graphite
| graphite | |
|---|---|
| Very pure graphite from the former Ceylon , today Sri Lanka | |
| General and classification | |
| other names |
graphite |
| chemical formula | C. |
|
Mineral class (and possibly department) |
Elements - semi-metals (metalloids) and non-metals |
|
System no. to Strunz and to Dana |
1.CB.05a ( 8th edition : I / B.02a) 01.03.06.02 |
| Similar minerals | Molybdenite (molybdenum luster) |
| Crystallographic Data | |
| Crystal system | hexagonal |
| Crystal class ; symbol | dihexagonal-dipyramidal; 6 / m 2 / m 2 / m |
| Space group | P 6 3 / mmc (No. 194) |
| Lattice parameters | a = 2.46 Å ; c = 6.71 Å |
| Frequent crystal faces | {001} |
| Physical Properties | |
| Mohs hardness | 1 to 2 |
| Density (g / cm 3 ) | 2.1 to 2.3, for the ideal single crystal 2.26 |
| Cleavage | perfect, layer spacing 3.35 Å for the ideal single crystal |
| Break ; Tenacity | uneven, pliable |
| colour | gray to black |
| Line color | grey black |
| transparency | opaque |
| shine | Metal gloss, matt |
| magnetism | diamagnetic |
| Crystal optics | |
| Refractive index | n = 1.93 to 2.07 (red) |
| Optical character | uniaxial negative |
| Pleochroism | strong red |
| Other properties | |
| Chemical behavior | insoluble in non-oxidizing acids |
| Special features | high anisotropy (e.g. hardness, conductivity) |
The graphite , according to the new German spelling and graphite is a very common mineral from the mineral class of the "elements". It is one of the natural manifestations of the chemical element carbon in its pure form and externally crystallizes in the hexagonal crystal system (for more details see crystal structure ).
Graphite forms opaque, gray to black crystals in hexagonal, tabular, scaly or stem-like shape, which have a metallic sheen on the crystal surfaces. Bulky or grainy aggregates , on the other hand, are matt. Its Mohs hardness is between 1 and 2, its density about 2.1 to 2.3 g / cm³, and it has a gray-black line color .
Etymology and history
The name graphite is derived from the ancient Greek γράφειν (graphein) , which means to write . He is alluding to the fact that graphite easily leaves a gray deposit on paper or other rough surfaces by rubbing off the individual leaves, which is used in pencils (out of date also tear lead or water lead ). Abraham Gottlob Werner coined the name in 1789, which then established itself internationally in the mineralogical specialist world and was also adopted by the International Mineralogical Association (IMA).
According to the new German spelling , the spelling Grafit is the recommended spelling according to Duden . However, according to the recommendation of the German Spelling Council , the spellings graphite and graphite are equivalent. However, the spelling graphite is still technically correct.
The use of graphite can look back on a long tradition in Europe in prehistoric times. The first indications of its use are known from the Mesolithic in northern Italy. Pieces of raw graphite were used as a coloring agent and given to the dead in the graves. There are numerous examples of graphite clay and graphitized ceramics in Bohemia for the Neolithic . In Bavaria, in the Early Bronze Age , the Straubing culture is particularly noticeable with its heavy use of graphite.
In the late Iron Age in Central Europe ( Latène period ), graphite was often used to make vessels, especially saucepans, more fireproof. Large-scale trade took place during this time, which included the entire spread of the Latène culture. The deposits near Passau and Český Krumlov (formerly Krummau ) were particularly significant here. After the collapse of the Celtic culture in Central Europe in the course of the Roman conquest and the Germanic expansion, it took about 800 years until the early Middle Ages until graphite was used again on a larger scale in Slavic East Central Europe. In Asia (especially China, which was literate at an early stage), graphite surprisingly played no role as a writing material.
In the 16th century, the English discovered a large amount of pure graphite, which they considered to be a form of lead mineral, galena , and called it plumbago . It was not until Carl Wilhelm Scheele in 1779 that it was possible to prove that graphite is pure carbon. Despite Scheele's evidence, the term pencil is still used today. Since graphite turned out not only to be a good writing material, but also a perfect material for casting molds of cannonballs, it also had a certain military significance. So was therefore z. For example, during the Napoleonic Wars at the beginning of the 19th century, the export of pencils from Great Britain to France was banned.
classification
Already in the outdated 8th edition of the mineral classification according to Strunz , graphite belonged to the mineral class of "elements" and there to the department of "semi-metals and non-metals", where it was the only member of group I / B.02a .
In the last revised and updated Lapis mineral directory by Stefan Weiß in 2018 , which, out of consideration for private collectors and institutional collections, is still based on this classic system of Karl Hugo Strunz , the mineral was given the system and mineral number. I / B.02-10 . In the “Lapis system”, this also corresponds to the “Semi-Metals and Non-Metals” department, where graphite, together with chaoite , diamond , fullerite (mineral status so far doubtful), lonsdalite and moissanite forms an independent but unnamed group.
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and was updated by the IMA until 2009, also classifies graphite in the division of "semi-metals (metalloids) and non-metals". However, this is further subdivided according to the main elements of the grouped minerals, so that graphite can be found in the sub-section “Carbon-Silicon Family”, where it is the only member of the unnamed group 1.CB.05a .
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , also assigns graphite to the class and there in the department of the same name of "elements". Here it is together with diamond, lonsdaleit, chaoite and fullerite in the group of " carbon polymorphs " with the system no. 01.03.06 can be found in the sub-section "Elements: Semi-Metals and Non-Metals".
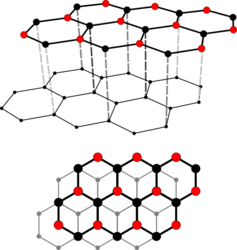
Crystal structure
Graphite occurs in two polytype crystal structures , called graphite-2H and graphite-3R .
Graphite-2H is oriented in hexagonal symmetry in the space group P 6 3 / mmc (space group no. 194) with the lattice parameters a = 2.46 Å and c = 6.71 Å as well as 4 formula units per unit cell .
With graphite 3R, on the other hand, the stratification is trigonal oriented with the lattice parameters a = 2.46 Å and c = 10.06 Å as well as 6 formula units per unit cell.
The crystalline, flat layers run parallel and lie as so-called “basal planes” or “ graphene layers”. A layer consists of covalently linked hexagons, the carbon atoms of which are sp 2 -hybridized . Within these levels the binding energy between the carbon atoms is 4.3 electron volts , while between them it is only 0.07 electron volts. This extreme directional dependence of the binding forces results in a clear anisotropy of the mechanical, electrical and thermal properties of graphite:
- easy cleavage of the pure graphite along the basal planes, significantly higher strength along the crystal layers;
- thermal and electrical insulation orthogonal to the basal planes against an almost metallic conductivity along the planes.
The conductivity within a plane is made possible by the delocalization of the π electrons.
The well-known lubricant properties of graphite are not only due to structure, because they only occur in the presence of traces of moisture.
If the levels do not show a fixed correlation to one another, one speaks of turbostratic carbon.
The transmission electron microscope (TEM) image shows stacks of basal planes in graphite. The superimposition of tilted stacks creates moiré stripes; the basal plane spacing of 3.35 Å (0.335 nm) is not resolved here.
In the so-called glassy carbon, on the other hand, the planes are not plane-parallel like the pages of a book , but like crumpled paper. This carbon is hard and isotropic like glass, hence its name. A special treatment (stretching of plastic fibers and subsequent graphitization) makes it possible to orient the planes in the direction of the fibers. The result is high-strength carbon fibers .
Fullerenes and nanotubes have only one basal plane, which in the first case are curved into a sphere, in the second case they are curved into tubes. Here, too, the transitions to graphite are fluid. Further layers can accumulate like onions and form soot-like powder.
Scanning tunnel microscope image of a graphite surface. The surface atoms (marked in red in the adjacent picture) that do not have a direct neighboring atom in the next layer below (atomic positions connected with dashed lines) are clearly visible, since the electronic density of states is higher here. A lower carbon atom changes the electronic structure of the atom above.
properties
At a temperature of over 2500 ° C, graphite becomes plastically deformable and sublimates in an oxygen-free environment at a temperature of 3750 ° C. When exposed to oxygen, graphite ignites at around 600 ° C.
Graphite is resistant to non-oxidizing acids and is diamagnetic . The strongly anisotropic behavior of graphite, especially with regard to hardness and electrical conductivity, is striking .
Magnetic susceptibility
Graphite has a negative magnetic susceptibility and is therefore diamagnetic. The amount of susceptibility and thus the extent of diamagnetism of graphite depends on the quality of the graphite and its orientation in the magnetic field.
When aligned perpendicular to the atomic layers, it is = −450 · 10 −6 for pyrolytic graphite (cf. pyrolitic graphite (s) ) up to = −595 · 10 −6 for highly oriented forms. When aligned parallel to the atomic layers, the susceptibility is = −85 · 10 −6 for pyrolytic graphite. In the case of polycrystalline graphite (e.g. soot , glassy carbon ), the susceptibility is isotropic and the values are averaged over the differently oriented single crystals . For soot z. B. a susceptibility of = −204 · 10 −6 .
Graphite is therefore the most diamagnetic element when aligned vertically . But it is still around 2000 times weaker than ideal diamond magnets - such as B. Superconductors - with a magnetic susceptibility of = −1. Depending on the shape, graphite is even more diamagnetic in polycrystalline form than the next strongest diamagnetic element bismuth .
Modifications and varieties
Along with diamond and fullerene, graphite is the third form ( modification ) of carbon that is stable under normal earth conditions . Another modification, the Lonsdaleit , only arises from extraordinary shock events such as a meteorite impact .
Education and Locations
Graphite occurs naturally in the form of isolated flakes and grains in carbon-rich metamorphic rock and as veins and aggregates in pegmatite .
There are numerous graphite sites around the world. The People's Republic of China , Korea , Madagascar , Zimbabwe , Brazil and India are of particular economic importance, both in open-cast mining and underground. Around 1,200,000 tons were mined in 2016.
There are currently only a few active graphite mines left in Europe. In the Ukraine , Norway and the Czech Republic , macrocrystalline graphite of varying quality is mined underground. With the macrocrystalline graphite, the individual graphite crystallite packages (flakes) are well preserved and visible. In Austria , on the other hand, microcrystalline graphites were obtained, the crystals of which are not as distinct.
In the 1960s, Austria took second place after South Korea among the graphite-producing countries (peak in 1964 with around 100,000 tons of production). The largest mining was in Kaisersberg near Sankt Stefan ob Leoben in Styria . It was shut down in 1997; Since spring 2008, work has been going on again in the local “Marie-Stollen”. Until 1991 there was a mining industry in Sunk bei Trieben im Paltental (Styria) in which graphite with a very high carbon content of over 85% was extracted. Further small graphite mines existed until the 1970s in Semmering , in Liesingtal (Styria), in the Dunkelsteiner Wald ( Lower Austria ) and in the Waldviertel , where the deposits in Mühldorf , which had been mined since 1831, were the most important.
In Germany, graphite mining in Kropfmühl / Passau district was and is significant. The graphite mine at the Kropfmühl location was officially reopened on June 21, 2012. According to a press release by the company, mining has become profitable again due to the increasing demand for graphite and the price development on the world market.
Synthetic manufacture
By coking graphitizable carbons produced carbonaceous materials. Starting substances are, for example, lignite , hard coal , crude oil and pitch, but also plastics. During graphitization, the amorphous carbon is converted to polycrystalline graphite by heating in the absence of air to around 3000 ° C.
Man-made graphite is also known as Acheson graphite . Important manufacturers include Showa Denko Carbon , SGL Carbon , Schunk Kohlenstofftechnik (Germany), Imerys (Switzerland), Tōkai Carbon (Japan) and Morgan Advanced Materials (Great Britain).
Highly ordered pyrolytic graphite (HOPG) is a very pure form of graphite.
use
Graphite electrodes
In 2011, 42% of all synthetic graphites were processed into electrodes. An average of 2–2.5 kg of graphite are used to manufacture one ton of electrical steel.
- Electrode in the electric arc furnace (for electric steel production)
- Electrode in carbon arc lamps
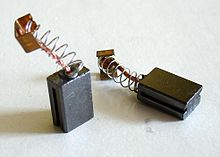
- Carbon brush in electric motors
- negative electrode of lithium-ion cells
- positive electrode of zinc-carbon primary cells
- Electrode for die sinking
- Contact strips on the pantograph of rail vehicles
Nuclear graphite
Graphite was and is used in a highly purified form as a moderator in some types of nuclear reactors . In the German, now decommissioned “ pebble bed reactors ” test reactor Jülich and THTR-300 , graphite served both as a moderator and fuel element matrix . The good moderation properties and the high temperature stability were decisive for use in nuclear technology.
However, the graphite fires of the British Windscale reactor in Sellafield in 1957 and the RBMK reactor in Chernobyl in 1986 raised concerns about the safety of graphite in reactors. Other problems are the ability to react with water vapor (> 900 ° C) with the formation of flammable gases and the tendency to energetic instabilities (see Wigner energy ).
In 2006 there were 250,000 t of irradiated nuclear graphite worldwide (Germany approx. 1000 t), for which there is still no economically acceptable disposal strategy due to its high content of C-14 (radioactive isotope with a half-life of 5700 years).
Other uses
Graphite is widely used as
- Pencil lead , also without a wooden cover, for artistic graphics
- Solid lubricant
- Material for self-lubricating bearings and seals
- Filler to improve the electrical conductivity and to reduce the coefficient of friction of plastics
- Melting pot
- Mold
- thermally highly resilient furnace lining
- Ablator (e.g. in the SpaceX Merlin 1A rocket engine)
- Monochromator in the single crystal diffractometer
- Standard substrate in scanning tunneling microscopy under ambient conditions.
- Weapon in the form of graphite threads for short-circuiting the enemy's power supply ( graphite bomb )
- corrosion-resistant material in the chemical industry
- Tub and electrodes in aluminum production ( melt flow electrolysis )
- Alloying element in cast iron - metallurgical precipitation phase ( spheroidal graphite , lamellar graphite)
- Phase change material
- Diabon is a material made of graphite
- Absorber material for high-energy particles (e.g. at the LHC)
- To improve the thermal insulation of EPS , such as Neopor .
See also
literature
- Ernst H. Weinschenk: The graphite, its most important occurrences and its technical utilization . Verl.-Anst. and Dr. A.-G., Hamburg 1898, urn : nbn: de: hbz: 061: 1-86250 .
- Eugen Ryschkewitsch: Graphite. Characteristics, production, processing and use . S. Hirzel, Leipzig 1926.
- Irene Kappel: The graphite clay ceramics from Manching . F. Steiner, Wiesbaden 1969.
- Wolfgang Delle et al .: Graphitic materials for use in nuclear reactors. 2. polycrystalline graphite and fuel element matrix . Thiemig, Munich 1983.
- Petr Korbel, Milan Novák: Mineral Encyclopedia (= Villager Nature ). Edition Dörfler im Nebel-Verlag, Eggolsheim 2002, ISBN 978-3-89555-076-8 , p. 15 .
Web links
- Mineral Atlas: Graphite (Wiki)
- Graphite. In: mindat.org. Hudson Institute of Mineralogy, accessed October 22, 2019 .
- David Barthelmy: Graphite Mineral Data. In: webmineral.com. Retrieved October 22, 2019 .
- World map with graphite mines and producers. In: mineral-exploration.de. Mineral & Exploration, 2012, accessed October 22, 2019 .
- Achim Breitruck, Harry E. Hoster, R. Jürgen Behm: Metal organic coordination networks of oligopyridines and Cu on graphite. In: uni-ulm.de. Archived from the original on June 11, 2016 ; Retrieved October 22, 2019 (2D organometallic networks on a graphite surface shown with a scanning tunneling microscope).
Individual evidence
- ↑ graphite. In: CanooNet.eu (spelling according to the new spelling allowed as a minor variant)
- ↑ a b German spelling - dictionary of words. In: grammis.ids-mannheim.de. German Spelling Council , accessed on August 9, 2020 .
- ↑ a b c d Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 51 .
- ^ A b Arnold Frederick Holleman, Nils Wiberg: Basics and main group elements . 103rd edition. tape 1 . De Gruyter, Berlin, Boston 2017, ISBN 978-3-11-026932-1 , pp. 997–998 ( limited preview in Google Book Search).
- ↑ Hans Lüschen: The names of the stones. The mineral kingdom in the mirror of language . 2nd Edition. Ott Verlag, Thun 1979, ISBN 3-7225-6265-1 , p. 232 .
- ^ AG Werner : Mineralsystem of Mr. Inspector Werner with his permission published by CAS Hoffmann . In: Alexander Wilhelm Köhler (Ed.): Bergmännisches Journal . tape 1 . Crazische Buchhandlung, Freyberg 1789, p. 369–386 ( rruff.info [PDF; 1.9 MB ; accessed on September 28, 2018] graphite p. 13).
- ↑ a b Malcolm Back, William D. Birch, Michel Blondieau and others: The New IMA List of Minerals - A Work in Progress - Updated: March 2020. (PDF 2436 kB) In: cnmnc.main.jp. IMA / CNMNC, Marco Pasero, March 2020, accessed on October 22, 2019 .
- ↑ Grafit at duden.de
- ↑ a b Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ Andre K. Geim, Philip Kim: Wonder material from the pencil . In: Spectrum of Science . tape 8 , 2008, p. 86–93 ( Spektrum.de [accessed on September 28, 2018]).
- ↑ Ernest H. Nickel, Monte C. Nichols: IMA / CNMNC List of Minerals 2009. (PDF 1703 kB) In: cnmnc.main.jp. IMA / CNMNC, January 2009, accessed October 22, 2019 .
- ↑ Bing K. Yen, Birgit E. Schwickert: Origin of low-friction behavior in graphite investigated by surface x-ray diffraction. (PDF 215 kB) In: slac.stanford.edu. Stanford Linear Accelerator Center , May 2004, accessed August 9, 2020 .
- ^ Harry Marsh, Francisco Rodríguez-Reinoso: Science of Carbon Materials . 2000. Quoted in: Christian Anton Rottmair: Influence of thermal process control on the properties of graphite molded parts , produced by powder injection molding of mesophase carbon . 2007, p. 10–11 ( (PDF) - Dissertation, University of Erlangen-Nuremberg, 2007). urn : nbn: de: bvb: 29-opus-11781
- ↑ Entry on graphite. In: Römpp Online . Georg Thieme Verlag, accessed April 10, 2011.
- ↑ Safety data sheet graphite (PDF; 22 kB) ( Memento from August 18, 2017 in the Internet Archive )
- ↑ a b MD Simon, AK Geim: Diamagnetic levitation: Flying frogs and floating magnets . In: Journal of Applied Physics . tape 87 , no. 9 , 2000, pp. 6200–6204 , doi : 10.1063 / 1.372654 (English, ucla.edu [PDF; 479 kB ; accessed on September 28, 2018]).
- ↑ a b Erich Wintermantel, Suk-Woo Ha: Medical technology: Life Science Engineering . Springer-Verlag, May 13, 2009, p. 1052 ( full text in Google Book Search).
- ↑ Find location list for graphite in the Mineralienatlas and Mindat
- ↑ Mineral Commodity Summaries 2017. US Geological Survey, January 2017, accessed August 17, 2017 .
- ↑ 1 1/2 centuries of graphite mining in Mühldorf. In: familie-wimmer.com. The Wimmer family, January 28, 2008, accessed September 28, 2018 .
- ↑ Resumption of graphite mining in Kropfmühl. Press release. Graphit Kropfmühl AG, June 21, 2012, accessed on July 18, 2012 .
- ^ Martin Bertau, Armin Müller, Peter Fröhlich, Michael Katzberg (4th); Karl Heinz Büchel, Hans-Heinrich Moretto, Dietmar Werner (3rd): Industrial Inorganic Chemistry . 4th edition. Wiley-VCH, 2013, ISBN 978-3-527-33019-5 , pp. 633 ( limited preview in Google Book search).
- ^ Author = Johannes Fachinger, Werner von Lensa, Tatjana Podruhzina: Decontamination of nuclear graphite . In: Proceedings HTR2006: 3rd International Topical Meeting on High Temperature Reactor Technology . October 2006, doi : 10.1016 / j.nucengdes.2008.02.010 .
- ↑ Katie Yurkewicz: Protecting the LHC from itself . In: Symmetry Magazine . tape 4 , no. 10 , December 2007, p. 18–23 ( symmetrymagazine.org [PDF; 843 kB ; accessed on August 21, 2018]).