Rutile
| Rutile | |
|---|---|
| Rutile on quartz from Kapujuk, Azerbaijan (size of the step: 5 cm × 3.2 cm × 3.5 cm) | |
| General and classification | |
| chemical formula | TiO 2 |
|
Mineral class (and possibly department) |
Oxides and hydroxides |
|
System no. to Strunz and to Dana |
4.DB.05 ( 8th edition : IV / D.02) 04.04.01.01 |
| Crystallographic Data | |
| Crystal system | tetragonal |
| Crystal class ; symbol | ditetragonal-dipyramidal; 4 / m 2 / m 2 / m |
| Space group | P 4 2 / mnm (No. 136) |
| Lattice parameters | a = 4.59 Å ; c = 2.96 Å |
| Formula units | Z = 2 |
| Frequent crystal faces | {110}, {010} and many others |
| Twinning | polysynthetic, lamellar and cyclic triplets and quadruplets |
| Physical Properties | |
| Mohs hardness | 6 to 6.5 (corresponds to VHN 894 to 974 (with a test force of 100 g) |
| Density (g / cm 3 ) | measured: 4.23; calculated: 4.25 |
| Cleavage | perfect according to (110), good according to (100) |
| Break ; Tenacity | shell-like, uneven |
| colour | reddish brown to strong red, also yellow, bluish or purple |
| Line color | yellow to brown |
| transparency | transparent to opaque |
| shine | Diamond luster |
| magnetism | paramagnetic, specific magnetic susceptibility (mass susceptibility ) 7.7 · 10 −7 emu / Oe · mg |
| Crystal optics | |
| Refractive indices |
n ω = 2.605 to 2.613 n ε = 2.899 to 2.901 |
| Birefringence | δ = 0.294 |
| Optical character | uniaxial positive |
| Axis angle | 2V = strong |
| Pleochroism | visible: ε = red or yellow ω = brown or green |
| Other properties | |
| Special features | very high refraction, comparable to that of diamond |
Rutile is a frequently occurring mineral from the mineral class of " oxides and hydroxides " with the chemical composition TiO 2 and thus, chemically speaking, titanium dioxide .
Rutile crystallizes in the tetragonal crystal system and develops mostly short to long prismatic, vertically striped crystals and very often crystal twins in the form of polysynthetic, lamellar and cyclic triplets, quadruplets and sextuplets, but also granular to massive mineral aggregates . Most rutile crystals are between a few millimeters and a few centimeters in size. However, crystals up to 25 cm in length could also be found.
The color of the mostly transparent to translucent crystals varies between reddish brown and strong red, but can also be golden yellow, bluish or purple. Colorful temper colors are also possible. The surfaces of the crystals have a diamond-like sheen .
Etymology and history
Rutil was described in 1803 by Abraham Gottlob Werner , who named the mineral based on its frequently occurring reddish color after the Latin word rutilus for red or reddish.
Until 1795, when its chemical composition became known, rutile was mistaken for a tourmaline -group mineral .
classification
In the 8th edition of Strunz's mineral systematics , which has been out of date since 1982 , rutile belonged to the mineral class of "oxides and hydroxides" and there to the department of oxides with the general formula "MO 2 - and related compounds", where it was named after "rutile Series "with the system no. IV / D.02 and the other members cassiterite , plattnerite and varlamoffite .
In the last revised and updated Lapis mineral directory by Stefan Weiß in 2018 , which, out of consideration for private collectors and institutional collections, is still based on this classic system of Karl Hugo Strunz , the mineral was given the system and mineral number. IV / D.02-10 . In the "Lapis system" corresponds to the department "Oxides with the molar ratio of metal: oxygen = 1: 2 (MO 2 - and relatives)", where rutile together with argutite , cassiterite, paratellurite , plattnerite, pyrolusite and tripuhyite form the "rutile Group "forms.
The 9th edition of Strunz's mineral systematics , valid since 2001 and updated by the International Mineralogical Association (IMA) until 2009, also assigns rutile to the department of "Oxides with the molar ratio of metal: oxygen = 1: 2 and comparable". However, this is further subdivided according to the size of the cations involved and the crystal structure, so that the mineral can be classified in the sub-section “With medium-sized cations; Chains edge-linked octahedra "can be found, where the" rutile group "with the system no. 4.DB.05 and the other members argutite, cassiterite, plattnerite , pyrolusite, tripuhyite, tugarinovite and varlamoffite.
The systematics of minerals according to Dana also assigns rutile to the class of "oxides and hydroxides" and there into the category of "oxides". Here it is together with ilmenorutil, strüverite, pyrolusite, cassiterite, plattnerite, argutite, squawcreekite and stishovite in the "rutile group (tetragonal: P 4 2 / mnm )" with the system no. 04/04/01 to be found in the subsection “ Simple oxides with a cation charge of 4 + (AO 2 ) ”.
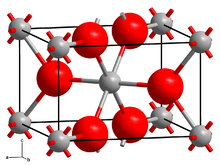
Crystal structure
Rutile crystallizes tetragonally in the space group P 4 2 / mnm (space group no. 136) with the lattice parameters a = 4.59 Å and c = 2.96 Å as well as two formula units per unit cell .
The rutile structure is a frequently occurring type of structure for AB 2 compounds and, in contrast to the fluorite structure, is not based on the closest packing of spheres. The oxide anions are arranged in the manner of distorted and corrugated "hexagonal" layers, with half of the octahedral gaps between them being occupied by the titanium cations, but due to the tetragonal symmetry these corrugated layers do not form the closest packing of spheres. The crystal structure can therefore be better described as a tetragonal rod packing made of strands of edge-sharing [TiO 6 ] octahedra (according to the Niggli notation : [TiO 4/2 O 2/1 ]), which run parallel to the crystallographic c axis. The strands are also linked via common corners to form a three-dimensional [TiO 6/3 ] network, which in abbreviated form results in the sum formula TiO 2 . The titanium cations, surrounded by oxygen atoms in an octahedral fashion, have the coordination number 6, while the oxide anions are surrounded by three titanium atoms in a slightly distorted trigonal planar arrangement (coordination number 3).
A number of other inorganic compounds also crystallize in the rutile structure, including the oxides NbO 2 , TaO 2 , MnO 2 and SnO 2 as well as the fluorides CrF 2 , MnF 2 , FeF 2 , CoF 2 , NiF 2 , CuF 2 and ZnF 2 .
properties
morphology
Rutile often forms prismatic crystals with a thick columnar to fine needle habit , on which the flat shapes {110} and {010} often predominate and whose crystal faces are parallel [001] stretched and striped. In addition to many other shapes, there are also ditetragonal prisms.
In feinnadeliger included to fibrous form, rutile is for, inter alia, for the at sapphires and rubies occurring Asterismus ( Light star responsible). In microscopic inclusions, in addition to hematite and other mineral inclusions, it can emphasize the "phantom crystals" observed especially in quartz.
Twins are generally found in rutile, which can be formed according to two laws: Twins, triplets and polysynthetic multifilaments are particularly common in lamellar or cyclic form according to (101), whereby the individuals meet at an angle of 65 ° 35 ′. Characteristic are knee -shaped or visor -shaped and V-shaped twin formations and even sextuplets that form closed rings. Twins according to (301) in the shape of a heart, whose vertical axes meet at 54 ° 44 ′, are less common. Both laws can also occur at the same time and thereby form a grid-like or reticulated aggregate, which is called sagenite .
colour
Rutile can appear in different colors, but most often it is found in reddish brown to strong red and black colors. As inclusions (inclusions) in other minerals - such as quartz - rutile also shines in a strong golden yellow color and is called Venus hair in this form and is often processed into gemstones . On the other hand, bluish or purple hues rarely occur.
Chemical and physical properties
Rutile is insoluble in acids and infusible before the soldering tube. In its pure form it is weakly paramagnetic with a specific magnetic susceptibility (mass susceptibility ) of 7.7 · 10 −7 emu / Oe · mg, but if it also contains iron , it becomes antiferromagnetic .
Modifications and varieties
Rutile is the most important and only modification of titanium dioxide that is stable at high temperatures . The other two are anatase and brookite .
Sagenite is a rutile variety called, which has flat, reticulated to lattice-like adhesions of needle-like fine rutile twins. It is also known as epitaxial (oriented) intergrowth.
Nigrin is the name of a ferrous , black rutile.
Education and Locations
Educational conditions
As a high-temperature and high-pressure mineral, rutile forms both magmatic and metamorphic and is found as an accessory component in many rocks , including as a soap mineral in river sediments . According rutile with many other minerals associated to be found, in addition to those already mentioned, other TiO 2 modifications anatase and brookite, you still Adular , albite , apatite , calcite , chlorite , ilmenite , pyrophyllite , titanite and quartz. In addition, rutile forms epitaxial adhesions with hematite .
Rutile occurs in eclogites and is the dominant Ti phase in garnet amphibolites.
Locations
As a frequent mineral formation, rutile can be found in many places around the world, with around 5900 sites being documented so far (as of 2019).
The “ Graves Mountain Mine” in Lincoln County (Georgia) , in the USA , where crystals up to 15 cm in size came to light, are worth mentioning because of its extraordinary mineral finds. From the "Cavradi Gorge" at Sedrun in the Swiss canton of Grisons and the community Ibitiara in the Brazilian state of Bahia is known particularly beautiful rutile hematite epitaxies . In addition, rutile inclusions in smoky quartz are frequently discovered in Ibitiara and in the municipality of Itabira, which belongs to the state of Minas Gerais . Large knee-shaped or visor-shaped crystal twins up to about 7 cm in size were found at Golčův Jeníkov and Soběslav in the Czech Republic. Crystals up to 3 cm in diameter and 5 cm in length were found in the Paragachay deposit on Mount Kapujuk, Nakhchivan Autonomous Republic in Azerbaijan .
In Germany, rutile was found mainly in some regions of the Black Forest (Baden-Württemberg), in the Fichtel Mountains , Spessart , Bavarian Forest and Upper Palatinate Forest (Bavaria), Hesse , Lower Saxony , in the North Rhine-Westphalian Siebengebirge , the Eifel (Rhineland-Palatinate), in the Saarland , in the Saxon Ore Mountains , Schleswig-Holstein and Thuringia .
In Austria, the mineral was found in Burgenland , on alpine fissures in many regions of Carinthia , Salzburg and Styria , in some regions of Lower Austria and Tyrol as well as in Upper Austria and Vorarlberg .
In Switzerland, rutile occurred primarily on alpine fissures in the cantons of Graubünden , Ticino and Valais .
Rutile could also be found in rock samples from the Mid-Atlantic Ridge and the Southwest Indian Ridge and outside the Earth on the moon , more precisely in the Fra Mauro highlands .
Largest producing countries
Worldwide, the mining reserves for the most important titanium minerals ilmenite and rutile are estimated at 692.58 million tons, with the largest regional concentrations in China with 28.9%, Australia with 17.0% and India with 13.3% ( As of November 2014).
use
As a raw material
With a metal content of around 60%, rutile is the most important titanium mineral after ilmenite .
Titanium dioxide in the rutile modification is used as a white pigment due to its high light refraction . In addition, it serves alone or in conjunction with cellulose as a coating for electrodes for arc welding , which improves welding or makes it possible in the first place.
Due to its semiconductor properties , rutile is used in dye solar cells , the so-called Grätzel cells . Its band gap is around 3.0 eV, so it can absorb light with a wavelength smaller than around 400 nm.
As a gem

Natural rutile is only occasionally processed into gemstones by collectors , as it usually forms crystals that are too small. Synthetic rutile, on the other hand, has been sold as an imitation diamond since 1948 under the trade name "Titania" or "Diamonite" (not to be confused with diamondite !) , Whereby it even exceeds its gloss by six times as high dispersion ( fire ).
Rutile needles enclosed in other minerals are also popular , which, in addition to the golden shine, also provide various optical effects such as asterism (star-shaped light reflections) and chatoyance (cat's eye effect ).
See also
literature
- Friedrich Klockmann : Klockmann's textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 529-531 (first edition: 1891).
- Martin Okrusch, Siegfried Matthes: Mineralogy. An introduction to special mineralogy, petrology and geology . 7th, completely revised and updated edition. Springer, Berlin [a. a.] 2005, ISBN 3-540-23812-3 , pp. 55-56 .
Web links
- Mineral Atlas: Rutile (Wiki)
Individual evidence
- ^ A b David Barthelmy: Rutile Mineral Data. In: webmineral.com. Retrieved February 14, 2019 .
- ^ A b Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 206 .
- ↑ a b rutile . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 (English, handbookofmineralogy.org [PDF; 68 kB ; accessed on February 14, 2019]).
- ↑ a b Georg Talut: Ferromagnetism in GaN and TiO 2 implanted with Fe . Technical University of Dresden, Dresden December 2009 ( qucosa.de [PDF; 11.7 MB ; accessed on February 14, 2019] Dissertation to obtain the academic degree doctor rerum naturalium (Dr. rer. nat.)).
- ↑ a b c rutile. In: mindat.org. Hudson Institute of Mineralogy, accessed February 14, 2019 .
- ↑ Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and expanded edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ Ernest H. Nickel, Monte C. Nichols: IMA / CNMNC List of Minerals 2009. (PDF 1703 kB) In: cnmnc.main.jp. IMA / CNMNC, January 2009, accessed April 25, 2019 .
- ↑ Localities for Rutile. In: mindat.org. Hudson Institute of Mineralogy, accessed October 3, 2019 .
- ↑ Petr Korbel, Milan Novák: Mineral Encyclopedia (= Dörfler Natur ). Edition Dörfler im Nebel-Verlag, Eggolsheim 2002, ISBN 978-3-89555-076-8 , p. 100 .
- ↑ Find location list for rutile in the Mineralienatlas and Mindat (accessed on October 3, 2019)
- ↑ Titan. Raw materials management profiles. (PDF 3.46 MB) Federal Institute for Geosciences and Natural Resources (BGR), November 3, 2014, accessed on February 14, 2019 .
- ^ Walter Schumann: Precious stones and gemstones. All kinds and varieties. 1900 unique pieces . 16th, revised edition. BLV Verlag, Munich 2014, ISBN 978-3-8354-1171-5 , pp. 220 .
- ↑ Jörg Plaar: Imitation of precious stones - Verneuil process. In: goldschmiede-plaar.de. Goldschmiede Plaar, July 29, 2012, accessed on May 20, 2019 .