Polyamides
| General structure of polyamides |
| Repeating units of polyamides, which are made from lactams or aminocarboxylic acids. The carboxamide group is marked in blue . R stands for the remainder of the compound used for the synthesis. |
| Repeating units of polyamides, which are made from a diamine and a dicarboxylic acid. R 1 stands for the remainder of the dicarboxylic acid used, R 2 for the remainder of the diamine compound used. |
Polyamides ( abbreviation PA ) are linear polymers with regularly repeating amide bonds along the main chain. The amide group can be understood as a condensation product of a carboxylic acid and an amine . The resulting bond is an amide bond that can be split again hydrolytically .
Polyamides are often used as construction materials because of their excellent strength and toughness. There is good chemical resistance to organic solvents, but they can easily be attacked by acids and oxidizing chemicals.
The term polyamides is usually as a term for synthetic, technically usable thermoplastic plastics used and borders this class so that the chemically related proteins from. Almost all important polyamides are derived from primary amines, because the repeating unit consists of the functional group –CO – NH–. There are also polyamides of secondary amines (–CO – NR–, R = organic residue). Aminocarboxylic acids , lactams and / or diamines and dicarboxylic acids are particularly used as monomers for the polyamides .
Chemical constitution
| Polyamide chains |
| Schematic representation: The marked amide groups (three in two chains in the schematic example) give the polyamides their name. |
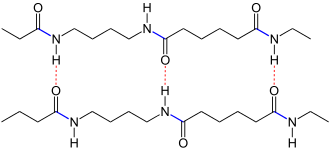
| Schematic representation: The chains of polyamide 4.6 running in opposite directions from left to right are linked to one another via hydrogen bonds (shown in red dashed lines). The amide bonds are marked in blue . |
Polyamides can be classified into the following classes:
- According to the type of monomers
- Aliphatic polyamides: the monomers are derived from aliphatic basic elements, PA from ε- caprolactam ( polycaprolactam , PA 6 for short) or from hexamethylenediamine and adipic acid (PA 6.6).
- Partly aromatic polyamides: some of the monomers are derived from aromatic base materials, PA from hexamethylenediamine and terephthalic acid (PA 6T).
- Aromatic polyamides ( polyaramides ): the monomers are derived from purely aromatic basic substances, p - phenylenediamine and terephthalic acid
- According to the type of monomer composition
- Homopolyamides: the polyamide is derived from an aminocarboxylic acid or a lactam or a diamine and a dicarboxylic acid. Such polyamides can be described by a single repeating unit. Examples are the PA from caprolactam [NH- (CH 2 ) 5 -CO] n (PA 6) or the PA from hexamethylenediamine and adipic acid [NH- (CH 2 ) 6 -NH-CO- (CH 2 ) 4 -CO ] n (PA 6.6).
- Copolyamides: the polyamide is derived from several different monomers. Such polyamides can only be described by specifying several repeat units. Examples of this are the PA from caprolactam, hexamethylenediamine and adipic acid [NH- (CH 2 ) 6 –NH – CO– (CH 2 ) 4 –CO] n - [NH– (CH 2 ) 5 –CO] m (PA 6 / 66), or PA from hexamethylenediamine, adipic acid and sebacic acid [NH- (CH 2 ) 6 -NH-CO- (CH 2 ) 4 -CO] n - [NH- (CH 2 ) 6 -NH-CO- (CH 2 ) 8 -CO] m (PA 66/610). It should be noted that the formulas given only describe the polymer composition, but not the sequence of the monomer units; these are usually distributed randomly over the polymer chains.
- According to the type of softening / solidification behavior
- Partly crystalline polyamides: form crystalline domains when the melt cools ( first order phase transition ). As a rule, the entire melt does not solidify in a crystalline manner, but amorphous domains also form (see below). The ratio between crystalline and amorphous domains is determined by the chemical nature of the polyamide and the cooling conditions. In addition, the crystallization can be promoted or hindered by nucleating or anti-nucleating additives. Polyamides that crystallize easily are PA 4.6 or PA 6.6, polyamides that are difficult to crystallize are PA mXD6 made of m- xylylenediamine and adipic acid or certain copolyamides.
- Amorphous polyamides: solidify like glass from the melt. In the solid state there is no long-range order of the repeat units. The transition between solid and liquid is described by the glass transition temperature ( 2nd order phase transition ). Examples are the PA made from hexamethylenediamine and isophthalic acid (PA 6I) and certain copolyamides. In general, amorphous polyamides contain monomer units that make a regular, crystalline arrangement of the chains impossible. Under extreme cooling conditions, otherwise partially crystalline polyamides can solidify amorphously.
According to these classifications, PA 6.6, for example, is an aliphatic, partially crystalline homopolyamide.
presentation
When representing homopolyamides, a distinction must be made between the aminocarboxylic acid type (AS) and the diamine-dicarboxylic acid type (AA-SS), A stands for an amino group and S for a carboxy group .
Homopolyamides of the AS type are produced either by polycondensation (chain-like monomer, example: ε-aminocaproic acid , above) or ring-opening polymerization (ring-shaped monomer, example: ε-caprolactam, below):
In contrast, polymers of the AA-SS type are produced by the polycondensation of a diamine and a dicarboxylic acid:
For the conversion to high molar masses, it is necessary with polymers of the AA-SS type to convert both monomers in a ratio of 1: 1. Even slight deviations from this ratio can greatly reduce the molar mass of the product. To prevent this, the monomers hexane-1,6-diamine (the AA component) and adipic acid (the SS component) are first converted into the AH salt in the PA-6.6 synthesis (nylon) . Both monomers are present in the AH salt in a ratio of 1: 1, so that weighing errors can no longer lead to a disproportion between the monomers. The AH salt is then converted into the product.
Abbreviation
For the rational designation of the polyamides there are abbreviations consisting of the letters PA and the numbers and letters that follow. Some important representatives are standardized in DIN EN ISO 1043-1. As a rule, the numbers result from the number of carbon atoms of the monomer or monomers. Letters are used to represent monomers with an aromatic base. For example, T stands for terephthalic acid and I for isophthalic acid .
| Abbreviation | Aminocarboxylic acid / lactam |
|---|---|
| PA 6 | Caprolactam ( C6 ) |
| PA 11 | Aminoundecanoic acid ( C11 ) |
The AS type
Polyamides which can be derived from aminocarboxylic acids of the H 2 N– (CH 2 ) x –COOH type or the corresponding lactams are identified as PA Z, where the number of carbon atoms in the monomer is designated ( ). PA 6 stands for the polymer made from ε-caprolactam or ω-aminocaproic acid, [NH– (CH 2 ) 5 –CO] n .
The AA-SS type
| Abbreviation | Diamine | Dicarboxylic acid |
|---|---|---|
| PA 6.4 | Hexamethylene diamine ( C6 ) | Succinic acid ( C4 ) |
| PA 5.9 | Pentamethylene diamine ( C5 ) | Azelaic acid ( C9 ) |
Polyamides which can be derived from diamines and dicarboxylic acids of the types H 2 N– (CH 2 ) x –NH 2 and HOOC– (CH 2 ) y –COOH are identified as PA Z1.Z2, where Z1 is the number of carbon atoms in the Diamine and Z2 denotes the number of carbon atoms in the dicarboxylic acid ( , ). PA 6.6 stands for the polymer made of hexamethylenediamine (contains a chain of 6 carbon atoms) and adipic acid (contains a chain of 6 carbon atoms), ie [NH– (CH 2 ) 6 –NH – CO– (CH 2 ) 4 –CO] n .
The point as a separator between the numbers is not used consistently. Sometimes it is completely left out, which can lead to misunderstandings, or it is replaced by a slash. For example, the following terms can be used synonymously: PA 6.10 = PA 6/10 = PA 610
Copolymers
Copolyamides can also be referred to analogously . The possible homopolyamide combinations are formed from the monomers present in the copolyamide and then linked together. The dividing point is often left out and instead a slash is placed between the possible combinations. An example already mentioned is PA 66/610, which is formed from three monomers [hexamethylenediamine (C6), adipic acid (C6), sebacic acid (C10)]. The homopolymer combinations PA 6.6 (hexamethylenediamine and adipic acid) and PA 6.10 (hexamethylenediamine and sebacic acid) are possible from these three monomers, from which PA 66/610 is the name for the copolyamide.
The most commonly used polyamide is usually written PA 6.6 or PA6.6. Knowing how the name depends on the molecular structure of the components, it becomes clear that it should never be spoken PA sixty-six, but always PA six six.
More examples of less common polyamides:
- PA 6.9 ( hexamethylenediamine / azelaic acid )
- PA 6.12 (hexamethylenediamine / dodecanedioic acid )
- PA 11 ( 11-aminoundecanoic acid )
- PA 12 ( laurolactam or ω-aminododecanoic acid)
- PA 4.6 ( tetramethylenediamine / adipic acid)
- PA 12.12 ( dodecanediamine / dodecanedioic acid)
- PA 6.12 ( caprolactam / laurolactam)
- PA 10.10 ( 1,10-decamethylenediamine / 1,10-decanedioic acid )
PA 6.6 versus PA 6
The two technically most frequently used polyamides are PA 6.6 and PA 6. Their manufacturing process is fundamentally different:
- Polyamide 6.6 is the original nylon and is made from hexamethylene diamine (HMD) and adipic acid. It is created by a polycondensation with elimination of water.
n H 2 N- (CH 2 ) 6 -NH 2 + n HOOC- (CH 2 ) 4 -COOH → (-NH- (CH 2 ) 6 -NH-CO- (CH 2 ) 4 -CO-) n + 2n H 2 O - Polyamide 6 ((–NH– (CH 2 ) 5 –CO–) n ) is produced by ring-opening polymerization from ε-caprolactam with water as a starter.
- In the polyamide 6.10 variant, the HMD is reacted with the sebacic acid HOOC (CH 2 ) 8 COOH. The formula is: (OOC (CH 2 ) 8 CONH (CH 2 ) 6 NH) n
PA 6.6 and PA 6 are chemically very similar, as they only differ in the mirrored arrangement of a –CH 2 –NH – CO group. They have similar physical properties.
The modulus of elasticity and degree of crystallization of PA 6.6 are higher than those of PA 6, which is due to the point symmetry of the macromolecule of PA 6.6, which increases the frequency of hydrogen bonds already in the melt as a short-range order and solidifies as a long-range order during crystallization. In the case of the non-symmetrical macromolecule of PA 6, the distances for the formation of hydrogen bonds are only suitable if the neighboring macromolecules are arranged in opposite directions, which is statistically less often the case.
Trade names
Fibers
- Dederon , brand name for PA 6 fibers from the GDR
- Nylon (unprotected), DuPont de Nemours
- Perlon , IG Farbenindustrie
- Timbrelle , brand name for PA 6.6 filament yarns from TWD Fibers
Further trade names were or are: Polycaprolactam; Caprolan ( Honeywell ); Silon; Danamid; Nivion; Enka; Hydrofil (Honeywell); Dorlon (later Bayer- Perlon); Lamigamide (Schwartz); Anjamid (almaak); Radilon (Radici Plastics); Schulamid ( A. Schulman ).
Well-known synthetic representatives of the polyamides are commercially available under the names nylon (PA 6.6), Cordura , Kevlar and Perlon (PA 6). In the GDR , the latter plastic was known as Dederon . From a chemical point of view, proteins also belong to the polyamides, even if this name is not common.
Perlon, nylon and Dederon are trademarks for chemically related synthetic fiber products. Perlon (PA 6) (also: nylon 6 ) is produced by polymerizing caprolactam . It is very similar to nylon (PA 6.6) made from adipic acid , but it absorbs dyes more easily and has a lower melting point .
nylon
Nylon (chemical name: polyhexamethylene adipamide) was developed by Wallace Hume Carothers and Julian Werner Hill at EI du Pont de Nemours and Company in Wilmington ( Delaware , United States ) on February 28, 1935 , and was patented almost two years later on February 16, 1937 . It was the first fiber to be produced entirely synthetically.
Nylon was first used for toothbrushes , not nylon stockings . Dupont sold the first five million pairs of nylon stockings on May 15, 1940 (N-Day) in selected stores in major US cities.
The name nylon was coined by DuPont for fibers made of polyamide 6.6 with the aim of establishing it as a synonym for stockings . For corporate policy reasons, it was not protected as a trademark . Later it was used as a generic name for linear aliphatic polyamides, especially in the Anglo-Saxon language area . Contrary to popular belief, the name nylon does not come from NY ( New York ) and Lon ( London ), the first places where nylon was produced. In 1940, John W. Eckelberry (DuPont) said nyl was a random syllable and on was a common ending for fibers (like Cotton ). DuPont later stated the name was originally intended to be No-Run (an allusion to no ladder ), but was changed over false allegations out of fear of legal battles. The renaming from Norun to Nylon went through several intermediate steps, including Nuron and Niron .
In addition, there is also a rumor that explains the name nylon, the inventor of the material, Wallace Carothers, triumphed over the success of the fiber with the exclamation Now You Lousy Old Nipponese (or Now You Look Old Nippon ) - in Schadenfreude, at last himself to have developed a fiber to compete with Japanese natural silk . The name nylon however, the fiber was only after Carothers' death, so this is probably a legend, probably during the Second World War was, as it just at this time for the Allies was particularly important, a silk substitute in the production of parachutes available to have.
Nyltest was a trademark of the NYLTEST easy dress for knitted fabrics made from the polyamide fiber nylon for blouses and shirts.
Perlon
Perlon is the trademark of a plastic fiber developed in 1938 by Paul Schlack for I. G.-Farbenindustrie AG in Berlin . It consisted of polyamide 6 and, as a German alternative to nylon (polyamide 6.6), was quickly declared an essential material for warfare. The name was derived from the original code name perlurane of the secret project to develop an alternative to nylon. During the Second World War, perlon was used to make parachutes, bristles to clean small arms and in aircraft tires. It was marketed under the name Perlon L from 1939 . It was not until 1943 that the civilian use of women's stockings began . Perlon is obtained from the monomer ε- caprolactam .
Dederon
Dederon (mostly DEDERON as a brand ) was the trade name for polyamide fibers in the GDR from 1959 . Dederon fibers were manufactured in the VEB Chemiefaserkombinat Wilhelm Pieck in Rudolstadt-Schwarza , in the VEB Chemiefaserwerk Herbert Warnke in Wilhelm-Pieck-Stadt Guben and in the VEB Chemiefaserwerk (until 1960 VEB Kunstseidenwerk) Friedrich Engels Premnitz . Dederon is a made-up word based on the Perlon model, made up of GDR and on. Dederon achieved particular fame through the famous smock aprons and shopping bags; Also on March 12, 1963, a block of stamps Chemistry for Peace and Socialism made of Dederon foil was issued.
Compact polyamide
Important trade names for non-fibrous polyamides are Leona (Asahi Kasei) , Alphalon ( Grupa Azoty ATT Polymers ), Akulon ( DSM ), Altech ( Albis Plastic ), Durethan ( Lanxess ), Frianyl (NILIT Plastics Europe, formerly Frisetta Polymer), Grilon ( EMS-CHEMIE ), Akromid , Akroloy , Schulamid ( A. Schulman ), MK-PAC6 ( Mertl Kunststoffe ), Technyl ( Solvay ), Torzen ( Invista ), Ultramid ( BASF ), Miramid (BASF), Vestamid ( Evonik Industries ), Polimid ( Poliblend Germany ) and Zytel ( DuPont ). In addition, numerous smaller compounders offer compact polyamide under their own trade names. All of these materials can turn with fibers, usually glass fibers , reinforced be.
Most of the non-fibrous polyamides are sold as plastic granules and are processed using injection molding .
properties
Many technically important polyamides are semi-crystalline thermoplastic polymers and are characterized by high strength , rigidity and toughness , and have good chemical resistance and processability. Many of the properties of the polyamides are largely dominated by the amide groups that interact with one another via hydrogen bonds .
Exact values for the properties of the polyamides depend, among other things, on their crystalline structure and in particular on their water content . Polyamides react to the moisture content of the environment with reversible water absorption or release. The water is stored in the amorphous areas of the polyamide. The water uptake depends very much on the concentration of the amide groups. In the ambient air, PA 6 absorbs approx. 2.5–3.5% water, while PA 12 only absorbs approx. 0.2–0.5%. Polyolefin- based additives have been developed to ensure high impact strength even when dry.
Here are some key properties:
| PA 6 | PA 6.6 | PA 6.10 | PA 6.12 | PA 11 | PA 12 | |
|---|---|---|---|---|---|---|
| designation | Polycaprolactam | Poly ( N , N ′ -hexamethylene adipinediamide) poly (hexamethylene adipamide) | Poly (hexamethylene sebacamide) | Poly (hexamethylene dodecanediamide) | Polyundecanolactam | Polylauryl lactam |
| CAS number | 25038-54-4 | 32131-17-2 | 9011-52-3 | 26098-55-5 | 25035-04-5 | 24937-16-4 |
| Melting point in ° C | 220 | 260 | 240 | 218 | 198 | 178 |
| Glass temperature 1) in ° C (dry) | 50 ... 60 | 50 ... 60 | 40 | 46 | 46 | 37 |
|
Density in g / cm³ - partially crystalline (typical value) - crystalline phase (a modification) - amorphous phase |
1.130 1.235 1.084 |
1.13 ... 1.14 |
1.04 | 1.06 | 1.03 | 1.01 |
| Moisture absorption in% (23 ° C, 50% humidity) | 2.6 ... 3.4 | 2.5 ... 3.1 | ||||
| Tensile modulus in MPa (dry / humid) | 2700… 3500/900… 1200 | 2700… 3500/1000… 1600 |
1) The glass temperature drops sharply with increasing humidity and can then be below 0 ° C
Compact polyamides have high wear resistance and good sliding properties . The mechanical properties can be further improved by fiber composites with glass or carbon fibers , so that strength and impact strength can be matched to the application. However, the addition of fibers increases the sensitivity of the materials to hydrolysis, as a microscopic gap remains between the matrix and the fiber through which moisture is drawn in by the capillary effect. However, this effect turns out differently depending on the size of the glass fiber and the associated connection between fiber and matrix.
recognition
Polyamides can be identified easily with just a few tools. The firing test is the simplest . A small section of the plastic part to be examined is ignited. PA burns with a blue flame with a yellowish edge, whereby the burning material foams a little and forms brown-black edges. If you blow out the flame, the smoke smells slightly horny. PA can be loosened with formic acid and thus also glued, depending on the type of polyamide, different concentrations are required (PA 6 70%, PA 6.6 80%).
Dyeing of polyamide fibers
Fibers come either as a spun-dyed material or as an off-white fiber material. The raw white fiber material can be colored in various stages of presentation (flake, yarn, piece). Acid or metal complex dyes are used. Polyamide can also be colored with disperse and direct dyes, but the fastness properties achieved are generally significantly poorer.
More recently, reactive dyes have also been used, which clearly exceed the fastness properties of acid, disperse and direct dyes.
use
| polyamide | amount | Share of total production |
|---|---|---|
| PA 6 | 2500 kt fibers / 1100 kt materials / 300 kt foils | 57% |
| PA 6.6 | 1600 kt fibers / 1000 kt materials | 38% |
| rest | 300 kt | 5% |
Most of the polyamide production is used as synthetic fibers for textiles . Examples are …
- clothing
- Parachutes , hang gliders , balloons , sails
- technical fabrics (e.g. screen fabrics for paper production)
- Ropes
- Fishing line
- Cutting line for grass trimmers
- Stringing of tennis rackets
- Strings for string instruments and plucked instruments
It is also used for the production of household items and technical parts that have to be very wear-resistant, such as dowels , screws , housings , slide bearings , insulators in the electrical engineering sector , cable ties , adhesive bases , junction pieces for medical tents , kitchen utensils (ladles, spoons), machine parts (covers , Gears , bearings , rollers) and toothbrush bristles.
Due to its resistance to lubricants and fuels at temperatures of over 150 ° C, it is also used in vehicle construction for engine components such as intake systems, fuel lines, engine covers, oil pans and for compressed air systems such as chassis and brakes. When used as solid material tires for industrial trucks , polyamide with a Shore A hardness of over 75 exceeds the load-bearing capacity of other plastics such as polyurethane or Vulkollan and other elastomers .
PA12 is used as an inexpensive standard material for 3D printing of components and housings. The powder wetted with water is baked with a laser beam.
Due to their uniformly smooth surface, polyamides are well suited as sutures in surgery . The polyamide sutures are particularly characterized by their excellent knotting properties and high tensile strength . It is a monofilament, non-absorbable surgical material made of polyamide 6 and polyamide 6.6.
In 2013 global sales of around $ 20.5 billion were achieved.
Bio-based polyamides
Depending on whether one or more monomers for a polyamide were made from renewable raw materials , this is considered to be partially bio-based or bio-based . The monomers for bio-based polyamides do not differ structurally from the monomers for petrochemical polyamides, so that the polymers themselves do not differ structurally either.
The following table provides an overview of polyamides that can be technically partially and completely bio-based (e.g. from castor oil ). It should be noted that the bio-based variants do not necessarily have to be on the market and may be significantly more expensive than conventional competing products. In addition, conventional monomers for products can only be partially replaced by bio-based ones, which means that the actual biogenic proportion can be lower than the value in the table. The monomers that can be produced bio-based are marked in green . The maximum biogenic proportion is calculated from the proportions of the molar masses of the monomers.
| Short name polyamide |
Diamine | Dicarboxylic acid | Aminocarboxylic acid or lactam |
Biogenic share up to (%) |
|---|---|---|---|---|
| PA 6 | - | - | Caprolactam ( C6 ) | 100 |
| PA 11 | - | - | Aminoundecanoic acid ( C11 ) | 100 |
| PA 6 4 | Hexamethylene diamine ( C6 ) | Succinic acid ( C4 ) | - | 42.4 |
| PA 6. 6 | Hexamethylene diamine ( C6 ) | Adipic acid ( C6 ) | - | 49.5 |
| PA 5 . 9 | Pentamethylene diamine ( C5 ) | Azelaic acid ( C9 ) | - | 100 |
| PA 6. 9 | Hexamethylene diamine ( C6 ) | Azelaic acid ( C9 ) | - | 57.5 |
| PA 4 10 | Tetramethylene diamine ( C4 ) | Sebacic acid ( C10 ) | - | 66.1 |
| PA 5 . 10 | Pentamethylene diamine ( C5 ) | Sebacic acid ( C10 ) | - | 100 |
| PA 6. 10 | Hexamethylene diamine ( C6 ) | Sebacic acid ( C10 ) | - | 59.6 |
| PA 10 . 10 | Decamethylene diamine ( C10 ) | Sebacic acid ( C10 ) | - | 100 |
| PA 10 . 12 | Decamethylene diamine ( C10 ) | Dodecanedicarboxylic acid ( C12 ) | - | 100 |
recycling

The recycling code for polyamides is 07.
literature
- Ludwig Bottenbruch, Rudolf Binsack (Hrsg.): Polyamide, Kunststoff-Handbuch Volume 3/4: Technical thermoplastics. Hanser, Munich 1998, ISBN 3-446-16486-3 .
- House of the history of the FRG (ed.): Artificial temptation: Nylon, Perlon, Dederon. Wienand-Verlag, 1999, ISBN 3-87909-640-6 , ISBN 978-3-87909-640-4 .
- Susanne Buck: Wrought miracles, delicate dreams. Of women's legs and pearl stockings. Jonas-Verlag Marburg, 1996, ISBN 3-89445-199-8 , ISBN 978-3-89445-199-8 .
- Shaul M. Aharoni: N-nylons. John Wiley and Sons, 1997, ISBN 0-471-96068-3 .
- Melvin I. Kohan: Nylon Plastics Handbook. Hanser, Munich 1995, ISBN 3-446-17048-0 .
- Richard Vieweg , Alfred Müller (ed.): Plastic manual. Volume 6: polyamides. Hanser, 1966, DNB 457323302 .
- Hans-Georg Elias: Macromolecules. Volume 2 - Technology. 5th edition. Hüthig & Wepf Verlag, 1992, ISBN 3-527-29959-9 .
- Otto Schwarz, Friedrich-Wolfhard Ebeling (Hrsg.): Plastic science: structure, properties, processing, applications of thermoplastics, thermosets and elastomers. 9th edition. Vogel, 2007, ISBN 978-3-8343-3105-2 .
- Paul Schlack: The development of polyamide fibers from a historical perspective In: Magazine for the entire textile industry. Volume 56, 1954, pp. 823–825.
- Hermann Klare: The discovery of polyamides and the beginning of their technical development In: Man-made fibers. Volume 38/90, 1988, pp. 540-544.
- Herbert Bode: Development of the production of polyamide fibers and their raw materials. In: Chemical Fibers International. Volume 50, 2000, pp. 128-131.
Web links
Individual evidence
- ↑ Hans Domininghaus, Peter Elsner, Peter Eyerer, Thomas Hirth: plastics. Properties and uses . Ed .: Peter Elsner, Peter Eyerer, Thomas Hirth. 8th edition. Springer-Verlag, Heidelberg 2012.
- ^ N24 History. aired July 31, 2008 at 10:05 pm.
- ↑ k. A. In: Context (DuPont company magazine) . tape 7 , no. 2 , 1978.
- ↑ Poliblend Germany . Last accessed on February 22, 2019.
- ^ "Technical information" from the 2002 price list of "Deutsche Steinzeug" or "AgrobBuchtal" ceramics, page 228.
- ↑ A. Thiede , D. Geiger: Sutures. In: Commentary on the PH.EUR. 1997, 9th edition
- ↑ Market Report: Global Polyamide Market. Acmite Market Intelligence , accessed February 16, 2015 .
- ↑ a b Oliver Türk: Material use of renewable raw materials . 1st edition. Springer Vieweg, Wiesbaden 2014, ISBN 978-3-8348-1763-1 , p. 455-466 .