids to gluconeogenesis . Furthermore, various degradation products with biological activity (e.g. neurotransmitters ) are formed from amino acids in the metabolism . Tryptophan is the precursor to serotonin . Tyrosine and its precursor phenylalanine are the precursors of the catecholamines dopamine , epinephrine (synonymous with adrenaline) and norepinephrine (synonymous with noradrenaline). Phenylalanine is the precursor to phenethylamine in humans. In plants, phenylalanine is the precursor to the phenylpropanoids . Glycine is the starting material for porphyrin synthesis ( heme ). The secondary messenger substance nitrogen monoxide is formed from arginine . Ornithine and S-adenosylmethionine are precursors to the polyamines . Aspartate, glycine and glutamine are the starting materials for the biosynthesis of nucleotides.
In various human infections with pathogens , competition with the host for the amino acids asparagine, arginine and tryptophan has been described.
literature
Books
- Harold Hart: Organic Chemistry: A Short Textbook. VCH, 1989, ISBN 3-527-26480-9 .
- Jeremy M. Berg, Lubert Stryer, John L. Tymoczko, Gregory J. Gatto: Biochemistry. Macmillan Learning, 2015, ISBN 978-1-464-12610-9 .
- GC Barrett: Amino Acids and Peptides. Cambridge University Press, 1998, ISBN 978-0-521-46827-5 .
- Uwe Meierhenrich : Amino Acids and the Asymmetry of Life. Springer-Verlag, Heidelberg and Berlin 2008, ISBN 978-3-540-76885-2 .
- Hubert Rehm, Thomas Letzel: The Experimenter: Protein Biochemistry / Proteomics . 6th edition, Spektrum Akademischer Verlag, Heidelberg 2009, ISBN 978-3-8274-2312-2 .
Magazine articles
- Lei Wang, Peter G. Schultz : The Extension of the Genetic Code . In: Angewandte Chemie 117, No. 1, 2005, pp. 34-68.
- H. Uneyama, H. Kobayashi, N. Tonouchi: New Functions and Potential Applications of Amino Acids. In: Advances in Biochemical Engineering / Biotechnology . Volume 159, 2017, pp. 273-287, doi : 10.1007 / 10_2016_35 , PMID 27872968 .
- Bernd Hoppe, Jürgen Martens: Amino acids - building blocks of life , chemistry in our time , 17th year 1983, No. 2, pp. 41–53.
- Bernd Hoppe, Jürgen Martens: Amino acids - production and extraction , chemistry in our time , 18th year 1984, no. 3, pp. 73-86.
Web links
Individual evidence
- ^ Georg Löffler: Biochemistry and Pathobiochemistry. Springer-Verlag, 2013, ISBN 978-3-662-06062-9 , p. 25.
- ↑ a b , Katharina Munk (HRsg.): Biochemistry - Cell Biology. Georg Thieme Verlag, Stuttgart 2008, ISBN 3-13-151991-6 , p. 122, Google Books .
- ↑ a b Peter Nuhn: Naturstoffchemie , S. Hirzel Wissenschaftliche Verlagsgesellschaft Stuttgart, 1990, ISBN 3-7776-0473-9 , p. 70.
- ↑ G. Genchi: An overview on D-amino acids. In: Amino Acids. Volume 49, Number 9, September 2017, pp. 1521-1533, doi : 10.1007 / s00726-017-2459-5 , PMID 28681245 .
- ↑ NASA Researchers Make First Discovery of Life's Building Block in Comet . nasa.gov, August 2009; Chiral amino acids in meteorites strengthen evidence for extraterrestrial life . spie.org, September 2010 (accessed October 4, 2010).
- ↑ L. Vauquelin, P. Robiquet: The discovery of a new plant principle in Asparagus sativus. In: Annales de Chimie. Volume 57, 1806, pp. 88-93.
- ^ W. Rose et al .: Feeding Experiments with Mixtures of Highly Purified Amino Acids. VIII. Isolation and Identification of a New Essential Amino Acid. In: Journal of Biological Chemistry. Volume 112, 1935, pp. 283-302.
- ^ R. Simoni, R. Hill, M. Vaughan: The Discovery of the Amino Acid Threonine: the Work of William C. Rose. In: Journal of Biological Chemistry. Volume 277, No. 37, September 13, 2002, pp. 56-58.
- ↑ Sabine Hansen: The discovery of proteinogenic amino acids from 1805 in Paris to 1935 in Illinois. Berlin 2015.
- ^ Theodor Wieland: History of Peptide Chemistry, in: Bernd Gutte (Ed.), Peptides, Academic Press 1995, p. 2
- ↑ a b Wissenschaft-Online-Lexika: Entry on amino acids in the Lexikon der Biologie , accessed on April 25, 2009.
- ^ G. Löffler, PE Petrides and PC Heinrich: Biochemie & Pathobiochemie. 8th edition, Springer, Heidelberg 2006, ISBN 978-3-540-32680-9 .
- ↑ Hao Wang, David Fewer, Liisa Holm, Leo Rouhiainen, Kaarina Sivonena: Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes . In: Proc Natl Acad Sci USA . Volume 111, No. 25, June 2014, pp. 9259-9264. PMC 4078802 (free full text).
- ^ A b International Union of Pure and Applied Chemistry and International Union of Biochemistry: Nomenclature and Symbolism for Amino Acids and Peptides (Recommendations 1983) . In: Pure & Appl. Chem . tape 56 , no. 5 , 1984, pp. 595-624 , doi : 10.1351 / pac198456050595 .
- ↑ Paula Yurkanis Bruice: Organic Chemistry. Pearson Education Inc., 2004, 4th Edition, ISBN 0-13-121730-5 , pp. 960-962.
- ↑ Katsura Asano: Why is start codon selection so precise in eukaryotes ?. In: Translation. Volume 2, No. 1, March 2014, doi : 10.4161 / trla.28387 , PMC 4705826 (free full text).
- ↑ Y. Fan, CR Evans, J. Ling: Rewiring protein synthesis: From natural to synthetic amino acids. In: Biochimica et Biophysica Acta . Volume 1861, number 11 Pt B, 11 2017, pp. 3024–3029, doi : 10.1016 / j.bbagen.2017.01.014 , PMID 28095316 , PMC 5511583 (free full text).
- ↑ Kathrin Lang, Lloyd Davis et al .: Genetically encoded norbornene directs site-specific cellular protein labeling via a rapid bioorthogonal reaction. In: Nature Chemistry . 2012, pp. 298-304, doi : 10.1038 / nchem.1250 .
- ↑ Scientific report on biological value - What amino acids are there: Essential amino acids
- ↑ a b c WR Taylor: The classification of amino acid conservation. In: Journal of Theoretical Biology. Volume 119, year 1986, pp. 205-218. doi : 10.1016 / S0022-5193 (86) 80075-3 .
- ^ Löffler: Basic knowledge of biochemistry. Springer textbook, Heidelberg 2005, ISBN 3-540-23885-9 , p. 24.
- ↑ Alberts, Johnson, Lewis, Raff, Roberts, Walter: Molecular Biology of the Cell. WILEY-VCH Verlag GmbH, Weinheim 2004, ISBN 3-527-30492-4 , p. 152.
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, pp. 506–507, ISBN 3-342-00280-8 .
- ↑ J. Kyte, RF Doolittle: A simple method for displaying the hydropathic character of a protein . In: Journal of Molecular Biology . tape 157 , no. 1 , 1982, pp. 105-132 , PMID 7108955 .
- ↑ Representation not available because with proline on the peptide backbone there is one less hydrogen atom on the nitrogen (a secondary amine ), because the side chain forms a ring with the nitrogen atom (–NHCH 2 CH 2 CH 2 -).
- ↑ Jean-Pierre Mothet, Angèle T. Parent, Herman Wolosker, Roscoe O., Jr. Brady, David J. Linden, Christopher D. Ferris, Michael A. Rogawski, Solomon H. Snyder: d-Serine is an endogenous ligand for the glycine site of the N -methyl-d-aspartate receptor . In: Proc. Natl. Acad. Sci. USA . 97, No. 9, 2000, pp. 4926-4931. doi : 10.1073 / pnas.97.9.4926 . PMID 10781100 . PMC 18334 (free full text).
- ↑ Karlheinz Drauz, Hans Günter Koban, Jürgen Martens , Werner Schwarze : Phosphonic and Phosphinic Acid Analogs of Penicillamine . In: Liebig's annals of chemistry . tape 1985 , no. 3 , 1985, pp. 448-452 , doi : 10.1002 / jlac.198519850303 .
- ↑ DA Wellings, E. Atherton: Standard Fmoc protocols. In: Methods in enzymology. Volume 289, 1997, pp. 44-67, PMID 9353717
- ^ Bing Yan: Analytical Methods in Combinatorial Chemistry, Second Edition. CRC Press, 2011, ISBN 978-1-439-85760-1 .
- ↑ Y. Song, C. Xu, H. Kuroki, Y. Liao, M. Tsunoda: Recent trends in analytical methods for the determination of amino acids in biological samples. In: Journal of pharmaceutical and biomedical analysis. Volume 147, January 2018, pp. 35-49, doi : 10.1016 / j.jpba.2017.08.050 , PMID 28927726 .
- ^ A b Zdzislaw E. Sikorski: Chemical and Functional Properties of Food Proteins. CRC Press, 2001, ISBN 978-1-566-76960-0 , pp. 71, 219.
- ↑ Mebus A. Geyh, Helmut Schleicher: Absolute Age Determination - Physical and Chemical Dating Methods and Their Application. Springer-Verlag, Berlin · Heidelberg 1990, ISBN 978-3-540-51276-9 , pp. 345-371.
- ↑ N. Fujii, T. Takata, N. Fujii, K. Aki, H. Sakaue: D-Amino acids in protein: The mirror of life as a molecular index of aging. In: Biochimica et Biophysica Acta . [Electronic publication before printing] March 2018, doi : 10.1016 / j.bbapap.2018.03.001 , PMID 29530565 .
- ↑ a b c LF Fieser, M. Fieser: Textbook of organic chemistry. 3rd edition, Verlag Chemie, 1957, p. 506.
- ^ A b LF Fieser, M. Fieser: Textbook of organic chemistry. 3rd edition, Verlag Chemie, 1957, p. 507.
- ↑ LF Fieser, M. Fieser: Textbook of organic chemistry. 3rd edition, Verlag Chemie, 1957, p. 511.
- ↑ LF Fieser, M. Fieser: Textbook of organic chemistry. 3rd edition, Verlag Chemie, 1957, p. 516.
- ^ A b LF Fieser, M. Fieser: Textbook of organic chemistry. 3rd edition, Verlag Chemie, 1957, p. 508.
- ^ A b LF Fieser, M. Fieser: Textbook of organic chemistry. 3rd edition, Verlag Chemie, 1957, p. 510.
- ↑ Bernd Hoppe, Jürgen Martens : Amino acids - production and extraction , Chemistry in our time , 18th year 1984, No. 3, pp. 73–86.
- ↑ N. Tonouchi, H. Ito: Present Global Situation of Amino Acids in Industry. In: Advances in Biochemical Engineering / Biotechnology . Volume 159, 2017, pp. 3-14, doi : 10.1007 / 10_2016_23 , PMID 27832295 .
- ↑ a b M. D'Este, M. Alvarado-Morales, I. Angelidaki: Amino acids production focusing on fermentation technologies - A review. In: Biotechnology Advances . Volume 36, Number 1, 2018 Jan - Feb, pp. 14-25, doi : 10.1016 / j.biotechadv.2017.09.001 , PMID 28888551 .
- ↑ JH Lee, VF Wendisch: Production of amino acids - Genetic and metabolic engineering approaches. In: Bioresource Technology . Volume 245, Pt B December 2017, pp. 1575–1587, doi : 10.1016 / j.biortech.2017.05.065 , PMID 28552565 .
- ↑ Entry on protein value. In: Römpp Online . Georg Thieme Verlag, accessed on January 18, 2013.
- ↑ K. Yamamoto, A. Tsuchisaka, H. Yukawa: Branched-Chain Amino Acids. In: Advances in Biochemical Engineering / Biotechnology . Volume 159, 2017, pp. 103-128, doi : 10.1007 / 10_2016_28 , PMID 27872960 .
- ↑ Wolfgang Legrum: Fragrances, Between Stink And Scent: Occurrence, Properties and Use of Fragrances and Their Mixtures , Gabler Wissenschaftsverlage (2011), p. 165 ( limited preview in the Google book search).
- ↑ Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH: Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants . In: PLOS ONE . 3, No. 10, 2008, p. E3301. bibcode : 2008PLoSO ... 3.3301S . doi : 10.1371 / journal.pone.0003301 . PMID 18923670 . PMC 2565062 (free full text).
- ↑ Shemin D , Rittenberg D: The biological utilization of glycine for the synthesis of the protoporphyrin of hemoglobin . In: The Journal of Biological Chemistry . 166, No. 2, December 1946, pp. 621-5. PMID 20276176 .
- ↑ Tejero J, Biswas A, Wang ZQ, Page RC, Haque MM, Hemann C, Zweier JL, Misra S, Stuehr DJ: Stabilization and characterization of a heme-oxy reaction intermediate in inducible nitric-oxide synthase . In: The Journal of Biological Chemistry . 283, No. 48, November 2008, pp. 33498-507. doi : 10.1074 / jbc.M806122200 . PMID 18815130 . PMC 2586280 (free full text).
- ↑ Rodríguez-Caso C, Montañez R, Cascante M, Sánchez-Jiménez F, Medina MA: Mathematical modeling of polyamine metabolism in mammals . In: The Journal of Biological Chemistry . 281, No. 31, August 2006, pp. 21799-812. doi : 10.1074 / jbc.M602756200 . PMID 16709566 .
- ↑ Lubert Stryer, Jeremy M. Berg, John L. Tymoczko: Biochemistry , 5th. Edition, WH Freeman, New York 2002, ISBN 978-0-7167-4684-3 , pp. 693-8.
- ↑ W. Ren, R. Rajendran, Y. Zhao, B. Tan, G. Wu, FW Bazer, G. Zhu, Y. Peng, X. Huang, J. Deng, Y. Yin: Amino Acids As Mediators of Metabolic Cross talk between host and pathogen. In: Frontiers in immunology. Volume 9, 2018, p. 319, doi : 10.3389 / fimmu.2018.00319 , PMID 29535717 , PMC 5835074 (free full text).