adhesive
An adhesive is a non-metallic substance that is able to connect materials through surface adhesion and its internal strength ( cohesion ). It is therefore a process material that is used in the bonding process to connect different materials. In addition to the load-transferring effect, adhesives can take on other functions in the connections , such as B. Vibration damping, sealing against liquids and gases, compensation of different component dynamics, corrosion protection, thermal and electrical insulation or conductivity.
Due to the many advantages of the bonding process , adhesives have become an integral part of everyday life. With their numerous applications in a wide variety of industries, they have become practically indispensable for today's modern life. Many products, such as B. books, cell phones, floors, e-readers, mattresses, cars and countless more would not be possible in their current form without adhesives.
history
Gluing is one of the oldest and most important cultural techniques known to man. Adhesives made weapons and tools possible and helped early humans to assert themselves against a hostile environment. Material evidence is rare, however, as organic materials - which also include the adhesives used at the time - have not withstood the course of time, with a few exceptions.
The oldest adhesive and sealant used by humans was probably clayey soil, with the help of which z. B. caves and huts of prehistoric times were made more livable. The more progressive Neolithic people noticed the adhesive power of asphalt (pitch), tree resins and wood tar. In Saxony-Anhalt, for example, birch pitch that was at least 115,000 years old was found as an adhesive for blade inserts in stocks (knives, spears). In southern Italy, scientists found birch pitch clinging to a stone tool that was at least 180,000 years old. And Ötzi had also made his arrows with birch pitch over 5000 years ago. This early glue does not occur naturally, but has to be specifically made from birch bark by dry distillation . Studies from 2019 came to the conclusion that usable amounts of birch pitch can be produced by simply burning birch bark near stone or bone surfaces. The birch pitch can be scraped off the surfaces after the burn.
6000 years ago, houses in Mesopotamia were built with air-dried mud bricks and asphalt was used for other building purposes. The ancient historian Herodotus reports that the walls of Babylon were glued with pitch. Around 1000 years later, the Sumerians were already familiar with making glue from boiled animal hides.
At least since about 1500 BC. The Egyptians used a broth made from sinews, cartilage and other animal waste as a suitable adhesive for veneered carpentry. The resulting adhesive was applied warm and has so far survived more than three millennia undamaged, as a plaque from Tutankhamun's tomb shows. The Egyptians also used beeswax for artisanal gluing by mixing it with pulverized stone flour and thus z. B. connected metal blades of razors with their handle.
The ancient Greeks and Romans also used different adhesives. While there was already the profession of glue boiler in Greece and their mostly protein-containing glue was called “Kolla”, the Romans called their glues made from flour paste, soured bread or cheese-lime mixtures as “Glutinum”. It is said that the Romans produced the first isinglass made from swim bladders around 1000 years before the Germans.
In the period that followed, there was initially little development in adhesives. In addition to their poor heat and cold resistance, the adhesives of that time also formed an ideal breeding ground for bacteria and fungi due to their composition, which represented a health risk. It was not until the late Middle Ages that the first independent glue boilers gradually formed in Europe, as paper processing developed into a growth market after Gutenberg's invention of the printing press. The increasing number of books had to be bound and provided with sturdy covers and spines.
The materials (wood, leather, paper, cardboard), the areas of application and thus also the adhesives remained essentially unchanged for centuries. The production was a monotonous job, so it is not surprising that the term “glue boiler” has long been a dirty word for particularly dull people.
In the course of industrialization, the scorned profession experienced an upswing that went hand in hand with the furniture and wallpaper industry. The new, rational production methods made it possible for ordinary people to have comfortable furniture and elegantly designed walls. The first artisanal glue factory was founded in Holland in 1690. In 1754, more than 60 years later, the first patent for isinglass for carpentry was granted in England and in 1789 Germany's first wallpaper printing company opened in Kassel. Although the amount of wood glue and paste required rose sharply, there was initially no significant change in the product category.
It was not until the end of the 19th century that the increasing demand for goods of all kinds accelerated the development of production technology, and packaging problems grew with mass production. The existing adhesives ultimately no longer met the qualitative requirements. This was the beginning of a systematic search for modern successor products.
After Otto Ring developed the first ready-to-use isinglass " Syndetikon " in 1880 and Ferdinand Sichel developed the first ready-to-use vegetable glue in 1889 , the age of adhesives based on synthetically produced raw materials began in 1909 with the patent for phenolic resin curing by Leo Hendrik Baekeland . Chemists, physicists and engineers sat down with the effects of adhesion - and cohesive forces apart, studied the macromolecular structure of the adhesives, perfected the known resources and eventually found in the synthetic resins , the starting point for creating more powerful synthetic adhesives.
The synthetic raw material most commonly used to manufacture adhesives today, polyvinyl acetate , was patented by Rollet and Klatte in 1914. In 1928, the first production of polyvinyl chloride (PVC) and polymethyl methacrylate ( Plexiglas ) took place in the USA . In 1929 a process for hardening the urea resin , discovered in 1919, was developed, making it usable for glue.
In the 1930s, the first technical production of polyvinyl acetate , polystyrene and polyacrylonitrile and the first stable plastic dispersion based on acrylic acid esters and vinyl acetate succeeded . The Swiss P. Castan used polyaddition to build plastics and invented epoxy resins , which he patented in 1939. In addition to the development of the first transparent adhesive tape, the manufacture of the raw material polychlorobutadiene and the polyurethanes patented by Bayer are of enormous market importance for the adhesives industry.
The adhesive found its way into aircraft construction in 1943 through the use of phenolic resin polyvinyl acetates and epoxy resin formulations. When the production of anaerobic and cyanoacrylate adhesives started in 1960 , the adhesives industry achieved a decisive breakthrough in the field of metal and plastic compounds.
With the discovery of the first temperature -resistant polyimide adhesives and moisture-curing polyurethanes in the 1970s, there was a rapid further development of polyurethane chemistry with a variety of 1- and 2-component formulations, UV light-curing acrylate formulations and the development of MS polymers. Reactive hot melt adhesives and anisotropically conductive adhesives are the greatest achievements of the 1980s.
From 1990 the development of adhesives with multiple curing mechanisms (e.g. UV radiation, humidity, oxygen access) begins. The silane-crosslinking polyurethane prepolymers (S-PUR) developed in 1995 show an improved balance between reactivity and storage stability, no bubble formation during curing and no longer have an isocyanate-based reaction mechanism. In 2000, the development of removable adhesive systems for repair and recycling began, based on the methods of changing temperature, voltage, current and pH.
Further growth opportunities can be seen in the future. The reasons for this are the assumption of additional functions that were previously performed by other materials, the increasing lightweight construction and, last but not least, the miniaturization of electronic components.
Economical meaning
In Germany, around 1.5 million tons of adhesives, sealants and cementitious construction adhesives as well as 1 billion square meters of carrier-bound adhesives (adhesive tapes / adhesive films) are produced annually, generating total industry sales of 3.7 billion euros. The added value generated through the use of adhesive technology is - conservatively calculated - well over 360 billion euros. This amount corresponds to around 50% of the contribution made by the manufacturing and construction industries to the German gross domestic product (GDP). Thus around 50% of the goods and construction services produced in Germany are associated with adhesives.
It is interesting to compare the development of the gross domestic product and the growth of the adhesives industry. Over the past ten years it has been clearly observed that the adhesives industry has also been subject to the macroeconomic business cycle - albeit with growth rates that are on average 2.5–5% above the growth rates of GDP.
The global market volume for adhesives for 2016 is estimated at 49.5 billion USD. In 2015, paper and packaging was the largest application segment in the market for adhesives and sealants. Of the various adhesive technologies, water-based adhesives represented the largest segment in 2015 in terms of both value and quantity. Growing populations and increasing demand for consumer goods in several countries around the globe are a key factor that will continue to drive the growth of the adhesives industry in the coming years. Countries like China, Japan and India will continue to gain in importance. In 2021, global sales of adhesives and sealants are expected to reach a good $ 63 billion.
Classification
Due to the wide range of applications for adhesives and the various requirements associated with them, it is not surprising that an almost immense number of adhesives is available. It therefore makes sense to classify adhesives in order to better recognize and understand similarities, but also differences. An initial classification can be made according to their chemical basis.
A distinction is made between adhesives made on the basis of organic substances and those based on inorganic substances. In between are the silicones, which contain both organic and inorganic components. In addition, a distinction can be made between adhesives that have a natural or a synthetic organic base. The latter is often not clearly recognizable, as many adhesives contain both synthetic and natural raw materials. Those on a purely natural basis, such as B. beeswax or tree resin are quite rare because of their inadequate properties for most applications. For some years now, not least as a result of increasing environmental awareness, the demand for more sustainability and a reduction in the carbon footprint, an increasing use of adhesive components based on renewable raw materials has been observed .
Another, more detailed classification is that according to the solidification mechanism. A distinction is made between solidification by a physical process and that by a chemical reaction. There is also the group of adhesives that are not subject to any solidification mechanism. These three types of adhesive listed in Table 1 are explained in more detail below.
The further subdivision of the division into thermosets , thermoplastics and elastomers , as is common with plastics, gives the user valuable information on the properties of the respective adhesive in its solidified state. However, it must be taken into account that adhesives of the same type of polymer, depending on the added additives or components, harden to form thermosets as well as elastomers or thermoplastics or can be present as such. Polyurethane adhesives are a good example of this.
| ADHESIVES | |||
|---|---|---|---|
| chemically curing | physically setting | Adhesives with combined solidification mechanisms | Adhesive tapes |
Curing through polymerization
|
Solidification by drying
|
Combination of different chemical mechanisms |
No solidification
|
Hardening by polyaddition
|
Solidification by cooling
|
Combination of a physical and a chemical solidification mechanism
|
|
Hardening through polycondensation
|
Solidification through gel formation
|
Combination of different chemical mechanisms
|
|
Physically setting adhesives
This refers to adhesives in which the polymer chains in the adhesive supplied by the adhesive manufacturer are already in their final composition and size. This means that the polymer itself is not subject to any chemical changes when the adhesive solidifies. Since adhesives adhere to an adherend at all, i. H. In order to be able to build up adhesion in liquid form, only polymers that can be liquefied are suitable for this class of adhesives. Thermoplastics can be liquefied by heating and then solidifying again by cooling. Other possibilities are thermoplastics that are soluble in solvents and the conversion into a dispersion . The solidification of the adhesive thus takes place through a physical process, the solidification or evaporation of the solvent or the dispersion medium water. The cooling of a hotmelt adhesive has the consequence that the mobility of the polymer chains is restricted and physical interactions, mind you, no chemical bonds are formed between the polymer chains, which in their type and extent ultimately reduce the internal strength, i.e. H. determine the cohesion of the adhesive in the solidified state. In the case of adhesives in which the liquid state already occurred during production by dissolving a polymer in a solvent or converting it into a dispersion, solidification occurs as a result of the evaporation of the solvent or the dispersion medium. The evaporation of the solvent or the dispersion medium has the consequence that the polymer chains approach one another and physical interactions develop. Illustrated, the polymer chains can be compared to spaghetti. As long as they are in hot water, they are relatively easy to move. However, if the water evaporates, they come closer together, form interactions with one another and thus acquire a certain internal strength.
In the following, the most important types of physically solidifying adhesives are characterized and described with regard to their typical properties and areas of application.
Solvent-based wet adhesives
In the case of solvent-based wet adhesives, the polymer is dissolved in organic solvents. The adhesive is usually applied to one of the parts to be joined and the joining takes place at a point in time when a large part of the solvent is still present in the adhesive. This ensures sufficient wetting of the second part surface. When the solvent evaporates, the adhesive sets, which means that it initially becomes tougher and then solidifies through the formation of physical interactions between the polymer chains. While with some of these adhesives the joining process can take place immediately after the adhesive has been applied, others require compliance with a product-dependent minimum drying time. H. Before joining, a certain flash-off time must be observed in order to allow some of the solvent to evaporate. This minimum drying time is followed by the wet bonding time . This is the period of time within which the adhesive still contains sufficient solvent, thus being sufficiently liquid and allowing the second part to be well wetted. If the wet bonding time is exceeded, this is usually associated with a loss of quality in terms of bond strength. The period of minimum drying time and wet bonding time is often referred to as the open waiting time . The subsequent so-called closed waiting time describes the period of time within which the adhesive sets to such an extent that an initial strength is achieved that allows further handling of the adhesive. During this period, the bond must not be loaded, which usually requires fixing. While the open waiting time and the wet bonding time essentially depend on the respective adhesive and the ambient conditions, the closed waiting time also depends on the materials to be joined, i.e. H. their ability to let the solvent escape from the glue joint and the requirements for the level of initial strength.
As polymers, for. B. Polyurethanes , polyvinyl acetate , synthetic or natural rubber and acrylates are used. The type of solvent, mixtures often also being used, depends on the particular polymer and its solubility. Typical solvents include esters (e.g. ethyl acetate ), ketones (e.g. 2-butanone ) or tetrahydrofuran .
Solvent-based wet adhesives can also be used for diffusion bonding ( cold welding ) of thermoplastics. Both adhesive surfaces are coated with the adhesive, which contains a solvent which is able to loosen the surface of the parts to be joined. After a short exposure time, the two parts to be joined are joined under pressure, as a result of which the polymer chains of the loosened surface exposed by the solvent penetrate and become entangled, similar to the bristles of two brushes that are pressed into one another. After the solvent has escaped, a connection is created after some time that is based purely on cohesive forces. Colloquially, this process is also known as cold welding or solvent welding.
application areas
For a long time, examples of solvent-based wet adhesives were the so-called “all-round adhesives”. It should be noted that it should actually be called “all-purpose adhesive”, but that there has never been such a thing in the narrower sense. Similar to how, for example, a "universal screw" designates a screw with a wide range of applications, an "all-purpose adhesive" is suitable for gluing a wide variety of materials, even if not all materials.
While in the past solvent adhesives etc. a. were also used in the hobby and household sector, for ecological and occupational safety reasons these have been replaced here and in many fields of application by other systems that do not contain any flammable and / or harmful solvents. Today they are used in particular for bonding paper and cardboard and for diffusion bonding, especially PVC.
When using solvent-based wet adhesives, in addition to their flammability and / or harmful effects, note that
- Particularly in the case of large-area bonds, at least one substrate must be solvent-permeable, since otherwise the complete escape of the solvent from the adhesive joint, which is necessary for setting, can take a very long time (up to a few days or weeks).
- The suitability of solvent-based wet adhesives for bonding materials that are sensitive to stress cracks such as polycarbonate must be viewed critically, as the solvents they contain can trigger stress cracks.
Solvent-based wet adhesives enable strengths of up to 10 MPa, depending on the adhesive, with elongation at break in the range of approx. 5 to 400%. Since the polymers used as adhesive raw materials are thermoplastics, the bonds have a limited heat resistance, show a tendency to creep under load and are sensitive to solvents.
Contact adhesives
Contact adhesives are polymers dissolved in solvents (especially polychloroprene and polyurethanes ) or, as a more environmentally friendly alternative, dispersions in water. However, they differ in their processing in that contact adhesives are applied to both parts to be joined. Only after a large part of the solvent or water has evaporated, i. H. When the adhesive film is more or less dry, the components are joined. Here the contact pressure, it should be at least 0.5 MPa, is of decisive importance, the contact time of minor importance. As a result of the pressing together, the two adhesive layers present in the amorphous state flow into one another in order to then solidify further, forming crystalline structures. The bond is resilient immediately after joining, so a closed waiting period is not required for typical contact adhesives.
The minimum drying time here describes the period of time that the adhesive film needs until it still has a certain residual tack when it is touched with the fingertip, but no longer pulls threads and thus the joining can take place. In order to avoid skin contact with the residual solvents, suitable gloves should be worn during this so-called finger test. In the technical data sheets for contact adhesives, the contact adhesive time, i.e. H. the period after the end of the minimum drying time within which the joint must be carried out is specified. If the bond is only joined after the contact bonding time has been exceeded, losses in the strength of the bond are to be expected. With many contact adhesives, if the contact bonding time is exceeded, the adhesive film can be reactivated by heat. Information on this can also be found in the technical data sheets for the respective adhesives.
In order to use the solvent-free, water-based contact adhesives, however, it must be noted that in particular the long minimum drying times and the often only limited moisture resistance prevent their use. Drying can be accelerated by applying appropriate heat in drying tunnels and the lack of moisture resistance by two-component work, i.e. H. the addition of a crosslinking agent before use (see adhesives with combined solidification mechanisms ).
application areas
Contact adhesives are z. B. used for gluing floor coverings, in the manufacture of mattresses and shoes and for attaching decorative and rubbing strips. Since the solvent has almost completely escaped from the adhesive film before joining, contact adhesives are also suitable for bonding two solvent-impermeable parts to be joined.
When using contact adhesives it should be noted that
- the product-specific process times, minimum drying time and contact bonding time are observed
- the required contact pressure is guaranteed when joining
If solvent-based contact adhesives are used, their flammability and / or harmful effects must be taken into account,
- that the solvents contained in plastics can trigger stress corrosion cracking.
Dispersion adhesives
In the case of dispersion adhesives, water is generally used to convert the adhesive polymers into the liquid state required for bonding. However, the polymers are not dissolved, as is the case with solvent-based adhesives , but in the form of a dispersion . The polymer particles are in the form of tiny particles and float, so to speak, in the water (known as the mobile phase). A dispersion adhesive is therefore an adhesive in which the adhesive molecules are kept apart by the surrounding water molecules with the aid of emulsifiers and other auxiliaries, so they cannot accumulate to form larger agglomerates.
Dispersion adhesives can be formulated both for processing as wet adhesives, analogous to the solvent-based wet adhesives described above, and as contact adhesives. In both forms, the setting takes place by removing the mobile phase, the water. This can be done either by evaporation or by absorption in the adherend. The concentration of the polymer particles increases and that of the water molecules that hold them apart decreases. As a result, the polymer particles get closer and closer until they finally flow together. During this process, known as film formation, interactions develop (formation of cohesion ) and the surface of the parts to be joined (formation of adhesion ).
It also applies to both forms that dispersion adhesives are generally sensitive to frost, i.e. H. the dispersion can be destroyed by the action of frost, causing what is known as breaking of the dispersion, the polymer particles coagulate to agglomerates so that a uniform adhesive film can no longer be applied. This also has a disadvantageous influence on the formation of adhesion to the part to be joined. The shear forces that occur when the adhesive is conveyed through hoses or pipelines or in the pumps used for this purpose can also lead to the dispersion breaking. Furthermore, dispersion adhesives are susceptible to mold growth in the storage container. For this reason, preservatives are usually added by the adhesive manufacturer, but nevertheless, especially when storing in tanks, care must be taken to ensure cleanliness and, if necessary, re-preserved.
In the case of wet adhesives, their open time, i.e. H. the period, beginning with the application of the adhesive, within which the joining process must take place, must be observed. It is determined by the temperature, relative humidity of the ambient air, thickness of the adhesive layer and the water absorption capacity of the part to be joined. For joining, the parts to be joined are pressed together. The pressing time in turn depends on the temperature and the water absorption capacity of the parts to be joined. In the case of contact adhesives, the minimum flash-off time, which in turn depends on the temperature, the relative humidity of the ambient air, the thickness of the adhesive layer and the water absorption capacity of the component to be joined, and the contact adhesive time must be observed and a sufficiently high contact pressure must be applied when joining.
In general, the strengths that can be achieved with dispersion adhesives are limited, as is the heat resistance due to the thermoplastic character of the polymers used. Because of the emulsifiers required to stabilize the dispersion, they show limited moisture resistance even if the film formation is not reversible. Both heat form and moisture resistance can be significantly improved by adding a crosslinking agent, i.e. a two-component work (see also adhesives with combined solidification mechanisms ).
Dispersion adhesives for use as wet adhesives are particularly suitable for large-area bonding of water-permeable materials such as wood, paper and cardboard. Accordingly, they are widely used in paper processing, in the manufacture of packaging and in the furniture industry. Dispersion adhesives are widely used as contact adhesives in the automotive industry for the lamination of interior trim parts with decorative films, textiles or leather. Because of the good temperature and moisture resistance required for the application, processing is usually carried out as a 2K system (see also adhesives with combined solidification mechanisms ).
Hot melt adhesives
Hotmelts - often also referred to as hotmelts - are solid at room temperature and can be processed by melting. The hot adhesive melt is applied to the part to be bonded and immediately joined to the second part within the open time. Immediately after the adhesive has cooled and solidified, the connection is firm and functional. This enables very fast cycle times and immediate further processing in production processes.
For hobby and small users, hot melt adhesives are available in the form of glue candles (glue sticks) that can be processed with hot melt glue guns. In technical applications, they are also processed in the form of foils, granulates or blocks with the help of melters and downstream application heads.
Hot melt adhesives are solvent-free, but their use is limited to temperature-resistant materials due to the high processing temperatures. On the other hand, the adhesive behaves reversibly, i.e. when the temperature rises it becomes soft again and therefore has only limited heat resistance (see also reactive hot melt adhesive ).
Plastisols
The term plastisol refers to dispersions , i.e. two-phase systems consisting of a powdery thermoplastic polymer in a high-boiling organic liquid ( plasticizer ). Other recipe components are fillers , pigments and additives such as B. adhesion promoter . PVC and acrylates are mainly used as polymers, and phthalic acid esters are mostly used as plasticizers .
When selecting the polymer and the plasticizer, it must be taken into account that the polymer is soluble in the plasticizer, but the rate of dissolution at room temperature must be negligible. Only at higher temperatures does the plasticizer diffuse into the polymer and the initially two-phase system turns into a single-phase gel , the plastisol gels. Temperatures of 150–180 ° C are required for this. This gel, made of a plasticized polymer, solidifies when it cools, creating a mass of extremely high viscosity that is no longer flowable at room temperature. The plastisols used as adhesives or sealants have high flexibility and good peel strengths, but have the disadvantage that they show a tendency to creep under load. Furthermore, as thermoplastics, they have only a limited temperature resistance.
Plastisols as adhesives and sealants
The use of plastisols in bonding technology is naturally limited to processes in which the temperatures required for gelation can be used. The main area of application is the body shop in automobile construction , whereby PVC plastisols are mostly used. Corresponding temperatures are available here with the oven processes for curing the paint. In addition to a joining function, the plastisols also perform a jointing function, often in connection with an increase in the rigidity of the body, as well as sealing joints against the ingress of moisture (avoiding corrosion ), as well as a damping function. The ability of the plastisols to absorb corrosion protection and drawing oils on the sheet metal surface during gelation is a further advantage. These oils therefore only have to be removed immediately before the painting process, so that the body is protected from corrosion during its production, which consists of a large number of individual steps is protected.
Another advantage is that the gelation process can take place in stages. Thus, by pre-gelation taking place at a lower temperature, the pasty plastisol can be solidified to such an extent that it can be removed during the washing processes that are absolutely necessary before painting. a. the corrosion protection and drawing oils are not washed out.
Disadvantages are e.g. B. in the already mentioned limited temperature resistance, limited strength, the release of corrosive hydrochloric acid when overheating z. B. when spot welding through the PVC plastisol or in its immediate vicinity, the risk of slow exudation of the plasticizers during the life of the vehicle, which leads to a certain embrittlement of the plastisol and also to an interior pollution of the vehicle interior with plasticizers classified as hazardous to health can as well as in the general "PVC problem" in vehicle recycling . Nonetheless, PVC-free alternatives, as long as they show no further advantages, could not replace PVC plastisols due to their significantly higher material costs. The development of adhesives that cure chemically through the application of heat, which began in the eighties of the last century, has opened up new areas of application for bonding in car body construction. In some cases, these adhesives have replaced the mostly cheaper plastisols in some applications due to their improved property profile. These systems and their applications are described in more detail below in the description of the chemically curing adhesives.
Chemically curing adhesives
In contrast to the physically setting adhesives described above, in which the polymer chains in the adhesive supplied by the adhesive manufacturer are already present in their final composition and size, this is not the case with chemically curing adhesives. The polymer that makes up the cured adhesives is only formed during curing through a chemical reaction from smaller components, the so-called monomers or prepolymers .
Suitable measures must be taken to ensure that the hardening only takes place in the glue joint and not prematurely in the delivery container. In the case of two-component adhesives, referred to for short as 2K adhesives, this is done by the basic components (often referred to as resin and hardener) being in two separate containers and only mixed together in the correct ratio shortly before application to the component to be bonded . As the reaction progresses, the viscosity of the mixture increases steadily and after the “pot life”, which is also known as the “open time” is exceeded, the surfaces to be connected are no longer adequately wetted. This means that when using 2K adhesives, the dosing and mixing of the adhesive components, the application to the component and the joining must take place within the pot life. After joining the components to be glued together, the curing time follows, in which the final strength of the glue builds up. Both the pot life and the curing time are strongly influenced by external influences, especially the temperature. An increase in temperature leads to accelerated hardening and often also to higher strength, while lower temperatures extend the pot life, but also the hardening time or even stop the hardening reaction. In this context, it should be pointed out that the instructions given by the respective adhesive manufacturer in the data sheets regarding the mixing ratio and the processing and curing conditions must be strictly observed. Deviations can lead to incorrect gluing. High demands are also made on the quality of the mixture. The 2K adhesives are often processed from special double cartridges with static mixers. The double cartridge ensures that the mixing ratio is maintained and the static mixer ensures homogeneous mixing. For series applications with a correspondingly high adhesive requirement, special dosing and mixing systems that enable processing from large containers are used.
In contrast, the one-component adhesives, or 1K adhesives for short, are already mixed ready for use and the adhesive can be used directly. Here a chemical blockage prevents premature hardening or the hardening reaction requires the addition of additional substances. Due to special conditions, e.g. B. the effect of heat, light of a certain wavelength, the access of humidity or metal ions in combination with the absence of oxygen, the blockage is lifted and the solidification reaction started. In order to prevent premature solidification in the delivery container, appropriate conditions are placed on the packaging, but also on transport and storage.
Regardless of whether it is a 2K or 1K adhesive, the curing reaction is exothermic, i.e. H. associated with the release of heat. Therefore, in the case of 2K adhesives, the adhesive quantities specified by the manufacturer in the technical data sheet should under no circumstances be exceeded. Too large quantities shorten the pot life and can lead to overheating, combined with the release of gaseous hazardous substances and even self-ignition. This also applies accordingly to 1K adhesives. In view of the fact that hardened adhesives are generally not hazardous substances, i.e. they do not have to be disposed of as hazardous waste, in the past the hardening of larger residues, including hot-hardening 1-component adhesives, resulted in overheating and even fires with the release of hazardous substances.
Even if the differentiation according to the type of chemical reaction - polymerization , polycondensation or polyaddition - appears to be of little importance to the user at first glance, knowledge of this does provide some important information. The polymers formed when polyaddition adhesives cure are characterized by an alternating arrangement of resin and hardener components, the stoichiometric ratio of the reactive groups in the starting monomers or prepolymers determining the mixing ratio. Not only must exactly one hardener be available for each reactive group of the resin, but these must also be able to react with one another, i. H. are close enough to each other. The mixing ratio must be adhered to exactly and good, homogeneous mixing is also required. Any excess resin or hardener building blocks not built into the adhesive polymer act like a plasticizer and have a detrimental effect on the mechanical properties of the hardened adhesive.
With polycondensation adhesives , as with polyaddition adhesives, one of the hardeners is required for each reactive group of the resin components, so that the mixing ratio and mixing quality are of great importance here as well. In addition, by-products are released during hardening. Depending on their type, these can attack the surfaces of the parts to be joined or cause the adhesive layer to foam.
Unlike the polyaddition - or Polykondensationsklebstoffen at will polymerization adhesives , curing started by hardener molecules and then passes without hardener further need in the form of a chain reaction starting. Thus, the requirements placed on compliance with the mixing ratio and the mixing quality are lower. There are special 2K polymerization adhesives on the market, known as no-mix systems, which can be processed without mixing the components. The resin component is applied to one part and the hardener component to the other. The thorough mixing that takes place during joining is sufficient to start the hardening reaction and to achieve reproducible results.
Polyaddition adhesives
Important representatives from the group formed by a polyaddition reaction curing adhesives are epoxy resin adhesives (short epoxy adhesives ), the polyurethane adhesives and some silicone adhesives
Epoxy resin adhesives
The ability of substances with reactive hydrogen atoms, including z. Reacting , for example, amines , amides , carboxylic acid anhydrides and mercaptans with the epoxy group of epoxy resins by opening the tensioned 3-ring is the basis of epoxy adhesive chemistry. In most cases, bisphenol-A-based epoxy resins, so-called bisphenol-A diglycidyl ethers, are used. A small number of repeating molecular units (n = 0 to 2) is a viscous, clear liquid (liquid resin), while more molecular units (n = 2 to 30) are colorless solids (solid resin). A liquid bisphenol A resin that is widely used in epoxy resin adhesives has an average n of 0.1. The course of the reaction of a 2K epoxy resin adhesive is described on slide 23 of the information series of the Fonds der Chemischen Industrie - Kleben / Klebstoffe
For hardened epoxy adhesives always are thermosets what their high strength explains its rather low flexibility, as well as their relatively good resistance to moisture, many chemicals and environmental influences.
The properties of epoxy resin adhesives are essentially determined by the hardeners used. By replacing at least part of the bisphenol-A-based resin with other epoxy resins, properties of the cured adhesive, such as e.g. B. the resistance to high temperatures and to chemicals, the flexibility and their electrical properties can be further adapted to the respective requirements. There are various other recipe components
· Fillers for the adjustment of the flow behavior during use, to reduce the Härtungsschrumpfs, to increase the thermal or electrical conductivity,
· Adhesion promoter to improve the adhesion to certain materials and
· Reactive or non-reactive thinners to reduce viscosity
for use. A reduction in the brittle character can be achieved by using elasticizing hardeners, appropriately modified epoxy resins or by adding components that have a flexibilizing effect. A further improvement that allows the adhesive to be used even in crash-prone areas in automobile bodies is achieved through a two-stage system. Small viscoplastic particles with a size of approx. 500-2000 nm are mostly reactively incorporated into the adhesive. If a sudden load is applied, the energy density and the crack growth are reduced and energy is absorbed. In the event of a crash, there is no sudden failure of the bond, but energy absorption is supported by sheet metal deformation, which ultimately contributes to the safety of the vehicle occupants.
Epoxy resin adhesives are available as both 2K and 1K systems. Some 2K systems cure completely relatively quickly in a few minutes at room temperature, others require several hours to a few days. The hardening process is accelerated while the strength is usually increased at the same time. However, it is essential to comply with the specifications of the respective manufacturer. One-component adhesives use hardeners that do not react or react only extremely slowly with the epoxy groups of the resin at room temperature. Dicyandiamide is a widely used hardener . This is a solid which is practically insoluble in epoxy resins at room temperature. It only begins to dissolve in the epoxy resin at an elevated temperature above 100 ° C and the hardening reaction begins. Usual curing temperatures are 160-200 ° C. The curing temperature can be reduced with special additives. However, the associated reduction in shelf life at room temperature should be noted, so that refrigerated storage may be necessary.
Areas of application Epoxy resin adhesives with their consistently high strengths, relatively high glass transition temperatures and very good long-term resistance are often used for so-called structural bonds, e.g. B. in vehicle construction, in the manufacture of rotor blades for wind turbines but u. a. also used for applications in the electronics industry. Depending on the formulation, epoxy resin adhesives are suitable for both adhesive connections on very large components, such as B. when gluing the two half-shells of rotor blades for wind turbines - depending on the rotor blade length, several hundred kilograms of adhesive are required per rotor blade - as well as on the smallest components with an adhesive requirement of a few milligrams or micrograms, such as B. the chip card production, or the gluing of ferrites in the production of electric motors in the electronics industry. Both highly automated large series applications with short cycle times and manual small series with long cycle times can be implemented. In modern automotive engineering, epoxy resin adhesives have made a significant contribution to ensuring that the legal requirements for reducing CO 2 emissions can be met. The high voltage peaks of a pure spot-welded connection during spot- weld bonding are leveled out by the additional adhesive located between the welding points, which allows the use of thinner sheets. In general, the component stiffness is increased by gluing, which also enables the use of thinner metal sheets, as already mentioned above, also of crash-prone components. Likewise, connections between different types of metals or types of steel, which cannot be welded due to their structure, can be realized by gluing. Today's lightweight automotive construction would not be possible without the use of adhesives and especially epoxy resin adhesives.
Occupational safety When using epoxy resin adhesives, it must be taken into account that these systems, when not cured, are usually hazardous substances in accordance with the European CLP Regulation (EC) No. 1272/2008 and the working and working conditions described by the respective adhesive manufacturers in the safety data sheets Environmental precautions must be observed. Due to its eye and skin irritation as well as sensitizing epoxy resin effect, any skin contact must be avoided and the long-term, harmful effect on aquatic organisms requires appropriate environmentally conscious handling. Even if carcinogenic amines have now been replaced by other hardeners, most of the amines used as hardeners cause serious eye irritation on contact with the eyes and can also cause irritation and sensitization on skin contact.
Polyurethane adhesives (PUR)
Both 1K and 2K adhesives can be produced on the basis of polyurethane chemistry. The basis of the reaction leading to the hardening of the adhesives is the ability of isocyanates , i.e. substances that are characterized by the -NCO group with hydrogen-active compounds, these are essentially substances with hydroxyl (-OH) or amine groups (NH-), or to react with yourself.
The group of 1K polyurethane adhesives includes, as is the case with 1K epoxy resin adhesives, those that require heat for curing, i.e. to activate the blocked hardener already contained in the adhesive. These contain prepolymers with terminal OH groups and blocked isocyanates as reactive components . After the blocking of the isocyanate groups has been lifted by supplying heat, they react with the OH groups of the prepolymer .
There are also moisture-curing 1K systems that contain a non-volatile polyurethane prepolymer with isocyanate end groups as a reactive component. The curing takes place through a reaction of the isocyanate groups with moisture, i. H. Water from the air or the parts to be joined. In order to prevent premature hardening, these products must be protected from the ingress of moisture by appropriate packaging during storage. After the adhesive has been applied to the part to be joined, in a first step moisture from the ambient air or the parts to be joined reacts in a polycondensation reaction with the elimination of small amounts of CO 2 to form an amine . Only in the second step does the polyaddition reaction of the amine formed with further isocyanate groups lead to the hardening of the adhesive with the formation of urea groups .
The curing of these systems takes place in the temperature range from about 5 to 40 ° C, with a relative humidity of 40 to 70% is required. Since the hardening takes place from the outside in, i. H. if a skin first forms on the surface of the adhesive, the period within which the joining process must be completed is limited by the so-called skin formation time. If the joining takes place later, the wetting of the second joining part, which is necessary for the formation of good adhesion, is no longer given. It should also be noted that the hardening speed of initially a few millimeters per day decreases with increasing thickness of the skin and possibly even comes to a standstill. So-called “booster systems” are available on the market in order to nevertheless enable large-area bonding of moisture-impermeable parts to be bonded or to be able to perform bonding when the relative humidity is too low. Here a moisture- containing gel is added as a second component, so to speak, and mixed with the actual adhesive via a static mixer . The hardening then takes place uniformly over the entire cross section of the adhesive layer.
After curing, these adhesives are rubber-elastic and flexible and are used when materials with very different load-temperature expansion behavior have to be connected to one another. Examples are the gluing of the panes in the automotive industry or the connection of glass fiber reinforced plastics on metal supports, e.g. B. in the manufacture of thermal trucks.
In a third variant, the reactive polyurethane hotmelt adhesives , the crosslinking of isocyanate groups-containing, thermoplastic prepolymers that are solid at room temperature is used with atmospheric humidity to convert the adhesive, which is initially liquefied and then solidified, into a no longer meltable elastomer or to transfer thermosets. This post-crosslinking significantly improves the bond's resistance to higher temperatures and various media. These adhesives thus show the advantage of a rapid initial strength of the hot melt adhesives, but eliminate the disadvantage of the heat resistance limited by the softening range of the adhesives.
As is generally the case with 2K adhesives, curing with 2K polyurethane adhesives is started by combining the two components, the resin (mixture of different polyols or polyurethane prepolymers with terminal OH groups) and the hardener (isocyanate). In addition to the reactive components in each case, the two components
· Fillers for the adjustment of the flow behavior during the application, reducing the Härtungsschrumpfs, increasing the thermal conductivity or electrical conductivity,
· Adhesion promoter to improve the adhesion to certain materials and
· Contains catalysts to adjust the curing speed. Due to the variety of raw materials that are suitable for the formulation of 2K polyurethane adhesives, and here in particular the polyols, adhesives with different mechanical properties, from low-modulus and high-elasticity to high-modulus but nonetheless viscoplastic, are available.
application areas
Due to the mechanical properties of polyurethane adhesives, which are largely determined by the adhesive formulation, representatives of this class of adhesives are used in a wide variety of industries for different applications. The elastic moisture-curing systems are often used as sealing compounds; one advantage over silicone sealing compounds is that they can be painted over. Another very broad area of application, both for moisture-curing 1K adhesives and for low-modulus, elastic 2K systems, is so-called elastic bonding.
The elastic properties of these adhesives are used to dampen vibrations or to compensate for forced relative movements of the parts to be joined, for example due to different thermal expansion behavior or changing mechanical loads in a large number of applications. As early as 1969, the front and rear windows in the VW-Porsche 914 were no longer attached with a rubber seal, but glued into the body, thus making a significant contribution to rigidity; today this is state of the art. Only sufficiently elastic adhesives are able to compensate for the mechanical stresses that occur during driving and also for the stresses that occur due to the different thermal expansion behavior of the pane and the body, and thus prevent glass breakage. Further applications can be found in bus, boat and rail vehicle construction, in the production of refrigerated containers, in the construction industry and in the production of “white goods” such as washing machines, refrigerators and stoves. In civil engineering , moisture-hardening systems to stabilize fragile rock formations are injected into the affected rock layers through boreholes.
Moisture post-curing polyurethane hotmelts are among others
- in the graphic industry for binding books,
- in the packaging industry for the production of folding boxes,
- in the textile industry for the production of functional textiles,
- in furniture production for edge gluing, the production of 3D furniture fronts and for flooring production
- in the automotive industry for surface lamination of interior parts,
- in the construction industry for sheathing window profiles and the production of sandwich elements
used.
Two-component polyurethane adhesives are used wherever elastic, but faster curing is required compared to the 1-component moisture-curing polyurethane adhesives, or where higher strengths and, at the same time, greater elasticity than epoxy adhesives are required. Examples can be found in the automotive industry in the manufacture of plastic add-on parts such as trunk lids, fenders, bumpers and spoilers as well as in the manufacture of automotive headlights when the lens is glued onto the housing. Two-component polyurethane adhesives are also used in the electronics industry, in the manufacture of filters and in the household appliance industry.
Occupational safety
For reasons of occupational safety, methylenediphenyl isocyanate (MDI) or prepolymers based on MDI are usually used as the isocyanate for the production of polyurethane adhesives . Like other monomeric isocyanates, MDI has a very low occupational exposure limit, currently 0.005 ppm, due to its health hazard potential, which should not be underestimated . Due to its significantly lower vapor pressure compared to other monomeric isocyanates, such as isophorone diisocyanate (IPDI), tolylene diisocyanate (TDI) or hexamethylene diisocyanate (HDI) , the occupational exposure limit can neither be reached nor exceeded if the adhesive is not heated. However, if the adhesive is processed warm or in a spray process, as is the case with reactive polyurethane hot melt adhesives, an effective suction system must be provided.
Polycondensation adhesives
Important representatives from the group of adhesives that cure by a polycondensation reaction are phenolic resin adhesives, moisture-curing silicone adhesives and the very new group of adhesives based on silane-modified polymers, such as. B. the MS polymer adhesives
Phenol-formaldehyde resin adhesives
The phenolic resins are polymers that are based on the various polycondensation reactions between phenols and aldehydes , in particular formaldehyde . The phenol-formaldehyde resins are of technical importance and are referred to as resol resins (> 1) or novolak resins (<1) depending on the molar ratio of aldehyde / phenol during the precondensation . Resole resins are initially meltable and soluble polymers that can be crosslinked to form duromers by heating, depending on the time, temperature and pH value . Novolok resins are also meltable and soluble, but require, in addition to temperature, additional formaldehyde and a crosslinker for further crosslinking. Frequently this will paraformaldehyde and hexamethylenetetramine is used (the so-called. "Hexa"). Pure phenol-formaldehyde resins are characterized by a high degree of brittleness and the bonds made with them are therefore quite sensitive to peel loads. They are therefore often modified by copolymerization or interpolymerization with monomers which produce thermoplastic polymers. On the other hand, due to the high crosslinking density, they offer high temperature and good chemical resistance.
Resole and novolak resins are used as adhesives in the form of a solution, powder or film. In the adhesive joint, the quasi-interrupted condensation reaction to form the insoluble and no longer meltable, highly crosslinked polymer is continued by increasing the temperature to around 140 to 180 ° C. The water released during hardening is in gaseous form due to the high hardening temperature. In order to prevent the adhesive from foaming, phenolic resin adhesives are usually cured under pressure.
application areas
The adhesives have good temperature resistance, which is why they are particularly used for temperature-loaded metal bonds. The good reputation of phenol-formaldehyde adhesives for metal bonds goes back to their use in aircraft construction as early as the 1940s. Despite the comparably high production costs (fixing the parts to be joined until hardening, hardening under pressure in an autoclave, etc.), this group of adhesives is still used today. a. Because of their good long-term resistance, also used against corrosive influences in aircraft construction for metal bonds.
Other major areas of application are the production of sandpaper and friction linings, e.g. B. for use in automatic transmissions. Phenol-formaldehyde resins are often used as binders for fixing the abrasive particles or the friction agent on a carrier. During the production of clutches, automatic transmissions and brake linings, the friction linings are bonded to metal substrates with phenol-formaldehyde adhesives. To avoid squeaking noises when braking, the metallic supports now consist of several thin metal sheets connected to one another by means of phenolic resin adhesive. The adhesive causes an acoustic decoupling through its damping effect, so that vibrations no longer lead to squeaking.
Phenol-formaldehyde resin adhesives are also used to reinforce the impregnation of filter papers, e.g. B. used for use in oil filters and prevent tearing of the filter papers under the high temperatures and pressures occurring during operation. Phenol-formaldehyde adhesives and the like are used in electronics. a. Used for lamination in the manufacture of printed circuit boards and for lamination of the carrier material with copper foils. High demands are made on the so-called adhesion promoter adhesives. On the one hand, in addition to good adhesive properties, they must also have good electrical properties and, on the other hand, they must be accessible to micro-roughening through oxidative attack, which in turn results in a significant improvement in the adhesive strength of the deposited copper layer on the carrier.
Occupational safety From the point of view of occupational safety, in addition to the respective solvents , in addition to the non-critical solvent water and ethanol , methanol is also often used, the content of free phenol and formaldehyde is important and leads to corresponding labeling of the adhesives in accordance with Regulation (EC) No. 1272/2008 ( CLP) . For the bonded products, in particular from the furniture industry, the residual amounts of monomeric formaldehyde in the cured adhesive are also important. Strict limit values apply here in order to avoid exposure of customers to formaldehyde evaporation.
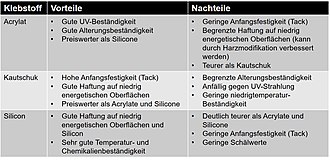
Silicones
Silicone polymers occupy a certain special position because their molecular structure, unlike the other adhesives described here, is not made up of carbon chains, but instead alternates in the main chain of silicon and oxygen atoms. These polymers have organic structures made up of carbon chains only in the side chains . The special properties of the silicone are based on this. The high chain mobility of the silicone polymers results in the high elasticity of the silicone, which is retained down to -70 to -90 ° C. Due to the higher binding energy of the silicon-oxygen bond compared to the carbon-carbon bond, silicones have a high temperature resistance. Silicones are permanently resistant up to approx. 200 ° C, and even up to 300 ° C for a short time. Another advantage is the high resistance to UV radiation . Under the influence of UV radiation, atmospheric oxygen (O 2 ) is converted into partially active oxygen, i.e. H. Ozone (O 3 ) and free radical oxygen (O), which attack the carbon chains of organic adhesives at defects, oxidize the carbon and thus destroy the chain. Such an attack is not possible with the silicon-oxygen chains of the silicones, since the silicon is already oxidized. Silicones are also quite stable to other chemically aggressive substances and show good moisture and weather resistance.
However, the mechanical load capacity that can be achieved with silicones is limited, so the tensile shear strengths that can be achieved with silicone adhesives are usually a maximum of 1 MPa . Another disadvantage is that, due to their very low surface tension, they can not be painted or coated and subsequent bonding in their surroundings is made significantly more difficult or impossible. In addition, depending on the condensation product released during hardening, they are susceptible to mold growth.
Silicone adhesives are offered as both 1K and 2K systems. Both systems are based on polyorgano-, mostly polydimethylsiloxanes and harden through polycondensation , the 1K systems through the ingress of moisture, the 2K systems through reaction with silicic acid esters .
In the ready -to- use 1K moisture-curing silicones (RTV-1), the terminal hydroxyl groups of the polydimethylsiloxanes are blocked by a so-called crosslinker. When moisture enters , this blockage is lifted by hydrolysis with splitting off of a condensation product, which enables the further crosslinking of the siloxane chains with one another. Depending on the type of crosslinker used to block the hydroxyl groups, one speaks of acidic, alkaline or neutral crosslinking systems.
The best known are the acid-crosslinking acetate systems , which split off acetic acid when it hardens . They are characterized by good adhesion to mineral substrates such as glass , enamel , porcelain and anodized aluminum and are used for bonding and sealing these materials, for example. B. used in the interior construction of houses and especially in the sanitary area. Due to their widespread use in the DIY sector, they are probably the epitome of a silicone for the layperson. When using these systems for metal bonds, the possible risk of acid corrosion and for plastic bonds that of stress corrosion cracking due to the acetic acid produced must be taken into account.
The alkaline crosslinking systems are suitable for bonding concrete, plaster, masonry and metals. The amine compounds released during curing are responsible for the characteristic, fish-like odor that occurs during curing and the risk of discoloration. Although amine or amineoxy systems still harden significantly even in cold weather and lead to extremely stable products, they have lost their importance, not least because of the unpleasant odor.
Corrosion or problems due to discoloration do not occur with the neutrally crosslinking systems. Oxime systems have long been the term for neutrally crosslinking silicones. Due to the toxicity of the cleavage products released during curing ( 2-butanone oxime (MEKO), 2-propanone oxime (DMKO) and / or 2-pentanone oxime (MPKO)), the use of oxime silicones is now to be viewed as critical. Among other things, the construction industry professional association (BG BAU) recommends using other silicone systems or other technologies and avoiding the use of oxime silicones. Ester systems are one of the more recent developments in the field of neutrally crosslinking silicones. In contrast to oxime silicones, ester silicones do not release any toxic cleavage products, but rather split off ester molecules during the curing process, which, as with other silicone systems, have a characteristic odor. They are suitable for sensitive substrates and show a wide range of adhesion on many materials. Alkoxy systems are also neutrally crosslinking silicones and have been on the market for many years. Alkoxysilicones split off lower alcohols (methanol and / or ethanol) during curing and therefore have a barely perceptible odor during curing. They are suitable for sensitive substrates and show a wide range of adhesion on many materials, including various plastics, paints and coatings.
The basis of the 2K condensation-curing silicones (RTV-2) is that produced by catalysts , e.g. B. a tin catalyst accelerated crosslinking reaction of hydroxypolysiloxanes with silicic acid esters . Here, an alcohol corresponding to the silicic acid ester is split off. As a 2K system, these adhesives cure independently of the ambient humidity and are therefore used when large-area bonds, moisture-impermeable materials or bonds with high adhesive layer thicknesses are required. As usual with 2K systems, the components must be mixed homogeneously in the correct ratio to one another. In addition, it must be ensured that no air is stirred in that would hinder the hardening process.
application areas
Silicones are used wherever their special properties, high elasticity at low temperatures, high temperature, chemical, weather and UV resistance are required and the relatively low strength is not important or can be compensated for by a correspondingly larger adhesive surface. Even if silicone applications are often seals, they are also used for bonds in various areas of application. Examples can be found in construction z. B. in the manufacture of windows, in glass facade construction or the manufacture of partition wall elements for interior work. Through the use of adhesives, completely smooth glass surfaces can be realized. In air conditioning and ventilation technology, silicone adhesives are used to be able to connect components made of materials with different thermal expansion behavior. In the manufacture of photovoltaic modules , silicones are used to glue the module frame and the junction box due to their weather and UV resistance. In the household appliance industry, temperature resistance and elasticity are the reasons. With almost all bonds in this area, e.g. B. the gluing of viewing windows in oven doors, materials with different thermal expansion coefficients such as glass, metals and plastics are connected to one another.
Occupational safety As a rule, silicone adhesives that cross-link through polycondensation are not hazardous substances within the meaning of Regulation (EC) No. 1272/2008 (CLP). The ketoxime cleavage products (2-butanone oxime (MEKO), 2-propanone oxime (DMKO) and / or 2-pentanone oxime (MPKO)) released during the crosslinking of oxime systems are now viewed as critical due to the suspicion of carcinogenic effects. The organotin catalysts often used in the 2K condensation-crosslinking silicones (RTV-2) are viewed as critical due to their reproductive and teratogenic effects and are now often replaced by alternative catalysts.
Adhesives based on silane-modified polymers (SMP adhesives)
At the end of the 1980s, a new group of moisture-curing systems came onto the market with adhesives and sealants based on silane-modified polymers. They combine the positive properties of moisture-curing silicones with those of polyurethanes. They show good adhesion to many substrates, have good weather and UV resistance, high elasticity, can be used in the temperature range from −40 to 100 ° C, can be painted over when not yet cured and are usually not hazardous substances as defined by Regulation (EC) No. 1272/2008 (CLP).
The silane-modified polymers used for the production of the SMP adhesives are mostly based on a high molecular weight polypropylene glycol (PPG) backbone, which is terminated at its ends either directly or via a urethane group with silane groups. As with the RTV1 silicones and the moisture-curing polyurethanes, curing takes place at room temperature through exposure to atmospheric moisture. The same prerequisites apply to their processing. Most of the time , methanol is released as the elimination product of the polycondensation reaction.
In order to enable them to be used even when there is insufficient moisture (low air humidity, thick-film bonding or large-area bonding of moisture-impermeable parts), these adhesives are also available as 2K systems, the second component essentially serving as a source of moisture.
A new process enables the polymer backbone to be specifically adapted. So you are no longer limited to a pure PPG structure, the installation of z. B. polyesters or polycarbonates is possible, additional functionalities can be built into the polymer backbone and the molecular weight of the polymer backbone can be adjusted over a wide range. The ability to structure these new polymers in such a way that they only split off ethanol during crosslinking without sacrificing the curing speed means that adhesives can be formulated that do not release the harmful methanol during curing . Since the silane functionality, which is decisive for crosslinking, is not exclusively terminal, as in previous systems, but specifically laterally distributed over the polymer chain, the crosslinking density achieved through curing and the polarity of the structure can be specifically adapted. Thus, with these new polymers, an improved resilience, an improved deep hardening, i. H. Improved curing of thicker layers, improved initial tack and higher final strengths, as previously only achieved with polyurethane adhesive sealants, but not with silicones.
application areas
The area of application in which SMP adhesives are used is by and large the same as that of moisture-curing polyurethanes and silicones and is actually only due to the limited temperature range compared to silicones and to a certain extent due to the somewhat lower weather, UV and chemical resistance limited. But that doesn't mean that a one-to-one exchange is possible. There are different systems with z. Some of the properties differ from one another and the entire range of requirements placed on the bond must be taken into account. This includes the processing properties and thus the requirements of the bonding process.
Another disadvantage of the SMP adhesive should not go unmentioned at this point. Use of the moisture-crosslinking SMP adhesives for gluing the panes in automobile construction is possible in principle, but is problematic because of the repair options required. The possibility of gluing on residues of the old adhesive after activation, which is given with the polyurethane systems, has not been possible with the SMP adhesives up to now. In the event of a repair, the old adhesive would have to be completely removed from the adhesive flange, whereby damage to the paint layer would not be permissible for reasons of corrosion protection.
Occupational safety As a rule, SMP adhesives are not hazardous substances in terms of Regulation (EC) No. 1272/2008 (CLP). However, due to the release of harmful methanol during hardening, appropriate ventilation should be provided during hardening. Since organic tin catalysts are often used for SMP adhesives, as is the case with condensation-curing RTV-2 silicones , the information given there also applies to SMP adhesives.
Polymerization adhesives
This group of adhesives as by a chain reaction running polymerization z include cure. For example, the under its colloquial name seconds adhesive or Super Glue (eingedeutscht Super glue) rather known cyanoacrylate adhesives. Further examples are the anaerobic adhesives that cure with exclusion of atmospheric oxygen. B. can be used to secure screws from unintentional loosening as well as the radiation-curing adhesives in which the polymerization is started by activating the hardener molecules by radiation, usually light of a certain wavelength. These systems are known from dentistry, where they are used as filling material for carious teeth.
Cyanoacrylate adhesives
This class of adhesives owes its common colloquial name “instant glue” to the ability to achieve sturdy bonds within seconds. However, it should not be forgotten that the final strength is only reached after several hours.
The cyanoacrylate adhesives are one- component reactive adhesives based on cyanoacrylic acid esters with alkyl chains of different lengths. Frequently, 2-cyanoacrylic acid methyl ester , n-butyl cyanoacrylate and 2-octyl cyanoacrylate are used as recipe components. The polymerization reaction leading to hardening is started by even small amounts of hydroxide ions . For example, the hydroxide concentration of 10 −7 mol / L present as a result of the autoprotolysis of untreated water is sufficient to start the hardening reaction (see figure) to form the poly (alkyl cyanoacrylate), which proceeds in an anionic polymerization . For this purpose, the hydroxide ion attacks the carbon atom of the CC double bond, which is positively polarized due to the electron-withdrawing effect of the cyano and ester groups. The carbanion formed in turn attacks the next cyanoacrylate monomer as a nucleophile, etc.
The hardening of the cyanoacrylates is started by the natural humidity or, more precisely, by the water molecules adsorbed from the ambient air on the surfaces of the parts to be joined . Since the subsequent polymerization of the cyanoacrylic acid ester monomers only takes place over a short range, cyanoacrylate adhesives can only be fully polymerized in layers of up to approx. 0.2 mm. In the case of thicker adhesive layers, complete crosslinking is not achieved, which is noticeable in reduced strengths.
Basic surfaces can also start or accelerate the polymerization, while acidic surfaces slow down the polymerization due to the low concentration of hydroxide ions . Strong acids lead to protonation of the carbanions and thus to the termination of the chain reaction . In a neutral or basic environment, the strongly exothermic reaction continues until all monomers are consumed. If the supply of hydroxide ions is too high, be it as a result of excessive moisture or a strongly alkaline surface, what is known as shock hardening can occur, with a consequent reduced bond strength.
The undeniable advantage of an almost instantaneous handling strength is offset by some disadvantages:
- When cured, cyanoacrylate adhesives are usually quite brittle and not very flexible.
- The achievable adhesive layer thicknesses are limited due to the low viscosity, which otherwise gives the adhesives a good capillary action, and the inability to fully cure in thicker layers.
- As a thermoplastic , they also have limited heat resistance.
- Even if they need water to cure, the cured adhesives have a certain sensitivity to high humidity.
- Depending on the formulation, cyanoacrylate adhesives can cause stress corrosion cracking on the parts to be joined
More recent developments have led to partially flexible cyanoacrylate adhesives, which are better able to reduce stress peaks and often also have better heat resistance than standard systems. The water resistance could also be improved.
Areas of application Due to the rapid hardening, cyanoacrylate adhesives are used in particular for gluing small parts with thin adhesive layers, e.g. B. used in the field of optics, microelectronics, medical and vehicle technology. So they are z. B. used for gluing glasses, however, it must be noted that strongly alkaline glasses accelerate the polymerization and the shock hardening mentioned above can occur. These adhesives are used u. a. For gluing vibration dampers in hi-fi systems, gluing the membrane in the production of loudspeakers, gluing the spirit levels in spirit levels, fixing the coil wire, fixing cover flaps and gluing elastomer seals. By butt gluing of elastomer round profiles, z. B. easy O-rings can be made.
Special cyanoacrylate adhesives with medical approval are also used in medicine as a replacement for sutures, for example for wound closure. Due to the warm and humid ambient conditions, these adhesions slowly dissolve again after the wound has healed.
Occupational safety
The rapid curing of cyanoacrylate adhesives also involves a certain safety risk when using them. If, despite the mandatory use of suitable personal protective equipment (including gloves, protective goggles, etc.) when handling chemicals , there is skin contact with cyanoacrylate adhesive or even a sticking, the leaflet published by the Industrieverband Klebstoffe eV gives first aid in the event of accidents with superglues important instructions. In any case, no attempt should be made to pull the adhesive off the skin or to forcibly loosen the bond.
Methyl methacrylate adhesives
The methyl methacrylate adhesives (MMA adhesives) are 2-component reactive adhesives in which the monomer used (typically the methyl ester of methacrylic acid , possibly mixed with other polymerizable acrylate and / or methacrylate monomers) polymerizes in a radical chain reaction becomes. To start the polymerization reaction , a reactive radical is required, which usually arises from a peroxide when it comes into contact with a suitable accelerator.
In order to achieve a correspondingly good shelf life of the adhesives, the peroxide in the methyl methacrylate monomer is marketed as one component and the accelerator dissolved in the base monomer is marketed as the second component. By adding fillers , pigments, etc., the processing properties, the color and, through toughening polymers, the mechanical properties of the cured adhesive can be adapted to the respective requirements and the mixing ratio can be varied within limits. Mixing ratios of 10: 1 or 1: 1 are common. By mixing both components, the radical chain reaction is initiated and the adhesive hardens. Of course, the product-specific pot life must also be observed here
Likewise, the entire monomer and the peroxide can be present in one component, the second then only containing the accelerator. This means that the previous mixing of the two components (and the associated pot life) can be dispensed with. In this so-called no-mix or A / B process, one component of the adhesive is applied to one part and the second to the other part. By joining the surfaces, the two components come into contact and the hardening reaction starts. Naturally, this method is limited to bonds with low adhesive layer thicknesses.
The main typical properties of MMA adhesives are:
- high strength (up to 25 N / mm²),
- high elasticity (up to 120% elongation),
- fast curing (compared to polyaddition crosslinking 2K polyurethane or 2K epoxy adhesives, MMA adhesives show a faster build-up of strength with the same pot life, i.e. the handling strength and final strength are reached more quickly).
- good adhesion spectrum and comparatively better tolerance to slightly soiled surfaces of the parts to be joined
- good weather resistance
- temperature resistant in the range of −40 - 120 ° C
- MMA adhesives show the typical odor of acrylates, which is often assessed as unpleasant.
- the exothermic , d. H. A hardening reaction that takes place when heated leads to strong heating in some 2K MMA adhesives, so that thick adhesive layers can cause the adhesive to "boil up". During this process, unreacted monomers evaporate, forming bubbles in the adhesive. This not only leads to a blistered adhesive layer with a corresponding loss of strength, but also to a health hazard during processing due to the released monomers and, in extreme cases, can lead to spontaneous combustion.
application areas
Typical applications include a. in the automotive industry e.g. B. in the manufacture of plastic attachments for passenger cars, in the manufacture of commercial vehicles, buses, agricultural machinery and railway wagons, in boat building z. B. with the gluing of the hull and deck and in the production of traffic signs with the gluing of reinforcement profiles.
Anaerobic curing adhesives
Similar to MMA adhesives, anaerobic adhesives over-harden through radical polymerization . However, these are one-component adhesives, whereby the fact that oxygen inhibits reactions occurring via radicals is used to prevent hardening in the delivery container. In addition to the polymerizable methacrylate monomers (e.g. tetraethylene glycol dimetharylate, TEGMA), these adhesives contain a complex hardener system consisting of radical formers (e.g. cumene hydroperoxide ), accelerators (e.g. N, N-dimethyl-p-toluidine ) and saccharin , which serves as a metal complexing agent and reducing agent for metal ions . The hardener system causes the oxygen contained in the adhesive to be chemically removed after the adhesive has been applied and joined. At the same time, the reaction product formed in this way releases metal ions from the parts to be joined and reduces them to a low oxidation level, which in turn catalyzes the decomposition of the radical generator into reactive radicals . The reactive radicals then initiate the curing of the adhesive, which takes place as radical polymerization.
The following boundary conditions for the use of anaerobic adhesives result from the curing process:
- To prevent premature hardening in the delivery container, the adhesive in the delivery container must be in contact with oxygen . This is achieved through the use of air-permeable plastic bottles, which are flushed with oxygen before filling and are only half filled.
- Oxygen ingress must be prevented in the adhesive gap, so that the application is limited to narrow adhesive gaps of a maximum of 0.3 mm.
- The narrower the bond gap, the faster the curing takes place.
- Metal contact (iron, copper) of the adhesive is required. If this is not the case, the surfaces to be joined can be activated using special accelerators containing iron or copper ions before bonding. The use of the accelerator is also recommended for metals with highly passive properties such as chrome and stainless steels.
When cured, these adhesives are thermosets and can therefore have high and temperature-resistant (−60 - to 200 ° C) strengths. However, the high-temperature-resistant systems are quite brittle and are therefore not suitable for flexible joining parts.
application areas
One of the main areas of application for anaerobic adhesives is screw locking, i.e. avoiding unintentional loosening of the screw, e.g. B. by vibration. These screw locks are offered in different strengths, so that by selecting an adhesive with a strength adapted to the screw diameter, releasability can be ensured. In addition, anaerobic adhesives are used to connect rotationally symmetrical parts ( shaft-hub connections ), e.g. B. used in electric motors. Due to the good resistance to oil, solvents and moisture, the sealing function of these adhesives is often used in addition to the joining function. Examples of this can be found u. a. in engine and gear assembly.
Radiation curing adhesives
These 1K adhesives also cure to solid polymers through polymerization , by the curing reaction by irradiation with UV light , whereby light from the visible range with wavelengths around 400 nm is also used in some cases or, much less often, by other radiation sources such as electron beams is initiated. The wavelength of the light emitted by the radiator used for curing must match the absorption range of the initiator used in the adhesive as closely as possible. The usual wavelengths used for curing are 365, 400 and 460 nm. Electron beam curing has not caught on, not least because of the high outlay on equipment.
In principle, a distinction is made between UV-curing adhesives between those that crosslink through radical and those that crosslink through cationic polymerization . In both cases it is a 1K adhesive that only needs short exposure times for curing. The radically crosslinking systems cure only during exposure. This must therefore take place after joining up to complete crosslinking, so at least one joining part must be permeable to the light used and sufficiently transparent. In contrast, some cationically curing systems remain sufficiently liquid for a short time after exposure and continue to cure even after the exposure has ended. These systems can therefore be activated by exposure before joining and are therefore also suitable for bonding non-UV-transparent parts to be joined. Of course, the open time, i.e. the time window within which the viscosity still allows wetting of the second part to be joined, must be observed here.
The radical crosslinking systems are based on acrylate monomers, the cationic curing systems on epoxy resins. In both cases, corresponding to photoinitiators needed, in the case of radical crosslinking systems either when exposed directly, the polymerization starting radicals form, or a hydrogen atom abstraction from an adjacent molecule, which then initiates the polymerization. The cationic photoinitiators generate a Brönsted or Lewis acid when they decompose, which starts the polymerization in the next step.
application areas
Well-known examples of UV-curing adhesives are gluing artificial fingernails or plastic tooth fillings. In industrial production, UV-curing adhesives are particularly used where rapid curing is required. You can find these adhesives in different areas such as B .:
- consumer electronics where z. B. the mini speakers for cell phones but also the phone housings are glued with UV-curing adhesives
- the furniture industry in the manufacture of glass furniture and the gluing of hinges to the glass doors of cupboards or shower cubicles
- the electronics for various potting tasks (switches, plugs, relays etc.) to protect the components from the weather, to fix the coil wire and to glue the housing
- the automotive industry z. B. in the manufacture of camera systems and various sensors
- the manufacture of LED lamps
There are also adhesives that, in addition to curing through UV light, also cure through the influence of humidity or heat. These systems are suitable for applications in which due to existing geometries and the resulting shadow zones not all areas of the bond are accessible to UV light. The light curing in the accessible area offers the advantage of rapid strength build-up and thus the realization of short cycle times. The hardening in the shadow areas then takes place at a later point in time either through humidity or heat.
Adhesive tapes (pressure sensitive adhesive systems)
Although adhesive tapes , in particular the so-called double-sided adhesive tapes, are used to connect parts to be joined, they are not actually adhesives. Pressure-sensitive adhesive systems, such as e.g. B. adhesive tapes, labels, self-adhesive stamped parts, etc. usually consist of a functional carrier that is coated on one or both sides with a pressure sensitive adhesive. The functional carrier serves u. a. the mechanical stability and strength of the adhesive mass and can also perform various tasks within a bond, such as B. mechanical damping, the compensation of different material expansions, thermal or electrical insulation or conductivity, etc., take over. Typical materials are paper, polymer foams and foils, metal foils, and semi-finished metal and plastic products. The so-called adhesive transfer tapes are another form of application and consist only of the pressure-sensitive adhesive film, so they are without a carrier. An essential component of the pressure-sensitive adhesive systems is the so-called pressure-sensitive adhesive, i.e. the substance that exhibits the adhesive effect.
Since the tapes are covered in a separate article, this section is limited to the pressure sensitive adhesives used to make tapes.
Pressure sensitive adhesives
Pressure-sensitive adhesives are adhesives that have a more or less pronounced permanent tack at room temperature and develop good adhesion to various surfaces under slight pressure . From a physical point of view, these are extremely highly viscous elastic liquids with a glass transition temperature of <−10 ° C. In order to develop adhesion to the surface to be bonded, the pressure-sensitive adhesive must wet this surface quickly and completely. The quality of the wetting depends u. a. on the viscoelastic behavior of the pressure sensitive adhesive. It must have sufficient flowability in order to be able to adapt to the surface structure of the part to be joined. This is supported by applying slight pressure. Because of this property, the internal strength ( cohesion ) of the pressure-sensitive adhesives is relatively low and the strengths that can be achieved with adhesive tapes are therefore significantly lower than those of other adhesive systems. A dynamic build-up of adhesion is typical for pressure-sensitive adhesives; Depending on the type of adhesive, temperature, contact pressure and duration, it takes a few minutes to several days until the final strength is achieved.
For a long time, natural rubber ( 1,4-cis-polyisoprene ), together with tackifying resins, was the dominant raw material for pressure-sensitive adhesives. Today, in addition to natural rubber, synthetically produced elastomers such as block copolymers made from isoprene and styrene (the so-called SIS block copolymers) and from butadiene with styrene (the so-called SBS block copolymers ) mixed with natural resins (e.g. rosin ) are modified natural resins (Rosin esters) or synthetic resins are used in the manufacture of pressure-sensitive adhesives. Resins with a low molecular weight and a glass transition temperature of −20 ° C to +70 ° C are mostly used. Another important group is that of the acrylate copolymers. Such pressure-sensitive adhesives are preferably produced from 2-ethylhexyl acrylate and isooctyl acrylate with the addition of acrylic acid and optionally other acrylate monomers. Acrylate-based pressure sensitive adhesives only require the addition of tackifying resins if adhesion to low-energy surfaces such as e.g. B. polyethylene or polypropylene is required. Like the natural rubber-based systems, acrylate systems have to be chemically crosslinked in order to achieve a certain internal strength ( cohesion ). This crosslinking usually takes place during the production of the adhesive tape, i. H. after the pressure-sensitive adhesive layer has been applied to the carrier. In contrast, this is not necessary with the SIS and SBS-based pressure sensitive adhesives.
In addition to the aforementioned pressure-sensitive adhesives based on natural or synthetic rubber and acrylates, there are also those based on polyurethanes and silicones, but these systems are of minor importance in terms of quantity and are only used if special properties are required. Polyurethane-based pressure sensitive adhesives are used e.g. B. used in wound coverings and silicone systems for high temperature applications or when high chemical resistance is required.
The aim of the formulation of pressure-sensitive adhesives is to achieve a ratio of initial tack, peel strength and internal strength that corresponds to the respective application by selecting the appropriate raw materials and the polymer design. H. To achieve shear and creep strength ( cohesion ).
In order to achieve a higher bond strength, relatively new pressure-sensitive adhesives are available, which are converted into a solid, higher force-transmitting adhesive by subsequent chemical curing. These then represent chemically curing adhesives. It is therefore not surprising that the technologies already known from chemically curing adhesives, such as B. polyurethane, epoxy resin, cyanoacrylate can be used.
application areas
In addition to being used as an adhesive layer for the production of double-sided and single-sided adhesive tapes and labels, pressure-sensitive adhesives are also used
- as transfer adhesive (double-sided, carrier-free adhesive tape );
- as a spray adhesive
- as an adhesive putty (e.g. Blu-Tack );
- as adhesive strips on sticky notes that can be removed without leaving any residue ;
- as an adhesive for dentures
Depending on the type of application, one can differentiate between the following product types:
- re-adhesive products:
- low adhesive strength,
- the adhesive system is not damaged when it is removed,
- z. B. sticky notes, adhesive seals (e.g. for paper tissue packaging) etc.
- detachable products:
- medium to high adhesive strength,
- can be removed without leaving any residue,
- mostly single use,
- Hook and poster fastening systems,
- Adhesive plasters and transdermal therapeutic systems (e.g. nicotine plasters),
- Sticker,
- Labels,
- self-adhesive foils,
- Packing tapes etc.
- permanent adhesive products:
- Bond strength is for semi-structural applications
- Application examples:
- Car exterior mirrors,
- Car protective and decorative strips,
- Damping stiffening elements in vehicle construction,
- Mirror bonding,
- Applications in window and facade construction,
- Mobile phones
- self-adhesive products that are deliberately visibly changed or destroyed when removed, such as B. Nameplates, TÜV stickers, etc.
Adhesive selection - testing adhesives
At the beginning of an adhesive selection, this applies to industrial use as well as to the handyman and do-it-yourselfer, a requirement profile should be drawn up on the basis of which potentially suitable adhesive technologies should then be identified. In the further course, manufacturers or retailers of such adhesives should be contacted in order to receive specific product recommendations and select adhesives for initial tests. In the adjacent illustration, without claiming to be exhaustive, the various criteria to be considered when choosing an adhesive are compiled. At the end of the process, there is evidence that the bonded joint produced under the process conditions specified by the user over the entire intended service life, the load capacity is always greater than the real possible loads. For more information, see.
All adhesives are characterized by different physico-chemical properties. In order to enable comparability between different adhesives, standardized test methods and test bodies were developed and described in standards. The list of standards of the Industrieverband Klebstoffe eV provides a compilation of a large number of standards and guidelines relevant to adhesive technology and is intended to help users of adhesives etc. a. assist in the selection of methods for testing adhesives and bonded joints.
Abuse as a drug
Volatile components of certain solvent-based adhesives can also be inhaled as snuff . The organic solvents have a psychoactive effect. There is a risk of long-term physical damage or death. Adhesives are used as a cheap and easily accessible substitute for illegal drugs, especially by children and young people.
literature
- Industrieverband Klebstoffe eV: Gluing guidelines - but correct, https://leitfaden.klebstoffe.com/
- Marlene Doobe: Successfully bonding plastics - basics, adhesive technologies, best practice examples. 1st edition. Springer Vieweg Verlag, Wiesbaden 2018, ISBN 978-3-658-18444-5 .
- Hermann Onusseit: Adhesive Technology - Basic Principles. 1st edition. Beuth Verlag, Berlin 2012
- Hermann Onusseit: Adhesives from nature. Application and perspectives for technology. In: Biology in Our Time. 5/34/2004, ISSN 0045-205X , pp. 307-314.
- Manfred Rasche: Manual Bonding Technology. 1st edition. Carl Hanser Verlag Munich Vienna 2012, ISBN 978-3-446-42402-9 .
- Wilhelm Finally: Adhesives and sealants in modern technology. A practical handbook of adhesive and sealant application. Vulkan-Verlag, Essen 1998, ISBN 3-8027-2183-7 .
- Gerhard Gierenz, F. Röhmer: Workbook gluing and adhesives. Cornelsen and Schwann, Düsseldorf 1989, ISBN 3-590-12939-5 .
- Adhesives. Theories and experiments. In: Practice of Natural Sciences - Chemistry. 7/38/1989, Aulis-Verlag, Cologne, ISSN 0177-9516 .
- Eckehardt Wistuba: Glues and adhesives. In: Chemistry in Our Time . 14th year 1980, No. 4, ISSN 0009-2851 , pp. 124-133.
- Gerd habenicht: Gluing. Guide to practical application and training. Vieweg-Verlag, Wiesbaden 1995, ISBN 3-528-04969-3 .
- Gerd habenicht: Gluing. Basics, technology, applications. 3. Edition. Springer-Verlag, Heidelberg 1997, ISBN 3-540-62445-7 .
- Gerhard Fauner, Wilhelm Endlich: Applied adhesive technology. Munich / Vienna 1979, ISBN 3-446-12767-4 .
- Eduardo H. Schindel-Bidinelli: Basics of structural bonding and sealing, types of adhesive, bonding and sealing technology. Hinterwaldner, Munich 1988, ISBN 3-927235-01-6 . (Structural Bonding and Sealing, Volume 1)
- Irving Skeist (Ed.): Handbook of Adhesives. 3. Edition. Van Nostrand Reinhold, New York 1990, ISBN 0-442-28013-0 .
- Author collective: adhesives and sealants. In: Ullmanns Encyclopedia of Industrial Chemistry. 4th edition. Volume 14 1977, ISBN 3-527-20014-2 , pp. 227-268.
- Bodo Müller, Walter Rath: Formulation of adhesives and sealants. 2nd Edition. Hanover 2009, ISBN 978-3-86630-862-6 .
- Bodo Müller, Walter Rath: Formulating Adhesives and Sealants - Chemistry, Physics and Applications. Hanover 2010, ISBN 978-3-86630-858-9 .
- Thomas Stotten, Martin Majolo: Assembly adhesives in practice. DRW-Verlag, Leinfelden-Echterdingen, 1st edition 2010, ISBN 978-3-87181-785-4 .
- Industrieverband Klebstoffe e. V .: Handbook Adhesive Bonding Technology 2016 . ISBN 978-3-658-14529-3 .
Broadcast reports
- Martina Preiner : Gluing - whatever it takes , SWR2 - "Knowledge" from December 17, 2014 (manuscript)
Web links
- Training, advice, research and development in bonding technology on the website of the Fraunhofer Institute for Manufacturing Technology and Applied Materials Research - "Bonding Technology and Surfaces"
- Gluing & mechanical joining at the Institute for Joining and Welding Technology at the TU Braunschweig
Individual evidence
- ↑ DIN EN 923: 2016-03 Adhesives - Terms and definitions; German version EN 923: 2015 . Beuth Verlag, Berlin.
- ↑ industry Klebstoffe eV: History of sticking. Retrieved February 16, 2018 .
- ↑ Schmidt, P., Blessing, M., Rageot, M., Iovita, R., Pfleging, J., Nickel, KG; Righetti, L. & Tennie, C .: Birch tar extraction does not prove Neanderthal behavioral complexity . In: PNAS . August 19, 2019, doi : 10.1073 / pnas.1911137116 .
- ↑ a b Industrieverband Klebstoffe eV: Economic data. Retrieved February 16, 2018 .
- ↑ MarketsandMarkets: Adhesives & Sealants Market worth 63.50 Billion USD by 2021 . In: MarketsandMarkets (ed.): Newsletter . 22nd December 2017.
- ↑ Entry on plastisols. In: Römpp Online . Georg Thieme Verlag, accessed on March 19, 2018.
- ↑ Problematic polyvinyl chloride . umwelthaushalt.de. Retrieved June 11, 2019.
- ↑ N. Banduhn et al: Gluing / glue . In: Fonds der Chemischen Industrie / Industrieverband Klebstoffe eV (Hrsg.): Information series of the Fonds der Chemischen Industrie . tape 27 . Frankfurt 2001, p. 39 .
- ↑ Gluing / Adhesives. (PDF) In: Information series of the Fonds der Chemischen Industrie. Fund of the Chemical Industry / Industrial Association of Adhesives, accessed on July 7, 2018 .
- ↑ D. Symitz, A. Lutz: Structural bonding in vehicle construction . Modern industry publishing house, Munich 2006.
- ↑ a b H. Stepanski: Spot weld bonding in automobile construction - How it began . In: adhesion - bonding and sealing . No. 5/210 . Springer Verlag, 2010.
- ↑ a b M. Pröbster: Elastic bonding from practice for practice . Springer Fachmedien, Wiesbaden.
- ↑ B. Burchard et al .: Elastic bonding . Modern industry publishing house, Munich 2005.
- ^ R. Heinzmann, S. Koch, H.-C. Thielemann, J. Wolf: Elastic bonding in construction . Modern industry publishing house, Munich 2001.
- ↑ Hartwig Lohse: Thermoplastic system solutions in automobile construction . In: Marlene Doobe (Ed.): Gluing plastics successfully . Springer Vieweg, Wiesbaden 2018, ISBN 978-3-658-18444-5 , p. 289 ff .
- ↑ Technical rules for hazardous substances, isocyanates - risk assessment and protective measures, TRGS 430. BAUA, 2009, accessed on July 7, 2018 .
- ↑ GISCHEM: data sheet 4,4'-MDI. Retrieved July 7, 2018 .
- ↑ GISCHEM: data sheet P-MDI. Retrieved July 7, 2018 .
- ↑ M. Wirts, R. Lüschen, OD Hennemann: Emissions during the processing of polyurethane adhesives . In: adhesion - bonding and sealing . No. 6/2001 . Springer Fachmedien, Wiesbaden 2001.
- ↑ N. Banduhn include: bonding / adhesives . In: Fund of the chemical industry / industrial association of adhesives eV (Hrsg.): Information series of the fund of the chemical industry . tape 27 . Frankfurt 2001, p. 33 ff .
- ↑ Gert habenicht: Gluing . Springer Verlag, Berlin 2002, p. 103 ff .
- ^ J. Kramer: Handbook Manufacturing Technology Gluing . Ed .: O. Hennemann. Carl Hanser Verlag, Munich / Vienna 1992, p. 48 ff .
- ↑ N. Banduhn include: bonding / adhesives . In: Fopnds of the chemical industry / industrial association of adhesives eV (Hrsg.): Information series of the fund of the chemical industry . No. 27 . Frankfurt 2001, p. 35 ff .
- ↑ B. Brede et al.: The art of gluing. Fund of the chemical industry / Industrieverband Klebstoffe eV, accessed on August 7, 2018 .
- ↑ Gluing guidelines - the right way. Industry Association for Adhesives, accessed on April 23, 2020 .
- ↑ bgbau.de ( Memento of the original from October 20, 2018 in the Internet Archive ) Info: The archive link was inserted automatically and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ bgbau.de ( Memento of the original from October 20, 2018 in the Internet Archive ) Info: The archive link was inserted automatically and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ wingisonline.de
- ↑ M. Roessing, B. Brugger: Silane-modified adhesives and sealants-new polymers . In: adhesion - bonding and sealing . No. 3/2011 . Springer Fachmedien, Wiesbaden 2011.
- ↑ M. Roessing, B. Brugger: New silane-modified polymers - flexible design for adhesives and sealants . In: adhesion, bonding and sealing . No. 4/2012 . Springer Fachmedien, Wiesbaden.
- ↑ a b Delo industrial adhesives (ed.): Delo Bond it - reference work on adhesive technology . 2015.
- ↑ Leaflet on first aid in the event of accidents involving superglues. (PDF) Industrieverband Klebstoffe eV, accessed on July 8, 2018 .
- ↑ N. Banduhn: bonding / adhesives . In: Fund of the chemical industry, Industrieverband Klebstoffe eV (Hrsg.): Information series of the fund of the chemical industry . tape 27 . Frankfurt 2001, p. 30th ff .
- ↑ W. Brockmann et al .: Bonding technology - adhesives, applications and processes . Wiley-VCH, Weinheim 2005, ISBN 978-3-527-31091-3 , pp. 41 ff .
- ^ Industrial Association of Adhesives eV: Pressure sensitive adhesives. Retrieved July 8, 2018 .
- ↑ Andreas Groß, Hartwig Lohse: Quality assurance in adhesive technology . In: Marlene Doobe (Ed.): Gluing plastics successfully - basics, adhesive technologies, best-practice examples . Springer Vieweg, Wiesbaden 2018, ISBN 978-3-658-18444-5 , p. 141 ff .
- ↑ Henning Gleich, Hartwig Lohse: Lifetime Prediction - Aging Effects in the Time Lapse Test . In: Marlene Doobe (Hrsg.): Gluing plastics successfully - Basics, adhesive technologies - Best practice examples . Springer Vieweg, Wiesbaden 2018, ISBN 978-3-658-18444-5 , p. 153 ff .
- ↑ Industrieverband Klebstoffe eV: List of standards. Retrieved July 8, 2018 .
- ↑ Drug profile "Volatile Substances" of the European Monitoring Center for Drugs and Drug Addiction , accessed on February 10, 2019.