Cafarsit
| Cafarsit | |
|---|---|
| Cuboctahedral cafarsite crystals from the Wannigletscher - Scherbadung (Monte Cervandone) area, Kriegalptal, Binntal , Valais , Switzerland (size: 3.5 cm × 1.8 cm × 1.3 cm) | |
| General and classification | |
| other names |
|
| chemical formula |
|
|
Mineral class (and possibly department) |
Oxides and hydroxides |
|
System no. to Strunz and to Dana |
4.JC.05 ( 8th edition : missing) 45.01.04.01 |
| Similar minerals | goethitized pyrite |
| Crystallographic Data | |
| Crystal system | cubic |
| Crystal class ; symbol | cubic-disdodecahedral; 2 / m 3 |
| Space group | Pn 3 (No. 201) |
| Lattice parameters | a = 15.9614 Å |
| Formula units | Z = 4 |
| Frequent crystal faces | {100}, {111}, {310}, {110} |
| Twinning | no |
| Physical Properties | |
| Mohs hardness | 5.5 to 6 |
| Density (g / cm 3 ) | 3.90 (measured); 3.60 (calculated) |
| Cleavage | no |
| Break ; Tenacity | shell-like to splintery; brittle |
| colour | dark brown, light red translucent in thin splinters, blackish (fresh) to brown (weathered), dark red translucent in thin splinters and small fresh crystals; Deep red in transmitted light |
| Line color | yellow-brown, brown-yellow |
| transparency | opaque, translucent in thin splinters |
| shine | Semi-metallic shine (fresh), earthy (weathered) |
| Crystal optics | |
| Refractive index | n = 2.0; n ≥ 2.20 |
| Optical character | anisotropic due to stress birefringence (?) |
| Other properties | |
| Chemical behavior | Slightly soluble in hydrochloric acid and oxalic acid |
Cafarsite is a very rarely occurring mineral from the mineral class of " oxides and hydroxides ". It crystallizes in the cubic crystal system with the idealized chemical composition (Ca 7.8 Na 0.8 Mn 0.5 REE 0.4 ) Σ = 9.5 (Ti 3.9 Fe 3+ 2.1 Fe 2+ 0.9 Mn 2+ 0.1 ) Σ = 7.0 (AsO 3 ) 14 F 0.5 and is therefore chemically a calcium - sodium - titanium - iron - arsenite , more precisely an arsenite without additional anions, but with additional fluorine ions .
The type locality of the Cafarsit is the Wannigletscher area - western flank of the Scherbadung (Monte Cervandone) ( coordinates of the Wannigletscher-Scherbadung area ) in the Kriegalptal, a side valley of the Binntal in the canton of Valais in Switzerland . Cafarsite forms up to 4.5 cm large, extensive crystals , the supporting shape of which is formed by the hexahedron {100}, the octahedron {111} or the pentagonal dodecahedron {310}, and which generally sit as single crystals on the cleft wall or on other minerals . In the “Hemlo” gold deposit in Ontario / Canada , however, the cafarsite occurs in the form of coarse, fine-grained aggregates .
Etymology and history
The mineral, later named cafarsite, was first described in 1880 from the Lärcheltini zone, Binntal, Valais, Switzerland:
"A gniss specimen from Alp Lercheltiny, which our collection owes to Mr Seligmann in Coblenz, shows iron luster and adulara, grown up as a younger generation, a rust-brown crystal 1–1½ cm in diameter, a combination of the octahedron with the hexahedron, the former somewhat predominant, and from the appearance of a pseudomorphism of brown iron ore, for example after pyrite. In the qualitative test, however, a small sample of the substance revealed, in addition to iron oxide and water, a content of arsenic, probably contained in the mineral as arsenic acid. Since it does not contain any trace of sulfuric acid, the assumption is that it was formed from arsenic iron by oxidation, that is, from the compound FeAs 2, which has not yet been found and which crystallographically corresponds to pyrite . Whether this assumption is correct, of course, can only be decided by finding the substance that has not yet been converted, which this brief note may suggest. "
In 1913 Heinrich Adolph Baumhauer proposed the name arsenoferrite for the cubic crystallizing precursor mineral of the rust-brown mineral, which he also regarded as a pseudomorphism . Even Henri Balder described arsenoferrite in the form of maximum 2 cm large crystals, which are difficult to distinguish from somewhat modified pyrite and are associated with quartz , adulara and hematite . This mineral was also believed by William F. Foshag & Maxwell Naylor Short in 1930 in ores from Jáchymov , Czech Republic - but Martin J. Buerger was able to identify the mineral they described as Löllingite , which is known to crystallize in the orthorhombic system. The name arsenoferrite became obsolete and the hypothetical mineral disappeared from the mineralogical map.
On September 16, 1963, the Swiss mineralogist and later a professor at the thought of Mineralogy and Petrography Institute of the University of Basel Stefan Grasses in crevices of the two-mica gneisses of after Monteleone named Monte Leone ceiling in the area Wannigletscher - Scherbadung two unknown minerals, of which up 3 cm large, dark brown cubic crystals with rough surfaces and the other forms lemon yellow, 0.5 mm large tablets. After the first chemical and X-ray diffractometric investigations, both turned out to be new minerals. This work was related to investigations to clarify the origin of the arsenic-containing solutions that were involved in the formation of the arsenic sulfosalts in the Lengenbach mine .
After intensive further investigation, the dark brown, cubic crystal-forming mineral was submitted to the International Mineralogical Association (IMA), which recognized it as a new mineral in 1965. In 1966, the first scientific description of this mineral was carried out by Stefan Grasses in the Swiss science magazine Swiss mineralogical and petrographic releases (as Cafarsit English Cafarsite ). The author named it CaFArs-it after the most important chemical elements calcium (Ca), iron ( Latin ferrum ) (F) and arsenic (Ars) involved in the composition of the new mineral . The identity of Cafarsit with the arsenoferrite from the Kollergraben (Chollergraben) of the Lärcheltini zone had already been assumed by Stefan Graeser in the type publication - it was finally proven in investigations of arsenoferrite crystals from museum collections in the 1970s.
In the type publication, the cafarsite was still viewed as a hydrous arsenate. It was not until 1977 that Andreas Edenharter and colleagues were able to show that cafarsite is not an arsenate with the functional [As 5+ O 4 ] 3− group, but an arsenite with the lower oxygen functional [As 3+ O 3 ] 3− group. That the mineral does not contain crystal water but fluorine ions was only shown in 2018 by two different working groups.
The type material for Cafarsit (holotype) is stored under catalog number SG749 in the collection of the Natural History Museum Basel , Switzerland. Another type level is in the collection of the Natural History Museum in London , United Kingdom (collection number 1966,434).
As early as the summer of 1965, the emitter Anton Imhof from Binn found crystals with an edge length of over 3 cm and a maximum weight of 48 grams on the Italian south-east side of the Scherbadung (correctly on Monte Cervandone).
classification
In the outdated, but partly still in use 8th edition of the mineral classification according to Strunz , the cafarsite belonged to the mineral class of "phosphates, arsenates and vanadates", but could not be classified in any department because it was not known whether the alleged water content was OH or H. 2 O was present.
The 9th edition of Strunz's mineral systematics, which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), assigns cafarsite to the class of "oxides and hydroxides" and there to the category of "arsenites, antimonites, bismuthites, sulfites, Selenite and Tellurite ”. This is further subdivided according to the possible presence of crystal water and / or additional anions , so that the mineral can be found in the sub-section “Arsenite, Antimonide, Bismutide, without additional anions, with H 2 O”, where it is the only one Member forms the unnamed group 4.JC.05 .
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , classifies the cafarsite as the outdated Strunz'sche systematics in the class of "phosphates, arsenates and vanadates" and there in the category of "acidic and normal antimonites, arsenites and phosphites". Here he is the only member of the unnamed group 45.01.04 within the sub-section “ Acid and normal antimonites, arsenites and phosphites with various formulas ”.
Chemism
Eighteen microprobe analyzes on cafarsite from the type locality revealed 11.91% TiO 2 ; 0.07% V 2 O 3 ; 8.06% FeO; 1.62% MnO; 16.65% CaO; 0.95% Na 2 O; 0.12% SnO 2 ; 56.87% As 2 O 3 ; 0.56% Y 2 O 3 ; 0.17% La 2 O 3 ; 0.95% Ce 2 O 3 ; 0.15% Pr 2 O 3 ; 0.46% Nd 2 O 3 (total 98.56%), which on the basis of 16.5 cations or 14 Arsenic - atoms per formula unit of the empirical formula: (Ca 7.79 Na 0.81 Mn 0.50 REE 0.40 ) Σ = 9.50 (Ti 3.91 Fe 3+ 2.1 Fe 2+ 0.9 Mn 2+ 0.10 ) Σ = 7.0 (AsO 3 ) 14 F 0.5 can be calculated .
The first analysis carried out at the Institute for Crystallography and Petrology at ETH Zurich by P. Thommen and Max Weibel revealed 13.0% CaO; 5.0% MnO; 11.0% Fe 2 O 3 ; 8.5% TiO 2 ; 60.0% As 2 O 5 and 2.4% H 2 O (total 99.9%). This analysis had been simplified by Graeser to the theoretical composition Ca 5.6 Fe 3.3 Ti 2.5 Mn 1.7 (AsO 4 ) 12 · 4H 2 O, which contains 13.14% CaO; 5.15% MnO; 11.31% Fe 2 O 3 ; 8.52% TiO 2 ; 58.81% As 2 O 5 and 3.07% H 2 O (total 100.00%). In contrast to the type publication and the first work on the crystal structure of the cafarsite by Andreas Edenharter and colleagues in 1977, Georgia Cametti and colleagues (2018) found around 2.5% by weight of REE 2 O 3 with mainly cerium as well as the type locality on the cafarsite Yttrium , lanthanum , praseodymium and neodymium detected. Yttrium was determined qualitatively in the type publication via X-ray fluorescence analysis , but not in the quantitative wet-chemical analysis .
In the course of the numerous works on the chemical composition of the cafarsite, it has been shown that cafarsites from different locations sometimes show considerable variations in their chemical composition. In contrast to the cafarsites from Swiss and Italian sites, cafarsite from "Hemlo" in Ontario / Canada has high contents of vanadium and manganese (4.7% V 2 O 3 ; 6.3% MnO), some aluminum (0.7% Al 2 O 3 ) and no detectable REE contents. The cafarsite from the Alps, on the other hand, show significant REE contents and hardly any vanadium. They are also lower in manganese than cafarsites from “Hemlo” - however, the cafarsites from the Mättital have a significantly higher manganese content than those from the Lärcheltini zone. Furthermore, the alpine cafarsites are not chemically homogeneous. With two growth generations, each with different REE contents, they reveal a characteristic zonal structure.
The combination of elements Ca – Na – Ti – Fe – As is unique among the currently known minerals; this means that there are no minerals with a chemical composition similar to that of cafarsite.
Crystal structure
Cafaesit was already recognized as cubic-disdodecahedral in the type publication. The latest investigations also show that cafarsite crystallizes in the cubic crystal system in the space group Pn 3 (space group no. 201) with the lattice parameter a = 15.9614 Å and four formula units per unit cell .
According to the first idea was the crystal structure of the Cafarsits trigonal AsO 3 - pyramids , with the Mø 4-6 - polyhedra are connected, stand for "M" Ca, Ti, Fe and Mn. Although the mineral was considered hydrated, no position for the H 2 O molecule could be located in the refined structure; In addition, there was no space in the corresponding structural model in which potential H 2 O molecules could find a place.
In the latest, of the IMA used model the structure of the Cafarsits also consists of trigonal AsO 3 pyramids, with the MO 6 - polyhedra are linked (M = Ca, Mn, Fe, Ti). In contrast to earlier work, the structural model contains an F-position. Four Ca1 atoms form a pseudo- tetrahedron around the fluorine ion. The MO 6–8 polyhedra are connected to the trigonal AsO 3 pyramids . The Ca1 position is coordinated sevenfold with six O positions (3 × O7 and 3 × O2) and an F position that has not yet been demonstrated in the previous structural refinements. The Ca2 and Ca3 positions are eight-fold coordinated. The Ca3 position is similar to the dodecahedral X position in a garnet structure, with the central cation shifted parallel to the long Ca3-O5 bonds. With the exception of the new, incompletely occupied Ca1A position belonging to Ca1, all other crystallographic positions are completely occupied. According to the chemical analyzes, Ca can be substituted by REE - however, of the three different Ca atoms Ca1, Ca2 and Ca3, only Ca1 substitutes the REE, by around 14%. Small amounts of manganese and sodium are assumed for the positions Ca2 and Ca3, respectively. The octahedral positions Ti1 and Fe2 are mainly occupied by titanium with minor substitution by Fe, whereas Mn1 is occupied by Mn 2+ and possibly also by Fe 2+ . The dense arrangement of the cations and anions in the cafarsite structure does not offer enough space for H 2 O molecules, which is why the hydrated character of the structural model by Andreas Edenharter and colleagues (1977) is not supported by the current structure refinements.
In the work by Giacomo Diego Gatta and colleagues, also published in 2018, three independent trigonal AsO 3 pyramids, one CaO 6 F polyhedron, one CaO 8 polyhedron, two independent (Ti , Fe) O 6 octahedron, a (Na, Fe, REE) O 8 polyhedron and a (Mn, Fe) O 6 octahedron are listed. The connections between the polyhedra are mainly made via common edges and corners, whereas the trigonal AsO 3 pyramids are not directly connected to one another.
properties
morphology
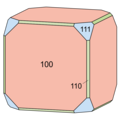
Cafarsite is mostly found in the form of grown single crystals grown on crevices in the gneiss. It forms isometric, large-area crystals with a maximum size of 4.5 cm, the supporting shape of which is formed by the hexahedron {100}, the octahedron {111} or the pentagonal dodecahedron {310}. So far, only the rhombic dodecahedron {110} and another pentagonal dodecahedron , which may be {510}, have been identified on other surface shapes . The different and z. The habitus of the cafarsite crystals, which in some cases varies greatly, is due to the fact that {100}, {111}, {310} and {110} vary in size and that a surface shape is sometimes strongly predominant and thus determines the costume. There are cafarsite crystals available as pure cubes, octahedra or pentagon dodecahedra. Only the rhombic dodecahedron has not yet been found to be the dominant form. The most common are combinations between cube and octahedron ( cuboctahedron ). Twins are unknown.
- Costume and habitus of cafarsite crystals (the same colors mean the same surface shapes)
The actually high-gloss cafarsite weathered relatively easily with the formation of a matt, X-ray amorphous brown oxidation crust, which covers the crystals in a more or less thick layer. This crust is very similar to limonitized ( goethitized) pyrite. Another weathering product of the cafarsite is the yttrium - arsenate agardite- (Y) , which arises during weathering from the arsenic of the arsenite group and the REE content of the cafarsite.
In addition, Cafarsit also forms aggregates. The first cafarsites recovered by Stefan Graeser on the Wanni Glacier were rough, reddish brown, bulbous mineral aggregates about 1 cm in diameter. In the "Hemlo" gold deposit in Ontario / Canada, the cafarsite was found in a drill core in the form of rough, fine-grained, maximum 1 mm large aggregates.
physical and chemical properties
Cafarsite crystals are described as dark brown, light red translucent in thin splinters or as blackish (fresh) to brown (weathered) and dark red translucent in thin splinters and small fresh crystals. The coarse aggregates from the "Hemlo" gold deposit in Canada are dark reddish brown. The line color of the cafarsite crystals, however, is given as yellow-brown or brown-yellow. The surfaces of the opaque crystals, which only shine through in thin splinters, show a semi-metallic sheen when fresh and are earthy when weathered. Cafarsit has a very high refraction ( n ≥ 2.0; n ≥ 2.20) , corresponding to its strong gloss . As a cubic mineral, cafarsite is not birefringent, but the mineral occasionally behaves anisotropically, possibly due to stress birefringence. In transmitted light, the mineral is deep red.
Cafarsit has no cleavability , breaks but because of its brittleness like quartz or Vesuvianite , wherein the fractured surfaces are formed conchoidal or splintery. The mineral has a Mohs hardness of 5.5 to 6 and is therefore one of the medium-hard minerals that can be scratched with a pocket knife or orthoclase ( feldspar ) with a steel file, just as easily as the reference mineral apatite (hardness 5) . The measured density for cafarsite is 3.90 g / cm³, the calculated density is 3.60 g / cm³.
Cafarsit is sparingly soluble in hydrochloric acid , HCl, and in oxalic acid.
Education and Locations
Cafarsite is a secondary mineral that was able to form due to an arsenic anomaly in the two mica gneisses on the Wanni Glacier. A pre-alpine, possibly also Variscan , Cu-As mineralization with tennantite and chalcopyrite located in the gneiss of the Monte Leone Nappe was sunk during the unfolding of the Alps and was overprinted by an amphibolite facial regional metamorphosis. Hot hydrothermal solutions partially dissolved the ores again. The arsenic-containing ore minerals of the primary mineralization in the Monte Leone Nappe reacted with a Cl - and F - -rich CO 2 -H 2 O fluid, the origin of which is traced back to the Mesozoic metasediments in the Monte Leone Nappe . The arsenic oxidized and was probably transported as H 3 AsO 3 0 complexes. These solutions became heavily enriched with arsenic and migrated to the north, probably along north-east-south-west trending fault systems. The resulting cooling led to supersaturation and consequently to the crystallization of arsenic-rich minerals. Owing to the lower oxygen content in the depths, these were often arsenites which, in contrast to arsenates with the functional [As 5+ O 4 ] group, contain the lower oxygen functional [As 3+ O 3 ] group. Along with asbecasite , fetiasite and cervandonite (Ce), cafarsite is one of these arsenite minerals that only rarely occur in nature.
In addition to alpine-type fissures, cafarsite has also been found in the Hemlo gold deposit in Ontario / Canada.
Typical accompanying minerals in cafarsite are asbecasite, gas parite (Ce) , chernovite and synchisite (Ce) as well as slightly smoke-colored quartz , chlorite (clinochlor), tourmaline and tilasite . In the type publication, magnetite , hematite , titanite , apatite , anatase , malachite , azurite , a pale ore ( tennantite ) and molybdenite (polytype molybdenite-6 H ) are given as parageneseminals of cafarsite . The photo documentation within the database Mindat.org According Cafarsit of quartz, is Agardit- (Y) , magnetite, hematite, Asbecasit, fluorite , muscovite , Senait , chlorite ( Rhipidolith ) and Crichtonit accompanied. Cafarsite can crystallize directly on large rock crystals or on asbecasite and is then younger than these. Finds are also known in which anatase sits on cafarsite and meta-kahlerite directly on or next to cafarsite. During the crystallization of agardite (Y) on cafarsite crystals, the yttrium necessary for the agardite was very likely released during the weathering of the cafarsite. These three minerals are therefore younger than the cafarsite. In "Hemlo" (Ontario / Canada) the cafarsite is accompanied by molybdenite, pyrite , tennantite, sphalerite and green muscovite containing vanadium.
As a very rare mineral formation, the cafarsite could so far (as of 2019) only be described from about ten sites. The type locality for Cafarsit is the Wannigletscher area - western flank of the Scherbadung in the Kriegalptal, a tributary valley of the Binntal , Valais , Switzerland that extends to the southeast . The type locality is thus on the Swiss side of the mountain Scherbadung - Monte Cervandone.
There are a number of other sites, the majority of which are also in the closer or further vicinity of the shear bath - Monte Cervandone and also go back to the remobilization of the pre-alpine Cu-As mineralization described above:
- the area of the "Monte Cervandone" (including "Conca del Cervandone" and "Ghiacciaio della Rossa"), in particular the Alpe Devero in the area of the municipality of Baceno , Valle di Devero - Valle Antigorio - Ossola Valley , Province of Verbano-Cusio-Ossola , Region Piedmont , Italy
- the "Pizzo Bandiera" also located in the area of the "Monte Cervandone"
- the northeast slope of the " Hillehorn " with the locality Chummibort , Binntal, Valais, Switzerland
- the "Gischi Glacier" including the "Gischihorn" ( Italian: Pizzo Cornera ), Kriegalptal, Binntal, Valais, Switzerland
- the “Gorb” in the Lärcheltini Zone, Binntal, Valais, Switzerland
- the Mättital, a small side valley of the Binntal, Valais, Switzerland, which extends from the hamlet or Maiensäss Heiligkreuz in a south-westerly direction to the 2631 m high Steinenjoch
- discovered in 1982, located near the northeastern shore of Lake Superior and 35 km east of Marathon , the huge Hemlo gold deposit, Ontario , Canada
Sites for asbecasite from Germany and Austria are therefore unknown.
use
Due to its rarity, cafarsite is only of interest to the collector of minerals.
See also
literature
- Stefan Graeser: Asbecasite and Cafarsite, two new minerals from the Binnatal (canton of Valais) . In: Swiss mineralogical and petrographic messages . tape 46 , no. 2 , 1966, p. 367–375 , doi : 10.5169 / seals-36131 ( e-periodica.ch [PDF; 11.1 MB ; accessed on January 20, 2019]).
- Andreas Edenharter, Werner Nowacki, Max Weibel: On the structure and composition of cafarsite: cafarsite is an As (III) oxide, not arsenate . In: Swiss mineralogical and petrographic messages . tape 57 , no. 1 , 1977, pp. 1–16 , doi : 10.5169 / seals-44418 ( e-periodica.ch [PDF; 22.5 MB ; accessed on January 20, 2019]).
- Georgia Cametti, Mariko Nagashima, Martin Fisch, Thomas Armbruster: New data on cafarsite: reinvestigation of its crystal structure and chemical composition . In: European Journal of Mineralogy . tape 30 , no. 4 , 2018, p. 859–868 , doi : 10.1127 / ejm / 2018 / 0030-2756 .
- Giacomo Diego Gatta, Nicola Rotiroti, Fernando Cámara, Martin Meven: On the labyrinthine world of arsenites: a single-crystal neutron and X-ray diffraction study of cafarsite . In: Physics and Chemistry of Minerals . tape 45 , no. 9 , 2018, p. 819-829 , doi : 10.1007 / s00269-018-0964-z .
- Cafarsite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 (English, handbookofmineralogy.org [PDF; 70 kB ; accessed on January 20, 2019]).
Web links
- Mineral Atlas: Cafarsit (Wiki)
- Mindat - Cafarsite (English)
- Webmineral - Cafarsite (English)
- RRUFF - Cafarsite (English)
- American-Mineralogist-Crystal-Structure-Database - Cafarsite (English)
Individual evidence
- ↑ a b c d e f g h i Georgia Cametti, Mariko Nagashima, Martin Fisch, Thomas Armbruster: New data on cafarsite: reinvestigation of its crystal structure and chemical composition . In: European Journal of Mineralogy . tape 30 , no. 4 , 2018, p. 859–868 , doi : 10.1127 / ejm / 2018 / 0030-2756 (English).
- ↑ a b c Giacomo Diego Gatta, Nicola Rotiroti, Fernando Cámara, Martin Meven: On the labyrinthine world of arsenites: a single-crystal neutron and X-ray diffraction study of cafarsite . In: Physics and Chemistry of Minerals . tape 45 , no. 9 , 2018, p. 819–829 , doi : 10.1007 / s00269-018-0964-z (English).
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah Stefan Graeser: Asbecasite and cafarsite, two new minerals from the Binnatal (Kt . Wallis) . In: Swiss mineralogical and petrographic messages . tape 46 , no. 2 , 1966, p. 367–375 , doi : 10.5169 / seals-36131 ( e-periodica.ch [PDF; 11.1 MB ; accessed on January 20, 2019]).
- ^ Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 269 (English).
- ^ Philippe Roth: Minerals first discovered in Switzerland and minerals named after Swiss individuals . 1st edition. Kristallografik Verlag, Achberg 2007, ISBN 978-3-9807561-8-1 , p. 48–49 (English, limited preview in Google Book Search).
- ↑ a b c d e Cafarsite. In: mindat.org. Hudson Institute of Mineralogy, accessed January 30, 2019 .
- ↑ a b c d Cafarsite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 (English, handbookofmineralogy.org [PDF; 70 kB ; accessed on January 20, 2019]).
- ↑ a b IMA / CNMNC List of Mineral Names; November 2018 (PDF 1.65 MB)
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y Stefan Graeser: Profile Cafarsite Ca 8 (Ti, Fe, Mn) 6–7 (AsO 3 ) 12 · 4H 2 O . In: Lapis . tape 20 , no. 7-8 , 1995, pp. 8-11 .
- ↑ a b Rudolf Duthaler, Stefan Weiß: Clean, prepare and store minerals. The workbook for the collector . 1st edition. Christian Weise Verlag, Munich 2008, ISBN 978-3-921656-70-9 , p. 162 .
- ^ Paul Heinrich von Groth : A pseudomorphosis from the Binnenthal . In: Journal for Crystallography and Mineralogy . tape 5 , no. 2–3 , 1880, pp. 253 .
- ↑ a b Heinrich Adolph Baumhauer : X. Arsenoferrite, a new member of the pyrite group . In: Journal for Crystallography and Mineralogy . tape 5 , no. 1 , 1913, pp. 143-145 , doi : 10.1524 / zkri.1913.51.1.143 .
- ↑ Henri Bader: Contribution to the knowledge of the rocks and mineral deposits of the inland valley (at the same time inaugural dissertation for obtaining a doctorate in philosophy submitted to the Philosophical Faculty II of the University of Zurich) . In: Swiss mineralogical and petrographic messages . tape XIV , no. 2 , 1934, p. 319–441 , doi : 10.5169 / seals-14644 ( e-periodica.ch [PDF; 17.3 MB ; accessed on January 20, 2019]).
- ^ William F. Foshag, Maxwell Naylor Short: Arsenoferrit from Jachymov, Czechoslovakia . In: The American Mineralogist . tape 15 , no. 9 , 1930, p. 428–429 (English, minsocam.org [PDF; 123 kB ; accessed on February 10, 2019]).
- ↑ Martin J. Buerger : The probable non-existence of arsenoferrite . In: The American Mineralogist . tape 21 , no. 1 , 1936, pp. 70–71 (English, minsocam.org [PDF; 141 kB ; accessed on February 10, 2019]).
- ↑ Stefan Grasses: Cherbadung September 1963: The discovery of the first arsenite zone . In: Lapis . tape 20 , no. 7-8 , 1995, pp. 33-35 .
- ↑ a b c Mischa Crumbach, Michael Praeger: Large-area cafarsit from Gorb in the Lärcheltinizone, Binntal . In: Lapis . tape 29 , no. 4 , 2004, p. 34-39 .
- ↑ a b c d e Andreas Edenharter, Werner Nowacki, Max Weibel: On the structure and composition of cafarsite: cafarsite an As (III) oxide, no arsenate . In: Swiss mineralogical and petrographic messages . tape 57 , no. 1 , 1977, pp. 1–16 , doi : 10.5169 / seals-44418 ( e-periodica.ch [PDF; 22.5 MB ; accessed on January 20, 2019]).
- ↑ Catalog of Type Mineral Specimens - C. (PDF 131 kB) In: docs.wixstatic.com. Commission on Museums (IMA), December 12, 2018, accessed August 29, 2019 .
- ^ Hugo Strunz : Mineralogical tables. A classification of minerals based on crystal chemistry . 8th edition. Academic publishing company Geest & Portig, Leipzig 1982, p. 324 .
- ^ A b c d e f Donald C. Harris: The mineralogy and geochemistry of the Hemlo gold deposit, Ontario (Economic Geology Report (Geological Survey of Canada) No. 38) . 1st edition. Energy, Mines and Resources Canada: Canadian Govt. Pub. Center, Supply and Services Canada, Ottawa, Canada 1989, ISBN 978-0-660-13269-3 , pp. 23 (English).
- ↑ a b c d Michael Krzemnicki, Stefan Graeser: Das Mättital . In: Lapis . tape 20 , no. 7-8 , 1995, pp. 68-71 .
- ↑ a b Stefan Graeser: New mineral finds from the Cherbadung / Cervandone region (Switzerland / Italy) . In: Swiss emitters . tape 7 , no. 11 , 1987, pp. 473-486 .
- ↑ a b c Stefan Graeser: Wannigletscher and Conca Cervandone . In: Lapis . tape 20 , no. 7-8 , 1995, pp. 41-64 .
- ↑ a b Michael Krzemnicki: As-Bi mineralizations in the Mte Leone Nappe of the Mattitales, Binntal region (CH) . In: Communications from the Austrian Mineralogical Society . tape 137 , 1992, pp. 163–164 ( geologie.ac.at [PDF; 6.1 MB ; accessed on January 20, 2019]).
- ↑ a b Stefan Graeser: New: Arsenic minerals from the Lärcheltini zone . In: Lapis . tape 20 , no. 7-8 , 1995, pp. 36-40 .
- ↑ Localities for Cafarsite. In: mindat.org. Hudson Institute of Mineralogy, accessed January 30, 2019 .
- ↑ Find location list for cafarsite at the Mineralienatlas and at Mindat (accessed on February 10, 2019)
- ↑ a b Alessandro Guastoni, Federico Pezzotta, Pietro Vignola: Characterization and genetic inferences of arsenates, sulfates and vanadates of Fe, Cu, Pb, Zn from Mount Cervandone (Western Alps, Italy) . In: Periodico di Mineralogia . tape 75 , no. 2–3 , 2006, pp. 141–150 (English, researchgate.net [PDF; 341 kB ; accessed on January 20, 2019]).
- ^ A b Claudio Albertini: L'Alpe Devero ed i suoi minerali . 1st edition. Edizioni Grafica PGA, Dormelletto (Novara) 1991, p. 1-299 (Italian).
- ↑ Stephane Cuchet, Ate van der Burgt, Nicolas Meisser: Chummibort, a new site for arsenic minerals in the Binn valley . In: Swiss emitters . tape 2005 , no. 2 , 2005, p. 19-29 .
- ^ Stefan Graeser, Hans Schwander, Francesco Demartin, Carlo M. Gramaccioli, Tullio Pilati, Eric Reusser: Fetiasite (Fe 2+ , Fe 3+ , Ti) 3 O 2 [As 2 O 5 ], a new arsenite mineral: its description and structure determination . In: The American Mineralogist . tape 79 , 1994, pp. 996–1002 (English, rruff.info [PDF; 867 kB ; accessed on January 20, 2019]).