Mustard gas
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Mustard gas | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 8 Cl 2 S | ||||||||||||||||||
| Brief description |
colorless to yellowish, oily liquid, in its pure form almost odorless. In technical purity, a garlic or mustard-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 159.07 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.27 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
13-14 ° C |
||||||||||||||||||
| boiling point |
217 ° C |
||||||||||||||||||
| Vapor pressure |
8.7 Pa (20 ° C) |
||||||||||||||||||
| solubility |
very heavy in water (0.48 g l −1 at 20 ° C) |
||||||||||||||||||
| Refractive index |
1.5313 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
No MAK value is generally assigned for carcinogenic substances. |
||||||||||||||||||
| Toxicological data |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Mustard gas is a common name for the chemical bis (2-chloroethyl) sulfide , a skin-damaging chemical warfare agent from the group of mustards . Other names are Lost , sulfur Lost , mustard gas , mustard gas , yperite or Schwefelyperit , in English usage sulfur mustard , mustard gas or shortly mustard . The NATO code is HD . The name "mustard gas" comes from the typical smell of mustard or garlic of the not highly purified product . In their pure form, mustards are colorless and odorless liquids at room temperature. The designation as a gas for these substances does not apply in the strict sense. Presumably, after the first use of chlorine gas as a chemical weapon (1915) , “ poison gas ” was initially adopted indiscriminately for all other chemical warfare agents.
history
The production was first achieved in 1822 by the Belgian chemist César-Mansuète Despretz , who observed the formation of a foul-smelling liquid while experimenting with ethene and sulfur dichloride . The French Alfred Riche produced mustard gas from chlorine and diethyl sulfide in 1854 . In 1886, the chemical was first fully described by the German chemist Victor Meyer . The proposal to use it as a warfare agent came from the two German chemists Wilhelm Lo mmel and Wilhelm St einkopf , both of Fritz Haber's employees at the Kaiser Wilhelm Institute , in 1916. The name Lost came from the first two letters of their surnames.
According to the memories of Wilhelm Westphal , Haber explicitly warned at a meeting in the War Ministry, in which Erich Ludendorff also participated, against the use of mustard gas if one was not sure that the war would be won in a year, as the opponent would then develop the poison gas himself would have, whatever happened. After a gas attack, according to Haber, clothes would have to be changed, which would not cause a problem for the opponent, but for the Germans.
First World War
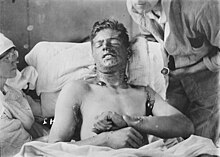
For the first time during the First World War, Schwefellost was used by the German troops on the night of July 12th to 13th, 1917. The tactical goal was to improve the German starting position for the expected British attack near Ypres (hence the name Yperit). Sulfur must became one of the most feared weapons in the final year of World War I because of the disfiguring injuries it caused.
"Of the German yellow gas attacks, in which the troops themselves and not the empty ground were shot at, Professor Meyer himself states :" The effect of the Yellow Cross in the Battle of Flanders in 1917 increased more and more, and it happened repeatedly that the Opponent was happy when he was able to keep a quarter of his team undamaged. ”The three quarters of the others, the damaged ones, may have consoled themselves with his famous paraphrase of the mustard gas effect, which reads:“ The wounds are not fatal in and of themselves 'But it is often because the breathing process in the lungs is stopped.' So that means if you have someone's throat constricted, it is not in and of itself fatal. You only die because you can no longer breathe ! - and the honor of mustard gas is saved. "
However, the phosgene used from 1915 onwards killed even more soldiers in the First World War than sulfur mustard.
Rif War in Morocco (1921–1926)
The Rif War was rushed by Spanish troops and started without securing the supply lines.
The leader of the Berber tribes, Mohammed Abd al-Karim , attacked the Spanish positions at Annual (Morocco) in northeastern Morocco on July 22, 1921 . Between 8,000 and 10,000 Spanish soldiers were killed in the three weeks of the Battle of Annual .
Then the Spaniards decided, with the help of Hugo Stoltzenberg, to use mustard gas on a large scale in this area. To this end, they cleared the central Rif Mountains by early 1925.
France also intervened in the war in 1925: the French Minister of War Paul Painlevé agreed on June 17, 1925 in Madrid with the dictator Miguel Primo de Rivera to erect an effective sea blockade . On July 13, 1925, Philippe Pétain was appointed Commander-in-Chief of the French Rif Army. He had more than a hundred battalions, not counting the more than 350,000 Harkas des Majzen , the administration of the Sultan Mulai Yusuf . From 1925 onwards, 250,000 men under Pétain occupied the fertile areas in French Morocco and prevented the Rif Republic from being supplied with food. Chemical weapons were used on a massive scale against the area controlled by the Rif Republic (main article: Use of chemical weapons in the Rif War ).
The use of mustard gas was a violation of the Hague Land Warfare Regulations . The Geneva Convention of June 1925 expressly prohibited the use of chemical and biological weapons.
The contamination with mustard resulted in the area around Al-Hoceima still leading the lung cancer statistics in Morocco.
Italo-Ethiopian War (1935-1936)
On October 3, 1935, Mussolini started the Italo-Ethiopian War . About 200,000 Italian soldiers and black shirts advanced into Ethiopia . When the advance stalled after a while, the fascist war command used poison gas and large-scale aerial bombardment to quickly win the war. There were massive air strikes with skin-damaging mustard gas, which led to high casualties among the poorly equipped and lightly dressed Ethiopian soldiers. In doing so, Italy broke the Geneva Protocol , to which it was bound under international law. The warfare agent was used against Ethiopian soldiers and against the civilian population. Agricultural areas were also contaminated with mustard gas and entire villages were cremated. There were mass shootings among the local population. The Italian associations also bombed hospitals of the Red Cross and the Red Crescent . To do this, they used cartographic material that the Red Cross had sent to Rome at the start of the war, in order to prevent (accidental) attacks on hospitals.
The targeted attacks made international headlines. The sanctions were not significantly tightened; France and Great Britain tried not to push Mussolini into Hitler's arms. In vain did Emperor Haile Selassie appear personally before the League of Nations and demand support. On May 5, 1936, the Italian Field Marshal Pietro Badoglio finally moved into the Ethiopian capital Addis Ababa ; so the war ended. Italy took the protests as an opportunity to withdraw from the League of Nations.
Second World War
During the Second World War, ammunitioned sulfur mustard was used, as far as is known, only once. This happened when a bridge was blown up and a roadblock was mined by Polish troops near Jasło . On September 8, 1939, two German soldiers were killed and twelve wounded. It is believed, however, that this was the decision of a single Polish officer. For this reason, the German troops did not retaliate.
On December 2, 1943, the German Air Force bombed the port of Bari in Italy. The US freighter John Harvey , loaded with sulfur mustard grenades, was hit and sunk. Part of the cargo ran into the water, another part was dispersed in the air by the explosions and fires. Since only a few people in Bari knew of the existence of this cargo due to the secrecy and they all perished, the wounded could not be treated properly at first. Exact numbers about the victims do not exist. It is estimated that over 600 soldiers and merchant marine personnel were burned, of whom about 100 died. The number of civilians killed is thought to be around 1,000. This incident almost triggered a further escalation of the war, since the Allies initially assumed that the warfare agent had been dropped by the Germans. A gas bomb found in the harbor basin was identified as an American model in good time, so that the Allies did not carry out a "counter strike".
During the Nazi era, S-Lost was produced in Munster and in Ammendorf near Halle in Germany by ORGACID GmbH until 1942 , but was no longer used during World War II . Under the former Ammendorfer company premises on today's Camillo-Irmscher-Straße there are eight widely ramified green-tiled cisterns that were difficult to detoxify due to the lack of building plans and were hermetically sealed after the fall of the Wall . Nevertheless, after 1990, 30 tons of toxins came to the surface through the groundwater.
After 1945

After the two world wars, a large part of the remaining German stocks of sulfur mustard was sunk in the Baltic Sea . Since the mustard gradually emerges from the now leaky barrels, small lumps of mustard that look like amber but are quite soft can be found on the beaches of the Baltic Sea . Chemical burns can occur on skin contact. The unsunked part has been disposed of in a dismantling and incineration facility in Munster by the German Association for Combat Disposal (GEKA) for several years .
Operations in further conflicts
Lost was used in the following conflicts:
- Great Britain during an intervention in the Russian Civil War in 1919
- Italy versus Libya in 1930
- Soviet Union in Xinjiang , a province of the Republic of China , in 1934 against Muslim and national Chinese insurgents
- Italy versus Ethiopia in 1935-1940
- Japan versus the Republic of China from 1937 to 1945
- Egypt versus the Yemeni Arab Republic from 1963 to 1967
- Iraq against Iran in the years 1983 to 1988, for long-term consequences see
- Iraq against members of the Kurdish minority in the poison gas attack on Halabja in 1988
- suspected use in Syria against Kurds in the attack on a small town near Kobane on July 12, 2014
- Confirmed use against Syrian Kurds during fighting in al-Hasakah by the terrorist militia Islamic State in summer 2015
- Confirmed operation against Iraqi Kurds in Machmur by the Islamic State terrorist militia in early August 2015
- According to reports not only from the US Department of Defense, on September 20, 2016, a mustard gas grenade shot down by the Islamic State terrorist group hit the al-Qayyara West air force base . No victims are known.
Production of S-Mustard
Original procedure
In Germany, S-mustard was produced in both world wars using a process developed by Victor Meyer by reacting thiodiglycol with dry hydrogen chloride at 50 ° C.
During the First World War, the Allies chose the electrophilic addition of sulfur chlorides to ethene as a synthesis . Initially, mixtures of disulphur dichloride and sulfur dichloride were used, resulting in a product that was heavily contaminated with other thioethers, which was regarded as a pure substance. Later, the reaction was also carried out with the pure substances disulfur dichloride (first synthesis of mustard in 1822 by César-Mansuète Despretz ) or sulfur dichloride (introduced in 1922 by William Jackson Pope ).
Depending on the starting material, the process is also called the Levinstein process or the Depretz method. In the reaction with disulfur dichloride, sulfur is formed as a by-product, which was regarded as an intermediate reaction of disulfur dichloride to form sulfur dichloride. When pure sulfur dichloride reacts, sulfur is also formed, as it is usually split into monochloride and sulfur and chemically behaves like a solution of chlorine in a sulfur monochloride and sulfur dichloride mixture. The crude product (Prochlerite) can be stored for years and contains about 70% S must, small amounts of dithio- and polythioethers (Levinsteinyperite) and other impurities. With newer process methods, about 92% S-mustard occurs.
In large-scale production, mostly cast-iron, lead-lined tanks with built-in agitators were used. It was filled with S 2 Cl 2 and ethene was blown in through a tube at the bottom while stirring. After the reaction was complete, the dichlorodiethyl sulfide was run through a settling tank to remove the sulfur formed. There was no further concentration.
In the USA, mustard was also produced by the radical addition of hydrogen sulfide to vinyl chloride under UV light using organic peroxides as a catalyst.
Modern process
The reaction of sodium hydrogen sulfide with ethylene oxide produces thiodiglycol as an intermediate . This is then chlorinated to mustard with thionyl chloride (SOCl 2 ) in a further reaction step .
toxicity
The main routes of exposure are percutaneous or inhalation ingestion of vapors. Lost is a powerful skin toxin and has been shown to be carcinogenic . The effect on the skin is comparable to severe burns or chemical burns . Large, very painful blisters form. The injuries heal badly. The tissue is permanently destroyed and cell division is inhibited. Extensively affected limbs usually have to be amputated. If the vapors are inhaled, the bronchi are destroyed.
Protective measures
Because of the high skin penetration and the delayed onset of action, the protection of the body surface is of particular importance. The absorption through the skin takes place easily and without any noticeable signs such as a sensation of wetness or cold. As a rule, the victim does not notice the poisoning.
Sulfur must, both in liquid and in the gas phase, can penetrate commercially available textiles relatively quickly; this ability, combined with the long latency period before the onset of action, greatly increases the danger posed by sulfur mustard. The common protective equipment introduced by most of the armed forces - masks, protective gloves, overshoes and protective suits - offer safe protection against the effects for a period of at least six hours. In the future z. For example, the German Armed Forces require secure protection over a period of at least 24 hours.
For decontamination, oxidizing agents (e.g. chlorinated lime or calcium hypochlorite ), alkaline solutions and non-aqueous media, e.g. B. amino alcoholates can be used because mustard is sensitive to oxidizing agents on the one hand and the hydrolysis of mustards once dissolved is very rapid on the other hand .
Analytics
The reliable determination of mustard gas in air samples is made possible by specially equipped mass spectrometers .
International controls
As a chemical on List 1 in the international disarmament treaty, S-Mustard is controlled by the responsible UN agency OPCW , based in The Hague. Development or possession for military purposes is prohibited. In Germany, any civil handling of S-Lost must be approved by the Federal Office of Economics and Export Control (BAFA) and reported to the OPCW.
literature
- K. Kehe, L. Szinicz: Medical aspects of sulfur mustard poisoning . In: Toxicology 214 (3), 2005, pp. 198-209. PMID 16084004 .
- Henning Sietz: It smells like mustard! At the invitation of the Soviets, the German military tested chemical warfare agents on the Volga between 1926 and 1933 . In: Die Zeit , No. 26/2006.
- Holger Schulz, Martin Weber: 100 years of chemical warfare agents as a means of warfare - review and current state of knowledge on toxicology and forensics of sulfur mustard . (PDF) In: Toxichem Krimtech , 2015, 82 (1), p. 5
Web links
- Mustard: An Ambiguous Term (English, PDF)
- Civil Defense Research - The Behavior of Environmental Chemicals in Soil and Groundwater . (PDF, 9.36 MB) Federal Office for Civil Protection
Individual evidence
- ↑ a b c d e f g h entry to bis (2-chloroethyl) sulfide in the GESTIS database of IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-50.
- ↑ There is not yet a harmonized classification for this substance . A labeling of bis (2-chloroethyl) sulfide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on November 1, 2016, is shown, which is derived from a self-classification by the distributor .
- ^ Günter Hommel: Handbook of dangerous goods. Volume 6 Springer Berlin Heidelberg, 2012, ISBN 978-3-642-25051-4 , p. 2298.
- ↑ a b c Entry on mustard gas in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ SPIEZ LABORATORY: Mustard gas fact sheet (PDF; 244 kB), accessed on February 4, 2017.
- ↑ Markus Schnedlitz: Chemical warfare agents: history, properties, effects. GRIN Verlag, 2008, ISBN 978-3-640-23360-1 , p. 30.
- ^ Wilhelm Heinrich Westphal: 68 years as a physicist in Berlin. In: Physical sheets. 28, 1972, pp. 258-265, doi: 10.1002 / phbl.19720280603 .
- ↑ Otto Jekel: Poison clouds . Toxic gases in World War I and today. In: Neues Wiener Tagblatt . June 15, 1935, p. 12 ( ANNO - AustriaN Newspapers Online [accessed on May 18, 2020]).
- ↑ Gertrud Woker : Blossoms of war gas propaganda . In: The coming poison and fire war and its effects on the civilian population . 6-9 Edition. Ernst Oldenburg Verlag, Leipzig 1932, end of Chapter X, p. 249, 278 pages with illustrations.
- ↑ Death clouds over Europe . In: Der Spiegel . No. 8 , 1982 ( online ).
- ↑ L'histoire oubliée des surréalistes et la guerre du Rif ( Memento of February 12, 2008 in the Internet Archive )
- ^ Lina Grip, John Hart: The use of chemical weapons in the 1935-36 Italo-Ethiopian War. (PDF) ( Memento from September 24, 2015 in the Internet Archive ) SIPRI Arms Control and Non-proliferation Program, October 2009.
- ^ Frankfurter Allgemeine Zeitung: With poison gas to the empire. In: FAZ.net . April 19, 2007, accessed July 19, 2010 .
- ↑ Günther W. Gellermann : The war that did not take place Bernard & Graefe Verlag, Koblenz 1986, ISBN 3-7637-5804-6 , pp. 135-137 and appendix pp. 227-232.
- ^ Günther W. Gellermann: The war that did not take place. Bernard & Graefe Verlag, Koblenz 1986, ISBN 3-7637-5804-6 , pp. 160-165.
- ^ Robert Harris , Jeremy Paxman: The silent death - The history of biological and chemical weapons , Heyne Verlag , 2002, pp. 191–197.
- ↑ geschichtsspuren.de: Warfare agent in Munster-Nord - Heeresversuchsstelle robbery chamber .
- ↑ Uses of CW since the First World War. In: fas.org. Archived from the original on August 22, 2010 ; accessed on May 19, 2020 (English).
- ↑ SM Razavi, M. Ghanei, P. Salamati, M. Safiabadi: Long-term effects of mustard gas on respiratory system of Iranian veterans after Iraq-Iran war: a review. , Chin J Traumatol. 2013 Jun 1; 16 (3): 163-8, PMID 23735551 .
- ↑ MERIA Report: Did ISIS Use Chemical Weapons Against the Kurds in Kobani? dated October 12, 2014 (English), WARNING: contained images can be disturbing.
- ↑ Islamic State confirmed to have used mustard gas against Kurds in Syria .
- ↑ US reportedly sees possible pattern in ISIS chemical weapons attacks .
- ^ BREAKING: Tests prove ISIS using mustard gas against Kurds .
- ^ Islamic State Suspected of Using Chemical Weapon, US Says .
- ↑ Islamists fire mustard gas grenades at base in Iraq. In: Neue Zürcher Zeitung. nzz.ch, September 22, 2016, accessed on September 22, 2016 .
- ↑ Attack on US forces: IS is said to have used poison gas. In: n-tv . ntv.de, September 22, 2016, accessed November 1, 2016 .
- ↑ On the trail of poison gas in Syria - the Swiss chemical weapons expert Stefan Mogl looks back on a dangerous mission ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. , NZZ, January 12, 2018.
- ↑ a b c d e Siegfried Franke: Textbook of Military Chemistry Volume 1 . S. 280 .
- ^ P. David Josephy, Bengt Mannervik: Molecular Toxicology . Oxford University Press, 2006, ISBN 978-0-19-517620-9 , pp. 468 ( limited preview in Google Book search).
- ↑ FG Mann u. WJ Pope, J. Chem. Soc. 121, 1052 (1922).
- ↑ Mark Anthony Benvenuto: Industrial Inorganic Chemistry . Walter de Gruyter GmbH & Co KG, 2015, ISBN 978-3-11-033033-5 , p. 196 ( limited preview in Google Book search).
- ↑ T. Urabe, K. Takahashi, M. Kitagawa, T. Sato, T. Kondo, S. Enomoto, M. Kidera, Y. Seto: Development of portable mass spectrometer with electron cyclotron resonance ion source for detection of chemical warfare agents in air. Spectrochim Acta A Mol Biomol Spectrosc. 2013 Oct 22; 120C: pp. 437-444. PMID 24211802 .