Iridium
 | |||||||||||||||||||||||||||||||||
| Iridium | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ɪˈrɪdiəm/ | ||||||||||||||||||||||||||||||||
| Appearance | Silvery white | ||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Ir) | |||||||||||||||||||||||||||||||||
| Iridium in the periodic table | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Atomic number (Z) | 77 | ||||||||||||||||||||||||||||||||
| Group | group 9 | ||||||||||||||||||||||||||||||||
| Period | period 6 | ||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d7 6s2 | ||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 15, 2 | ||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||
| Melting point | 2719 K (2446 °C, 4435 °F) | ||||||||||||||||||||||||||||||||
| Boiling point | 4403 K (4130 °C, 7466 °F) | ||||||||||||||||||||||||||||||||
| Density (at 20° C) | 22.562 g/cm3 [3] | ||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 19 g/cm3 | ||||||||||||||||||||||||||||||||
| Heat of fusion | 41.12 kJ/mol | ||||||||||||||||||||||||||||||||
| Heat of vaporization | 564 kJ/mol | ||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.10 J/(mol·K) | ||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||
| Oxidation states | −3, –2, −1, 0, +1, +2, +3, +4, +5, +6, +7, +8, +9[4] | ||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.20 | ||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 136 pm | ||||||||||||||||||||||||||||||||
| Covalent radius | 141±6 pm | ||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) (cF4) | ||||||||||||||||||||||||||||||||
| Lattice constant | a = 383.92 pm (at 20 °C)[3] | ||||||||||||||||||||||||||||||||
| Thermal expansion | 6.47×10−6/K (at 20 °C)[3] | ||||||||||||||||||||||||||||||||
| Thermal conductivity | 147 W/(m⋅K) | ||||||||||||||||||||||||||||||||
| Electrical resistivity | 47.1 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[5] | ||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +25.6 × 10−6 cm3/mol (298 K)[6] | ||||||||||||||||||||||||||||||||
| Young's modulus | 528 GPa | ||||||||||||||||||||||||||||||||
| Shear modulus | 210 GPa | ||||||||||||||||||||||||||||||||
| Bulk modulus | 320 GPa | ||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 4825 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||
| Poisson ratio | 0.26 | ||||||||||||||||||||||||||||||||
| Mohs hardness | 6.5 | ||||||||||||||||||||||||||||||||
| Vickers hardness | 1760–2200 MPa | ||||||||||||||||||||||||||||||||
| Brinell hardness | 1670 MPa | ||||||||||||||||||||||||||||||||
| CAS Number | 7439-88-5 | ||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||
| Discovery and first isolation | Smithson Tennant (1803) | ||||||||||||||||||||||||||||||||
| Isotopes of iridium | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
Iridium (Template:PronEng) is a chemical element that has the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C. Although only certain molten salts and halogens are corrosive to solid iridium, finely divided iridium dust is much more reactive and can even be flammable. The most important iridium compounds in terms of use are the salts and acids it forms with chlorine, but iridium also forms a number of organometallic compounds that find application in catalysis and in research.
Iridium was discovered in 1803 among insoluble impurities in natural platinum found in South America. It is one of the rarest elements in the Earth's crust, and the annual production and consumption is just a few tonnes. However, iridium does find a number of specialized industrial and scientific applications. Iridium is employed when high corrosion resistance and high temperatures are needed, as in crucibles for recrystallization of semiconductors at high temperatures, electrodes for the production of chlorine in the chloralkali process, and radioisotope thermoelectric generators used in unmanned spacecraft. Iridium compounds also find applications as catalysts for the production of acetic acid.
Iridium has been implicated in the extinction of the dinosaurs and many other species 65 million years ago. The unusually high abundance of iridium in the clays of the K–T geologic boundary was a crucial clue that led to the theory that the extinction was caused by the impact of a massive extraterrestrial object with Earth—the so-called Alvarez hypothesis. Iridium is found in meteorites with an abundance much higher than its average abundance in the Earth's crust. It is thought that due to the the high density and siderophilic character of iridium, most of the iridium on Earth is found in the inner core of the planet.
Characteristics
A platinum group metal, iridium is white, resembling platinum, but with a slight yellowish cast. Due to its extreme hardness,[clarification needed] brittleness,[clarification needed] and very high melting point (the tenth highest of all elements), iridium is difficult to machine, form, or work, and powder metallurgy is commonly used.[citation needed] Iridium is the most corrosion-resistant metal known:[citation needed] it is not attacked by any acid, nor by aqua regia. It can, however, be attacked by some molten salts, such as NaCl and NaCN,[8] as well as oxygen and the halogens (particularly fluorine)[9] at higher temperatures.[10]
The measured density of iridium is only slightly lower (by about 0.1%) than that of osmium, the densest element known. There has been some ambiguity regarding which element is the densest due to the small size of the difference in density and the difficulty in measuring it accurately.[11] The best available calculations from X-ray crystallographic data give densities of 22.56 g/cm3 for iridium and 22.59 g/cm3 for osmium.[12]
Isotopes
Iridium has two naturally occurring isotopes, 191Ir and 193Ir, with natural abundances of 37.3% and 62.7%, respectively.[citation needed] A number of 34 radioisotopes have also been synthesized, ranging in atomic mass from 164 to 199. 27 of these are lighter than the stable isotopes, whereas only 6 are heavier than the stable isotopes. 192Ir, which falls between the two stable isotopes, is the most stable radioisotope, with a half-life of 73.827 d, and finds application in brachytherapy.[citation needed] Two other isotopes have half-times of at least a day—188Ir, 189Ir, 190Ir—while the rest usually have a half-time of at least 1 ms.[13] One of the least stable isotopes is 165Ir with a half-life of 1 µs. Isotopes with masses below 191 decay by some combination of β+ decay, α decay, and proton emission, with the exceptions of 189Ir, which decays by electron capture, and 190Ir, which decays by positron emission. Synthetic isotopes heavier than 191 decay by β- decay, although 192Ir also has a minor electron capture decay path.[13] All known isotopes of iridium were discovered between 1934 and 2001; the most recent is 171Ir.[14]
At least 32 metastable isomers have been characterized, ranging in atomic mass from 164 to 197. The most stable of these is 192m2Ir, which decays by isomeric transition with a half-life of 241 years,[13] making it more stable than any of iridium's synthetic isotopes in their ground states. The least stable isomer is 190m3Ir with a half-life of only 2 µs.[13]
Compounds

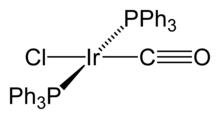
Iridium forms compounds in all oxidation states ranging from −1 to +6, but the most common oxidation states are +3 and +4.[citation needed] The highest oxidation states are only observed for the fluorides.[citation needed] Iridium hexafluoride, IrF6 is a volatile and highly reactive yellow solid, composed of octahedral molecules.[15] It decomposes in water and is reduced to IrF4 by the halogens.[9] The pentafluoride has similar properties but it is actually a tetramer, Ir
4F
20, formed by four corner-sharing octahedra. The tetrafluoride and trifluoride are also known.[15] Iridium dioxide, IrO2, a brown powder, is the only well-characterized oxide of iridium.[citation needed] A sesquioxide, Ir
2O
3, has also been described as a blue-black powder which is oxidized to IrO2 by HNO3.[9]
Hexachloroiridic(IV) acid, H
2IrCl
6, and its ammonium salt are industrially the most important iridium compounds.[citation needed] They are involved in the purification of iridium and used as precursors for most other iridium compounds, as well as in the preparation of anode coatings.[citation needed] The [IrCl6]2− ion has an intense dark brown color, and can be readily reduced to the lighter-colored [IrCl6]3− and vice versa.[16] Iridium(III) chloride IrCl3, which can be obtained directly from direct oxidation of iridium powder by chlorine at 650°C, is often used as a starting material for the synthesis of other Ir(III) compounds.[citation needed] Another compound used for this purpose is ammonium hexachloroiridate(III), (NH
4)
3IrCl
6. Iridium(III) complexes are diamagnetic (low-spin) and generally have an octahedral molecular geometry.[15][16]
Iridium forms organometallic compounds with lower oxidation states. For example, oxidation state zero is found in tetrairidium dodecacarbonyl, Ir
4(CO)
12, which is the most common and stable binary carbonyl of iridium.[15] In this compound, each of the iridium atoms is bonded to the other three, forming a tetrahedral cluster.[15] Some organometallic Ir(I) compounds are notable enough to be named after their discoverers. One is Vaska's complex, IrCl(CO)[P(C
6H
5)
3]
2, which has the unusual property of binding to dioxygen molecule, O2.[17] Another is Crabtree's catalyst, a homogeneous catalyst for hydrogenation reactions.[18] These compounds are both square planar, d8 complexes, with a total of 16 valence electrons, which accounts for their reactivity.[19]
Occurrence
Iridium is one of the rarest elements in the Earth's crust; with an average abundance of 0.001 ppm in crustal rock, it is four times less abundant than gold, ten times less abundant than platinum, and eighty times less abundant than silver and mercury.[15] Tellurium is about as abundant as iridium, and only three naturally-occurring elements are less abundant: rhenium, ruthenium, and rhodium, the last two being ten times less abundant than iridium.[15] Despite its low abundance in crustal rock, iridium is relatively common in meteorites. It is thought that the concentration of iridium in meteorites matches the concentration of iridium in the Earth as a whole;[citation needed] because of the density and siderophilic nature of iridium, it descended below the Earth's crust and toward the inner core at a time when the Earth was young and still molten.[16]
In the nickel and copper deposits the platinum group metal occure as sulphides (i.e. (Pt,Pd)S)), tellurides (i. e. PtBiTe), antimonides (PdSb) and arsenides (i.e. PtAs2) and end alloy with the raw nickle or raw copper.[20]
The largest known primary reserves are in the Bushveld complex in South Africa,[21] the large copper–nickel deposits near Norilsk in Russian and the Sudbury Basin, Canada with its large ore deposits are the two other large deposits. Smaller reserves can be found in the United States.[21] Iridium is also found in secondary deposits, combined with platinum and other platinum group metals in alluvial deposits. The alluvial deposits used by pre-Columbian people in the Chocó Department, Colombia are still a source for platinum group metals. The second large alluvial deposit was found in the Ural mountains, Russia, which is still mined. In these places iridium occurs as the natural alloys include osmiridium and iridiosmium.[citation needed]
History
The discovery of iridium is intertwined with that of platinum and the other metals in the platinum group. Native platinum used by ancient Ethiopians and by South American cultures always contained a small amount of the other platinum group metals,[citation needed] including iridium. Platinum reached Europe as platina ("small silver"), found in the 17th century by the Spanish conquerors in the River Pinto near Papayan (today the Department of Chocó, Colombia).[22] The discovery that this metal was not an alloy of known elements, but instead a distinct new element, did not occur until 1748.[23]
Chemists who studied platinum usually dissolved it in aqua regia (a mixture of hydrochloric and nitric acids) to create soluble salts. They always observed a large amount of a dark, insoluble residue.[24] Joseph Louis Proust thought that the residue was graphite or plumbago.[25] The French chemists Victor Collet-Descotils, Antoine François, comte de Fourcroy, and Louis Nicolas Vauquelin also observed the black residue in 1803, but did not obtain enough for further experiments.[26]
In 1803, British scientist Smithson Tennant analyzed the insoluble residue and, after attempting to alloy it with lead,[citation needed] concluded that it contained a new metal. Vauqelin treated the powder with alkali and obtained a volatile new oxide, which he believed to be of this new metal—which he alter named Ptene, from the Greek word πτηνος (ptènos) for winged.[25] However, Tennant continued his research and identified the two previously undiscovered elements in the black residue, iridium and osmium. He obtained dark red crystals (probably of Na
2[IrCl
6].nH
2O) by a sequence of reactions with sodium hydroxide and hydrochloric acid.[25] He named iridium after the Greek winged godess of the rainbow and the messenger of the Olympian gods Iris (Ιρις),[note 1] because many of the salts he obtained were strongly colored.[27] Discovery of the new elements was documented in a letter to the Royal Society on June 21, 1804.[28][26]
K-T boundary
The K–T boundary of 65 million years ago, marking the temporal border between the Cretaceous and Tertiary periods of geological time, was identified by a thin stratum of iridium-rich clay.[citation needed] A team led by Luis Alvarez proposed in 1980 an extraterrestrial origin for this iridium, attributing it to an asteroid or comet impact.[29] Their theory is now widely accepted to explain the demise of the dinosaurs. A large buried impact crater structure with an estimated age of about 65 million years was later identified near what is now Yucatán Peninsula (the Chicxulub crater).[30][31] Dewey M. McLean and others argue that the iridium may have been of volcanic origin instead, as the Earth's core is rich in iridium, and Piton de la Fournaise on Réunion, for example, is still releasing iridium today.[citation needed]
Production
| Year | Price ($/oz)[32][33] |
|---|---|
| 2001 | 415.25 |
| 2002 | 294.62 |
| 2003 | 93.02 |
| 2004 | 185.33 |
| 2005 | 169.51 |
| 2006 | 349.45 |
| 2007 | 440.00 |
Iridium is obtained commercially as a by-product from nickel and copper mining and processing.[citation needed] During electrorefining of copper, more noble metals such as silver, gold and the platinum group metals as well as selenium and tellurium settle to the bottom of the cell as anode mud. From this the extraction of the platinum group metals starts.[32][34]
After ruthenium and osmium have been removed, iridium is separated by precipitating (NH4)2IrCl6 or by extracting [IrCl6]2− with organic amines. The first method is similar to the procedure Tennant and Wollastone used for their separation. The second method can be planned as continuous liquid liquid extraction and is therefore more suitable for industrial scale production. In either case, the product is reduced using hydrogen, yielding the metal as a powder or sponge that can be treated using powder metallurgy techniques.[35][36]
In 1996, only 3.8 tonnes of iridium were produced in the Western world (compared to 14.2 tonnes of rhodium and 20,000 tonnes of cobalt).[15] The price of iridium as of 2007 was $440 USD/oz, but the price fluctuates significantly due to supply and demand as shown on the table. A considerable part of iridium production comes from scrap recycling.[32]
Applications
The global demand for iridium in 2007 was 119,000 troy ounces (3,700 kg), out of which 25,000 oz (780 kg) were used for electrical applications such as spark plugs; 34,000 oz (1,100 kg) for electrochemical applications such as electrodes for the chloralkali process; 24,000 oz (750 kg) for catalysis; and 36,000 oz (1,100 kg) for other uses.[37]
The high melting point, hardness and corrosion resistance of iridium and its alloys determine most of its applications. Iridium and especially iridium–platinum alloys or osmium–iridium alloys have a low wear and are used for example for multi-pored spinnerets, through which a plastic polymer melt is extruded to form fibers, such as rayon.[38] Osmium-iridium is used for compass bearings and for balances.[39]
Iridium is also used as a hardening agent in platinum alloys. The Vickers hardness of pure platinum is 56 HV while platinum with 50% of iridium can reach over 500 HV.[40][41]
Devices that must withstand extremely high temperatures are often made from iridium; for example, high-temperature crucibles for the Czochralski process.[42][43] Its resistance to arc erosion makes iridium alloys ideal for electrical contacts for spark plugs).[43][44]

An alloy of 90% platinum and 10% iridium was used in 1889 to construct the standard metre bar and kilogramme mass, kept by the International Bureau of Weights and Measures near Paris. The metre bar was replaced as the definition of the fundamental unit of length in 1960 (see krypton), but the kilogram prototype is still the international standard of mass.[45]
Iridium has been used in the radioisotope thermoelectric generators of unmanned spacecraft such as the Voyager, Viking, Pioneer, Cassini, and Galileo. Iridium was chosen to encapsulate the plutonium-238 fuel in the generator because it can withstand the operating temperatures of up to 2000°C for many years.[26]
Other use
- A radiography source for non-destructive testing.[46]
- As a low valent metal catalyst for performing C-H activation chemical transformations.
- Iridium is commonly used in complexes like Ir(mppy)3 and other complexes in polymer LED technology to increase the efficiency from 25% to almost 100% due to triplet harvesting.[47]
- Used as a source of radioactivity (Ir-192) for the treatment of cancer (in particular High Dose Rate prostate brachytherapy, bilary duct brachytherapy, and intracavitary cervix brachytherapy)
- Iridium compounds are used as catalysts in the Cativa process for carbonylation of methanol to produce acetic acid[48]
- Iridium is used in supercolliders in the production of antimatter, specifically antiprotons.[49]
Historical uses

- As an alloy with platinum, in bushing the vents of heavy ordnance
- In a finely powdered condition (iridium black), for painting porcelain black
- Iridium-osmium alloys were used to tip early-twentieth-century fountain pen nibs. The tip material in modern fountain pens is still conventionally called "iridium," although there is seldom any iridium in it; other metals such as tungsten take its place.[50]
Precautions
Iridium in bulk metallic form is not hazardous to health due to its lack of reactivity. However, finely divided iridium powder can be hazardous to handle, as it is an irritant and may ignite in air.[21] Very little is known about the toxicity of iridium compounds because they are used in very small amounts, but soluble salts, such as the iridium halides, could be hazardous due to elements other than iridium or due to iridium itself.[51] The only reported injuries related to iridium concern accidental exposure to radiation from 192Ir used in brachytherapy.[51]
High-energy gamma radiation from 192Ir can increase the risk of cancer. External exposure can cause burns, radiation poisoning, and death. Ingestion of 192Ir can burn the linings of the stomach and the intestines.[52] 192Ir, 192mIr, and 194mIr tend to deposit in the liver, and can pose health hazards from both gamma and beta radiation.[53]
Notes
- ^ iridium means "of rainbows"
References
- ^ "Standard Atomic Weights: Iridium". CIAAW. 2017.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Wang, Guanjun; Zhou, Mingfei; Goettel, James T.; Schrobilgen, Gary G.; Su, Jing; Li, Jun; Schlöder, Tobias; Riedel, Sebastian (2014). "Identification of an iridium-containing compound with a formal oxidation state of IX". Nature. 514 (7523): 475–477. Bibcode:2014Natur.514..475W. doi:10.1038/nature13795. PMID 25341786. S2CID 4463905.
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Husted, Robert (2003-12-15). "Iridium". Periodic Table of the Elements. Los Alamos National Laboratory. Retrieved 2008-09-20.
- ^ a b c Dale L. Perry, ed. (1995). Handbook of Inorganic Compounds. CRC Press. pp. 203–204. ISBN 0-8492-8671-3.
{{cite book}}: Check|isbn=value: checksum (help) - ^ J. J. Lagowski, ed. (2004). Chemistry Foundations and Applications. Vol. 2. Thomson Gale. ISBN 0-02-865732-3.
{{cite book}}: Check|isbn=value: checksum (help) - ^ Lide, D. R. (Ed.) (1990). CRC Handbook of Chemistry and Physics (70th Edn.). Boca Raton (FL):CRC Press.
- ^ J. W. Arblaster (1989). "Densities of osmium and iridium: recalculations based upon a review of the latest crystallographic data" (PDF). Platinum Metals Review. 33 (1): 14–16.
- ^ a b c d Audi, Georges (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A. 729. Atomic Mass Data Center: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ J. W. Arblaster (2003). "The discoverers of the iridium isotopes: the thirty-six known iridium isotopes found between 1934 and 2001". Platinum Metals Review. 47 (4): 167–174.
- ^ a b c d e f g h Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b c Hermann Renner, Günther Schlamp, Ingo Kleinwächter, Ernst Drost, Hans Martin Lüschow, Peter Tews, Peter Panster, Manfred Diehl, Jutta Lang, Thomas Kreuzer, Alfons Knödler, Karl Anton Starz, Klaus Dermann, Josef Rothaut, Ralf Drieselman (2002). "Platinum group metals and compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a21_075.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Vaska, Lauri (1961). "Carbonyl and Hydrido-Carbonyl Complexes of Iridium by Reaction with Alcohols. Hydrido Complexes by Reaction with Acid". Journal of the American Chemical Society. 83: 2784–2785. doi:10.1021/ja01473a054.
{{cite journal}}: Unknown parameter|author link=ignored (|author-link=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Crabtree, Robert H. (1979). "Iridium compounds in catalysis". Acc. Chem. Res. 12: 331–337. doi:10.1021/ar50141a005.
- ^ Robert H. Crabtree (2005). The Organometallic Chemistry of the Transition Metals. Wiley. ISBN 978-0-471-66256-3.
- ^ Xiao, Z. (2004). "Characterizing and recovering the platinum group minerals—a review". Minerals Engineering. 17: 961––979. doi:10.1016/j.mineng.2004.04.001.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Seymour, Richard J. (2001). "Platinum-group metals". Kirk Othmer Encyclopedia of Chemical Technology. Wiley. doi:10.1002/0471238961.1612012019052513.a01.pub2.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "78 Platinum". Elements.vanderkrogt.net. Retrieved 2008-09-21.
- ^ Juan and De Ulloa (1748). Relación histórica del viage a la América Meridional. Vol. 1. p. 606.
- ^ "76 Osmium". Elements.vanderkrogt.net. Retrieved 2008-09-21.
- ^ a b c "77 Iridium". Elements.vanderkrogt.net. Retrieved 2008-09-21.
- ^ a b c Hunt, L. B. (1987). "A History of Iridium". Platinum Metals Review. 31 (1). Retrieved 2008-09-15.
{{cite journal}}: Text "pages 32–41" ignored (help) - ^ Weeks, Mary Elvira (1968). Discovery of the Elements (7 ed.). Journal of Chemical Education. pp. 414–418.
- ^ Tennant, Smithson (1804). "On Two Metals, Found in the Black Powder Remaining after the Solution of Platina". Philosophical Transactions of the Royal Society of London. 94: 411&ndash418.
- ^ Alvarez LW, Alvarez W, Asaro F, Michel HV (1980). "Extraterrestrial cause for the Cretaceous–Tertiary extinction". Science. 208 (4448): 1095–1108. doi:10.1126/science.208.4448.1095. PMID 17783054.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hildebrand, Alan R. (1991). "Chicxulub Crater; a possible Cretaceous/Tertiary boundary impact crater on the Yucatan Peninsula, Mexico". Geology. 19 (9): 867–871. doi:10.1130/0091-7613(1991)019<0867:CCAPCT>2.3.CO;2.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Frankel, Charles (1999). The End of the Dinosaurs: Chicxulub Crater and Mass Extinctions. Cambridge University Press. ISBN 0521474477.
- ^ a b c Micheal W. George (2008). "Platinum-group metals" (pdf). U.S. Geological Survey Mineral Commodity Summaries. USGS Mineral Resources Program.
- ^ Micheal W. George (2006). "Platinum-group metals" (pdf). U.S. Geological Survey Mineral Commodity Summaries. USGS Mineral Resources Program.
- ^ George, Micheal W. "2006 Minerals Yearbook: Platinum-Group Metals" (PDF). United States Geological Survey USGS. Retrieved 2008-09-16.
- ^ Ohriner, E. K. (2008). "Processing of Iridium and Iridium Alloys". Platinum Metals Review. 52 (3): 186–197. doi:10.1595/147106708X333827.
- ^ Hunt, L. B. (1969). "Platinum Metals: A Survey of Productive Resources to industrial Uses". Platinum Metals Review. 13 (4): 126–138.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Platinum 2008 (PDF). Johnson Matthey. 2008.
- ^ Egorova,, R. V. (1979). "Spinnerets for viscose rayon cord yarn". Fibre Chemistry. 10 (4): 377–378. doi:10.1007/BF00543390.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: extra punctuation (link) - ^ Emsley, John (2005-01-18). "Iridium" (PDF). Visual Elements Periodic Table. Royal Society of Chemistry. Retrieved 2008-09-17.
- ^ Darling, A. S. (1960). "Iridium Platinum Alloys" (PDF). Platinum Metals Review Platinum. 4 (l): 18–-26.
- ^ Biggs, T. (2005). "The Hardening of Platinum Alloys for Potential Jewellery Application". Platinum Metals Review. 49 (1): 2–15. doi:10.1595/147106705X24409.
{{cite journal}}: C1 control character in|pages=at position 2 (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Crookes, William (1908). "On the Use of Iridium Crucibles in Chemical Operations". Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character. 80 (541): 535–536.
- ^ a b Handley, J. R. (1986). "Increasing Applications for Iridium". Platinum Metals Review. 30 (1): 12–13.
- ^ H. Stallforth, Federation of European Materials Societies, Peter Allen Revell (2000). EUROMAT 99.
{{cite book}}: Text "publisher Wiley-VCH" ignored (help)CS1 maint: multiple names: authors list (link) - ^ Penzes, William B. (2001). "Time Line for the Definition of the Meter". NIST. Retrieved 2008-09-16.
- ^ Halmshaw, R. (1954). "The use and scope of Iridium 192 for the radiography of steel". Br. J. Appl. Phys. 5: 238–243. doi:10.1088/0508-3443/5/7/302.
- ^ Wang, Xiangjun (2004). "Electrophosphorescence from substituted poly(thiophene) doped with iridium or platinum complex". Thin Solid Films. 468 (1–2): 226–233. doi:doi:10.1016/j.tsf.2004.05.095.
{{cite journal}}: Check|doi=value (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Cheung, Hosea (2000). "Acetic acid". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a01_045.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Möhl, D. (1997). "Production of low-energy antiprotons". Zeitschrift Hyperfine Interactions. 109: 33–41. doi:10.1023/A:1012680728257.
- ^ John Mottishaw (1999). "Notes from the Nib Works -- Where's the Iridium?". The PENnant. XIII (2).
- ^ a b Mager Stellman, Jeanne (1998). "Iridium". Encyclopaedia of Occupational Health and Safety. International Labour Organization. p. 63.19. ISBN 9789221098164.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - ^ "Radioisotope Brief: Iridium-192 (Ir-192)" (PDF). Radiation Emergencies. Center for Disease Control and Prevention. 2004-08-18. Retrieved 2008-09-20.
- ^ "Iridium" (PDF). Human Health Fact Sheet. Argonne National Laboratory. March 2005. Retrieved 2008-09-20.


