Concepts for overcoming the blood-brain barrier
Concepts for overcoming the blood-brain barrier make it possible to supply the brain with active substances for therapeutic purposes . The blood-brain barrier is a dynamic interface that controls via influx (inflow, literally: inflow) and efflux (outflow) which nutrients, drugs , drugs , xenobiotics and other compounds can be supplied to the brain. This ensures that the central nervous system (CNS) has an optimal environment.
However, its protective function also makes the blood-brain barrier a barrier for many potential active substances and thus prevents their use in drug therapies. Around 98% of potential neuropharmaceuticals fail because of this. Only relatively few neurological and psychiatric diseases such as affective disorders such as depression , epilepsy or chronic pain can be treated with small lipophilic active ingredients.
In contrast, there is no therapy for neurodegenerative diseases such as Alzheimer's disease , Huntington 's disease and amyotrophic lateral sclerosis (ALS). No effective drug therapies are known for brain tumors , strokes , spinal cord injuries and traumatic brain injuries . The blood-brain barrier also represents a barrier in childhood syndromes such as autism , lysosomal storage diseases , fragile X syndrome or ataxia , which prevents previous drug therapy approaches. Even with diseases such as multiple sclerosis , the progression of the disease in the central nervous system cannot be stopped, as the drugs administered only work in the periphery. In principle, many of these diseases could be treated with active ingredients, for example based on enzymes , genes or biotechnologically produced proteins - if they could cross the blood-brain barrier. However, therapy is only possible if these substances can also reach the site of action - i.e. the central nervous system - in sufficient, i.e. therapeutically effective, concentration. For decades, intensive research has therefore been carried out on methods that are intended to enable the transport of active substances into the brain by bypassing or - ideally more selectively - opening the blood-brain barrier. A number of strategies for overcoming the blood-brain barrier have been developed or are still in the development stage.
Bypassing the blood-brain barrier - intrathecal and intraventricular drug application
The most obvious form of drug transport into the CNS bypassing the blood-brain barrier is injection directly into the cerebrospinal fluid ( intrathecal ) or directly into the cerebral ventricle ( intraventricular ). The active ingredient is injected directly into the liquor. This method is applied for example as intrathecal chemotherapy among others, the folic acid - antagonist methotrexate (MTX), with cytarabine (AraC), and cortisol ; especially for patients with acute lymphoblastic leukemia and aggressive lymphoma . In the triple intrathecal chemotherapy for the treatment of meningeal leukemia, the three active ingredients are applied together into the liquor.
The intrathecal application of active ingredients is - compared to the intravenous (systemic) administration of active ingredients - significantly more complex and also more unpleasant for many patients. In addition, due to the significantly increased risk of infection and injury, there are particularly strict requirements for hygiene and technical skills of the user in such forms of administration. By injecting active ingredients with a slow release effect , the treatment intervals can be extended to longer periods of time - for example, fortnightly. The use of an Ommaya reservoir , which is implanted under the scalp, is less complex . Implantable drug pumps offer a similar approach . In severe pain, this method can be chosen for the dosage of morphine, for example. The active substance can also be administered intrathecally via such a pump for the treatment of spasticity , for example in multiple sclerosis with baclofen . The method was first used in 1984 and has been established since then.
Active ingredients applied intrathecally are usually specially formulated for this dosage form. For example, they must not contain any bactericides or a number of other auxiliary substances that are common additives in intravenously administered drugs.
For a few diseases, intrathecal or intraventricular drug application enables effective therapy. However, these two methods of bypassing the blood-brain barrier are not suitable for the treatment of brain tumors. The reason for this lies in the diffusion of the active ingredients, which is limited to just a few millimeters, into the parenchyma of the brain.
A gap in the blood-brain barrier that can be used experimentally and therapeutically is the cranial nerves entering the brain . For example, it has been shown that neurotrophins , neuropeptides , insulin , cytokines and even DNA that were administered through the nose can enter the central nervous system via the olfactory nerve . It was also possible to successfully smuggle stem cells into the brain in this way .
Crossing the blood-brain barrier for therapeutic purposes
An intact blood-brain barrier is vital for every vertebrate. For many active ingredients that are supposed to develop their effect outside of the central nervous system, retention at the blood-brain barrier is an important criterion for approval in order to be able to safely rule out the sometimes considerable side effects that are otherwise to be expected, especially when a drug is taken continuously . On the other hand, the blood-brain barrier represents an insurmountable barrier for many connections in the treatment of neurological diseases.
Lipophilization
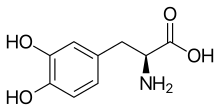
The diffusivity of a molecule through the endothelia of the blood-brain barrier is mainly determined by its fat solubility ( lipophilicity ) and size. Modifying the molecule with lipophilic groups can therefore improve brain penetration. A classic example of this is the di- acetylation of the natural substance morphine to diacetylmorphine ( heroin ). Heroin (log P = 1.12) shows an uptake in the brain that is over 25 times higher than morphine (log P = 0.2) (see table 1). Corresponding results are obtained with the brain uptake index (BUI) for radioactively labeled morphine, codeine and heroin which is injected into the carotid artery. The BUI is below the detection limit for morphine, 24% for codeine and 68% for heroin.
This prodrug concept can lead to an improvement in brain penetration even with peptide active ingredients.
However, the concept fails for molecules with a molar mass greater than 500 g · mol −1 , since such substances can no longer pass the blood-brain barrier by diffusion due to their size. In addition, lipophilization is accompanied by a significantly poorer solubility of the active ingredient. In the case of oral administration, however, only dissolved active substances can be absorbed in the gastrointestinal tract. The lipophilization naturally also causes an increased uptake in other, non-cerebral cells. Lipophilization is also ineffective against efflux transporters, which channel the diffused active substance out of the endothelium.
Exploitation of the transporter
There are several transport systems in the endothelium of the blood-brain barrier to supply the brain with essential hydrophilic substances. One approach to being able to smuggle active substances into the brain is to use these transporters. This is used, for example, in the therapy of Parkinson's disease . Patients suffering from it have a deficiency of the neurotransmitter dopamine in the brain . The administration of dopamine would be ineffective in this regard, as dopamine cannot cross the blood-brain barrier. If, on the other hand, levodopa , a non-proteinogenic α-amino acid, is administered, it is supplied to the brain via the LAT1 transporter and is then metabolized into dopamine. The LAT1 transporter belongs to the LNAA transporter family ( large neutral amino acid ).
Also, the antiepileptic drug gabapentin , the antihypertensive α-methyldopa and the cytostatic drugs melphalan and acivicin can LNAA transporter the blood-brain barrier.
The upper limit for using the existing transport systems is around 500 to 600 g · mol −1 .
Vectorization
Another way to cross the blood-brain barrier with an active ingredient is vectorization. This approach is based on the observation that some macromolecules, such as transferrin , low-density lipoprotein and insulin, can cross the blood-brain barrier through a multi-stage process known as receptor-mediated transcytosis . Via receptors located on the surface of the endothelial cells of the brain capillaries and protruding into the lumen of the blood vessels, the macromolecules are smuggled into the interior of the endothelial cells via vesicles, and then transported to the other side of the cell (abluminal side) and discharged . If an active substance molecule is bound to such a macromolecule, the receptor-mediated transcytosis can be used to overcome the blood-brain barrier.
An example of this is the transferrin receptor , which, with the help of monoclonal antibodies directed against it, can be used to transport active substances across the blood-brain barrier. This receptor is usually responsible for the transport of iron across the blood-brain barrier. Another target is the insulin receptor , which is also of the endothelial cells of the blood-brain barrier expressed is. With both vectors, different, also larger, peptides were successfully smuggled across the blood-brain barrier in the animal model. Vectorization is a very promising approach, especially for the therapy of neurodegenerative diseases for which only low concentrations of active ingredients are required. Cytostatics such as doxorubicin were also bound to transferrin receptor antibodies.
However, the phenomenon of transcytosis is not limited to macromolecules. Although the exact mechanism has not always been clarified, it has been shown that small peptides and low molecular weight substances can also enter and pass through the cell in this way. A vectorization for the purpose of crossing the blood-brain barrier is thus also possible with short peptide sequences. Basic protegrin derivatives such as Syn-B and penetratin derived from the homeodomain of Antennapedia , a transcription factor from Drosophila , were used as vectors for active substances such as doxorubicin . Another peptide vector is the HIV-TAT ( Trans-Activator of Transcription ) , which consists of eleven predominantly basic amino acids and is isolated from the transduction domain of the HI virus . A peptide with similar properties is Transportan , a cell-penetrating peptide, made up of 27 amino acids .
With transgenic macrophages , proteins can be funneled through the blood-brain barrier.
Cationization
Positively charged molecules (cations) can cross the blood-brain barrier with the help of adsorption-mediated transcytosis , also known as cationic transport. In adsorption-mediated transcytosis, electrostatic interactions between the cell surface negatively charged by glycoproteins and positively charged molecules cause a nonspecific binding to the surface of cells, as a result of which they are taken up and transported through the cytoplasm of the endothelia. Cationic transcytosis through the endothelium of the blood-brain barrier enables a higher degree of substance transport than receptor-mediated transcytosis.
The cationization of antibodies has been successfully used to cross the blood-brain barrier in a number of different studies and fields of application. For example, to make β-amyloid plaques visible or to target mitochondria.
Peptides and proteins whose isoelectric point is basic already have a positive charge . One approach to improve the uptake of non-basic peptides and proteins in the brain is to chemically modify them with the help of naturally occurring polyamines such as putrescine , spermidine or spermine . An alternative to this is the conjugation of active ingredient peptides and proteins to basic peptides such as Syn-B, as described in the chapter on vectorization. Synthetic polyamines, such as polyethyleneimine , can also be used to facilitate the transport of active substances and DNA through the blood-brain barrier.
The effect of the cationization allows active substances and diagnostics to pass through the blood-brain barrier, but at the same time causes a significantly increased absorption of the applied dose in the liver and kidneys - with the corresponding expected side effects.
Nanoparticles
In the 1990s, experiments with nanoparticles made up of biocompatible polymers found that these particles are able to cross the blood-brain barrier under certain circumstances. The diameter of these particles is usually 50 to 300 nm. The unfunctionalized, pure polymer particles in this form are not able to be transported through the endothelium to the brain. Receptor-mediated transport is only possible through special functionalization, usually with polysorbate 80 or poloxamers . The polymers used are mostly polylactides (PLA), polylactide-co-glycolide (PLGA) and various polycyanoacrylates , such as polybutyl cyanoacrylate (PBCA), which are pharmacologically safe and approved for other applications, for example as surgical sutures . Active substances trapped in the particles can be transported to the brain by means of receptor-mediated transcytosis.
The essential prerequisites for the brain penetration of the nanoparticles are - in addition to their size - the longest possible circulation time in the blood and the appropriate surface characteristics. The plasma half-life is mostly achieved through PEGylation and the interaction on the endothelium with the polysorbate already described. The exact transport mechanism has not yet been finally clarified. However, the polysorbate coating on the particles obviously leads to adsorption of apolipoprotein E or B onto the particles in the blood plasma . As a result, the nanoparticles are recognized as an LDL mimetic by the LDL receptor and transported into the interior of the endothelium. The active ingredient is then either released in the endothelium, which allows it to reach the brain by diffusion, or the particles are completely expelled through the abluminal side to the brain (transcytosis).
The nanoparticulate drug delivery is currently still in preclinical research. Promising results in the treatment of transplanted glioblastomas have been achieved in the animal model (rat) . The particles were loaded with doxorubicin. The transport of doxorubicin into the brain could be increased by a factor of 60. Chemotherapy for brain tumors, which is difficult to implement because of the extensive impermeability of the blood-brain barrier to chemotherapeutic agents, is one of the main goals in the development of these nanoparticulate active substance carrier systems.
Tissue- or receptor-specific targeting of the nanoparticles is also conceivable with special ligands .
In addition to the nanoparticulate approach with polymers, nanoscale liposomes and dendrimers are also in preclinical testing as potential drug carriers. Particular attention is paid to the discussion about its risks that takes place in the context of nanotechnology as a whole.
Solvents and surfactants
Compounds administered intravenously, such as ethanol , dimethyl sulfoxide or glycerine, can lead to a solvent-induced opening of the blood-brain barrier. In the animal model (chick) the concentration of solvent is above 1 mg per kg of body weight. These compounds presumably disrupt the function of the cell membrane in the endothelium, which enables the transport of substances through transcellular diffusion.
If short-chain alkyl glycerols , such as 1-O-hexyl diglycerol , are injected into the carotid arteries of mice or rats together with marker substances, the uptake of these markers in the brain increases significantly. Larger molecules that would otherwise not cross the blood-brain barrier, such as methotrexate , vancomycin or gentamicin , can - due to the presence of the alkylglycerol - diffuse into the brain. This effect is not observed with intravenous administration of alkylglycerol. The amphipathic glycerols open the blood-brain barrier for about 5 to 120 minutes. The concentrations of the alkyl glycerols are in the millimolar range. Obviously, these surfactant-like compounds form vesicular structures with the active ingredients or markers . Alkyl glycerols are largely non-toxic and pharmacologically safe. The mechanism by which the blood-brain barrier is overcome is largely unclear. But it is obviously a matter of a transport through the tight junctions .
When injected into the carotid artery, the surfactant sodium lauryl sulfate also significantly increases the permeability of the blood-brain barrier. Sodium lauryl sulfate is a pharmacological adjuvant that is used in various active ingredient formulations. The appropriate application of such formulations can therefore lead to unexpected results. The adjuvant sodium lauryl sulphate in a formulation with interleukin-2 caused the blood-brain barrier in cats to surprisingly become permeable to the marker substance horseradish peroxidase. Similar effects were also observed with the excipient polysorbate-80. For a mouse, doses in the range of 3 mg per kg of body weight are sufficient. Kyotorphin , a neurophysiologically active dipeptide, is unable to cross the blood-brain barrier and show a neurological effect. The neurological effect is only achieved in conjunction with polysorbate-80.
Efflux inhibition
Many molecules are able to cross the blood-brain barrier both because of their size and their lipophilicity. However, after diffusing into the cytoplasm of the endothelia, they are transported back into the lumen by efflux pumps such as P-glycoprotein. One strategy to still make these molecules accessible to the brain is to switch off these efflux transporters. In principle, this is possible through:
- Gene regulation in the transcriptional or translational phase
- Changes in membrane targeting after the synthesis of the transporters in the ribosomes
- Preventing the transport by inhibitors ( co-drugs )
While the first two methods are still at a very early stage of development at the cell culture level, extensive experience in animals and clinical studies in humans is available with the efflux inhibitors.
A number of substances are now known that inhibit the efflux - especially through P-glycoprotein.
Mice in which the MDR1 gene has been switched off ( knockout ) so that no P-glycoprotein is produced in the endothelium show a significantly increased uptake of a number of active substances in the brain via the blood-brain barrier. Compared to the wild-type of mouse , for example, the concentration ratio rose brain to the blood in the HIV protease inhibitors nelfinavir , indinavir and saquinavir by a factor of 7 to 36 at. With the taxanes docetaxel and paclitaxel , the concentration in the brain increases by a factor of 7 to 28 and with digoxin by a factor of 10. With verapamil, the absorption in the brain is increased by a factor of 8.5.
Comparable results were obtained in wild types of mice and rats to which selectively acting P-glycoprotein inhibitors, such as valspodar (PSC 833, a cyclosporine derivative), elacridar (GF120918) and zosuquidar (LY335979), were administered. In rats given ciclosporin, verapamil levels in the brain increased by a factor of 9.6.
Verapamil - a drug approved as a calcium antagonist - is itself an effective co-drug in animal experiments, which can significantly increase the absorption of subsequently applied active ingredients in the brain. This has been demonstrated in animal models, among other things, with cytostatic vinca alkaloids . Procyanidins have a similar effect .
The disadvantage of the efflux inhibition approach is that the administered inhibitors - especially the first generation, such as verapamil and ciclosporin - are themselves pharmacologically active and thus have a number of undesirable side effects. With the second and third generation of P-glycoprotein inhibitors, these effects are significantly reduced. In addition, all cells - which express P-glycoprotein - inhibit the same. Thus the apical side of intestinal epithelial cells, which are in the systemic administration of efflux inhibitors bile canaliculi ( Bilis canaliculi ) of the renal tubules and placenta , as well as on the luminal side of the seminiferous tubules affected.
BCRP ( Breast Cancer Resistance Protein ), the second most important efflux transporter of the blood-brain barrier, obviously has hardly any influence on the transport of active substances. This was found in experiments on knockout mice in which the BCRP-encoding ABCG2 gene was switched off.
Efflux inhibition is particularly pursued in cancer therapy, since many cancer cells express P-glycoprotein to a high degree during the course of therapy and can therefore largely escape the effects of cytostatics. The tumors then no longer respond to the administered cytostatics.
Opening the blood-brain barrier for therapeutic purposes
Opening the blood-brain barrier for therapeutic purposes is, in addition to the two principles shown above, another strategy for delivering active substances to the brain that are normally not able to cross the blood-brain barrier. The aim of this procedure is to open or at least loosen the tight junctions as reversibly as possible in order to enable paracellular drug transport into the brain. With the increasing understanding of the molecular structure of the blood-brain barrier - and especially the tight junctions - new ways and methods for pharmacological, but also physical, opening of the blood-brain barrier have been developed. Most of these procedures are still in preclinical testing.
When the blood-brain barrier is opened, there is a general risk that plasma proteins that are toxic to the brain can diffuse in and then trigger chronic neuropathologies .
Tight Junction Modulation
Connections that affect the tight junctions are known as tight junction modulators. As a result of advances in genomic drug development, high-throughput screening , combinatorial chemistry and bioinformatics , a number of substances have been developed or identified that are able to directly target the individual peptides of the tight junctions and adherens junctions and thus to modulate the cell-cell contact of the endothelia.
Modulators that directly target the tight junctions are derived, for example, from the enterotoxins of the bacteria Vibrio cholerae and Clostridium perfringens . Vibrio cholerae - a cholera pathogen - forms the zonula occludens toxin (ZOT, Zonula occludens = tight junction). ZOT is a 45 kDa protein made up of 399 amino acids that interacts in the intestine with a surface receptor - the ZOT receptor - of the endothelia there, thereby triggering an intracellular signal cascade that has not yet been fully understood. Among other things, the enzyme protein kinase A is activated, which catalyzes the breakdown of tight junctions. On individual layers of cerebral endothelia, ZOT causes a significant reduction in transendothelial electrical resistance (TEER) in vitro , which is reversible. For the marker molecules sucrose , inulin, paxlitaxel and doxorubicin, the paracellular permeability is significantly increased. The 12 kDa active ZOT fragment ΔG and the active ZOT domain (AT1002) consisting of only six amino acids (in the one-letter code: FCIGRL) also bind to the ZOT receptor.
The 44 amino acid OCC2 peptide binds selectively to the second domain of the tight junction protein occludin , which also facilitates paracellular transport.
Bradykinin , a vasodilating oligopeptide made up of nine amino acids , binds to the B 2 receptors on the luminal side of the endothelia. As a result, the concentration of free intracellular increases calcium - ion , and with the transmembrane Tight-junction proteins occludin and Claudin associated actin-myosin complex is activated, whereby the open tight junctions.
Osmotic opening of the blood-brain barrier
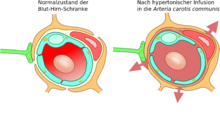
Shortly after the discovery of tight junctions in 1970, the thesis was put forward that the action of hyperosmotic solutions on the endothelial cells could open the blood-brain barrier. This method was used for the first time in 1980, and in 1984 electron microscopic images provided experimental evidence for this thesis. Electron-dense markers had diffused into the brain through the tight junctions.
Hyperosmolar solutions, for example mannitol or arabinose, are infused via the internal carotid artery . The different osmotic pressure between the endothelial cells and the infused solution causes a loss of fluid in the endothelial cells, which leads to their shrinkage. The shrinkage creates tensile forces between the cells, which leads to an opening of the tight junctions and thus to the opening of the blood-brain barrier.
Due to the concentration gradient between the intravascular and interstitial space, a large amount of water flows back from the plasma into the brain ( bulk flow ). As a result, molecules dissolved in the water are washed into the brain, causing edema .
The opening of the tight junctions caused by the shrinkage of the endothelial cells is around 20 nm. This allows molecules with a hydrodynamic diameter of around 20 nm to diffuse into the brain. The opening of the blood-brain barrier is reversible with this method. It is fully restored ten minutes to two hours after the infusion at the latest. The exposure time to the hyperosmolar solution is about 30 seconds. By pretreatment with a Na + / Ca 2+ - channel blockers the opening period, the blood-brain barrier to be extended.
The method was tested in animal models with a variety of water-soluble active ingredients, peptides, antibodies, enzymes and viral vectors for gene therapy. A number of clinical studies on the therapy of brain tumors in combination with chemotherapeutic agents are being carried out in various clinics. The results are promising for this application.
Ultrasonic
The blood-brain barrier can be opened by focused ultrasound . This effect was first demonstrated in 1956. The opening of the blood-brain barrier could be detected by staining the brain with trypan blue - a vital dye that normally cannot pass the blood-brain barrier - and by radioactively labeled phosphate . No changes in the endothelium could be observed microscopically. However, the use of ultrasound resulted in brain injuries. In 1960 the blood-brain barrier was opened by ultrasound for the first time with only minor damage to the surrounding parenchyma. All these experiments were carried out with high-intensity focused ultrasound , with powers in the range of 4000 watts / cm². This creates cavitation bubbles that can irreversibly destroy the tissue.
- Focusing ultrasound with microbubbles
The opening of the blood-brain barrier with ultrasound and simultaneously administered microbubbles ( microbubbles ) came in 2001 for the first time used. The approach is that no cavitation bubbles have to be generated, but injected microbubbles take over the function of the cavitation bubbles otherwise generated by the high ultrasonic power. This can significantly reduce the power of the ultrasound; there is no longer any risk of overheating the treated skull or the surrounding tissue. The technology has now developed so far that when the blood-brain barrier is opened, no apoptosis , ischemia or other long-term damage can be detected in the brain. A few hours after the treatment, the old state of the blood-brain barrier is restored.
The focus of the ultrasound can be directed to any area in the brain. This allows the blood-brain barrier to be opened selectively, limited to certain brain areas. In this way, applied active ingredients can diffuse specifically into these areas. The treated areas can be precisely followed by simultaneous magnetic resonance imaging (MRT). The contrast agent used for the MRI , for example gadopentetate dimeglumine , only penetrates the brain through the opened areas of the blood-brain barrier. These areas are clearly highlighted in the MRI. The highly polar gadopentetate dimeglumine is not able to pass through the unopened areas of the blood-brain barrier.
In the mouse animal model, when using focused ultrasound with microbubbles, frequencies in the range of 0.5 and 2 MHz with short pulse lengths in the millisecond range and repetition frequencies in the range of 1 Hz over a period of less than one minute are used. The optimal frequency range is below 1 MHz. The acoustic power is less than one watt . The microbubbles used are mostly approved contrast media from contrast-enhanced sonography . They typically have a diameter of 3 to 4.5 μm, consist for example of human albumin and are filled with perfluoropropane or similar heavy gases.
- mechanism
The mechanism of opening the blood-brain barrier through the use of focused ultrasound, along with microbubbles, is not fully understood. The interaction of ultrasound and microbubbles plays a major role here and leads to a number of biological effects in vivo . Shear forces that are generated by micro-currents seem to play an essential role. These micro-currents themselves come from oscillations of the micro-bubbles in the ultrasonic field. The endothelia themselves, in turn, are known to be able to react dynamically to shear forces and that shear forces are a critical variable for homeostasis. Electron micrographs of capillary vessels of test animals treated in this way show both a transcellular and a paracellular transport of the corresponding marker molecules (horseradish peroxidase). The transcellular transport is essentially transcytosis. Paracellular transport is initiated by a complex disintegration process in which the tight junctions lose their function.
The blood-brain barrier that is opened in this way is permeable to low-molecular chemotherapeutic agents such as doxorubicin and antibodies such as trastuzumab . The principle feasibility of transporting genes into the brain was also demonstrated using this method in an animal model. The process of opening the blood-brain barrier with ultrasound and simultaneously applied microbubbles is still a very young process. So far it has only been tested on laboratory animals. Experience has shown that it will take many years before the process can be approved for humans.
The non-focused ultrasound radiation (sonography) used for imaging in diagnostics does not affect the integrity of the blood-brain barrier - even when contrast media are administered.
literature
- AG De Boer, W. Sutanto: Drug Transport Across the Blood-brain Barrier. CRC Press, 1997, ISBN 90-5702-032-7
- DJ Begley et al. a .: The Blood-brain Barrier and Drug Delivery to the CNS. Informa Health Care, 2000, ISBN 0-8247-0394-4
- P. Ramge: Investigations into overcoming the blood-brain barrier with the help of nanoparticles. Shaker Verlag , 1999, ISBN 3-8265-4974-0
Web links
Individual evidence
- ^ A. Tsuji: Small Molecular Drug Transfer across the Blood-Brain Barrier via Carrier-Mediated Transport Systems. In: NeuroRx 2, 2005, pp. 54-62. PMID 15717057 (Review).
- ^ A b c W. M. Pardridge: Blood-brain barrier drug targeting: the future of brain drug development. In: Mol Interv 3, 2003, pp. 90-105. PMID 14993430 (Review).
- ↑ A. Ajay et al. a .: Designing libraries with CNS activity. In: J Med Chem 42, 1999, pp 4942-4951. PMID 10585204 .
- ^ AK Ghose: A knowledgebased approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. In: J Comb Chem 1, 1999, pp. 55-68. PMID 10746014 .
- ↑ a b c W. W. Pardridge: The blood-brain barrier: bottleneck in brain drug development. In: NeuroRx 2, 2005, pp. 3-14. PMID 15717053 (Review).
- ↑ a b c N. Vykhodtseva et al. a .: Progress and problems in the application of focused ultrasound for blood-brain barrier disruption . In: Ultrasonics , 48, 2008, pp. 279-296. PMID 18511095 .
- ^ A b D. J. Begley: Delivery of therapeutic agents to the central nervous system: the problems and the possibilities . In: Pharmacol Ther , 104, 2004, pp. 29-45. PMID 15500907 (Review).
- ↑ WM Pardridge: Why is the global CNS pharmaceutical market so under-penetrated? In: Drug Discovery Today , 7, 2002, pp. 5-7. PMID 11790589 .
- ^ AG de Boer, PJ Gaillard: Strategies to improve drug delivery across the blood-brain barrier . In: Clin Pharmacokinet , 46, 2007, pp. 553-576. PMID 17596102 (Review).
- ^ AG de Boer and PJ Gaillard: Drug targeting to the brain . In: Annu Rev Pharmacol Toxicol , 47, 2007, pp. 323-355. PMID 16961459 (Review).
- ↑ G. Fleischhack et al. a .: Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. In: Clin. Pharmacokinetics 44, 2005, pp. 1-31. PMID 15634030 .
- ↑ JZ Kerr et al. a .: Intrathecal chemotherapy . In: Crit Rev Oncol Hematol , 37, 2001, pp. 227-236. PMID 11248578 .
- ^ S. Stapleton, S. Blaney: New agents for intrathecal administration . In: Cancer Invest , 24, 2006, pp. 528-534. PMID 16939963 .
- ↑ YL Kwong et al. a .: Intrathecal chemotherapy for hematologic malignancies: drugs and toxicities . In: Ann Hematol , 88, 2009, pp. 193-201. PMID 19050889 .
- ↑ SL Berg, MC Chamberlain: Current treatment of leptomeningeal metastases: systemic chemotherapy, intrathecal chemotherapy and symptom management . In: Cancer Treat Res , 125, 2005, pp. 121-146. PMID 16211887 .
- ↑ a b A. Ruggiero u. a .: Intrathecal chemotherapy with antineoplastic agents in children . In: Pediatr Drugs , 3, 2001, pp. 237-246. PMID 11354696 .
- ↑ H. Schneider: Implantable drug pumps. ( Memento of the original from March 4, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF) In: Implant Catalog Part III (Medical Service of the Central Health Insurance Associations) April 2000.
- ↑ ES Krames: Intrathecal infusional therapies for Intractable Pain: Patient Management Guidelines . In: J Pain Symptom Manage , 8, 1993, pp. 36-46. PMID 8482892 .
- ↑ VL Ghafoor u. a .: Intrathecal drug therapy for long-term pain management . In: Am J Health Syst Pharm , 64, 2007, pp. 2447-2461. PMID 18029950 .
- ↑ G. Ochs u. a .: Intrathecal baclofen for long-term treatment of spasticity: a multi-center study . In: J Neurol Neurosurg Psychiatry , 52, 1989, pp. 933-939. PMID 2487035 .
- ↑ G. Ochs: Therapeutic possibilities of growth factors in neuromuscular diseases. Retrieved January 15, 2008.
- ^ PM Brennan, IR Whittle: Intrathecal baclofen therapy for neurological disorders: a sound knowledge base but many challenges remain . In: Br J Neurosurg , 22, 2008, pp. 508-519. PMID 18649160 .
- ↑ RD Penn: Intrathecal baclofen alleviates spinal cord spacicity . In: The Lancet , 8385, 1985, p. 1078. PMID 6144008 .
- ^ KS Lewis, WM Mueller: Intrathecal baclofen for severe spasticity secondary to spinal cord injury . In: Ann Pharmacother , 27, 1993, pp. 767-774. PMID 8329801 .
- ^ A. Dario and G. Tomei: A benefit-risk assessment of baclofen in severe spinal spasticity . In: Drug Saf , 27, 2004, pp. 799-818. PMID 15350152 (Review).
- ↑ YW Cheung et al. a .: Stability of cytarabine, methotrexate sodium, and hydrocortisone sodium succinate admixtures . In: Am J Hosp Pharm , 41, 1984, pp. 1802-1806. PMID 6496516 (Review).
- ↑ Q. Yan et al. a .: Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with trk receptor expression . In: Exp Neurol , 127, 1994, pp. 23-36. PMID 8200435 .
- ↑ World Cup Pardridge: CNS drug design based on principles of blood-brain barrier transport . In: J Neurochem , 70, 1998, pp. 1781-1792. PMID 9572261 (Review).
- ↑ EM Kemper et al. a .: Modulation of the blood-brain barrier in oncology: therapeutic opportunities for the treatment of brain tumors? In: Cancer Treat Rev 30, 2004, pp. 415-423. PMID 15245774 (Review).
- ↑ Hanson LR, Frey WH: Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease . In: BMC Neurosci . 9 Suppl 3, 2008, p. S5. doi : 10.1186 / 1471-2202-9-S3-S5 . PMID 19091002 . PMC 2604883 (free full text).
- ↑ Danielyan L, Schäfer R, from Ameln-Mayerhofer A, et al. : Intranasal delivery of cells to the brain . In: Eur J Cell Biol.. . 88, No. 6, June 2009, pp. 315-24. doi : 10.1016 / j.ejcb.2009.02.001 . PMID 19324456 .
- ^ G. Miller: Drug targeting. Breaking down barriers. In: Science 297, 2002, pp. 1116-1118. PMID 12183610 .
- ↑ MD Habgood et al. a .: Determinants of passive drug entry into the central nervous system. In: Cell Mol Neurobiol 20, 2000, pp. 231-253. PMID 10696512 .
- ^ WH Oldendorf: Lipid solubility and drug penetration of the blood-brain barrier. In: Proc Soc Exp Biol Med 147, 1974, pp. 813-816. PMID 4445171 .
- ^ WH Oldendorf u. a .: Blood-brain barrier: penetration of morphine, codeine, heroin, and methadone after carotid injection. In: Science 178, 1972, pp. 984-986. PMID 5084666 .
- ↑ D. Patel et al. a .: Peptide targeting and delivery across the blood-brain barrier utilizing synthetic triglyceride esters: design, synthesis, and bioactivity. In: Bioconjug Chem 8, 1997, pp. 434-441. PMID 9177851 .
- ↑ E. Mutschler u. a .: Mutschler drug effects. Wissenschaftliche Verlagsgesellschaft, Stuttgart, 2008, ISBN 3-8047-1952-X .
- ↑ Y. Takada et al. a .: Rapid high-affinity transport of a chemotherapeutic amino acid across the blood-brain barrier. In: Cancer Res 52, 1992, pp. 2191-2196. PMID 1559223 .
- ↑ DM Killian u. a .: Modulating blood-brain barrier interactions of amino acid-based anticancer agents. In: Drug Deliv 7, 2000, pp. 21-25. PMID 10895416 .
- ↑ Y. Takada et al. a .: Affinity of antineoplastic amino-acid drugs for the large neutral amino acid transporter of the blood-brain barrier. In: Cancer Chemother Pharmacol 30, 1991, pp. 89-94. PMID 1760863 .
- ↑ A. Bootz: Development, characterization and testing of nanoparticles to overcome the blood-brain barrier based on poly (butyl cyanoacrylate). Dissertation, Johann Wolfgang Goethe University, 2006.
- ↑ J. Temsamani et al. a .: Vector-mediated drug delivery to the brain. In: Expert Opin Biol Ther 1, 2001, pp. 773-782. doi : 10.1517 / 14712598.1.5.773 . PMID 11728213 (Review).
- ^ RL Roberts u. a .: Receptor-mediated endocytosis of transferrin at the blood-brain barrier. In: J Cell Sci 104, 1993, pp. 521-532. PMID 8505377 .
- ↑ B. Dehouck et al. a .: Upregulation of the low density lipoprotein receptor at the blood-brain barrier: intercommunications between brain capillary endothelial cells and astrocytes. In: J Cell Biol 126, 1994, pp. 465-473. PMID 8034745 .
- ↑ KR Duffy et al. a .: Human blood-brain barrier insulin-like growth factor receptor. In: Metabolism 37, 1988, pp. 136-140. PMID 2963191 .
- ↑ U. Bickel u. a .: Pharmacologic effects in vivo in brain by vector-mediated peptide drug delivery. In: PNAS 90, 1993, pp. 2618-2622. PMID 8385339 .
- ↑ T. Moos and EH Morgan: Restricted transport of anti-transferrin receptor antibody (OX26) through the blood-brain barrier in the rat. In: Journal of Neurochemistry 79, 2001, pp. 119-129. PMID 11595764 .
- ↑ WM Pardridge u. a .: Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. In: Pharm Res 12, 1995, pp. 807-816. PMID 7667183 .
- ↑ WM Pardridge: Non-invasive drug delivery to the human brain using endogenous blood-brain barrier transport system. In: Pharmacol Sci Technol Today 2, 1999, pp. 49-59. PMID 10234207
- ↑ WM Pardridge u. a .: Transport of human recombinant brain-derived neurotrophic factor (BDNF) through the rat blood-brain barrier in vivo using vector-mediated peptide drug delivery. In: Pharm Res 11, 1994, pp. 738-746. PMID 8058646 .
- ↑ a b c J. M. Scherrmann: Drug delivery to brain via the blood-brain barrier. In: Vascul Pharmacol 38, 2002, pp. 349-354. PMID 12529929 (Review).
- ↑ D. Karkan et al. a .: A unique carrier for delivery of therapeutic compounds beyond the blood-brain barrier. In: PLoS ONE 3, 2008, e2469. PMID 18575595 .
- ↑ C. Rousselle et al. a .: New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. In: Mol Pharmacol 57, 2000, pp. 679-686. PMID 10727512 .
- ↑ C. Rousselle et al. a .: Enhanced delivery of doxorubicin into the brain via a peptide vector-mediated strategy: saturation kinetics and specificity. In: J Pharmacol Exp Ther 296, 2001, pp. 124-131. PMID 11123372 .
- ↑ B. Christiaens et al. a .: Tryptophan fluorescence study of the interaction of penetratin peptides with model membranes In: FEBS Journal 269, 2002, pp. 2918-2926. PMID 12071955 .
- ↑ SR Schwarze u. a .: In vivo protein transduction: delivery of a biologically active protein into the mouse. In: Science 285, 1999, pp. 1569-1572. PMID 10477521 .
- ↑ M. Pooga et al. a .: Cell penetration by transportan. In: FASEB 12, 1998, pp. 67-77. PMID 9438412 .
- ↑ MJ Haney, Y. Zhao, EB Harrison, V. Mahajan, S. Ahmed, Z. He, P. Suresh, SD Hingtgen, NL Klyachko, RL Mosley, HE Gendelman, AV Kabanov, EV Batrakova: Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. In: PloS one. Volume 8, number 4, 2013, p. E61852, doi: 10.1371 / journal.pone.0061852 . PMID 23620794 . PMC 3631190 (free full text).
- ↑ EV Batrakova, HE Gendelman, AV Kabanov: Cell-mediated drug delivery. In: Expert opinion on drug delivery. Volume 8, Number 4, April 2011, pp. 415-433, doi: 10.1517 / 17425247.2011.559457 . PMID 21348773 . PMC 3062753 (free full text).
- Jump up ↑ Y. Zhao, MJ Haney, V. Mahajan, BC Reiner, A. Dunaevsky, RL Mosley, AV Kabanov, HE Gendelman, EV Batrakova: Active Targeted Macrophage-mediated Delivery of Catalase to Affected Brain Regions in Models of Parkinson's Disease. In: Journal of nanomedicine & nanotechnology. S4September 2011, S., doi: 10.4172 / 2157-7439.S4-003 . PMID 22288024 . PMC 3267477 (free full text).
- ↑ a b F. Hervé u. a .: CNS delivery via adsorptive transcytosis. In: AAPS J 10, 2008, pp. 455-472. PMID 18726697 (Review).
- ↑ MW Smith and M. Gumbleton: Endocytosis at the blood-brain barrier: from basic understanding to drug delivery strategies. In: J Drug Target 14, 2006, pp. 191-214. PMID 16777679 (Review).
- ^ I. Tamai et al. a .: Structure-internalization relationship for adsorptive-mediated endocytosis of basic peptides at the blood-brain barrier. In: J Pharmacol Exp Ther 280, 1997, pp 410-415. PMID 8996222 .
- ↑ T. Terasaki et al. a .: In vivo transport of a dynorphin-like analgesic peptide, E 2078, through the blood-brain barrier: an application of microdialysis. In: J Pharmacol Exp Ther 251, 1991, pp. 815-820. PMID 1681528 .
- ↑ S. Nobmann: Isolated brain capillaries as an in vitro model of the blood-brain barrier. Dissertation, Ruprecht-Karls-Universität Heidelberg, 2001.
- ↑ HH Szeto: Development of mitochondria-targeted aromatic-cationic peptides for neurodegenerative diseases. In: Ann NY Acad Sci 1147, 2008, pp. 112-121. PMID 19076436 .
- ↑ JF Poduslo et al. a .: Putrescine-modified nerve growth factor: bioactivity, plasma pharmacokinetics, blood-brain / nerve barrier permeability, and nervous system biodistribution. In: J Neurochem 71, 1998, pp. 1651-1660. PMID 9751199 .
- ↑ TM Wengenack u. a .: Putrescine-modified catalase with preserved enzymatic activity exhibits increased permeability at the blood-nerve and blood-brain barriers. In: Brain Res 767, 1997, pp. 128-135. PMID 9365024 .
- ↑ Vinogradov SV, Bronich TK, Kabanov AV: Nano Sized cationic hydrogels for drug delivery: preparation, properties and interactions with cells . In: Adv. Drug Deliv. Rev. . 54, No. 1, January 2002, pp. 135-47. PMID 11755709 .
- ^ J. Kreuter: Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. In: J Nanosci Nanotechnol 4, 2004, pp. 484-488. PMID 15503433 (Review).
- ↑ P. Blasi et al. a .: Solid lipid nanoparticles for targeted brain drug delivery. In: Adv Drug Deliv Rev 59, 2007, pp. 454-477. PMID 17570559 (Review).
- ↑ JC Oliver: Drug transport to brain with targeted nanoparticles. In: NeuroRx 2, 2005, pp. 108-119. PMID 15717062 (Review).
- ↑ P. Calvo et al. a .: Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. In: Pharm Res 18, 2001, pp. 1157-1166. PMID 11587488
- ↑ J. Kreuter: Nanoparticulate systems for brain delivery of drugs. In: Adv Drug Deliv Rev 47, 2001, pp. 65-81. PMID 11251246 (Review).
- ↑ J. Kreuter and S. Gelperina: Use of nanoparticles for cerebral cancer. In: Tumori 94, 2008, pp. 271-277. PMID 18564616 .
- ↑ AE Gulyaev et al. a .: Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. In: Pharm Res 16, 1999, pp. 1564-1569. PMID 10554098 .
- ↑ AJ Sawyer et al. a .: New methods for direct delivery of chemotherapy for treating brain tumors. In: Yale J Biol Med 79, 2006, pp. 141-152. PMID 17940624 (Review).
- ↑ E. Garcia-Garcia et al. a .: Colloidal carriers and blood-brain barrier (BBB) translocation: a way to deliver drugs to the brain? In: Int J Pharm 298, 2005, pp. 274-292. PMID 15896933 (Review).
- ↑ SB Tiwari and MM Amiji: A review of nano carrier-based CNS delivery systems. In: Curr Drug Deliv 3, 2006, pp. 219-232. PMID 16611008 (Review).
- ↑ IP Kaur u. a .: Potential of solid lipid nanoparticles in brain targeting. In: J Control Release 127, 2008, pp. 97-109. PMID 18313785 (Review).
- ↑ JL Gilmore et al. a .: Novel nanomaterials for clinical neuroscience. In: J Neuroimmune Pharmacol 3, 2008, pp. 83-94. PMID 18210200 (Review).
- ^ WH De Jong and PJ Borm: Drug delivery and nanoparticles: applications and hazards. In: Int J Nanomedicine 3, 2008, pp. 133-149. PMID 18686775 (Review).
- ↑ RD Broadwell et al. a .: Morphologic effect of dimethyl sulfoxide on the blood-brain barrier. In: Science 217, 1982, pp. 164-166. PMID 7089551 .
- ↑ a b c J. P. Hanig u. a .: Ethanol enhancement of blood-brain barrier permeability to catecholamines in chicks. In: Eur J Pharmacol 18, 1972, pp. 79-82. PMID 5031276 .
- ↑ B Erdlenbruch u. a .: Transient and controllable opening of the blood-brain barrier to cytostatic and antibiotic agents by alkylglycerols in rats. In: Exp. Brain Res. 135, 2000, pp. 417-422. PMID 11146820 .
- ↑ a b B. Erdlenbruch u. a .: Alkylglycerol opening of the blood-brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. In: British Journal of Pharmacology 140, 2003, pp. 1201-1210. PMID 14597599 .
- ↑ HJ Lee et al. a .: Blood-brain barrier disruption following the internal carotid arterial perfusion of alkyl glycerols. In: Journal of drug targeting 10, 2002, pp. 463-467. PMID 12575736 .
- ↑ B. Erdlenbruch u. a .: Intracarotid administration of short-chain alkylglycerols for increased delivery of methotrexate to the rat brain. In: British journal of pharmacology 139, 2003, pp. 685-694. PMID 12812991 .
- ↑ B. Erdlenbruch u. a .: Blood-brain barrier opening with alkylglycerols: Biodistribution of 1-O-pentylglycerol after intravenous and intracarotid administration in rats. In: J Drug Target 13, 2005, pp. 143-150. PMID 16036302 .
- ↑ A. Saija et al. a .: Changes in the permeability of the blood-brain barrier following sodium dodecyl sulphate administration in the rat. In: Exp Brain Res 115, 1997, pp. 546-551. PMID 9262210 .
- ^ MD Ellison et al. a .: Blood-brain barrier dysfunction in cats following recombinant interleukin-2 infusion. In: Cancer Res 47, 1987, pp. 5765-5770. PMID 3499219 .
- ↑ MN Azmin u. a .: The distribution and elimination of methotrexate in mouse blood and brain after concurrent administration of polysorbate 80. In: Cancer Chemother Pharmacol 14, 1985, pp. 238-242. PMID 3995684 .
- ↑ T. Sakane et al. a .: The effect of polysorbate 80 on brain uptake and analgesic effect of D-kyotorphin. In: Int J Pharm 57, 1989, pp. 77-83.
- ↑ a b c Y. Su and PJ Sinko: Drug delivery across the blood-brain barrier: why is it difficult? how to measure and improve it? In: Expert Opin Drug Deliv 3, 2006, pp. 419-435. PMID 16640501 (Review).
- ↑ AH Schinkel et al. a .: Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. In: Cell 77, 1994, pp. 491-502. PMID 7910522 .
- ↑ P. Jolliet-Riant and JP Tillement: Drug transfer across the blood-brain barrier and improvement of brain delivery. In: Fundam Clin Pharmacol 13, 1999, pp. 16-26. PMID 10027084 (Review).
- ↑ RB Kim et al. a .: The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. In: J Clin Investig 101, 1998, pp. 289-294. PMID 9435299 .
- ↑ EM Kemper et al. a .: Increased penetration of paclitaxel into the brain by inhibition of P-glycoprotein. In: Clin Cancer Res 9, 2003, pp. 2849-2855. PMID 12855665 .
- ↑ a b E. M. Kemper u. a .: The influence of the P-glycoprotein inhibitor zosuquidar trihydrochloride (LY335979) on the brain penetration of paclitaxel in mice. In: Cancer Chemother Pharmacol 53, 2004, pp. 173-178. PMID 14605863 .
- ↑ a b E. M. Kemper u. a .: Improved penetration of docetaxel into the brain by co-administration of inhibitors of P-glycoprotein. In: Eur J Cancer 40, 2004, pp. 1269-1274. PMID 15110893 .
- ↑ a b U. Mayer u. a .: Full blockade of intestinal P-glycoprotein and extensive inhibition of blood-brain barrier P-glycoprotein by oral treatment of mice with PSC833. In: J Clin Investig 100, 1997, pp. 2430-2436. PMID 9366556 .
- ↑ a b N. H. Hendrikse u. a .: Complete in vivo reversal of P-glycoprotein pump function in the blood-brain barrier visualized with positron emission tomography. In: Br J Pharmacol 124, 1998, pp. 1413-1418. PMID 9723952 .
- ↑ H. Kusuhara et al. a .: P-glycoprotein mediates the efflux of quinidine across the blood-brain barrier. In: J Pharmacol Exp Ther 283, 1997, pp. 574-580. PMID 9353372 .
- ↑ P. Hsiao et al. a .: Verapamil P-glycoprotein transport across the rat blood-brain barrier: cyclosporine, a concentration inhibition analysis, and comparison with human data. In: J Pharmacol Exp Ther . 317, 2006, pp. 704-710. PMID 16415090 .
- ↑ N. Drion et al. a .: Role of P-glycoprotein in the blood-brain transport of colchicine and vinblastine. In: J Neurochem 67, 1996, pp. 1688-1693. PMID 8858954 .
- ↑ I. Sauer: Apolipoprotein E derived peptides as vectors for overcoming the blood-brain barrier. Dissertation FU Berlin, 2004.
- ↑ L. He, C. Zhao, M. Yan, LY Zhang, YZ Xia: Inhibition of P-glycoprotein function by procyanidine on blood-brain barrier. In: Phytother Res 23, 2009, pp. 933-937 PMID 19172664 .
- ^ SF Zhou: Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. In: Xenobiotica 38, 2008, pp. 802-832. PMID 18668431 (Review).
- ↑ YJ Lee et al. a .: Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. In: J Pharmacol Exp Ther 312, 2005, pp. 44-52. PMID 15448171 .
- ↑ H. Yuan et al. a .: Strategies to overcome or circumvent P-glycoprotein mediated multidrug resistance. In: Curr Med Chem 15, 2008, pp. 470-476. PMID 18289002 (Review).
- ^ HM Coley: Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. In: Cancer Treat Rev 34, 2008, pp. 378-390. PMID 18367336 (Review).
- ↑ S. Nobili et al. a .: Pharmacological strategies for overcoming multidrug resistance. In: Curr Drug Targets 7, 2006, pp. 861-879. PMID 16842217 (Review).
- ↑ a b N. N. Salama et al. a .: Tight junction modulation and its relationship to drug delivery. In: Adv Drug Deliv 58, 2006, pp. 15-28. PMID 16517003 .
- ↑ NS Ningaraj Drug delivery to brain tumors: challenges and progress. In: Expert Opin Drug Deliv 3, 2006, pp. 499-509. PMID 16822225 .
- ↑ M. Kondoh and K. Yagi: Tight junction modulators: promising candidates for drug delivery. In: Curr Med Chem 14, 2007, pp. 2482-2488. PMID 17979701 (Review).
- ^ PH Johnson et al. a .: Discovery of tight junction modulators: significance for drug development and delivery. In: Drug Discov Today 13, 2008, pp. 261-267. PMID 18342803 (Review).
- ↑ A. Fasano et al. a .: Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. In: J Clin Invest 96, 1995, pp. 710-720. PMID 7635964 .
- ↑ MA Deli: Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. In: Biochim. Biophys. Acta 1788, 2009, pp. 892-910 PMID 18983815 (Review).
- ↑ CS Karyekar et al. a .: Zonula occludens toxin increases the permeability of molecular weight markers and chemotherapeutic agents across the bovine brain microvessel endothelial cells. In: J Pharm Sci 92, 2003, pp. 414-423. PMID 12532391 .
- ↑ K.-H. Song u. a .: Effect of the six-mer synthetic peptide (AT1002) fragment of zonula occludens toxin on the intestinal absorption of cyclosporin A. In: Int J Pharm 351, 2008, pp. 8-14. PMID 17954018 .
- ^ V. Wong and B. Gumbiner: A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. In: J Cell Biol 136, 1997, pp. 399-409. PMID 9015310 .
- ^ S. Zausinger: Bradykinin receptor antagonists in cerebral ischemia and trauma. In: IDrugs 6, 2003, pp. 970-975. PMID 14534854 (Review).
- ↑ a b N. Hettenbach: Influence of chronic electromagnetic exposure to mobile phone radiation (GSM and UMTS) on the integrity of the blood-brain barrier in rats. Dissertation, Ludwig Maximilians University Munich, 2008.
- ^ SI Rapoport u. a .: Testing of a hypothesis for osmotic opening of the blood-brain barrier. In: Am J Physiol 223, 1972, pp. 323-331. PMID 5046750 .
- ↑ a b S. I. Rapoport u. a .: Quantitative aspects of reversible osmotic opening of the blood-brain barrier. In: Am. J. Physiol. 238, 1980, pp. 421-431. PMID 7377381 .
- ↑ K. Dorovini-Zis: Hyperosmotic arabinose solutions open the tight junctions between brain capillary endothelial cells in tissue culture. In: Brain Res 302, 1984, pp. 383-386. PMID 6733518 .
- ^ SI Rapoport: Effect of concentrated solutions on the blood-brain barrier. In: Am J Physiol 219, 1970, pp. 270-274. PMID 5424853 .
- ^ SI Rapaport et al. a .: Reversible osmotic opening of the blood-brain barrier. In: Science 173, 1971, pp. 1026-1028. PMID 5098961 .
- ↑ EA Neuwelt u. a .: Use of enhanced computerized tomography to evaluate osmotic blood-brain barrier disruption. In: Neurosurgery 6, 1980, pp. 49-56. PMID 6153461 .
- ↑ YZ Zilyan et al. a .: Blood-brain barrier permeability to sucrose and dextran after osmotic opening. In: Am J Physiol 247, 1984, pp. R634-R638. PMID 6208789 .
- ^ PJ Robinson and SI Rapoport: Model for drug uptake by brain tumors: Effects of osmotic treatment and of diffusion in brain. In: J Cereb Blood Flow Metab 10, 1990, pp. 153-161. PMID 2303532 .
- ^ A b P. J. Robinson and SI Rapoport: Size selectivity of blood-brain barrier permeability at various times after osmotic opening. In: Am J Physiol 253, 1987, pp. R459-R466. PMID 2443025 .
- ↑ SI Rapoport: Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. In: Cell Mol Neurobiol 20, 2000, pp. 217-230. PMID 10696511 .
- ^ SI Rapoport: Modulation of the blood-brain barrier permeability. In: Journal of Drug Targeting 3, 1996, pp. 417-425. PMID 8863135 .
- ^ SI Rapoport: Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. In: Expert Opin Investig Drugs 10, 2001, pp. 1809-1818. PMID 11772287 (Review).
- ↑ K. Jahnke u. a .: Intraarterial chemotherapy and osmotic blood-brain barrier disruption for patients with embryonal and germ cell tumors of the central nervous system. In: Cancer 112, 2008, pp. 581-588. PMID 18072268 .
- ↑ L. Bakay et al. a .: Ultrasonically produced changes in the blood-brain barrier. In: Arch Neurol Psychiatry 76, 1956, pp. 457-467. PMID 13371961 .
- ↑ HT Ballantine u. a .: Progress and problems in the neurological applications of focused ultrasound. In: J Neurosurg 17, 1960, pp. 858-876. PMID 13686380 .
- ↑ K. Hynynen et al. a: Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. In: Radiology 220, 2001, pp. 640-646. PMID 11526261 .
- ↑ S. Meairs and A. Alonso: Ultrasound, microbubbles and the blood-brain barrier. In: Prog Biophys Mol Biol 93, 2007, pp. 354-362. PMID 16959303 (Review).
- ↑ HL Liu et al. a .: Magnetic resonance imaging enhanced by superparamagnetic iron oxide particles: usefulness for distinguishing between focused ultrasound-induced blood-brain barrier disruption and brain hemorrhage. In: J Magn Reson Imaging 29, 2009, pp. 31-38. PMID 19097103 .
- ↑ N. McDannold et al. a .: Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. In: Ultrasound Med Biol 34, 2008, pp. 930-937. PMID 18294757 .
- ↑ GT Clement and K. Hynynen: A non-invasive method for focusing ultrasound through the human skull. In: Phys Med Biol 47, 2002, pp. 1219-1236. PMID 12030552 .
- ↑ a b K. Hynynen: Ultrasound for drug and gene delivery to the brain. In: Advanced Drug Delivery Reviews 60, 2008, pp. 1209-1217. PMID 18486271 (Review).
- ^ A b W. L. Nyborg: Biological effects of ultrasound: development of safety guidelines. Part II: general review. In: Ultrasound Med Biol 27, 2001, pp. 301-333. PMID 11369117 .
- ↑ L. Krizanac-Bengez et al. a .: The cerebral vasculature as a therapeutic target for neurological disorders and the role of shear stress in vascular homeostatis and pathophysiology. In: Neurol Res 26, 2004, pp. 846-853. PMID 15727268 (Review).
- ↑ N. Sheikov et al. a .: Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. In: Ultrasound Med Biol 34, 2008, pp. 1093-1104. PMID 18378064 .
- ↑ LH Treat u. a .: Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. In: Int J Cancer 121, 2007, pp. 901-907. PMID 17437269 .
- ↑ M. Kinoshita u. a .: Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. In: PNAS 103, 2006, pp. 11719-11723. PMID 16868082 .
- ↑ M. Kinoshita u. a .: Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. In: Biochem Biophys Res Commun 340, 2006, pp. 1085-1090. PMID 16403441 .
- ↑ SB Raymond et al. a .: Ultrasound Enhanced Delivery of Molecular Imaging and Therapeutic Agents in Alzheimer's Disease Mouse Models. In: PLoS ONE 3, 2008, e2175. PMID 18478109 .
- ↑ N. Sheikov et al. a .: Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. In: Ultrasound Med Biol 32, 2006, pp. 1399-1409. PMID 16965980 .
- ↑ GJ Jungehulsing u. a .: Diagnostic transcranial ultrasound perfusion imaging at 2.5 MHz does not affect the blood-brain barrier. In: Ultrasound Med Biol 34, 2008, pp. 147-150. PMID 17854981 .