polymer
| Polymers (examples) |
|---|
| The repeating unit of the polymer polypropylene . |
| Cellulose is a polymer and the important structural element of plants. |
A polymer [ poliˈmeːɐ̯ ] (from ancient Greek πολύ , polý 'a lot' and μέρος , méros 'part') is a chemical substance that consists of macromolecules . The macromolecules of a substance are made up of one or more structural units, the so-called constitutional repeat units or repeat units. The adjective polymer accordingly means “made up of many (equal) parts”. In many cases, a polymer consists of non-identical macromolecules because the number of repeating units and thus the molecular mass of the molecules varies. Synthetic or semi-synthetic polymers are the main component in the manufacture of plastics . Polymers produced by living beings are called biopolymers and are of essential importance for life.
Classification
Polymers can be divided into natural and synthetic polymers.
- Natural polymers ( biopolymers ) are synthesized in living things and form the basic building blocks of organisms. These polymers include proteins ; they form hair and silk , for example . The polysaccharides include cellulose , starch, and chitin . Nucleic acids fulfill many biological functions, such as deoxyribonucleic acid as a genetic material.
- Synthetic polymers are substances produced industrially or on a laboratory scale through polyreactions , including polyethylene , polystyrene and polyvinyl chloride . Chemically modified polymers are created through the further processing of biopolymers, for example nitrocellulose , celluloid or starch derivatives.
Polymers can be classified according to the number of basic materials ( monomers ) from which they are built.
- Homopolymers consist of only one type of monomer such as polyethylene , polypropylene , polyvinyl chloride or polycaprolactam . A natural homopolymer is natural rubber as a polyisoprene .
- Copolymers are made up of various monomers, such as acrylonitrile-butadiene-styrene copolymer (ABS), styrene-acrylonitrile (SAN) or butyl rubber . Most biopolymers are copolymers.
- Polymer blends are created by mixing different homopolymers and / or copolymers. They are usually produced by intensive mechanical mixing of molten polymers, which results in a homogeneous material. A special form of a blend is a polymer alloy .
Furthermore, can be organic of inorganic different polymers. Unlike organic polymers, inorganic polymers do not contain any carbon atoms in the main chain of the polymer. The inorganic polymers include polysiloxanes , polyphosphazenes or polysilazanes . While glasses are not counted among the polymers in most chemical textbooks, glasses and in some cases ceramics and basalt are considered inorganic polymers in other textbooks and in textile technology .
Synthetic polymers
Polymer chemistry
The formation of polymers from individual monomers takes place via various types of polyreactions , such as chain polymerizations , polycondensation or polyaddition .
Structure of polymers
The macromolecules formed during synthesis have different basic structures that determine the physical properties of the polymer. Linear macromolecules that only consist of one polymer chain ( main chain ) can form. In the case of polyethylene , the main chain is a long chain n -alkane . Depending on the reaction conditions, branched macromolecules with a main chain and side chains are also formed ; in the case of polyethylene, these would be alkyl radicals . In addition to the chain length, the degree of branching also determines the density, strength and melting point of the polymer. Highly branched polymers are amorphous , the molecules in the solid interact with one another in a disorderly manner . Unbranched macromolecules in particular form a partially crystalline structure as a solid , marked in red in the figure below. While branched and unbranched polymers are usually thermoplastics , many elastomers have a wide-meshed network between the “main chains”. Close-meshed networking, on the other hand, leads to thermosets . Networks and branches are shown in the figures as red dots.

linear macromolecule
branched macromolecule
semi-crystalline structure of linear polymers
wide-meshed cross-linked polymer
close-meshed cross-linked polymer
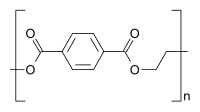
Polymers that are built up from only one (real or imaginary) type of monomer are called homopolymers . Polymers made from two or more different types of monomers are copolymers . Polymers such as polyethylene terephthalate , which are necessarily made from two different monomers, are usually regarded as homopolymers, as only one characteristic repeating unit can be formed at a time.
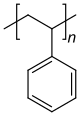
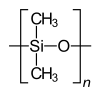
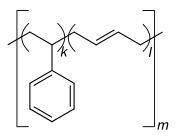
Homo- and copolymers (examples) Homopolymer Polystyrene : A constitutional repeating unit that is repeated n times in the macromolecule. Homopolymer poly (dimethylsiloxane) , a silicone . The main chain is made up of silicon and oxygen atoms. Copolymer styrene-butadiene rubber : The monomers styrene and 1,3-butadiene form two constitutional repeating units , which can alternate in any order in a macromolecule as shown in the figure. Polyethylene terephthalate has only one characteristic constitutional repeating unit, although two monomers have to be used for the synthesis.
In the case of copolymers, the sequence of the constitutional repeating units can be controlled via a synthetic route. It is divided into random and alternating copolymers, block copolymers, graft copolymers, and gradient copolymers. In the figure below, bipolymers are shown schematically, Ⓐ and Ⓑ symbolize the two repeating units.
Repeating units can carry substituents (“residues”) and are often identified with the letter R in figures. If the monomer units are asymmetrical, there is a tacticity of the arrangements in the macromolecule. The polymers can be divided into atactic, isotactic and syndiotactic polymers. An example of tacticities is polystyrene with a phenyl group as the remainder. While the classic synthesis route leads to atactic, amorphous plastics, syndiotactic synthesis results in a crystalline polystyrene of increasing economic importance.
In the case of polymers which are unsaturated in the main chain , analogous to the cis - trans -isomerism, cis - or trans -tactic polymers occur, as in the case of natural rubber or butadiene rubber . Stereospecific polymerizations often lead to higher mechanical strength, higher crystallinity, higher density and higher thermal stability.
Section from a polymer chain of cis -1,4- polybutadiene . A C 4 unit is marked in blue .
Section from a polymer chain of trans -1,4-polybutadiene. A C 4 unit is marked in blue .
Solid structures
The macroscopic physical properties of a polymer are closely linked to the interactions between the polymer chains.
 Statistical ball |
 Entanglement of several molecules with ball-like substructures |
- Atactic polymers, polymers with a high degree of branching and random copolymers form amorphous , glass-like structures in the solid state . In melt and solution, long-chain molecules tend to form a constantly changing “statistical tangle”, see Gaussian chain (Freely-Jointed-Chain-Model). In the solid state, the respective conformations of the molecules are frozen. Entanglement and entanglement of chain molecules with one another lead to a “mechanical bond” between the chains. Intermolecular and intramolecular minor valence bonds only occur at locations where molecular segments are close enough to one another. The irregular structure of the molecules prevents a closer arrangement. Such arrangements are sometimes called "spaghetti structure".
 Polyethylene: zigzag conformation of the molecules in close chain packing |
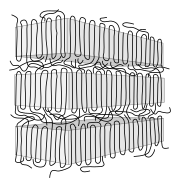 Lamellae with tie molecules |
 Spherulite |
 Helix |
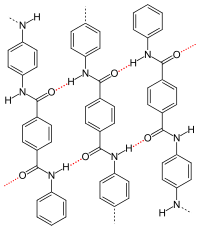 p -aramid , dotted red: hydrogen bonds |
- Linear polymers with a regular structure, with little branching and stereoregular (iso- and syndiotactic) polymers have a partially crystalline structure in the solid state . In the case of simply structured polymers, such as polyethylene , in an idealized conception, large areas are not present as a tangle, but in a zigzag conformation. Several zigzag conformations form dense chain packings and are therefore crystalline in these areas. Such crystallites are called lamellae and are much thinner (often around 10 nm) than the polymers are long. They are formed by more or less regular folds of one or more molecular chains. There are amorphous structures between the lamellae. Individual molecules can form entanglements between the lamellae, but they can also be involved in the formation of two (or more) lamellae (tie molecules). Several lamellae form a superstructure, a spherulite , often with a diameter in the range from 0.05 to 1 mm.
- The type and arrangement of (functional) residues of the repeat units influence or determine the crystallinity and strength of the secondary valence bonds. With isotactic polypropylene, the molecules form a helix . As with a zigzag conformation, helices allow close chain packing. Particularly strong intermolecular interactions occur when the remainder of the repeating units allow hydrogen bonds to form, as is the case with p -aramid , for example . The crystallinity and superstructure are always dependent on the conditions under which they are formed, see also crystallization of polymers . Compared to amorphous structures, semi-crystalline structures lead to a higher stiffness, density, melting temperature and durability of a polymer.
- Wide-meshed crosslinked polymers are elastomers and, like thermoplastics, cannot be melted without decomposition. Thermoplastic elastomers , on the other hand, are “physically cross-linked” via secondary valences and can be melted. Block copolymers, in which a hard segment of the polymer tends to crystallize, and a soft segment with an amorphous structure, are a variant. The hard segments ensure a wide-meshed, physical network.
Polymer physics
The classification of the polymer materials is based on DIN 7724 based on the temperature profile of the shear module and the tensile set at room temperature. It is based on the mechanical behavior in the service temperature range and the existence of a melting range (flow range):
In polymer physics one deals with, among other things
- Average molar mass , molar mass distribution
- Crystallization and Crystallization Kinetics
- Surface properties of polymers
- Rheology , i.e. the flow behavior and viscosity
Temperature resistant polymers
The temperature resistance of a polymer depends on the structure of the monomers used, the stability of the bonds between the monomers and the interactions between the polymer chains. High heat resistance can be achieved by increasing the enthalpy of fusion and lowering the entropy of fusion . In the case of amorphous polymers, the glass transition temperature and, in the case of partially crystalline polymers, the glass and melting temperature should be as high as possible. To achieve temperature resistance, CH bonds and CC bonds can be replaced by bonds between carbon and heteroatoms such as fluorine, nitrogen or oxygen, or by more stable aromatic bonds. Another possibility is the construction of polymers with two parallel and interconnected main chains (ladder polymers).
Conductive polymers
A prerequisite for the electrical conductivity of polymers is the presence of conjugated pi-electron systems . However, such polymers are initially still insulators, at best semiconductors. The conductivity, comparable to that of metallic conductors, only sets in when the polymers are oxidatively or reductively doped . The first investigations were carried out on polyacetylene , whose conductivity was achieved by doping with arsenic pentafluoride or iodine . In addition, the conductivity increases as the crystallinity of the polymer increases. Further examples of conductive polymers are doped polypyrrole , polyphenylene sulfide , polythiophene and organometallic complexes with macrocyclic ligands such as phthalocyanine . Oxidative doping can be achieved with arsenic pentafluoride, titanium tetrachloride , bromine or iodine, while reductive doping is achieved with sodium - potassium alloys or dilithium benzophenonate. Doping creates charges on the polymer chains, which are delocalized over the chains by the π conjugation. However, the explanation for the conductivity of polymers is very complex. Attempts have been made to describe the charge transport along a polyene chain with the soliton concept or with the model of bipolarons ( charge pairs held together in a small space).
Conducting, i.e. electrically active, polymers are used to create polytronic applications. In contrast to molecular electronics , the information is not processed in individual molecules, but in differently doped volumes.
Such electronic applications are:
- Displays: OFETs , OLEDs
- RFID tags
- Solar cells
- Sensors and actuators
- Fuel cells
- Lithium polymer batteries
- Electrolytic capacitors
Another application is the processing of polymers with the help of electronics in electrospinning .
Health assessment
Polymers are generally classified as harmless to health. Plastics can therefore in principle be used in the medical sector (e.g. as implants ) or in the food sector (as packaging ). However, it must be ensured that only harmless catalysts are used in the production, no harmful monomers are left behind, etc.
Supramolecular Polymers
A relatively new field of polymer chemistry comprising supramolecular polymers, ie polymers in which the blocks do not by covalent bonds , but by relatively weak intermolecular bonds such as hydrogen bonds , ionic bonds , metal-ligand interactions , Van der Waals or hydrophobic interactions are held together . These intermolecular bonds can be broken easily (at elevated temperature), but can also recede quickly (on cooling). Because of this reversibility, supramolecular polymers are a new class of self-healing materials. Another consequence of the weak intermolecular bonds is the low viscosity of melts of supramolecular polymers, which can be advantageous in production and processing, but also in certain applications such as inkjet printing.
While covalently bound polymers play a major role in nature (DNA, polypeptides, cellulose), relatively few naturally occurring supramolecular polymers are known. An example of supramolecular polymerization in nature is the self-assembly of the tobacco mosaic virus .
Examples and symbols
- Synthetic Polymers:
- Polyethylene (PE)
- Polypropylene (PP)
- Polyvinyl chloride (PVC)
- Polystyrene (PS), better known in the foamed state as Styropor ® (trade name of BASF )
- Polytetrafluoroethylene (PTFE), trade name is Teflon ® (E. I. Du Pont de Nemours and Company) or Tefal ®
- Polymethyl methacrylate (PMMA), under the trade name Plexiglas ® ( Evonik Industries AG )
- Polyacrylonitrile (PAN), as a copolymer with polymethyl methacrylate for the production of textile fibers
- Polyacrylamide (PAA) as a gel (former), flocculant and the like a.
- the group of polyamides , as PA66 under the trade name Nylon ® , as PA6 under the trade name Perlon ® or as PA12G under the trade name Lauramid ®
- the group of aramids (polyaramids, aromatic polyamides), including the textile fibers poly ( p -phenylene terephthalamide) (PPTA, trade names: Kevlar ® , Twaron ® ) and poly ( m -phenylene terephthalamide) (PMPI, trade names: Nomex ® , Teijinconex ® )
- Polyketones, such as polyetherketones (PEK)
-
Polyesters also belong to this product group
- Polycarbonates (PC) with trade names Lexan or Makrolon ® ( Covestro )
- Polyethylene terephthalate (PET)
- Polyethylene glycol (PEG)
- the group of polyurethanes (PU)
- Silicones , more precisely poly (organo) siloxanes
- Melamine resin (MF), a polymer based on melamine and formaldehyde
- Biopolymers:
literature
- Manfred D. Lechner, Klaus Gehrke, Eckhard H. Nordmeier: Macromolecular Chemistry. A textbook for chemists, physicists, materials scientists and process engineers. 5th edition, Springer-Verlag, Berlin, Heidelberg 2014, ISBN 978-3-642-41768-9 .
- J. Kahovec, RB Fox, K. Hatada: Nomenclature of regular single-strand organic polymers (IUPAC Recommendations 2002). In: Pure and Applied Chemistry . 74, 10, 2002, pp. 1921-1956, doi: 10.1351 / pac200274101921 , online version .
- Ulf W. Gedde: Polymer Physics. Chapman & Hall, London a. a. 1995, ISBN 0-412-62640-3 .
- H. Cherdron, F. Herold, A. Schneller: Technically important temperature-resistant polymers , Chemistry in our time , 23rd year 1989, No. 6, pp. 181-192, ISSN 0009-2851 .
- Klaus Menke, Siegmar Roth: Metallic conductive polymers I and II , Chemistry in Our Time, 20th year 1986, No. 1, pp. 1-10, No. 2, pp. 33-43, ISSN 0009-2851 .
- Michael Dröscher: States of Order in Polymers . In: Chemistry in Our Time . tape 10 , no. 4 , 1976, p. 106-113 , doi : 10.1002 / ciuz.19760100403 .
- Dietrich Braun: The long road to the macromolecule - polymer research before Hermann Staudinger . In: Chemistry in Our Time . tape 46 , no. 5 , 2012, p. 310-319 , doi : 10.1002 / ciuz.201200566 .
Web links
- VDMA polymer electronics
- Chemical background
- Introduction and view in the chemistry of the polymers (English)
Individual evidence
- ↑ Entry on polymer . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.P04735 Version: 2.3.3.
- ↑ Entry on polymer blend . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.P04736 Version: 2.3.1.
- ↑ Hans-Georg Elias: Macromolecules. John Wiley & Sons, 2003, ISBN 978-3-527-62654-0 . Volume 4, p. 165.
- ↑ Chokri Cherif (Ed.): Textile materials for lightweight construction Springer-Verlag, Berlin / Heidelberg 2011, ISBN 978-3-642-17991-4 , p. 14f.
- ↑ Hilmar Fuchs , Wilhelm Albrecht (Ed.): Nonwovens - Raw materials, production, application, properties, testing. 2nd edition, Wiley-VCH Verlag, Weinheim 2012, ISBN 978-3-527-31519-2 , p. 42.
- ↑ Wolfgang Bobeth (Ed.): Textile fibers. Texture and properties . Springer-Verlag, Berlin / Heidelberg / New York 1993, ISBN 3-540-55697-4 , types of textile fibers in Fig. 1.1; s. rear intent
- ↑ a b Bernd Tieke: Makromolekulare Chemie. 3rd edition, Wiley-VCH, Weinheim 2014, p. 295f.
- ^ A b Wolfgang Kaiser : Synthetic chemistry for engineers. 3rd edition, Carl Hanser, Munich 2011, p. 84.
- ↑ Harald Cherdron, Friedrich Herold, Arnold Schneller: Technically important temperature-resistant polymers. In: Chemistry in Our Time. 23, 1989, pp. 181-192, doi: 10.1002 / ciuz.19890230602 .
- ↑ CK Chiang, YW Park, AJ Heeger, H. Shirakawa, EJ Louis, AG MacDiarmid: Conducting polymers: Halogen doped polyacetylene . In: The Journal of Chemical Physics . tape 69 , no. 11 , 1978, p. 5098-5104 , doi : 10.1063 / 1.436503 .
- ^ GG Wallace, TE Campbell, PC Innis: Putting function into fashion: Organic conducting polymer fibers and textiles . In: Fibers and Polymers . tape 8 , no. 2 , 2007, p. 135-142 , doi : 10.1007 / BF02875782 .
- ↑ Peter Elsner, Peter Eyerer: Domininghaus - plastics: properties and applications . Ed .: Thomas Hirth. Springer, 2012, ISBN 978-3-642-16173-5 .
- ↑ Plastic recommendations of the BfR. Retrieved November 13, 2019 .
- ^ TFA de Greef, EW Meijer: Materials Science: Supramolecular Polymers . In: Nature. 453, pp. 171-173.
- ↑ orf.at: materials research - self-healing through light , accessed on April 21, 2011; Mark Burnworth, et al .: Optically healable supramolecular polymers. in: Nature, Volume 472, pp. 334-337, doi: 10.1038 / nature09963