Cyst kidney
| Classification according to ICD-10 | |
|---|---|
| Q61.1 | Polycystic kidney, autosomal recessive infantile type |
| Q61.2 | Polycystic kidney, autosomal dominant adult type |
| Q61.3 | Polycystic kidney, unspecified |
| ICD-10 online (WHO version 2019) | |

Polycystic kidney disease , also known as polycystic kidney designated ( English kidney disease polycystic , PCD are a group of serious, mostly) hereditary -related diseases of the kidney . Due to the formation of a multitude ( ancient Greek πολύς polys 'much') of fluid-filled chambers or vesicles, the so-called cysts , the kidneys are considerably restricted in their filtering function . A kidney cyst , on the other hand, is a single oneCyst mentioned in an examination as a generally harmless chance finding .
Genetically determined kidney cysts are the most common life-threatening hereditary disease in humans and one of the main causes of chronic kidney failure . A cure is only possible through a kidney transplant .
Symptoms
The first symptoms that may indicate cystic kidneys are high blood pressure , bloody urine ( hematuria ), repeated urinary tract infections , an increase in the size of the abdomen, and pain in the abdomen. As long as the kidneys can compensate for any functional restrictions, there are often no symptoms. About a third of patients remain symptom- free, even up to the point of end-stage renal failure (ESRF). This makes an early diagnosis much more difficult. The increasing destruction of the kidney tissue leads to more and more complaints in various organs due to the retained waste products and the water. These include decreased performance and malaise, the yellowing and itching of the skin due to the stored urine toxins, sleep and concentration disorders, headaches, calf cramps, nausea, vomiting, diarrhea and taste disorders. High blood pressure, cardiac arrhythmias or inflammation, and breathing problems also occur. In addition, there is anemia (due to the reduction in the erythropoietin produced by the kidney, which is used for blood formation), coagulation disorders, increased susceptibility to infections, cerebral bleeding and bone softening (since the kidney is also involved in the vitamin D metabolism).
Patients with polycystic kidneys often complain of pain in the side flank of their back or abdomen . The pain can be temporary or permanent, dull and excruciating. The pain is probably due to the extensive cyst growth. In addition, the surrounding organs are displaced by the extreme expansion of the kidney capsule ( Capsula fibrosa renalis ).
The pain can be relieved briefly by puncturing the cyst, for example percutaneously , i.e. through the skin, or minimally invasively by laparoscopic decortication of the cysts. Due to the formation of new cysts, these measures are not sustainable, so that the corresponding interventions have to be repeated. The treatment also does not change the course of the disease.
In around 30 to 50% of patients, the initial diagnosis of “polycystic kidneys” is made from bloody urine ( hematuria ). The cause of the bleeding is usually a crack in the cyst. The bleeding itself is largely harmless and stops by itself. Due to the impaired kidney function, patients with cystic kidneys excrete increased amounts of endogenous proteins in the urine . If the body excretes 30 to 300 milligrams of albumin per day , it is called microalbuminuria . If even larger amounts of albumin are excreted, this is called macroalbuminuria . If proteins larger than albumin can be detected in the urine, proteinuria is present. The latter can be easily detected with test strips that are held in the urine. Microalbuminuria is much more difficult to detect. Protein and microalbuminuria are an indication of impaired kidney function. Polycystic kidneys are just one of several possible conditions that can lead to this dysfunction.
An arterial hypertension ( "hypertension") is located in front of patients with polycystic kidney at 50 to 75%. The blood pressure of those affected is often already significantly increased before a decline in kidney function ( glomerular filtration rate , GFR).
Diagnosis

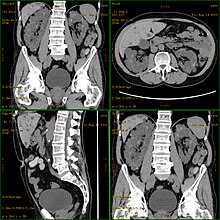
The diagnosis is usually made by sonography ("ultrasound") or other imaging methods , such as magnetic resonance imaging . With ultrasonography, cysts down to a size of 5 mm can be diagnosed with modern devices. The early detection rate in 20-year-old patients is around 90%. The computer tomography while providing a higher resolution with better image quality, but it is mainly because of the radiation exposure not for patient screening used, but only for special diagnostic problems.
The biopsy , in which a small amount of kidney and liver tissue is removed, is used in childhood to differentiate between ARPKD and ADPKD ( early-onset ). This means that morphological changes in the basement membrane can be detected at a very early stage. The diagnosis of ARPKD is made by determining congenital liver fibrosis .
According to Osathanondh and Potter , the cyst kidneys are pathologically and anatomically divided into the following types:
| Type | Infestation | Kidney size | Cyst size | Glomeruli | Bile duct cysts | Survival time |
|---|---|---|---|---|---|---|
| I. | both sides | enlarged to greatly enlarged | evenly wide (12 mm) | normal | available | Neonatal period |
| II | double-sided, one-sided or partially | enlarged or reduced | different sized | diminished and abnormal | unavailable | Adulthood |
| III | mostly bilateral | enlarged | different sizes, sometimes very far | glomerular cysts | occasionally, but then only in circumscribed areas | Adulthood |
| IV | both sides | scaled down | small, subcapsular | Glomeruli diminished, glomular cysts | unavailable | Adulthood |
However, the types defined in this way often do not allow a clear classification in practice . In addition to the pathoanatomical description of the kidneys and liver, the family history ( anamnesis ) therefore plays an important role. For hereditary cases of kidney cysts, the genetically based terms autosomal - dominant and autosomal- recessive are usually used.
By identifying potentially affected genes, a non-invasive demonstrative molecular genetic diagnosis is possible. In many cases, this procedure can replace invasive biopsy diagnostics and enable etiological classification. This classification in turn opens up possibilities for differential therapeutic options for treating the disease. The sensitivity for a really positive result is around 95%. A correlation between genotype and phenotype is only possible to a limited extent. Mutation analysis is difficult for the PKD1 gene due to its size (46 coding exons and 14.2 kb of the transcript ). In addition, the first 33 exons of PKD1 in three homologous copies of the affected chromosome 16 on gene locus p13.1 (HG-A ≈21 kb, HG-B ≈17 kb and HG-C ≈8.5 kb; HG = homologous gene). This makes specific multiplication by means of the polymerase chain reaction (PCR) considerably more difficult .
A particular problem arises from molecular genetic diagnostics. On the one hand, the early diagnosis of the patient's genetic disposition enables prophylactic measures and early supportive therapy. On the other hand, relatives and patients may be confronted with the likelihood of a life-threatening disease breaking out in several decades as early as childhood. The risks and benefits must therefore be carefully weighed before a diagnosis.
In patients with a familial disposition ( predisposition ), the diagnosis can be made by sonography from the age of 20 if at least two kidney cysts can be detected per kidney. Missing cysts, however, rule out the disease in those over 30 years of age.
Pathogenesis
The origin and development of cyst kidneys , the pathogenesis , is based on a cystic degeneration of the so-called tubules (urinary tubules ) in the kidneys. In the autosomal dominant inherited PKD, this leads to increasing kidney enlargement over the course of decades. This can lead to a functional impairment or even a complete loss of the kidney's filtering function. Both kidneys are equally affected. Several hundred cysts, which are bulky in their appearance, can develop per organ. As a result, the mass and volume of the kidneys can increase considerably. While a healthy kidney has an average mass of 160 g , polycystic kidneys can reach up to 8 kg with a volume of up to 40 × 25 × 20 cm³ (= 20 liters) (healthy kidney: 12 × 6 × 3 cm³ = 0.216 liters). Despite the significantly increased space requirement of the organ, functional disorders of the neighboring organs only occur relatively rarely.
The cysts are found on both the renal medulla ( medulla renis ) and the renal cortex ( cortex renalis ). In principle, any area of a nephron can develop a cyst. However, the glomeruli and the loop of Henle are primarily affected . The cysts are filled with what is known as tubular urine . The diameter of a single cyst can vary widely from a few millimeters to over 100 mm. Large cysts can contain several hundred milliliters of tubular urine. The inside of the cyst consists of a single-layer squamous epithelium or single-layer isoprismatic (cubic) epithelium . As the disease progresses, both the number and size of cysts present can increase.
etiology
Polycystic changes in the kidneys are a condition that occurs in a number of diseases. They can arise sporadically as a deviation from the normal development of the kidneys or can be acquired in adult life ( acquired cystic kidneys ). The far more common cause ( etiology ) for this disease are inherited defects in certain genes ( hereditary cystic kidneys ). By far the largest share is taken by the autosomal dominant polycystic kidney disease ( English autosomal dominant polycystic kidney disease , ADPKD ). This disease is the most common hereditary cause of chronic kidney failure : around 7% of all dialysis patients suffer from it.
In addition, various other - much rarer - hereditary diseases cause cystic kidneys. Acquired cystic kidneys can also develop - especially in dialysis patients. Since the vast majority of cyst kidneys are caused by ADPKD, the term “cyst kidney” is often used synonymously for ADPKD.
Hereditary cystic kidneys
The majority of polycystic kidney diseases are hereditary ( hereditary ). A large number of different genes can be affected and thus trigger the disease. The syndromes listed below represent a selection of the most important hereditary polycystic kidney diseases. Some of the diseases are included in the so-called NPH-MCKD complex .
| gene | Chromosome gene locus |
protein | illness | Incidence | Age ∗) |
|---|---|---|---|---|---|
| Autosomal dominant | |||||
| PKD1 | 16 p13.3 | Polycystin-1 | ADPKD | 1: 500-1000 | approx. 50 |
| PKD2 | 4 q21-q23 | Polycystine-2 | ADPKD | 1: 3500-7000 | approx. 70 |
| VHL | 3 p26-p25 | VHL30 | Von-Hippel-Lindau | 1: 35,000 | 20-30 |
| TSC1 | 9 q34 | Hamartin | Tuberous sclerosis | 1: 10,000 (both together) | 30-40 |
| TSC2 | 16 p13.3 | Tuberine | Tuberous sclerosis | ||
| ? | 1 q21 | Medullary cystic kidney disease type 1 | 1 to 9: 1,000,000 (type 1 + 2) | 62 | |
| UMOD | 16 p12.3 | Uromodulin | Medullary cystic kidney disease type 2 | 32 | |
| Autosomal recessive | |||||
| PKHD1 | 6 p21.2-p12 | Fibrocystin | ARPKD | 1: 20,000 | <20 |
| NPHP1 | 2 q13 | Nephrocystine-1 | Nephronophthisis (juvenile) | approx. 1: 100,000 (all NPHP) | 13 |
| NPHP2 | 9 q22-q31 | Inverse | Nephronophthisis (infantile) | <1 | |
| NPHP3 | 3 q22.1 | Nephrocystine-3 | Nephronophthisis (adolescent) | 19th | |
| NPHP4 | 1 p36.22 | Nephroretinin | Nephronophthisis | 21st | |
| NPHP5 | Nephrocystine 5 | Nephronophthisis | 13 | ||
| NPHP6 | Nephrocystine 6 | Nephronophthisis | |||
| GLIS2 | 16 p13.3 | GLI-Similar Protein 2 | Nephronophthisis | ||
| BBS1 | 11 q13.1 | BBS1 protein | Bardet-Biedl syndrome | 1: 140,000 (all BBS) | |
| BBS2 | 16 q21 | BBS2 protein | Bardet-Biedl syndrome | ||
| ARL6 | 3 p13-p12 |
BBS3 protein ADP-ribosylation factor-like protein 6 |
Bardet-Biedl syndrome | ||
| BBS4 | 15 q22.3-q23 | BBS4 protein | Bardet-Biedl syndrome | ||
| BBS5 | 2 q31.1 | BBS5 protein | Bardet-Biedl syndrome | ||
| MKKS | 20 p12 | BBS6 protein | McKusick-Kaufman syndrome | ||
| BBS7 | 4 q27 | BBS7 protein | Bardet-Biedl syndrome | ||
| TTC8 | 14 q31.3 | BBS8 protein | Tetratricopeptide Repeat Domain 8 | ||
| BBS9 | 7 p14 | PTHB1 | Bardet-Biedl syndrome | ||
| BBS10 | 12 q21.2 | BBS10 protein | Bardet-Biedl syndrome | ||
| TRIM32 | 9 q33.1 | Zinc finger protein HT2A | Tripartite motif-containing 32 | ||
| BBS12 | 4 q27 | BBS12 protein | Bardet-Biedl syndrome | ||
| X-linked dominant | |||||
| CXORF5 | X p22.3-p22.2 | OFD1 | Oro-facio-digital syndrome type 1 | 1: 250,000 | |
| Unknown inheritance | |||||
| ? | ? | Medullary cyst kidney | 1: 5000 | 40-50 | |
| ? | ? | Multicystic kidney dysplasia | <10 and 50-60 |
Autosomal dominant polycystic kidney disease
The autosomal dominant polycystic kidney disease (ADPKD), also known as Potter type III cystic kidney degeneration , is the most common life-threatening hereditary disease in humans. There are approximately 5 million people affected by ADPKD worldwide. The incidence is 1: 500 to 1: 1000. For example, in the United States, it is two times more common than multiple sclerosis and ten times more common than sickle cell anemia . Men and women are equally affected. Race and origin are also irrelevant. The symptoms are usually observed only in adulthood. The inheritance of ADPKD is autosomal dominant ( monogenic ) with complete penetrance . Due to the autosomal dominant inheritance, half of the children inherit the mutated gene from their parents on the statistical average and will develop ADPKD themselves. About 50% of all mutation carriers suffer from progressive renal insufficiency. At an average age of 58 years, kidney replacement therapy is indicated in half of ADPKD patients .
As a systemic disease in ADPKD, other organs - in most cases the liver - are often affected by cyst formation. Depending on the author, up to 75% of those affected by ADPKD have liver cysts.
genetics
So far, mutations in two different genes have been identified as the cause of the disease in ADPKD patients: the genes PKD1 and PKD2 . In humans, PKD1 is located on chromosome 16 locus 16p13.3-p13.12. It codes for the protein polycystin-1 . In patients with significant mutations in PKD1, renal insufficiency reaches its end stage at an average age of 50 years, so renal replacement therapy is indicated. Patients with mutations in PKD2, which is located on chromosome 4 gene locus q21-q23 and encodes polycystin-2, reach this stage considerably later, at an average age of 70 years ( late onset ). About 85% of patients with ADPKD carry one or more mutations in PKD1. The remaining 15% are due to mutations in PKD2.
At the cellular level, ADPKD is a recessive mechanism. The first condition for the disease is a germline mutation in one of the PKD1 or PKD2 alleles . Second, a somatic mutation , a so-called second hit, has to take place so that the cyst formation is initiated. This loss of heterozygosity ( loss of heterocygosity , LOH) found in ADPKD obviously takes forever. The initial somatic mutation can be on the other gene. In this case, called transheterozygosity , the germline mutation is on PKD1 and the somatic mutation on PKD2, or vice versa. In animal models it was found that germline mutations affecting both alleles of a PKD gene are perinatally fatal. With the second hit , the affected cell loses the ability to inhibit proliferation and thus becomes the starting point for the formation of a new cyst. An important indication of the correctness of the second hit theory are experiments with knockout mice in which PKD1 or PKD2 were switched off ( gene deletion ). Only homozygous animals become ill , while heterozygous animals develop almost normally. The second hit theory also explains why only about 1% of all nephrons in ADPKD form cysts, even though all cells carry the inherited mutation.
From 1995 onwards, a third gene, called PKD3, was suspected to be another possible cause of ADPKD. Mutations that were not caused by PKD1 or PKD2 were later observed in four other families with cystic kidneys from different countries. The existence of this gene is now being questioned.
| gene | Loci | Exons | Mutation type | Frequency (%) |
| PKD1 | 16p13.3 | 46 (14.1 kb) | Nonsense | 33 |
| Frameshift | 28 | |||
| In-frame | 6th | |||
| Splicing | 14th | |||
| Missense | 19th | |||
| PKD2 | 4q21-q23 | 15 (5 kb) | Nonsense | 37 |
| Frameshift | 39 | |||
| Splicing | 17th | |||
| Missense | 6th | |||
| Deletion | 1 |
Molecular causes and cyst formation
The proteins polycystin-1 and polycystin-2 encoded by the affected genes and the fibrocystin encoded by the PKHD1 gene are located at the base of the primary cilium of the cells of the renal tubule (renal tubule cells) . The primary cilium is a hair-thin cell extension, of which each cell only develops one. According to current knowledge, a malfunction of the primary cilium plays a decisive role in the formation of cysts in all diseases leading to cyst kidneys. The primary ciliates of the tubular cells protrude into the tubular lumen, where they probably serve to perceive the flow of fluid. In addition, the primary cilium is involved in the spatial alignment of the mitotic spindle during cell division . The two polycystines form a calcium- regulating ion channel that is permeable to calcium ions. The polycystine complex plays an important role in the primary cilium with several signaling pathways and mechano-sensory functions. The physiological function of this cell organelle is still largely not understood.
The origin of the cysts can be in any section of a nephron - from the glomerulum to the collecting ducts ( tubulus renalis colligens ). When the cysts reach a diameter of more than 0.2 mm, they no longer have any connection to the kidney tubules ( tubules ).
In order for the cysts to form, the number of cells inside the cyst wall must increase. This happens through excessive proliferation of the epithelial cells of the kidneys. The protein mTor ( mammalian target of rapamycin ) is upregulated . Fluid must also accumulate in the cyst lumen due to increased secretion and / or decreased drainage. This transepithelial fluid secretion is dependent on the secondary active chloride ion secretion. The secretion of chloride ions is regulated by the CFTR ( cystic fibrosis transmembrane conductance regulator ) or by a calcium-dependent chloride channel. Both are located in the apical cell membrane .
Course and prognosis
The course of ADPKD is slowly progressive (progressive). Even before the onset of renal insufficiency, a disturbance in urine concentration (water reabsorption) can be detected in the affected patients. In the early stages of the disease, kidney function is not restricted by the formation of cysts. The performance only decreases from a kidney size of 1000 cm³. If the kidney volume is above 1500 cm³, the glomerular filtration rate is reduced annually by about 4 to 5 ml · min −1 . On average, the volume of the kidneys increases by over 5% per year in patients with a kidney volume above 750 cm³. The first symptoms of the disease are usually noticed between the ages of 30 and 40 years. In general, however, there is a wide range of variation - often within a family.
In almost all cases, the disease leads to end-stage renal failure (ultimate kidney failure). Women reach this stage an average of six years later than men.
Further survival is then only guaranteed through kidney replacement therapy, i.e. dialysis or kidney transplantation. It is not yet fully understood why polycystic kidneys ultimately lead to terminal kidney disease. The mechanism cannot be explained by the pressure atrophy of the parenchyma alone. Surgical interventions such as punctures do not delay the course of the disease. From histological examinations it can be concluded that hypertension is an important factor in the progression of renal insufficiency.
In addition to genetics, the patient's environment and lifestyle also have an impact on the course of ADPKD. In women, for example, it was found that multiple deliveries and other estrogenic factors significantly worsen the course of the disease. The accelerated growth of the cysts compared to women and the earlier reaching of terminal kidney failure in men are also attributed to hormonal influences. Also, the tobacco smoking influences - especially among men - the progression of ADPKD negative. One possible explanation here are the known negative effects of smoking on the blood vessels .
Life expectancy
In a study, 333 patients from 31 families with PKD1 and 291 patients with PKD2 from 31 families were compared with a 398-strong, geographically identical control group. PKD1 patients reached a mean age of 53.0 years (± 1.8 years; 95% probability). PKD2 patients, on the other hand, came to an average of 69.1 years (± 2.2 years; 95%), while the persons from the control group were 78.0 years (± 4.2 years; 95%) (see adjacent graphic).
Causes of death
In a retrospective study, the cause of death of 129 patients with ADPKD was analyzed. After that, 36% died of heart disease and 24% of infections . 94% of the infections were sepsis (blood poisoning). At the autopsies , cardiac hypertrophy was found in 89% of all patients and coronary artery disease in 81% . A neurological event resulted in death in 12% of the patients and a rupture of a brain aneurysm in 6%. Cerebral hemorrhage caused by hypertension was the cause of death in 5% and an ischemic stroke in 1% of patients. No patient died of kidney cancer .
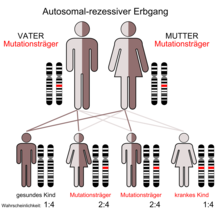
Autosomal recessive polycystic kidney disease
Autosomal recessive polycystic kidney disease ( ARPKD ), also known as spongy kidney or Potter I kidney , manifests itself in childhood. The prevalence of this disease in newborns is in the range of 1: 6,000 to 1: 40,000, with an average of 1: 20,000. The disease is therefore relatively rare. The penetrance is complete. Around every seventieth person is a carrier of the mutation (see diagram of autosomal recessive inheritance). Mutations in the PKHD1 gene - which in humans is located on chromosome 6 , gene locus p21.1-p12 - can lead to the formation of cystic kidneys. The protein fibrocystin encoded by PKHD1 is found together with polycystin-2 in the basal body of the primary cilia . In the apical domain of polarized epithelial cells, it is apparently involved in the formation of the tubules and / or the maintenance of the architecture of the lumen of the collecting tube . Accordingly, in ARPKD, the collecting ducts are essentially affected by the cyst formation. Autosomal recessive polycystic kidney disease is associated with congenital liver fibrosis.
ARPKD manifests itself in patients at a very young age ( early onset ). The age range is 0 to 20 years. The mean life expectancy of the affected children is six years. A distinction is made between perinatal (28th week of pregnancy up to seven days after birth), neonatal (newborn), infantile (childlike) and juvenile (adolescent) form. The lower the age at the onset, the worse the prognosis .
NPH-MCKD complex
The NPH-MCKD complex ( nephronophthisis-medullary cystic kidney disease ) is a group of genetic diseases of the kidney that lead to a cyst kidney. In the case of nephronophthisis, inheritance is autosomal recessive, while it is autosomal dominant in both forms of medullary cystic kidney disease. The common clinical picture of the diseases is the formation of cystic kidneys at the cortical-medullary border (corticomedullary border). All diseases of the NPH-MCKD complex lead to terminal kidney failure in certain age ranges, depending on the affected gene.
Bardet-Biedl syndrome
Bardet-Biedl syndrome (BBS) is a very rare oligogenetic hereditary disease with an autosomal dominant inheritance. The cause of the disease are mutations in the BBS genes 1 to 12. In addition to the formation of polycystic kidneys, there is degeneration of the retina , childhood obesity , intellectual disabilities , malformations of the urinary and sexual apparatus and polydactyly (multiple fingers).
Tuberous sclerosis
Individual kidney cysts are common in autosomal dominant inherited tuberous sclerosis. Polycystic kidney disease is also less common. This is mostly due to larger deletions affecting both the TSC2 gene, which is affected in tuberous sclerosis, and the PKD1 gene; both genes are located in close proximity on chromosome 16 .
Oro-facio-digital syndrome type 1 (OFD 1)
The oro-facio-digital syndrome type 1, also called Papillon-Leage-Psaume syndrome, is a very rare X-linked dominantly inherited disease. The prevalence in newborns is around 1: 250,000. The disease has a variety of symptoms, particularly of the face and mouth, and a tendency to have polycystic kidneys seen in many patients. The latter are usually diagnosed very late when the kidney failure is well advanced.
The disease is prenatally fatal for males .
With orofazio -digital syndrome type 2 , OFD2 or Mohr syndrome , no changes are observed in the kidneys.
Acquired cystic kidneys
A special form of Endstadiumniere, as polycystic secondary transform or as acquired cystic kidney (engl. Acquired cystic kidney disease , ACKD ) is referred to, to 50% of all patients developing at 40 after long-term dialysis. She is a very serious complication in ESRD ( English end-stage renal disease, ESRD ). In the case of transplant recipients , both their own kidneys and the transplant can be affected. The cause of the development of the acquired dialysis-related cyst kidneys is usually a long-term dialysis for analgesic nephropathy . Cysts on the kidneys are very common in patients with end-stage renal disease. The frequency and size of the cysts increase with the duration of dialysis. Both sexes are equally affected by the disease, regardless of the age of the patient. The likelihood that kidney cancer will develop as a further complication is significantly increased - especially in male patients.
therapy
There is currently only one drug approved for the treatment of polycystic kidney disease ( tolvaptan , see below). About 50% of all ADPKD patients - the majority of those with polycystic kidney disease - will require renal replacement therapy during their lifetime. A cure is only possible through a kidney transplant .
Adjuvant measures
The adjustment of the arterial blood pressure, usually with the help of ACE inhibitors , is of particular importance as an adjuvant measure in the case of polycystic kidneys. In addition, there are a number of recommendations for patients with cystic kidneys, which also do not allow a cure, but make the progression of the disease more favorable for the patient.
Since caffeine is suspected of accelerating cyst growth, patients should avoid drinks containing caffeine as much as possible. A low-salt diet helps lower blood pressure, which is associated with impaired excretion of sodium ions. Nonsteroidal anti-inflammatory drugs , mixed analgesics , certain antibiotics and other drugs that are toxic to the kidneys should be avoided as far as possible. On the other hand, cyst infections are treated as early as possible with antibiotics that can penetrate the cyst or bile .
Renal replacement therapy
Only kidney replacement therapy, i.e. dialysis or kidney transplantation, ensures the survival of the patient in terminal kidney failure. In most cases, dialysis is carried out in the form of hemodialysis , since the oversized kidneys - and often the liver - make the abdominal cavity very narrow and peritoneal dialysis is therefore not possible. If possible, kidney transplantation is preferable to dialysis. It enables the restoration of physical performance, quality of life and the social integration of patients. It also significantly improves life expectancy compared to dialysis. The long waiting times for donor kidneys are problematic due to the low number of available donor kidneys.
Polycystic kidneys are - in contrast to the previous practice - only removed pretransplant in exceptional cases, for example when the kidney volume has reached a critical size.
Future therapeutic approaches
The treatment of patients with cystic kidneys incurs annual costs of over US $ 1 billion in the USA alone. This sum essentially results from the costs for the renal replacement therapy necessary in terminal kidney failure.
The proliferation and size increase of the thin-walled, fluid-filled cysts depend on two processes: proliferation of cells of the cyst epithelium and secretion of fluid into the cysts. Both processes are dependent on cAMP . cAMP stimulates the Ras / MAP kinase pathway and thus leads to abnormal cell growth. In addition, cAMP activates the CFTR - chloride channel , thus promoting fluid secretion into cysts. Therapeutic approaches currently being tested target both cAMP-dependent processes in order to slow down cyst formation and growth.
Research is also being carried out into the possible involvement of the protein C-Met and, associated with this, a therapy with C-Met inhibitors , which have shown promising results in animal experiments with mice .
Imaging procedures and investigation of new therapeutic approaches
The average age at diagnosis of ADPKD is currently 27 years. If renal impairment occurs, there is a rapid decrease in GFR of ≈5.9 ml / min per year. So far, no randomized study in this late stage of the disease has been able to demonstrate the beneficial effect of a treatment. Because of the long presymptomatic phase and the late onset of renal insufficiency , the primary endpoints that are usually investigated in studies on chronic kidney disease, such as time to dialysis treatment, doubling of serum creatinine or death, are only of limited use in studies on polycystic kidney disease. This is why the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease was created. (CRISP), whose task it is to examine imaging procedures that enable statements to be made about the course of the disease in the early stages. An important finding of the investigations of CRISP using magnetic resonance imaging is that the cysts in patients with ACPKD grow continuously and quantifiable and that the cyst growth correlates with the decrease in kidney function. That is, a larger increase in cyst size is associated with a faster decrease in kidney function.
HALT-Polycystic Kidney Disease (HALT-PKD) is a prospective study that is currently investigating the effects of a blockade of the renin-angiotensin-aldosterone system and / or strict blood pressure control in the early stages of the disease on cyst growth or in later stages of the disease had an impact on serum creatinine doubling, onset of dialysis, and on death.
Inhibition of cell proliferation
In recent years, with increasing molecular biological knowledge about the causes of PKD, new therapeutic approaches have been found. Some of these approaches are currently in clinical testing . Initially, however, it was more of an incidental finding: in some patients who had received a foreign kidney, a retrospective study found that the remaining polycystic kidney did not increase in volume, but that the cysts partially regressed somewhat. The number of patients in the first study (n = 4) was not statistically meaningful, but the effect could be statistically proven in various animal models . The obvious cause of this improvement was taking sirolimus (rapamycin), which was given to patients as an immunosuppressant . Patients with a donor kidney have to take immunosuppressants for the rest of their life in order to avoid rejection of the foreign organ by the body's own immune system . In human studies, treatment with the mTOR inhibitors sirolimus and everolimus slowed the increase in kidney volume, but not the progressive decrease in kidney function.
In addition to sirolimus and derivatives of this compound, research is also being carried out on other potential substances, some of which use other signaling pathways. Thus, for example, cAMP - antagonists somatostatin and vasopressin potential drug because elevated levels of cAMP stimulate the proliferation and secretion of cystic epithelial cells.
Triptolide is a small molecule isolated from a traditional Chinese drug ( Thunder God Vine ) that has anti-proliferative and pro- apoptotic properties. Triptolide promotes calcium release through a polycystin-2 dependent metabolic pathway and inhibits cyst formation and growth in animal models.
Inhibition of fluid secretion
Antidiuretic hormone (vasopressin) levels are increased in patients with polycystic kidney disease . The V2 vasopressin receptor in the distal tubule and collecting duct expressed . These are the places on the nephron where cyst formation takes place. Vasopressin stimulates the formation of cAMP in the distal tubule via the V2 receptor.
In the animal model, V2 receptor antagonists inhibit the formation of cAMP, the increase in size of the kidneys and the formation of cysts and protect kidney function.
The V2 receptor antagonist tolvaptan was shown to be safe and well tolerated in patients with ADPKD in phase II / III studies . A double-blind, placebo-controlled study was performed in patients with ADPKD, with normal kidney function and with a kidney volume greater than 750 ml. Tolvaptan can slow the progression of the disease. Tolvaptan has been approved for the treatment of ADPKD in Europe since May 2015.
Complications
Typical complications with cystic kidneys are increased blood pressure due to stimulation of the renin-angiotensin-aldosterone system and urinary tract infections .
Due to the shorter urinary tract, urinary tract infections particularly affect female patients. In most cases it is an infection of the urinary bladder caused by gram-negative and nosocomial germs. Urinary tract infections are treated symptomatically, preferably with lipophilic antibiotics . Extreme infections, such as pyonephrosis (a purulent hydronephrosis ), can lead to the removal of the affected kidney ( nephrectomy ).
While the incidence of kidney stones in the population is around 5%, these deposits affect 10 to 34% of patients with polycystic kidneys. One possible cause of the increased incidence of kidney stones is the low pH level in the urine of those affected.
Depending on the author and the study carried out, 25 to 75% of all ADPKD patients with cystic kidneys also have liver cysts . The number of liver cysts increases with the age of the patient. Women have larger and higher numbers of cysts on the liver. Due to the cysts, the liver can be considerably enlarged and literally permeated with cysts. In most cases, however, the function of the parenchymal cells is not impaired. For example, liver enzyme and bilirubin levels are normal. More extensive complications arise from the space required by the sometimes extremely enlarged liver. For example, the diaphragm may be elevated , individual sections of the intestine narrowed, which can make it difficult to transport food, and larger blood vessels, such as the inferior vena cava , may be obstructed .
ARPKD leads to fibrosis, cirrhosis , and increased pressure in the portal vein ( portal hypertension ) in the liver .
In other organs such as the pancreas , spleen, or ovaries , cysts are much less common in patients with polycystic kidneys.
A connection between polycystic kidneys and cerebral aneurysms was described as early as 1904 . The data on the prevalence vary between 4.5 and 22.5%. A possible tear (rupture) of the affected blood vessel is one of the most feared complications in cystic kidneys and is fatal in almost 50% of cases.
history
The Paris surgeon Félix Lejars (1863-1932) first used the term polycystic kidneys in his dissertation in 1888 . The Canadian physician William Osler described it in 1915. Until the middle of the 20th century, only a few publications dealt with this clinical picture. In his dissertation in 1957, Dalgaard was the first to recognize the autosomal dominant inheritance of ADPKD. In 1985, Reeders and colleagues discovered the PKD1 gene locus on chromosome 16 in humans.
literature
Technical article
- W. Kühn, G. Walz: Autosomal dominant polycystic kidney disease. In: Dtsch Arztebl. , 104, 2007, pp. A3022-A3028.
- I. Ishikawa: Acquired renal cystic disease. In: The Cystic Kidney. Kluwer, 1990, ISBN 0-7923-0392-X , pp. 351-377.
- JJ Grantham, PA Gabow: Polycystic Kidney Disease. In: Diseases of the Kidney. Little Brown, 1988, pp. 583-615.
- Joachim Frey : Cystic kidneys and other congenital anomalies. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition ibid. 1961, p. 974 f.
Reference books
- ML Watson (Ed.): Polycystic kidney disease. Oxford Univ. Press, 1996, ISBN 0-19-262578-0 .
- HM Sass, P. Schröder (ed.): Patient education in the case of genetic risk. LIT Verlag, 2003, ISBN 3-8258-4987-2 , pp. 147-198.
Patient information
- AB Chapman, LM Guay-Woodford: The Family and ADPKD: A Guide for Children and Parents. Polycystic Kidney Research Foundation, 1997, ISBN 0-9614567-5-2 .
- Information sheets on cystic kidneys in a ring binder . PKDeV patient association
Popular science
- T. Kotlorz: New hope for kidney patients. In: Die Welt , July 23, 2007
- H. Jänz: Hoping for the kidney. In: Die Welt , June 3, 2006
Web links
- PKD familial cystic kidneys e. V.
- Urology Textbook: Autosomal Dominant Polycystic Kidney Disease (ADPKD)
- zystennieren.de Ruhr University Bochum
- PathoPic - Image database of the University of Basel: Postmortem angiogram of a cyst kidney (image of a specimen)
- PathoPic - Image database of the University of Basel: ADPKD (Potter Type I) (image of a specimen)
- PathoPic - image database of the University of Basel: Polycystic kidney after terminal renal failure (image of a preparation)
- PathoPic - Image database of the University of Basel: ARPKD (Potter Type I) (image of a specimen)
- PathoPic - Image database of the University of Basel: Two polycystic kidneys and one transplant kidney (image of a specimen)
Videos
- Professor Obermüller: Cystic kidneys explained clearly - attacks on a high-performance organ on YouTube
- Affected reports on YouTube
Individual evidence
- ↑ S. Wang et al. a .: The autosomal recessive polycystic kidney disease protein is localized to primary cilia, with concentration in the basal body area. In: J Am Soc Nephrol . , 15, 2004, pp. 592-602. PMID 14978161
- ↑ a b c d e f g h i j k l m n o p q r s t U. Faber: Long-term course in adult polycystic kidney degeneration after kidney transplantation. Dissertation, Heinrich Heine University Düsseldorf, 2000.
- ↑ J. Milutinovic et al. a .: Autosomal dominant polycystic kidney disease: symptoms and clinical findings. In: QJ Med. , 53, 1984, pp. 511-522. PMID 6240069
- ↑ LW Elzinga u. a .: Surgical management of painful polycystic kidneys. In: Am J Kidney Dis . , 22, 1993, pp. 532-527. PMID 8213792
- ↑ LW Elzinga u. a .: Surgery in the management of autosomal dominant polycystic kidney disease. In: Am. J. Kidney Dis. , 1992, pp. 89-92. PMID 1739090
- ↑ D. Frang et al. a .: A new approach to the treatment of polycystic kidneys. In: Int. Urol. Nephrol. , 20, 1988, pp. 13-21. PMID 3360583
- ↑ LW Elzinga u. a .: Cyst decompression surgery for autosomal dominant polycystic kidney disease . In: J Am Soc Nephrol. , 2, 1992, pp. 1219-1226. PMID 1591362 .
- ↑ BJ Lifson et al. a .: Role and long-term results of laparoscopic decortication in solitary cystic and autosomal dominant polycystic kidney disease. In: J Urol . , 159, 1998, pp. 702-705. PMID 9474129
- ↑ AB Chapman et al. a .: Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. In: J Am Soc Nephrol. , 5, 1994, pp. 1349-1354. PMID 7894001
- ↑ PA Gabow et al. a .: Renal structure and hypertension in autosomal dominant polycystic kidney disease. In: Kidney International , 38, 1990, pp. 1177-1180. PMID 2074659
- ↑ a b P. A. Gabow: Autosomal dominant polycystic kidney disease In: NEJM 329, 1993, pp. 332-342. PMID 8321262
- ^ AB Chapman, PA Gabow: Hypertension in autosomal dominant polycystic kidney disease. In: Kidney Int Suppl , 61, 1997, pp. 71-73. PMID 9328971
- ↑ PA Gabow et al. a .: Utility of ultrasonography in the diagnosis of autosomal dominant polycystic kidney disease in children. In: J Am Soc Nephrol. , 8, 1997, pp. 105-110. PMID 9013454
- ^ WC O'Neill et al. a .: Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: the Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP). In: Am J Kidney Dis. , 46, 2005, pp. 1058-1064. PMID 16310571
- ↑ KM Koch u. a .: Clinical Nephrology. Urban & Fischer-Verlag, 1999, ISBN 3-437-21730-5 , pp. 437-459.
- ↑ V. Osathanondh, EL Potter : Pathogenesis of polycystic kidneys. In: Arch. Path. , 77, 1964, pp. 459-465. PMID 14120681
- ↑ B. Hermanns u. a .: Pathology and genetics of hereditary cystic kidneys. In: Der Pathologe , 24, 2003, pp. 410-420. PMID 14605845
- ↑ ADPKD (Autosomal Dominant Polycystic Kidney Disease) ( page no longer available , search in web archives ) Aachen University Hospital; Retrieved September 30, 2008.
- ↑ a b c S. Helmig: Population genetic investigation on the PKD 1 gene of the cat with regard to polycystic syndrome. Dissertation, Justus Liebig University Giessen, 2005.
- ↑ F. Hildebrandt, M. Wolf: Pathology and genetics of hereditary cystic kidneys. In: Medical Therapy. Springer, 2005, ISBN 3-540-21226-4 , pp. 927-939.
- ↑ a b c d e f g W. Kühn, G. Walz: Autosomal dominant polycystic kidney disease. In: Ärzteblatt , 104/2007, pp. A3022 – A3028.
- ^ RG Elles u. a .: Diagnosis of adult polycystic kidney disease by genetic markers and ultrasonographic imaging in a voluntary family register. In: J Med Genet , 31, 1994, pp. 115-120. PMID 8182715 .
- ↑ LF Fried u. a .: Duodenal obstruction in polycystic kidney disease. Case report and review of the literature. In: Am. J. Nephrol. , 18, 1998, pp. 318-320. PMID 9653836
- ↑ a b P.D. Wilson: Polycystic kidney disease. In: N Engl J Med. , 350, 2004, pp. 151-164. PMID 14711914 .
- ^ U. Frei, HJ Schober-Halstenberg: Renal replacement therapy in Germany. (PDF; 1.4 MB) In: QuaSi-Niere annual report 2005/2006. Berlin
- ↑ GM Fick, PA Gabow: Hereditary and acquired cystic disease of the kidney. In: Kidney Int. , 46, 1994, pp. 951-964. PMID 7861721 .
- ^ R. Rohatgi: Clinical manifestations of hereditary cystic kidney disease. In: Front Biosci. , 13, 2008, pp. 4175-4197. PMID 18508505
- ↑ renal disease, medullary cystic, autosomal dominant, with or without hyperuricemia orpha.net; Retrieved October 4, 2008.
- ↑ M. Attanasio et al. a .: Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. In: Nature Genet . , 39, 2007, pp. 1018-1024. PMID 17618285
- ↑ a b B. Buchholz: Functional interaction of polycystin 2 and TRPV4. Dissertation, Albert-Ludwigs-Universität Freiburg, 2004.
- ^ AR Gallagher et al. a .: Molecular basis of autosomal dominant polycystic kidney disease. In: Cellular and Molecular Life Sciences , 59, 2002, pp. 682-693. PMID 12022474 .
- ↑ ADPKD (Autosomal Dominant Polycystic Kidney Disease) . ( Page no longer available , search in web archives ) University Clinic Aachen; Retrieved November 11, 2008.
- ↑ DW Bianchi et al. a .: Fetology. McGraw-Hill Professional, 2000, ISBN 0-8385-2570-9 , p. 632.
- ↑ PA Gabow et al. a .: Factors affecting the progression of renal disease in autosomal dominant polycystic kidney disease. In: Kidney Int. , 41, 1992, pp. 1311-1319. PMID 1614046
- ^ AC Ong, PC Harris: Molecular pathogenesis of ADPKD: the polycystin complex gets complex. In: Kidney Int. , 67, 2005, pp. 1234-1247. PMID 15780076
- ^ S. Rossetti et al. a .: Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. In: Am. J. Hum. Genet. , 68, 2001, pp. 46-63. PMID 11115377
- ^ GG Germino: Autosomal dominant polycystic kidney disease: a two-hit model. In: Hosp Pract. , 32, 1997, pp. 81-82, 85-88, 91-92. PMID 9078975
- ↑ Y. Pei et al. a .: Somatic PKD2 mutations in individual kidney and liver cysts support a "two-hit" model of cystogenesis in type 2 autosomal dominant polycystic kidney disease. In: J Am Soc Nephrol. , 10, 1999, pp. 1524-1529. PMID 10405208
- ↑ F. Qian et al. a .: The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. In: Cell , 87, 1996, pp. 979-987. PMID 8978603
- ↑ T. Watnick et al. a .: Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. In: Nature Genetics , 25, 2000, pp. 143-144. PMID 10835625
- ↑ K. Hackmann: Studies on the expression of the murine genes Pkd1 and Pkd2, the orthologous genes of the autosomal dominant polycystic kidney disease (ADPKD). (PDF) Dissertation, Bielefeld University, 2005.
- ↑ W. Lu u. a .: Late onset of renal and hepatic cysts in Pkd1-targeted heterozygotes. In: Nature Genetics , 21, 1999, pp. 160-161. PMID 9988265
- ↑ G. Wu et al. a .: Somatic inactivation of Pkd2 results in polycystic kidney disease. In: Cell , 93, 1998, pp. 177-188. PMID 9568711
- ↑ N. Bogdanova et al. a .: Genetic heterogeneity of polycystic kidney disease in Bulgaria. In: Hum Genet. , 95, 1995, pp. 645-650. PMID 7789949
- ↑ MC Daoust u. a .: Evidence for a third genetic locus for autosomal dominant polycystic kidney disease. In: Genomics , 25, 1995, pp. 733-736. PMID 7759112
- ^ AD Paterson, Y. Pei: Is there a third gene for autosomal dominant polycystic kidney disease? In: Kidney International , 54, 1998, pp. 1759-1761. PMID 9844156
- ↑ M. Koptides, CC Deltas: Autosomal dominant polycystic kidney disease: molecular genetics and molecular pathogenesis. In: Hum Genet. , 107, 2000, pp. 115-126. PMID 11030408
- ↑ M. Consugar et al. a .: PKD3 revisited with improved PKD1 and PKD2 haplotyping and mutation screening. In: J Am Soc Nephrol. , 16, 2005, p. 358A.
- ^ AD Paterson, Y. Pei: PKD3-to be or not to be? In: Nephrol Dial Transplant , 14, 1999, pp 631-614. PMID 10570111
- ↑ Y. Pei et al. a .: Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. In: Am J Hum Genet. , 68, 2001, pp. 355-363. PMID 11156533
- ^ A b S. Rosetti, PC Harris: Genotype-Phenotype Correlations in Autosomal Dominant and Autosomal Recessive Polycystic Kidney Disease. In: J Am Soc Nephrol. , 18, 2007, pp. 1374-1380. PMID 17429049
- ↑ a b C. Boucher, R. Sandford: Autosomal dominant polycystic kidney disease (ADPKD, MIM 173900, PKD1 and PKD2 genes, protein products known as polycystin-1 and polycystin-2). In: Eur J Hum Genet. , 12, 2004, pp. 347-354. PMID 14872199 .
- ↑ C. Stayner, J. Zhou: Polycystin channels and kidney disease. In: Trends in Pharmacological Sciences , 22, 2001, pp. 543-546. PMID 11698076
- ^ T. Watnick, G. Germino: From cilia to cyst. In: Nature Genetics , 34, 2003, pp. 355-356. PMID 12923538
- ^ BK Yoder: Role of Primary Cilia in the Pathogenesis of Polycystic Kidney Disease. In: J Am Soc Nephrol. , 18, 2007, pp. 1381-1388. PMID 17429051
- ↑ BK Yoder u. a .: Molecular pathogenesis of autosomal dominant polycystic kidney disease. In: Expert Rev Mol Med. , 17, 2006, pp. 1-22. PMID 16515728
- ↑ KD Gardner et al. a .: Why renal cysts grow. In: Am J Physiol. , 266, 1994, F353-359. PMID 8160782
- ↑ D. Rizk, AB Chapman: Cystic and inherited kidney diseases. In: Am J Kidney Dis. , 42, 2003, pp. 1305-1317. PMID 14655206
- ↑ MHK Shokeir: Expression of adult polycystic kidney disease in childhood: a longitudinal study. In: Clin. Genet. , 14, 1978, pp. 61-72. PMID 688689
- ↑ N. Gretz et al. a .: Is gender a determinant for evolution of renal failure? A study in autosomal dominant polycystic kidney disease. In: Am J Kidney Dis. , 14, 1989, pp. 178-183. PMID 2672797 .
- ↑ R. Sherstha et al. a .: Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. In: Hepatology , 26, 1997, pp. 1282-1286. PMID 9362373 .
- ↑ PC Harris et al. a .: Cyst number but not the rate of cystic growth is associated with the mutated gene in ADPKD. In: J Am Soc Nephrol. , 17, 2006, pp. 3013-3019. PMID 17035604
- ↑ R. Magistroni et al. a .: Genotypic function correlation in type 2 autosomal dominant polycystic kidney disease. In: J Am Soc Nephrol. , 14, 2003, pp. 1164-1174. PMID
- ↑ SR Orth u. a .: Smoking as a risk factor for end-stage renal failure in men with primary renal disease. In: Kidney Int. , 54, 1998, pp. 926-931. PMID 9734618
- ↑ SR Orth u. a .: Smoking as a risk factor for end-stage renal failure in patients with primary renal disease. In: Contrib Nephrol. , 130, 2000, pp. 109-123. PMID 10892557 .
- ↑ a b N. Hateboer: Comparison of phenotypes of polycystic kidney disease types 1 and 2. In: The Lancet , 353, 1999, pp 103-107. PMID 10023895
- ↑ GM Fick u. a .: Causes of death in autosomal dominant polycystic kidney disease. In: J Am Soc Nephrol. , 5, 1995, pp. 2048-2456. PMID 7579053
- ^ Autosomal Recessive Polycystic Kidney Disease (ARPKD), Full Gene Analysis. Mayo Medical Clinic; accessed on January 16, 2018.
- ^ R. Witzgall: New Developments in the Field of Cystic Kidney Diseases. (PDF; 250 kB) In: Current Molecular Medicine , 5, 2005, pp. 455-465. PMID 16101475 .
- ↑ K. Zerres et al. a .: Autosomal recessive polycystic kidney disease. In: J Mol Med. , 76, 1998, pp. 303-309. PMID 9587064
- ↑ ST Shaikewitz u. a .: Autosomal recessive polycystic kidney disease: Issues regarding the variability of clinical presentation. In: J Am Soc Nephrol. , 3, 1993, pp. 1858-1862. PMID 8338916
- ↑ EC cap: Molecular biological studies on the PKD1 gene in cats. Dissertation, Justus Liebig University Giessen, 2008.
- ↑ MZ Zhang u. a .: PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. In: Proc Natl Acad Sci USA , 101, 2004, pp. 2311-2316. PMID 14983006
- ↑ LM Guay-Woodford: Renal cystic diseases: diverse phenotypes converge on the cilium / centrosome complex. In: Pediatric Nephrology , 21, 2006, pp. 1369-1376. PMID 16823577
- ↑ T. Benzing et al. a .: Wnt signaling in polycystic kidney disease . In: J Am Soc Nephrol. , 18, 2007, pp. 1389-1398. PMID 17429050
- ↑ SJ Ansley et al. a .: Basal body dysfunction is a likely cause of pleiotropic Bardet – Biedl syndrome. In: Nature , 425, 2003, pp. 628-633. PMID 14520415
- ↑ Sampson et al. a .: Renal cystic disease in tuberous sclerosis: role of the polycystic kidney disease 1 gene. In: Am J Hum Genet. , 1997 Oct; 61 (4), pp. 843-851. PMID 9382094 .
- ↑ AA Connacher u. a .: Orofaciodigital syndrome type I associated with polycystic kidneys and agenesis of the corpus callosum. In: J. Med. Genet. , 24, 1987, pp. 116-122. PMID 3560170
- ↑ SA Feather u. a .: Oral-facial-digital syndrome type 1 is another dominant polycystic kidney disease: clinical, radiological and histopathological features of a new kindred. In: Nephrol Dial Transplant . , 12, 1997, pp. 1354-1361. PMID 9249769
- ^ E. Prati: Oro-facio-digital syndrome type 1. (PDF) In: Orphanet Encyclopedia. October 2004.
- ↑ K. Zerres, S. Rudnik-Schöneborn: Polycystic kidney diseases. In: Handbook of Molecular Medicine. Springer-Verlag, Volume 7 (Part 2), 2000, pp. 281-295.
- ↑ a b M. A. Matson, EP Cohen: Acquired cystic kidney disease: occurrence, prevalence, and renal cancers. In: Medicine (Baltimore) , 69, 1990, pp. 217-226. PMID 2374506
- ^ W. Remmele: Pathologie 5. Springer, 1997, ISBN 3-540-61098-7 , p. 172.
- ^ University of Basel: Secondary cyst kidney after dialysis because of analgesic nephropathy. Image of a histological specimen; Retrieved September 8, 2008.
- ↑ FA Belibi et al. a .: The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. In: J Am Soc Nephrol. , 13, 2002, pp. 2723-2729. PMID 12397042
- ↑ M. Schmid et al. a .: Natriuresis-pressure relationship in polycystic kidney disease. In: J. Hypertens. , 8, 1990, pp. 277-283. PMID 2159509
- ^ BZ Colleen: Polycystic Kidney Disease: An Overview and Commentary. In: Dialysis and Transplantation , 28, 1999, pp. 468-474.
- ↑ HH Knispel u. a .: Transplantation in autosomal dominant polycystic kidney disease without nephrectomy. In: Urol. Int. , 56, 1996, pp. 75-78. PMID 8659014
- ↑ Y. Pirson et al. a .: Outcome of renal replacement therapy in autosomal dominant polycystic kidney disease. In: Nephrol Dial Transplant. , 11, 1996, pp. 24-28. PMID 9044324
- ↑ JJ Grantham: Polycystic kidney disease: old disease in a new context. In: Trans Am Clin Climatol Assoc. , 113, 2002, pp. 211-224. PMID 12053711
- ↑ JP Calvet: Strategies to inhibit cyst formation in ADPKD. In .: Clin J Am Soc Nephrol . , 2008; 3 (4), pp. 1205-1211. PMID 18434615
- ↑ S. Qin, M. Taglienti and a .: Failure to ubiquitinate c-Met leads to hyperactivation of mTOR signaling in a mouse model of autosomal dominant polycystic kidney disease. In: The Journal of clinical investigation , Volume 120, Number 10, October 2010, pp. 3617-3628, doi: 10.1172 / JCI41531 , PMID 20852388 , PMC 2947217 (free full text).
- ↑ AB Chapman: Approaches to testing new treatments in autosomal dominant polycystic kidney disease: insights from the CRISP and HALT-PKD studies. In: Clin J Am Soc Nephrol. , 3, 2008, pp. 1197-1204. PMID 18579674
- ↑ JJ Grantham et al. a .: Volume progression in polycystic kidney disease. In: NEJM , 354, 2006, pp. 2122-2230. PMID 16707749
- ↑ JM Shillingford et al. a .: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. In: Proc Natl Acad Sci. , 103, 2006, pp. 5466-5471. PMID 16567633
- ↑ Y. Tao et al. a .: Rapamycin Markedly Slows Disease Progression in a Rat Model of Polycystic Kidney Disease. In: J Am Soc Nephrol. , 16, 2005, pp. 46-51. PMID 15563559
- ↑ PR election u. a .: Inhibition of mTOR with sirolimus slows disease progression in Han: SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). In: Nephrol Dial Transplant. , 21, 2006, pp. 598-604. PMID 16221708 .
- ↑ SM Flechner u. a .: Transplantation , 74, 2002, pp. 1070-1076. PMID 12438948
- ↑ AL Serra, D. Poster, AD Kistler, F. Krauer, S. Raina, J. Young, KM Rentsch, KS Spanaus, O. Senn, P. Kristanto, H. Scheffel, D. Weishaupt, RP Wüthrich: Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. In: The New England Journal of Medicine , Volume 363, Number 9, Aug 2010, pp. 820-829, ISSN 1533-4406 . doi: 10.1056 / NEJMoa0907419 . PMID 20581391 .
- ↑ G. Walz, K. Budde, M. Mannaa, J. Nürnberger, C. Wanner, C. Sommerer, U. Kunzendorf, B. Banas, WH Hörl, N. Obermüller, W. Arns, H. Pavenstädt, J. Gaedeke, M. Büchert, C. May, H. Gschaidmeier, S. Kramer, KU Eckardt: Everolimus in patients with autosomal dominant polycystic kidney disease. In: The New England Journal of Medicine , Volume 363, Number 9, Aug 2010, pp. 830-840, ISSN 1533-4406 . doi: 10.1056 / NEJMoa1003491 . PMID 20581392 .
- ↑ a b A. Masoumi et al. a .: Potential pharmacological interventions in polycystic kidney disease. In: Drugs , 67, 2007, pp. 2495-2510. PMID 18034588
- ↑ FA Belibi et al. a .: Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. In: Kidney Int. , 66, 2004, pp. 964-973. PMID 15327388
- ↑ T. Yamaguchi et al. a .: cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. In: Kidney Int. , 57, 2000, pp. 1460-1471. PMID 10760082
- ↑ SJ Leuenroth u. a .: Triptolide Reduces Cystogenesis in a Model of ADPKD. In: J Am Soc Nephrol. , 19, 2008, pp. 1659-1662. PMID 18650476
- ↑ SJ Leuenroth u. a .: Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. In: Proc Natl Acad Sci. , 104, 2007, pp. 4389-4394. PMID 17360534
- ↑ a b V. E. Torres: Role of vasopressin antagonists. In: Clinical Journal of the American Society of Nephrology , Volume 3, Number 4, July 2008, pp. 1212-1218. doi: 10.2215 / CJN.05281107 . PMID 18434616 . (Review).
- ↑ VE Torres u. a .: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. In: Nat Med , 10, 2004, pp. 363-364. PMID 14991049 .
- ↑ Clinical study (phase III): "TEMPO 3/4 Trial" Tolvaptan Efficacy and Safety in Management of Polycystic Kidney Disease and Its Outcomes (TEMPO3 / 4) at Clinicaltrials.gov of the NIH
- ↑ V. Patel, R. Chowdhury, P. Igarashi: Advances in the pathogenesis and treatment of polycystic kidney disease. In: Current Opinion in Nephrology and Hypertension Volume 18, Number 2, March 2009, pp. 99-106, ISSN 1535-3842 . doi: 10.1097 / MNH.0b013e3283262ab0 . PMID 19430332 . PMC 282027 (free full text). (Review).
- ^ VE Torres: Vasopressin antagonists in polycystic kidney disease. In: Seminars in Nephrology , 2008, 28, pp. 306-317. doi: 10.1016 / j.semnephrol.2008.03.003 . PMID 18519091 . (Review).
- ↑ VE Torres u. a .: Renal stone disease in autosomal dominant polycystic kidney disease. In: Am. J. Kidney Dis. , 22, 1993, pp. 513-519. PMID 8213789
- ↑ J. Milutinovic et al. a .: Liver cysts in patients with autosomal dominant polycystic kidney disease. In: Am. J. Med. , 68, 1980, pp. 741-744. PMID 7377224
- ↑ a b E. Higashihara et al. a .: Clinical aspects of polycystic kidney disease. In: J. Urol. , 147, 1992, pp. 329-332. PMID 1732586 .
- ↑ Y. Itai et al. a .: Hepatobiliary cysts in patients with autosomal dominant polycystic kidney disease: prevalence and CT findings. In: Am. J. Roentgenol. , 164, 1995, pp. 339-342. PMID 7839965
- ↑ PA Gabow et al. a .: Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. In: Hepatology , 11, 1990, pp. 1033-1037. PMID 2365280
- ↑ E. Levine et al. a .: Liver cysts in autosomal-dominant polycystic kidney disease: clinical and computed tomographic study. (PDF) In: Am. J. Roentgenol. , 145, 1985, pp. 229-233. PMID 3875218
- ↑ A. Telenti et al. a .: Hepatic cyst infection in autosomal dominant polycystic kidney disease. In: Mayo Clin. Proc. , 65, 1990, pp. 933-942. PMID 2198396
- ↑ WI Schievink u. a .: Saccular intracranial aneurysms in autosomal dominant polycystic kidney disease. In: J. Am. Soc. Nephrol. 3, 1992, pp. 88-95. PMID 1391712 .
- ↑ AB Chapman et al. a .: Intracranial aneurysms in autosomal dominant polycystic kidney disease. In: NEJM , 327, 1992, pp. 916-920. PMID 1513348
- ↑ F. Lejars: You gros reins polykystique de l'adult. Dissertation, 1888, Paris
- ↑ B. Schulze: Cystic kidneys: On the way to a treatable disease. (PDF) In: MedReport , 44, 2006, p. 5.
- ^ W. Osler: The diagnosis of polycystic kidney. In: Internat Clin. , Philadelphia, 2, 1915, pp. 1-5.
- ↑ LP Brendan et al. a .: Did Sir William Osler Perform an Autopsy at The Johns Hopkins Hospital? In: Archives of Pathology & Laboratory Medicine , 2, 132, 2007, pp. 261-264.
- ^ OZ Dalgaard: Bilateral polycystic disease of the kidneys. In: Acta Med Scand. , 328, 1957, pp. 1-255. PMID 13469269
- ↑ ST Reeders u. a .: A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. In: Nature , 317, 1985, pp. 542-544. PMID 2995836