Aerosol
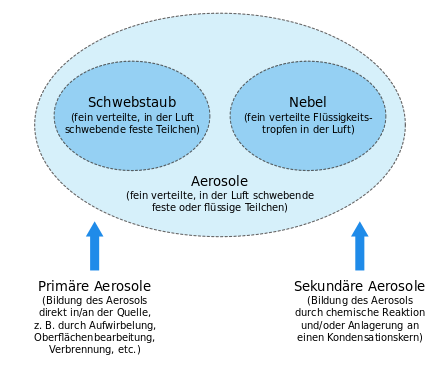
An aerosol [ aeʁozoːl ] ( Art word from ancient Greek ἀήρ AER , German , air ' and Latin solutio , solution' ) is a heterogeneous mixture ( dispersion ) of solid or liquid particles suspended in a gas .
The suspended particles are called aerosol particles or aerosol particles. The smallest particles are a few nanometers in size, there is no fixed upper limit, but particles that fail within a few seconds are rarely the subject of studies. An aerosol is a dynamic system and is subject to constant changes due to the condensation of vapors on existing particles, the evaporation of liquid components of the particles, the coagulation of small particles into large ones or the deposition of particles on surrounding objects.
Aerosol research deals, among other things, with the physical and chemical properties of aerosol particles, the formation and generation of aerosols, the effects on human health and the effects of atmospheric aerosols on visibility and the climate.
Species, origin and occurrence
Classification
Aerosols can be divided into categories in a number of ways. Criteria can be the formation of the aerosol particles, their size, material properties (solid or liquid, chemical composition) or their effect ( condensation nuclei ). It is also possible to distinguish between atmospheric aerosols, indoor aerosols and generated industrial or technical aerosols.
Aerosols can result from mechanical comminution of material or from condensation of material from precursor gases. Mechanical processes include attrition, crushing, or other grinding processes of solids and dispersion of liquids into small droplets. Nucleation and condensation, on the other hand, are processes in which solid or liquid material is formed from supersaturated gases. New particles can form in the process, or the material can condense on existing particles.
The classification according to origin differentiates u. a. between primary and secondary aerosols. Primary aerosols are introduced directly into the ambient air and mostly come from mechanical (e.g. road dust) or thermal (e.g. combustion engine) processes. Secondary aerosols are formed in the air from gaseous substances through chemical reactions and / or through the addition of the reaction products to condensation nuclei.
Examples
Aerosols can be found in many areas in the area:
- Dust in the indoor air
- Cigarette smoke
- Fog from a spray can
- Soot or oil smoke from a car exhaust
Our earth's atmosphere always contains aerosols and aerosol particles of different types and concentrations . These include
- natural bioaerosols : pollen , spores , bacteria and viruses
- natural inorganic components: desert or mineral dust caused by erosion , volcanic ash and sulfur dioxide as well as sea salt
- Parts brought in by humans: Combustion products such as smoke ( fire gas , flue gas) and ash or dust, industrially manufactured nanoparticles
distribution
The diameter of aerosol particles is in the order of magnitude between 0.1 nm and 10 μm. Individual aerosol particles are therefore invisible to the naked eye. Depending on the particle size, a number of aerosol particles are visible in air from concentrations of 10,000–100,000 particles per cubic centimeter, which is referred to as smog when it comes from anthropogenic sources .
At a certain humidity level, water in the air condenses on the particles and droplets begin to form. The higher the humidity, the larger the droplets will be. When the air humidity is high, they collide, clouds form and ultimately rain. Aerosol particles are therefore referred to as cloud condensation nuclei.
The concentration of the particles varies depending on the location and decreases with altitude. Ten kilometers above the ground there is only a ten-thousandth of the value on the ground, which is around ten micrograms of aerosol particles per kilogram of air. Volcanic eruptions can greatly increase the concentrations of aerosols right up into the stratosphere and, in addition to the weather, also affect air traffic. Desert dust also consists of comparatively large particles and forms a clearly defined aerosol layer several kilometers thick during transport. Measurements in the Sahara area showed clear influences on the atmospheric radiation transfer, but the calculated climate effects show great uncertainties.
Just as the wind , especially when it creates turbulence, mobilizes the soil ( aeolian soil erosion ), it can constantly mobilize anew aerosol particles that have sunk to the surface. Depending on the wind direction , wind strength and meteorological situation, the wind can distribute aerosols over a large area. An example of this is the distribution of radioactive aerosols after the Chernobyl nuclear disaster in 1986, which were transported in clouds by the wind and contaminated large parts of Europe as radioactive fallout.
Particle types and their origin
Aerosol particles have different compositions, which indicate their properties and origin. The smallest (sub-) particles are individual molecules , rarely larger than 1 nm, that arise from burns or as a metabolic product of plants and animals ( e.g. terpenes ). In the earth's atmosphere they react quickly with other molecules or larger particles. Aerosol particles are formed when several molecules combine to form a particle with a solid state of aggregation .
Blast furnace emissions consist largely of soot , but also of various sulfates and nitrates . The size range of these particles is between 1 and 1,000 nm. Soot particles are produced, for example, in the smelting of metals as well as in coal-fired power plants and internal combustion engines. Similar to blast furnace emissions, smoke particles, such as those produced by open fires and forest fires, are largely made up of soot.
Particles from mineral dust are mainly due to the erosion of rocks, large amounts of mineral dust particles are formed, for example, in sandstorms. Aerosol particles from sea salt are created when small droplets of salt water are blown up from the sea by the wind. The water then evaporates, leaving a sea salt particle behind.
In the standardization, bioaerosol refers to all accumulations of particles in the air space to which fungi , bacteria , viruses or pollen as well as their cell wall components and metabolic products (e.g. mycotoxins ) adhere. In a broader sense, all parts of biological origin, such as skin flakes or fiber parts, are counted among the bioaerosol particles. The larger suspended particles include pollen with aerodynamic diameters in the range from 10 µm to 100 µm, while viruses usually move in a size range from 0.02 µm to 0.4 µm. There are also numerous other types of aerosol particles. Some of them are radioactive , others come from the entry of cosmic dust .
In order to determine the origin of a particle, an analysis of its ingredients is required. During their time as an aerosol, the particles are constantly changing. If water condenses on the particles and larger droplets form from many small droplets, aerosol particles can react with one another or chemical processes in the air are catalyzed which change the composition of the particles.
Test aerosols are used for technical applications, such as the calibration of dust measuring devices or the implementation of filter tests. Depending on the application, these are generated by dispersing solids or liquids, condensation processes or chemical reactions.
properties
The property of particles to be able to be transported in gases over a longer period of time is that they behave more and more like gas molecules with decreasing diameter . Aerosols have their maximum rate of descent when there is an equilibrium of forces between weight and air resistance, provided the density of the particle is much greater than that of the fluid, which means that the buoyancy force is negligible. The following applies to the weight of a spherical particle with a radius in a gravitational field with a spatial factor :
Halving the diameter of a particle corresponds to a reduction in its mass and thus the gravitational force affecting it by a factor of eight. The frictional force of the particle with the air can first be described by Stokes' law , since it is the area of a laminar flow .
- dynamic viscosity of the fluid
- Speed of the particle
If the diameter of a particle is halved, the frictional force is only reduced by a factor of two. In the equilibrium of forces, the rate of descent would therefore decrease by a factor of four.
However, Stokes' law above only applies as long as the particles are significantly larger than the mean free path of the surrounding gas (68 nm in air) and the velocity of the fluid on the surface of the particle is zero. With decreasing particle size, however, there is a transition from the continuum to the range of a molecular flow , whereby the flow resistance of a particle falls more slowly than expected according to Stokes' law. The resulting rate of descent is therefore greater than in the above relationship and the Cunningham correction must be taken into account for its calculation .
If the frictional force is divided by the Cunningham correction factor , one obtains from for the rate of descent
For particles that are not spherical, an aerodynamic diameter is used as the equivalent diameter . This is the diameter that a spherical particle would have with the same sinking speed .
Measurement
Aerosol concentrations are determined with core counters . In the simplest case, a certain amount of air can act on a thin layer of vaseline and the evaluation is carried out microscopically. A distinction is made depending on the grain size .
- Aitken kernels: 0.01 to 0.1 µm
- large nuclei: 0.1 to 2 µm
- Giant nuclei: larger than 10 µm
Impactors or centrifuges are further measuring methods in which particles are separated out for weighing. Aerosol particles can be in an air stream by means of a radioactive source (usually krypton -85 or Americium -241) defines electrically charged and in a differential mobility analyzer ( English differential mobility analyzer , DMA) sorted according to size classes detect. When detectors are either condensation particle counter ( English condensation particle counter , CPC) in question, in which the particles enlarged by heterogeneous condensation processes and are then detected optically, or electric detectors, such as the Faraday cup electrometer (FCE).
In addition, aerosol particles can be measured using optical methods. The integrating nephelometer is used to detect all light (of a certain wavelength ) scattered by aerosol particles in a reference volume. Polar nephelometers also analyze the scattered light depending on the scattering angle. Single particle counters analyze the scattered light of individual aerosol particles in an air stream and can thus provide a size distribution.
LIDAR systems analyze the “light echo” of laser pulses sent into the atmosphere. The aerosol stratification in the atmosphere over several kilometers can be analyzed according to the intensity and the time interval to the emitted light pulse.
The aerosol optical thickness (AOD, function of the Ångström coefficient ) integrated over the entire atmosphere can be mapped by various remote sensing methods of its spatiotemporal distribution. For this purpose, assumptions have to be made regarding the reflection properties of the earth's surface (for example: reflection of deep, clear water surfaces in the near infrared is zero). Such procedures are used in remote sensing to correct the images recorded by the satellite .
meaning
Weather and climate
Hygroscopic aerosol particles, which act as condensation nuclei and thus stimulate the formation of droplets or clouds , play an important role in the weather . There are also aerosol particles that act as ice nuclei and lead to the formation of ice crystals (these can be aerosol particles from certain bacteria, such as those used in snow cannons ). Ice crystals are the initiator for precipitation formation in clouds; the principle is described by the Bergeron-Findeisen process . For this reason, silver iodide and other chemicals have long been used to cause clouds to rain down through artificial ice nuclei. Especially when there is a risk of hail , the hail pilots should defuse particularly "dangerous" cloud formations in this way. The absence of aerosols is used in cloud chambers and leads to a supersaturation of the water vapor of up to 800 percent.
The importance of aerosols for the climate or climate change is not fully understood. Anthropogenic emissions lead to local increases in the concentration of aerosols ( smog ). This can influence the earth's radiation budget directly or indirectly (through cloud formation) and is a focus of many research projects. Aerosol particles can scatter or reflect incoming solar radiation and thus reduce the radiation arriving on the earth's surface and thereby lower the mean surface temperature. This happened, for example, after the Krakatau eruption in 1883.
Some scientists suspect cosmic radiation to be an intensifying factor for the formation of cloud condensation nuclei . A research team led by the Dane Henrik Svensmark showed a strong correlation with the global cloud density, but this is doubted by other scientists. To investigate the influence of cosmic radiation on aerosol formation in the earth's atmosphere , the CLOUD experiment has been taking place at CERN since 2006, which was able to demonstrate a slight reinforcing effect on aerosol formation in higher atmospheric areas.
Effect on cloud formation
The aerosol particles play an important role in the formation of cloud droplets. Every particle has the ability to act as a condensation nucleus; the degree of this ability is determined by the composition and size of the particle. The larger a particle, the more water-soluble individual components it contains; This means that there is all the more hydrophilic mass that allows water vapor to condense on the particle. In the case of aerosol particles that do not contain any hydrophilic components, such as carbon black, it depends on how well water vapor can condense on the surface of the particle. The larger the surface of the aerosol particle, the more water can condense on it. Larger particles form cloud droplets earlier than smaller ones. But it also depends on the composition of the particles. Cloud condensation nuclei made from hydrophilic mineral salts such as ammonium sulfate or ammonium nitrate can form droplets from 70% humidity, while hydrophobic soot particles only form droplets when the humidity is oversaturated, i.e. above 100% humidity. As a rule, all aerosol particles form droplets from 103% humidity. If there were no aerosol particles, a supersaturation of up to 300% air humidity would be necessary in order to bring about the formation of droplets. As a rule, the term relative humidity is used in connection with aerosol particles. It is also investigated how the concentration of the particles affects cloud formation. As soon as the condensation nuclei of the clouds form droplets, the air humidity drops, as the water previously dissolved in the air has condensed on the particles, and the droplets eventually stop growing. So if there are only a few particles in the air, large droplets form, which are then very likely to collide, and rain quickly occurs. If, on the other hand, there are many particles, numerous small droplets form with a low probability of colliding. Large clouds form that hardly give off any rain. This effect is often observed in forest fires; Pyro clouds sometimes grow up to the stratosphere .
Effect on the climate
Thanks to concentrations of an average of 10,000 particles per cubic centimeter of air, aerosol particles have an impact on the climate. However, they have nothing to do with the greenhouse effect , for which only gases are responsible. How exactly the aerosols affect the climate has not yet been adequately researched. The mechanisms by which aerosols have an impact on the climate are diverse and sometimes in opposite directions. In addition to cloud formation, it also has an influence on the climate, whether the particles have the property of absorbing sunlight and releasing heat in the process (like soot), or whether they reflect or break the light like salt particles. It is not only a question of these properties, but also the extent to which they are effective. In the troposphere, for example, soot particles cause the temperature to rise because they absorb sunlight and thus emit thermal radiation . In the stratosphere, on the other hand, they absorb the light through their absorption , so that less UV light reaches the troposphere and the temperature there drops. This effect is exactly the opposite with mineral particles. They cool the troposphere, while they are responsible for warming them when they are in the stratosphere.
Influence on the ozone hole
The ozone hole is mainly caused by chlorofluorocarbons (CFCs). These substances, which are very stable in the troposphere, columns in the stratosphere fluorine - and chlorine radicals from which the reaction of ozone (O 3 ) to oxygen (O 2 ) catalyze . Aerosols are partly responsible for this splitting off of chlorine and fluorine radicals in the stratosphere, since the reaction can only take place on the surface of an aerosol particle.
acid rain
As acid rain is rain denotes the (due to excessive acidity sulfuric acid H 2 SO 4 and nitric acid, HNO 3 ) the pH decreases the precipitation water and the thus supported bottom acidification the Edaphon affected. The cause of the high acid content are certain aerosols, for example nitrates (R-NO 3 ), sulfates (RSO 4 ) and various nitrogen oxides . They react with other aerosols in the air, or during droplet formation, to form nitric and sulfuric acids. The main sources of such aerosols are the exhaust gases produced by humans. In addition, soot filters were installed in the chimneys of many factories in the 1970s, when less was known about the origin of acid rain. Although less visible soot was released, the invisible nitrogen oxides and other acid-forming aerosols continued to be emitted. In the 1990s, combustion gases were therefore additionally desulfurized (by FGD ) and freed of NO x components ( DeNOx process).
Significance in human medicine
Aerosols are inhaled by humans, and some of the inhaled aerosol particles are deposited in the respiratory tract . About 10% of all inhaled aerosol particles remain in the respiratory tract, the rest are exhaled again or excreted through the activity of the ciliated epithelium . Because the probability of a particle being separated depends heavily on its size, this can only be a rough guide. Particles that can penetrate at least into the bronchial area are called lung-accessible. This includes all aerosol particles below a diameter of approximately 10 micrometers (PM10). Larger particles are deposited in the nose or throat or cannot be inhaled at all. Particles with a diameter between 0.5 micrometer and 1 micrometer separate the least. This also means that they penetrate particularly deeply into the lungs. Significantly larger and smaller particles are already separated more strongly in the upper areas, thus penetrating less deeply and putting less strain on the sensitive alveoli .
Aerosol particles separated in the respiratory tract remain there for a certain time. Their residence time depends on the particle material and the deposition location. The substance of easily soluble particles is quickly distributed throughout the entire organism. Chemically very poorly soluble particles can remain in the alveolar area for up to several years. Nevertheless, the organism also fights these particles. Alveolar macrophages enclose the particles and in some cases can digest them or at least transport them to the lymph nodes . Cilia in the bronchial area mechanically transport deposited particles out of the respiratory tract very quickly. With the legal regulations for fine dust according to PM10 and PM2.5, an attempt is made to simulate the conditions in the respiratory tract in order to determine the limit values based on their harmful effects.
The effects of inhaled particles on humans range from poisoning and radioactive radiation from radon decay products to allergic reactions. Fibrous particles, especially asbestos and nanotubes , are particularly dangerous because fibers block the lungs from being cleaned by macrophages. If inhaled aerosols contain pathogens (see also bioaerosol ), a droplet infection occurs .
For inhalation therapies for medical purposes prepared aerosols are used. Inhalation devices atomize medication that the patient inhales into the body. In addition to bronchial treatment, this approach can avoid intolerance to tablets or syringes. A major problem with this application is the correct dosage of a drug.
Application and use
Are specifically manufactured and used aerosols to substances on surfaces uniformly applied , for example when painting or applying pesticides or lubricants . Aerosol cans with nasal spray , hairspray or room spray release aerosols that are intended to promote well-being, but also have risks and side effects. Fog fountains create an aerosol of air and water to humidify the air by evaporating the droplets. Cold or athlete spray cools by evaporation.
In electronic warfare , aerosols were sprayed to mask real targets, similar to chaff . Aerosol clouds could possibly be flown through better by combat aircraft than chaff clouds. The effect is rather small compared to modern radar devices. Aerosols are also used to cool engine exhaust gases in order to affect infrared homing heads of anti-aircraft missiles.
Classification in the scheme of chemical substances
| Schematic classification of the substances | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See also
literature
- J. Feichter: Aerosols and the climate system . In: Physics in our time , 2003, 34, pp. 72–79, doi: 10.1002 / piuz.200390034 .
- J. Schnelle-Kreis, M. Sklorz, H. Herrmann, R. Zimmermann: Atmospheric aerosols: sources, occurrences, composition . In: Chemistry in our time , 2007, 41, pp. 220–230, doi: 10.1002 / ciuz.200700414 .
- T. Hoffmann, C. Zetzsch, MJ Rossi: Chemistry of Aerosols . In: Chemistry in Our Time . 2007, 41, pp. 232-246, doi: 10.1002 / ciuz.200700417 .
- Robert Sturm: Biogenic suspended particles in the atmosphere. Bioaerosols - everything we breathe. In: Biology in our time 41 (4), 2011, pp. 256–261, doi: 10.1002 / biuz.201110456 .
- Ulrich Pöschl: Atmospheric aerosols: composition, transformation, climate and health effects . In: Angewandte Chemie , 2005, 117, pp. 7690-7712.
- Walter Roedel: Physics of our environment - the atmosphere . 3. Edition. Springer, Heidelberg 2000, ISBN 3-540-67180-3 , 9 Aerosols, pp. 395-439 .
Web links
- What are aerosols? Laboratory for Atmospheric Chemistry (LAC) of the Paul Scherrer Institute (PSI)
- Aerosols Education Server wiki - Climate Change (klimawiki.org)
- GAeF Homepage Society for Aerosol Research e. V.
- The greenhouse effect, Chapter 5. Aerosols (PDF; 261 kB) Webpage by H. Zickmann and B. Rakow
- Aerosols: Tiny Particles, Big Impact NASA Earth Observatory (English)
- Introduction to aerosol research ( Memento of May 13, 2012 in the Internet Archive ), Geographical Institute of the University of Bern
- Homepage of the AEROCOM project International project for the evaluation of aerosol models (English)
- Global Atmosphere Watch: GAW Aerosol Research (English)
Individual evidence
- ↑ Keyword “aerosol”. In: Lexicon of Biology. Spektrum.de, accessed on July 20, 2019 .
- ↑ Keyword "aer-, aero-". In: Lexicon of Biology. Spektrum.de, accessed on July 20, 2019 .
- ↑ a b c aerosols. In: Lexicon of Geography. Spektrum Akademischer Verlag, 2001, accessed on September 18, 2017 .
- ↑ according to Andreae 1994 (1)
- ↑ a b Saharastaub , DWD; accessed on June 16, 2016
- ↑ VDI 4250 sheet 1: 2014-08 Bioaerosols and biological agents; Environmental medical assessment of bioaerosol immissions; Effects of microbial air pollution on humans (bioaerosols and biological agents; Risk assessment of source-related ambient air measurements in the scope of environmental health; Effects of bioaerosol pollution on human health) . Beuth Verlag, Berlin, p. 5.
- ^ A b Wolfgang Mücke, Christa Lemmen: Bioaerosols and health. Effects of biological substances in the air and practical consequences . ecomed Medicine, 2008, ISBN 978-3-609-16371-0 , pp. 13-14.
- ↑ VDI 3491 sheet 1: 2016-07 Measurement of particles; Manufacturing process for test aerosols; Basics and overview (Measurement of particles; Methods for generating test aerosols; Principles and overview) . Beuth Verlag, Berlin, pp. 10–15.
- ↑ K. Scherer et al .: Interstellar-Terrestrial Relations: Variable Cosmic Environments, the dynamic heliosphere, and their Imprints on terrestrial archives and Climate . In: Space Science Reviews . tape 127 , no. 1-4 , August 25, 2007, pp. 467 , doi : 10.1007 / s11214-007-9167-5 .
- ↑ Jasper Kirkby et al .: Role of sulfuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation . In: Nature . tape 476 , August 25, 2011, p. 429-433 , doi : 10.1038 / nature10343 .
- ↑ Bob Yirka: Researchers find the cooling effect of aerosols in cumulus and MSC clouds twice as high as thought. In: Phys.org. January 18, 2019; Retrieved October 7, 2019 (American English).
- ↑ Dirt in moderation makes more rain . Max Planck Institute for Chemistry Mainz
- ^ Ashton B. Carter, David N. Schwartz: Ballistic Missile Defense . Brookings Institution Press, 1984, ISBN 0-8157-1311-8 ( limited preview in Google Book Search)