Ebola virus
| Ebola virus | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Ebola virus |
||||||||||||||||||
| Systematics | ||||||||||||||||||
|
||||||||||||||||||
| Taxonomic characteristics | ||||||||||||||||||
|
||||||||||||||||||
| Scientific name | ||||||||||||||||||
| Ebola virus | ||||||||||||||||||
| Short name | ||||||||||||||||||
| EboV | ||||||||||||||||||
| Left | ||||||||||||||||||
|
The Ebola virus is a genus from the Filoviridae family . This genus includes five species whose representatives enveloped single (-) - strand - RNA viruses are. The Ebola viruses cause Ebola fever . In addition to humans, they infect other primates ( gorillas , chimpanzees ) and trigger a hemorrhagic fever in them . Ebola viruses triggered the Ebola virus epidemics from 2014 to 2016 in West Africa and from 2018 to 2020 in the Democratic Republic of the Congo and Uganda .
features
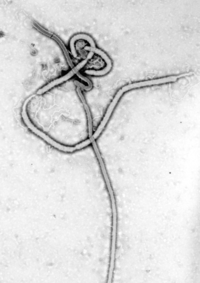
The Ebola virus has a thread-like ( Latin filum 'thread'), sometimes also bacillus-shaped shape. In its basic structure, however, it can occasionally also be bent into a U-shape.
Its length varies between 1 and 4 micrometers (µm), but its diameter is a constant 80 nm and, together with the genus Marburg virus from the same family, it is one of the largest known RNA viruses. Another special feature of this pathogen is the matrix proteins VP40 and VP24.
Ebola viruses are able to multiply in almost all cells of the host. Due to the rapid virus synthesis, a virus crystal (crystalloid) emerges that penetrates from the area of the cell nucleus and releases individual viruses after the cell has dissolved or disintegrated ( lysis ).
morphology
The interior of the Ebola virus has an electron- tight central body, the so-called nucleocapsid . The nucleocapsid has a diameter of 50 nm and is helically wound. In the outer virus envelope, which is comparable to the cell membrane, there are trimers of the surface protein GP, which form what are known as spikes . These are usually 8 nm in size and protrude from the particle.
Genome organization
The basic genetic makeup of the Ebola virus consists of approximately 19,000 nucleotides , which corresponds to a size of 19 kb ( kilobase pairs ). The gene sequence of the viral structural proteins that are located on the genome is arranged linearly as follows: 3′-NP-VP35-VP40-GP-VP30-VP24-L-5 ′
The ends of this gene sequence 3'-5 'contain sequences that contain important functions for replication and transcription of the genome and also control them. In addition, there are non-transcribing areas between the individual genes, which may influence the stability of the viral mRNA and have potential control signals from the virus’s own polymerase L.
The viral structural proteins comprise seven structuring and one non-structuring protein. Four of the seven structuring proteins are part of the nucleocapsid complex and enclose the nucleocapsid . These include the NP, the nucleoprotein that occurs most frequently in terms of quantity and consists of 715 amino acids . Its function is to take over the packaging of the viral genome. It is also connected to the proteins VP35 and VP30 and is connected to the polymerase L via VP35.
Another representative of the nucleocapsid complex is the protein VP35. It has structural elements that are important for the structure of the nucleocapsid. The VP30 is also part of this complex and has the task of acting as an activator during transcription, and this protein is also a strongly phosphorylated protein .
The last part of the complex is polymerase L, which is the largest protein. It is responsible for the transcription and replication of the viral genome and is also connected to the nucleocapsid via VP35. The nucleocapsid complex is in turn surrounded by the proteins VP40 and VP24 and thus separated from the outer virus envelope.
These two proteins thus form a matrix between the virus envelope and the nucleocapsid. VP40 is the main protein of the matrix and is used to release newly formed virions .
The second matrix protein VP24 plays an important role in the transport of nucleocapsids and may also be involved in the formation of functional nucleocapsids. Compared to the other proteins, however, it is the least understood so far.
The outer virus envelope represents the limit and contains the surface protein GP. A distinction is also made here between two types of surface proteins, on the one hand the soluble sGP, which is secreted in the cell supernatant, and on the other hand the GP, which is located directly in the outer virus envelope. The task of the GP is to attach or adsorb to the target molecule during infection.
Replication mechanism
The docking of the Ebola pathogen on the plasma membrane of the target cell within the organism represents the start of the replication mechanism. The docking is mediated via the surface protein GP. Then a receptor-mediated endocytosis takes place, whereby the pathogen reaches the target cell. The nucleocapsid that has now been channeled into the cytoplasm is used as a template for the initial transcription of the viral mRNA. This process is ensured by the virus' own polymerase L.
The initial transcription and replication can only take place via the genomic RNA, which is located in the nucleocapsid and thus functions as a template. The synthesis of nucleocapsid proteins is therefore essential for the formation of a new genome capable of replication in order to package the newly formed RNA. The viral mRNA is then translated by cellular ribosomes, as a result of which new proteins are formed which, following replication, assemble into new virions.
When the viral genome is replicated, a positive-stranded antigenome is first formed, which then serves as a template for the negative-stranded RNA. The now mature nucleocapsids are then channeled to the plasma membrane via the matrix proteins VP24 and VP40, where the virions are now released.
General origin
The viruses originate from the tropical rainforests of Central Africa and Southeast Asia (species Reston Ebola virus ). They were first discovered by scientists in 1976 in Yambuku , Zaire (since 1997 Democratic Republic of the Congo ); they occurred almost simultaneously in Sudan . The genus was named after the Congolese river Ebola , near which the first generally known outbreak occurred. In 55 villages along this river, 318 people fell ill, 280 of them died, representing a death rate of 88 percent. The first case occurred in a Belgian mission hospital of the attendant van het H. Hart van Maria and was initially described as " yellow fever with hemorrhagic features". Shortly afterwards, almost all of the nuns and nurses - as well as most of those who had visited the hospital or were still there - fell ill. The nurses only owned five hypodermic needles that they had used on hundreds of patients without disinfecting or sterilizing them. A virus related to the Ebola virus ( Marburg virus ) was introduced into scientific laboratories in Marburg with monkeys from Uganda in 1967 .
reservoir
The natural reservoir of the Ebola virus ( main host , reservoir host ) has not yet been found with certainty. Bat species came into the sights of the scientists because they had already been identified in other continents as possible reservoir hosts for equally unusual virus infections , for example for the Hendra virus in Australia and the Nipah virus in Malaysia . There is now strong evidence of various types of fruit bats that are widespread in Africa and survive infection with Ebola viruses.
In a large study of bats between 2003 and 2008. Scientists have also found that most likely the Egyptian fruit bat ( Rousettus aegyptiacus ) and the hammerhead ( Hypsignathus monstrosus ) as reservoir hosts for both the Ebola virus as the Marburg virus are used. However, Rousettus aegyptiacus was the only species in which antibodies against both Ebola and Marburg virus were detectable in high concentrations. In addition, antibodies against the Ebola virus were found in the flying fox species Epomops franqueti , narrow-necked flying fox ( Myonycteris torquata ), Micropteropus pusillus and Mops condylurus . In 2010, corresponding antibodies were also detected in palm bats ( Eidolon helvum ). Species living in caves are believed to be particularly affected, although further transmission by dropping fruit that has been eaten and then mostly eaten by monkeys appears likely. In 2019 it became known that Ebola pathogens of the Zaire type had been detected in a long-winged bat of the species Miniopterus inflatus in West Africa and the population in Liberia had been warned against hunting bats. Antibodies to the virus as well as genetic material from the virus were found. The same type of virus caused a major epidemic in West Africa in 2013/16. However, no Ebola virus was found in hundreds of other bat samples examined.
transmission
Sources of infection and routes of infection
Transmission of the virus from the reservoir host to humans has been a rare occurrence so far, and the exact route of transmission has not yet been fully clarified. According to the World Health Organization (WHO) virus transmission has occurred to humans by body contact with infected, sick or dead wild animals as examples are chimpanzees , gorillas and other monkeys , bats , African " forest antelope " and porcupines called. One type of such contact that is common in parts of Africa is the hunting, trade, preparation and consumption of wild animals (“ bush meat ”) that are considered reservoir hosts . We therefore advise against this.
A person-to-person transmission of the Ebola virus occurs through direct body contact and through direct contact infection in the event of contact with the blood , other body fluids or organs removed from infected people . Blood, feces and vomit are described by the WHO as particularly infectious. The virus was also found in breast milk , urine and semen of infected people. After the onset of symptoms, people with Ebola can transmit the virus for as long as the Ebola virus is detectable in their blood and other body fluids, including semen and breast milk. Men who survived the infection can still transmit the virus in their ejaculate for up to ten weeks after they have recovered . Transmission through saliva and tear fluid is also not ruled out by the WHO, although previous studies have not provided any clear results. According to the WHO, the Ebola virus has not yet been detected in sweat .
The Centers for Disease Control and Prevention (CDC) evaluated various reports on past epidemics (including in Kikwit , Democratic Republic of the Congo , 1995) with regard to the maximum duration of the detectability of the virus RNA in various body fluids. The virus RNA is examined in the laboratory using reverse transcriptase PCR . After the onset of symptoms, seminal fluid was up to 101 days, in vaginal swabs up to 33 days, in swabs of the rectum up to 29 days, in urine up to 23 days, in conjunctival swabs up to 22 days, in blood up to 21 days, in Breast milk up to 15 days, in saliva up to eight days and on the skin up to six days virus RNA can be found. However, the evidence in breast milk relates to only one documented case of illness, so that no recommendation can be derived from this as to the period after which the breast milk is again suitable as baby food .
Entry portals for Ebola virus - Virions (virus particles) are mucous membranes and injured skin areas. Transmission through sexual contact has been proven, but this transmission path plays a subordinate role. The Robert Koch Institute (RKI) reports, “So far there is no evidence of the transmission of filoviruses to humans through the breath” and refers to the recommendations issued by the WHO. This was also confirmed by the WHO and the CDC during the Ebola virus epidemic from 2014 to 2016 . The RKI sees a “high risk of exposure ” for a person who “had close contact without protective clothing to someone who was seriously ill with Ebola / Marburg virus, for example. Coughed as [...] had. "The WHO estimates that a transfer by splashes (" splashes ") can take place. The WHO recommends that medical staff and other contact persons observe appropriate hygiene measures and wear protective masks (face shield or mouth and nose protection and protective goggles ), long-sleeved protective gowns and protective gloves . The recommendations of the RKI provide for gloves, protective goggles, at least FFP3 half masks , hoods and water-repellent protective gowns. Disease hygiene isolation of the patient with "barrier nursing" is also recommended during treatment. As a precaution, transport of sick people should be carried out in "highly contagious ambulances".
Transmission through contaminated objects is also possible. The virus maintains its infectivity at room temperature and down to 4 ° C for several days. At −70 ° C it can last indefinitely. Infection can therefore also take place as a smear infection via syringes and other objects that have been contaminated with infectious body fluids. According to an assessment by the WHO, the risk of smear infection is low and can be further reduced by appropriate cleaning and disinfection measures.
Infection at the cellular level
After entering the body, the Ebola viruses infect primarily macrophages , liver cells and cells in the lymph nodes and the spleen in the early stages, and a number of other host cells in the later stages . At the cell biological level it has only recently been possible to decipher how the Ebola virus penetrates the cell interior . The Zaire Ebola virus activates the so-called phosphoinositide-3-kinase (PI3K) via a previously unknown membrane receptor of the receptor tyrosine kinase class and thus causes its internalization in the cell in the form of endosomes . Inhibitors of PI3K and downstream enzymes prevented the infection in cell culture experiments , which gives hope for future treatment options. It is also known that the expression of the glycoreceptor "liver and lymph node sinusoidal endothelial cell C-type lectin " (LSECtin) on myeloid cells gives the Ebola virus its binding capacity.
Possible sources of infection that have not been conclusively investigated
Antibodies against the Ebola virus have been found in rodents, dogs, horses, pigs, antelopes and bush pigs .
In a 2012 experiment, wild boars transmitted Ebola viruses to primates only through droplet infection and without direct contact, without becoming fatally ill themselves. They also came under suspicion of causing major, annual epidemics in Africa.
On the occasion of the Ebola virus infections in the USA and Spain in 2014 , a possible transmission through pets was also discussed. The Centers for Disease Control and Prevention (CDC) announced that there are no known reports that dogs or cats have developed Ebola or have transmitted the Ebola virus to humans or other animals. This also applies to areas in Africa where Ebola virus outbreaks have occurred. In an outbreak in Gabon (2001–2002), a study on dogs showed that between 10 and 30% of the animals examined - depending on the areas in which they lived - had developed antibodies against the Ebola virus . However, they did not show any symptoms of the disease. In the event that a person infected in the United States has a pet, the CDC recommends an exposure assessment of the animal and subsequent action to be taken with veterinarians . In the case of the nurse who was infected in Spain, her dog was put to sleep as a precaution.
incubation period
The incubation period usually varies between 2 and 21 days, most commonly 8-10 days. Studies carried out on the occasion of the Ebola virus epidemic in West Africa in 2014 have shown that the incubation period is between one and 21 days for 95% of laboratory-confirmed cases of illness, for a further 3% the incubation period is between 22 and 42 days, for the remaining 2% no information is given in the cases.
A comparison of the information from previous epidemics with reliable information during the 2014 epidemic shows that the assumption of an incubation period of a maximum of 21 days may not be sufficient for some of the infected. According to this comparison, the incubation time is longer in 0.2 to 12% of the cases examined. The statement that the incubation time is between one and 21 days in around 95% of laboratory-confirmed cases of illness is therefore interpreted as a compromise between the costs of the extended quarantine measures and the reduction of the risk of discharging patients who are still infectious. The Deutsches Ärzteblatt regards this 95 percent limit as a sensible compromise.
Infectious dose
This value indicates how many pathogens are necessary to trigger the infection of the host. The infectious dose for contracting hemorrhagic fever ranges from 1 to 10 virus particles. This value was determined by means of animal experiments on primates in the course of bioweapons research. The virus particles were supplied to the test animals in the form of an aerosol via the breath. In the case of anthrax , the infection dose is around 8,000–50,000 bacterial spores for comparison.
Risk group, official classification
In Germany, the Ebola virus infection is listed as a notifiable animal disease in the ordinance on notifiable animal diseases ( Section 1 TierSeuchAnzV). In the European Union , the Ebola virus is classified as a zoonotic agent by Directive 2003/99 / EC .
Due to the high mortality (50–90%) and the risk of infection, the pathogen is classified in the highest risk group 4 according to the Biological Agents Ordinance . The classification according to the Biological Agents Ordinance in conjunction with the TRBA ( Technical Rules for Biological Agents) 462 applies to the four species Bundibugyo Ebolavirus , Tai Forest Ebolavirus (formerly Côte d'Ivoire Ebolavirus ), Sudan Ebolavirus and Zaire Ebolavirus , while the Reston Ebolavirus of risk group 2 is assigned.
The Biological Agents Ordinance defines four risk groups for biological agents . The work must be carried out taking appropriate protective measures into account, which are divided into four biological protection levels ( BSL) by the Biological Agents Ordinance . As a result, work with the Ebola virus (exception: Reston Ebola virus , see above) must be carried out under the strict requirements of protection level 4. Filoviruses are researched in 20 laboratories worldwide (as of 2013). These must therefore comply with protection level 4 and are also referred to as BSL-4 laboratories . For Germany, this applies to the Bernhard Nocht Institute for Tropical Medicine and the Institute for Virology at the University of Marburg . The CDC classified Ebola virus as a possible biological weapon .
Virus species
In the genus Ebolavirus , five species were distinguished, each named after the place of their first known occurrence. In 2010 the ICTV Filoviridae Study Group proposed an updated systematics and nomenclature for the representatives of the Filoviridae , which was implemented in the 9th ICTV Report of 2011. Accordingly, the following nomenclature should be used, which is already used in corresponding publications of the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC):
- Genus Ebolavirus (formerly Ebola-like viruses , en. Ebola-like viruses )
- Species Ebola Bundibugyo Virus , en. Bundibugyo ebolavirus (formerly BEBOV)
- Virus: Bundibugyo Virus (BDBV)
- Species Ebola Reston Virus , en. Reston ebolavirus (formerly REBOV), 4 subtypes
- Virus: Reston Virus (RESTV)
- Species Ebola Sudan Virus , en. Sudan ebolavirus (formerly SEBOV), 3 subtypes
- Virus: Sudan Virus (SUDV)
- Species Ebola Taï Forest Virus , en. Tai Forest ebolavirus (also Taï Forest ebolavirus , obsolete Ebola-Côte d'Ivoire virus , en. Côte d'Ivoire ebolavirus , formerly CIEBOV), 1 subtype
- Virus: Taï Forest Virus (TAFV)
- Species Ebola-Zaire virus , en. Zaire ebola virus (also Zaire ebola virus ; formerly ZEBOV), type species with 6 subtypes
- Virus: Ebola Virus (EBOV)
- Species Ebola Bundibugyo Virus , en. Bundibugyo ebolavirus (formerly BEBOV)
Notes: The Côte d'Ivoire ebolavirus has been renamed Taï Forest ebolavirus , the official spelling is Tai Forest ebolavirus (without diacritical symbols). The species Zaire ebolavirus (also found with the spelling Zaïre ebolavirus ) is the only representative of the Ebola virus, which is abbreviated as EBOV. In English spelling Ebola virus (not set in italics for the virus, i. E. The subtype), the Separately case distinguishes this virus from the genus, which in English Ebola virus is written. Several variants of the Ebola virus (EBOV) are known, e.g. B. Mayinga (abbreviated as EBOV / May). In this case, the responsible expert group advises not to use the term “ strain ” (“virus strain”) or “subtype” and instead to name them as a variant.
Species differences
The four species Tai Forest ebolavirus , Sudan ebolavirus , Zaire ebolavirus and Bundibugyo ebolavirus cause hemorrhagic fever in humans with a mortality rate of around 50 to 90%. In individual cases z. B. in diseases caused by the Bundibugyo Virus (BDBV), the mortality rate is also lower. As with the Marburg viruses, this high mortality indicates that the Ebola viruses and their variants have not yet adapted to humans and have not yet penetrated the population . Damaging its host up to and including death is not beneficial for a virus, since it depends on this host for its own reproduction. The symptoms that are nevertheless triggered in the host are side effects of the infection. The strategy of spreading the pathogen virus in the case of Ebola viruses is known as hit and run . If a virus is better adapted to its host, its chance of spreading further is greater, because the host is no longer killed by such a virus in the acute phase of the disease. In the event that the host does not immediately develop effective antibodies that neutralize the virus, the virus can use the host for its own reproduction for much longer, using the so-called infect-and-persist strategy .
Zaire Ebola Virus
This species is the most dangerous of the Filoviridae family with a lethality rate of 60 to 90% . Like all Ebola viruses, the virus, formerly known as the Zaïre subtype, has a diameter of around 80 nm and a length of 990 to 1086 nm. The genome of the virus consists of around 19,000 bases.
The Zaire Ebola virus was observed for the first time in 1976 in Zaire (renamed the Democratic Republic of the Congo in 1997 ) and caused 280 deaths among 318 infected people (mortality 88%). A year later, another person fell ill and died in Zaire. In 1994 52 people fell ill, 31 of them died (60% mortality). In 1995 315 residents became infected, 250 of them died (mortality 81%). 1996–1997 in Gabon 21 of 37 people (mortality 57%) and 45 of 60 (mortality 75%) were killed in two waves. There was also a death from South Africa . 2001–2002 there was another epidemic in Gabon , which killed 53 out of 65 people (mortality 82%). At the same time there were 44 deaths among 59 infected people in the Democratic Republic of the Congo (mortality 75%). In 2002–2003, 128 of 143 people died from the virus when another epidemic broke out in the Democratic Republic of the Congo (89% mortality). From December 2008 to January 2009 there was an outbreak in the Democratic Republic of the Congo. 32 people became infected there (suspected, probable and confirmed cases), 15 of them died (mortality 46%). In May 2011, the WHO confirmed a death in Uganda . In the summer of 2014, 5400 people fell ill in Guinea , Liberia , Sierra Leone , Nigeria and Senegal (as of September 14, 2014, excluding cases in the DR Congo) , of which 2600 died (mortality 49%).
Reston Ebola Virus
This species causes disease in macaques and pigs. These two species are the main hosts of the virus. Due to the different geographical distribution of infected pigs and macaques, two independent adaptation steps of the virus to the two hosts are assumed. In humans there is only a subclinical infection , which means that there are no signs of disease. However, antibodies against the virus are produced.
Infectious diseases, epidemics and general risk of infection
The infectious disease that occurs in humans after a viral infection is known as Ebola . A typical symptom is a haemorrhagic fever , characterized by a high fever of > 38.5 ° C combined with bleeding. Ebola fever outbreaks have occurred repeatedly since 1976. In the tropical part of Africa in particular , these outbreaks cost the lives of numerous people, see Documented occurrence and epidemics of the Ebola virus disease . The largest documented Ebola epidemic to date occurred in West Africa in 2014. The second largest documented is that from the Democratic Republic of the Congo, which began in 2018.
Forms of therapy and diagnostics
There is currently no standardized form of therapy. The focus is on symptomatic treatment of the hemorrhagic fever caused by the infection. In this case, targeted action is taken against the symptoms that occur and, if possible, work is carried out under intensive medical conditions and isolation.
At the end of July 2015, the World Health Organization announced that the VSV-EBOV vaccine could be used successfully to fight Ebola. In a field test with 4,000 guineans, it was found that the vaccine protected the test subjects one hundred percent from the virus after 10 days. More studies are needed to find out more information about the vaccine and how to fight the virus.
One possible detection of the Ebola virus is the copying process of genetic information, also known as the polymerase chain reaction (PCR). This consists of a test kit, which is designed so that you can see the PCR running in real time on a screen using a special device ( Real Time Quantitative PCR ). Blood samples as well as urine, but also saliva samples are used, which can contain the Ebola pathogen. If the source material contains Ebola pathogens, these can be detected within 90 minutes using the method.
Further virus detection methods are carried out by means of virus cultivation or electron microscopic examinations. However, the detection of specific antibodies using IF, ELISA or NT can also provide information.
Disposal of Ebola waste
According to the regulations of the Biological Agents Ordinance (BioStoffV) in Germany, all waste produced in the event of a suspected Ebola fever must be thermally inactivated on site. If this cannot be achieved, special regulations apply to the packaging and transport of this hazardous waste. Under the waste code AS 180103 * in accordance with the Waste Catalog Ordinance , they must be brought to an approved hazardous waste incineration plant in accordance with packaging regulation P620 and with the UN number UN 2814.
The European Agreement on the International Carriage of Dangerous Goods by Road (ADR) and the Ordinance on Hazardous Goods Road, Rail and Inland Waterways (GGVSEB) are binding here. The Federal Institute for Materials Research and Testing (BAM) has approved appropriate containers for smaller amounts of material.
Reporting requirement
In Germany, direct or indirect evidence of the Ebola virus must be reported by name in accordance with Section 7 of the Infection Protection Act if the evidence indicates an acute infection.
In Switzerland, positive and negative laboratory analytical findings on the Ebola virus must be reported in accordance with the Epidemics Act (EpG) in conjunction with the Epidemics Ordinance and Annex 3 of the EDI Ordinance on the reporting of observations of communicable diseases in humans .
See also
literature
- Hans-Dieter Klenk (Ed.): Marburg and Ebola Viruses. In: Current Topics in Microbiology and Immunology. No. 235, Springer, 1999, ISBN 3-540-64729-5 .
- Jens H. Kuhn & Charles H. Calisher (Eds.): Filoviridae: The Marburgviruses and Ebolaviruses. (= Archives of Virology. Supplementa ). 1st edition. Springer, Vienna 2005, ISBN 3-211-20671-X .
- Jens Holger Kuhn, Charles H. Calisher (Eds.): Filoviruses, A Compendium of 40 Years of Epidemiological, Clinical, and Laboratory Studies. (= Archives of Virology. Supplement 20). Springer, Berlin / Heidelberg a. a. 2008, ISBN 978-3-211-20670-6 (Hardcover).
- Centers for Disease Control and Prevention - Special Pathogens Branch Division of High-Consequence Pathogens and Pathology: Ebola Hemorrhagic Fever Information Packet. dated April 9, 2010; Full text (PDF) accessed on October 19, 2011.
- David Quammen : Ebola: The Natural and Human History of a Deadly Virus. [including the events in West Africa]. Norton , New York 2014, ISBN 978-0-393-35155-2 (paperback); ISBN 978-0-393-35156-9 (eBook).
- Hans W. Doerr, Wolfram H. Gerlich (Ed.): Medical Virology . 2nd Edition. Thieme, Stuttgart 2002, ISBN 978-3-13-113962-7 .
- Hermann Feldmeier: Flying foxes - the reservoir of Ebola viruses . In: NZZ , December 9, 2009
Broadcast reports
- Franziska Badenschier : Medicine - trial and error - Ebola studies in an urgent procedure , Deutschlandfunk - “ Science in focus ” from February 15, 2015
- Franziska Badenschier: Early warning system against Ebola - Guardian of World Health , Deutschlandfunk - “Science in focus” from December 20, 2015
Web links
- Ebola virus infections. Robert Koch Institute , rki.de, accessed on November 3, 2014.
- Ebola virus. ViralZone, Swiss Institute of Bioinformatics (SIB), accessed on February 18, 2018.
- Virus taxonomy . Database of the International Committee on Taxonomy of Viruses , 2009 Release; Retrieved November 3, 2014.
- Global Alert and Response (GAR) - Ebola . Factsheet - Ebola haemorrhagic fever. who.int, as of September 2014; Retrieved November 2, 2014
- Outbreak of Ebola in Guinea and Liberia . Centers for Disease Control and Prevention (CDC), Outbreak Updates.
- Chronology of Ebola Hemorrhagic Fever Outbreaks . in tabular form, Centers for Disease Control and Prevention.
- Global Alert and Response (GAR) - Ebola virus disease (EVD) . WHO; including outbreaks updates .
- Georg Rüschemeyer: Ebola. Fatal in case of doubt . On: faz.net - Wissen, March 31, 2014; Retrieved November 2, 2014.
- Personal Protective Equipment in the Context of Filovirus Disease Outbreak Response. (PDF) Handout from the WHO on individual protection against Ebola viruses, status: October 2014, accessed on November 2, 2014.
Individual evidence
- ↑ ICTV Master Species List 2018b.v2 . MSL # 34, March 2019
- ↑ a b ICTV: ICTV Taxonomy history: Akabane orthobunyavirus , EC 51, Berlin, Germany, July 2019; Email ratification March 2020 (MSL # 35)
- ↑ HW Doerr, WH Gerlich: Medical Virology. Stuttgart 2002, p. 9.
- ↑ HW Doerr, WH Gerlich: Medical Virology. Stuttgart 2002, p. 574 f.
- ↑ HW Doerr, WH Gerlich: Medical Virology. Stuttgart 2002, p. 574 f.
- ^ A virologist's tale of Africa's first encounter with Ebola. On: sciencemag.org of August 11, 2014.
- ↑ K. Halpin, PL Young, HE Field, JS Mackenzie: Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus ( Memento of April 27, 2010 in the Internet Archive ). In: Journal of General Virology. 2000, Vol. 81, No. 8, pp. 1927-1932.
- ↑ Outbreak of Hendra-like virus – Malaysia and Singapore, 1998–1999. In: Morbidity and Mortality Weekly Report . (MMWR) April 9, 1999, Volume 48, No. 13, pp. 265-269.
- ↑ S. Wacharapluesadee, T. Hemachudha: Duplex nested RT-PCR for detection of Nipah virus RNA from urine specimens of bats. In: Journal of Virological Methods. April 2007, Volume 141, No. 1, pp. 97-101, doi: 10.1016 / j.jviromet.2006.11.023 .
- ↑ a b c Xavier Pourrut et al .: Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. In: BMC Infectious Diseases , September 28, 2009, No. 9, p. 159, doi: 10.1186 / 1471-2334-9-159 ; biomedcentral.com (PDF).
- ↑ Eric M. Leroy et al .: Fruit bats as reservoirs of Ebola virus. In: Nature . December 1, 2005, Volume 438, pp. 575-576, doi: 10.1038 / 438575a .
- ↑ Eric Leroy et al .: Recent Common Ancestry of Ebola Zaire Virus Found in a Bat Reservoir. In: PLoS Pathogens . October 27, 2006, Volume 2, No. 10, pp. 885-886, doi: 10.1371 / journal.ppat.0020090 .
- ↑ a b DT Hayman, P. Emmerich et al .: Long-term survival of an urban fruit bat seropositive for Ebola and Lagos bat viruses. In: PloS one. Volume 5, No. 8, 2010, p. E11978, ISSN 1932-6203 . doi: 10.1371 / journal.pone.0011978 . PMID 20694141 . PMC 2915915 (free full text).
- ↑ Researchers find Ebola virus in Fledermaus , Spiegel Online, January 26, 2019
- ↑ Scientists Discover Ebola Virus in West African Bat , Columbia University, January 24, 2019
- ^ A. Townsend Peterson, John T. Bauer, James N. Mills: Ecologic and Geographic Distribution of Filovirus Disease. In: Emerging infectious diseases. January 2004, Volume 10, No. 1, pp. 40-7, ISSN 1080-6040 , PMID 15078595 ; cdc.gov (PDF).
- ↑ F. A. Murphy, M. P. Kiley, S. P. Fisher-Hoch: Filoviridae: Marburg and Ebola viruses. In: B. N. Fields, D. M. Knipe (Eds.): Virology. Raven Press, New York 1990, pp. 933-42.
- ↑ a b c d e f g h i Ebola virus disease - WHO Fact Sheet No. 103rd WHO , April 2014, accessed August 12, 2014 .
- ↑ a b Frequently asked questions on Ebola virus disease. WHO, August 14, 2014, accessed October 14, 2014 .
- ↑ European Center for Disease Prevention and Control: Factsheet for health professionals. On: ecdc.europa.eu of August 21, 2012, accessed on July 17, 2014.
- ↑ Facts About Bushmeat and Ebola. (PDF; 68 kB) In: CDC: Ebola (Ebola Virus Disease), What's New. October 7, 2014, accessed October 7, 2014 .
- ↑ a b c WHO: Ebola virus disease. Fact sheet No. 103, Updated September 2014 . Section: Transmission. On: who.int ; Retrieved September 26, 2014.
- ↑ a b c d e f g What we know about transmission of the Ebola virus among humans. In: WHO: Situation assessments: Ebola virus disease . October 6, 2014, accessed October 6, 2014 .
- ↑ Ebola: sperm remain infectious for many months, Ärzteblatt, October 15, 2015
- ↑ a b c d Review of Human-to-Human Transmission of Ebola Virus. Centers for Disease Control and Prevention (CDC), October 17, 2014, accessed October 25, 2014 .
- ↑ Marc Silver: Why Is This Ebola Outbreak Spreading? On: National Geographic , March 27, 2014; Retrieved September 26, 2014.
- ↑ a b c d e RKI: Ebolavirus infections. ( Memento from August 10, 2014 in the Internet Archive ) On: rki.de Status: August 11, 2014, last accessed on August 14, 2014.
- ↑ Munich fire brigade for the transport of suspected Ebola cases ( memento from 23 August 2014 in the Internet Archive ). Bayerischer Rundfunk ( br.de/mediathek ), video, from August 12, 2014; accessed on August 24, 2014.
- ↑ Ebola Virus, Pathogen Data Sheet at: www.phac-aspc.gc.ca , last updated: August 1, 2014; accessed on August 14, 2014.
- ↑ a b c Ebola hemorrhagic fever. Center for Disease Control and Prevention (CDC), August 10, 2014, accessed August 12, 2014 .
- ↑ Graham Simmons et al .: DC-SIGN and DC-SIGNR Bind Ebola Glycoproteins and Enhance Infection of Macrophages and Endothelial Cells. In: Virology. Volume 305, No. 1, 2003, pp. 115-123, doi: 10.1006 / viro.2002.1730
- ↑ Asuka Nanbo, Masaki Imai, Shinji Watanabe et al .: Ebolavirus Is Internalized into Host Cells via Macropinocytosis in a Viral Glycoprotein-Dependent Manner. In: PLoS Pathog. Volume 6, No. 9, September 23, 2010, e1001121, doi: 10.1371 / journal.ppat.1001121
- ↑ RJ Wool-Lewis, P. Bates: Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. In: Journal of virology. (J Virol) Vol. 72, No. 4, April 1998, pp. 3155-3160, PMID 9525641 .
- ↑ M. Shimojima et al .: Tyro3 family-mediated cell entry of Ebola and Marburg viruses. In: Journal of virology. October 2006, Volume 80, No. 20, pp. 10109-10116, PMID 17005688 , doi: 10.1128 / JVI.01157-06 , PMC 1617303 (free full text).
- ↑ MF Saeed et al .: Phosphoinositide-3 Kinase-Akt Pathway Controls Cellular Entry of Ebola Virus . In: PLoS Pathogens. 2008, Volume 4, No. 8, e1000141, doi: 10.1371 / journal.ppat.1000141 ; plospathogens.org (PDF)
- ↑ A. Dominguez-Soto, L. Aragoneses-Fenoll et al .: The DC-SIGN-related lectin LSECtin mediates antigen capture and pathogen binding by human myeloid cells. In: Blood June 2007, Vol. 109, No. 12, pp. 5337-5345. doi: 10.1182 / blood-2006-09-048058 , PMID 17339424 .
- ↑ a b Animals spread Ebola better than humans - www.20min.ch
- ↑ Do dogs transmit Ebola? In: The world .
- ↑ Loïs Allela, Olivier Bourry, Régis Pouillot et al .: Ebola Virus Antibody Prevalence in Dogs and Human Risk. In: Emerging Infetious Deseases. (EID) Volume 11, No. 3, March 2005, Research doi: 10.3201 / eid1103.040981 .
- ↑ BP Krasnianskiĭ, VV Mikhailov, IV Borisevich et al .: Preparation of hyperimmune horse serum against Ebola virus. In: Voprosy virusologii. Volume 40, No. 3, May-June 1995, pp. 138-140, PMID 7676681 .
- ↑ Hana M. Weingartl, Carissa Embury-Hyatt, Charles Nfon et al .: Transmission of Ebola virus from pigs to non-human primates. In: Scientific Reports. No. 2, Article No. 811, doi: 10.1038 / srep00811 .
- ↑ Sherry Seethaler: Pattern of Human Ebola Outbreaks Linked to Wildlife and Climate ( Memento of October 17, 2014 in the Internet Archive ). On: biology.ucsd.edu , November 14, 2006; Retrieved October 17, 2014.
- ↑ Ebola transmitted by wild boars On: aerzteblatt.de , November 16, 2012; accessed on August 14, 2014.
- ↑ a b c Questions and Answers about Ebola and Pets. Centers for Disease Control and Prevention (CDC), October 13, 2014, accessed October 14, 2014 .
- ↑ L. Allela, O. Boury et al .: Ebola virus antibody prevalence in dogs and human risk. In: Emerging infectious diseases , Volume 11, No. 3, March 2005, pp. 385-390, ISSN 1080-6040 . doi: 10.3201 / eid1103.040981 . PMID 15757552 . PMC 3298261 (free full text).
- ↑ Spain: Ebola patient's dog euthanized as a precaution . On: Spiegel Online from October 9, 2014, accessed on October 13, 2014.
- ↑ Are the Ebola outbreaks in Nigeria and Senegal over? World Health Organization (WHO), October 14, 2014, accessed October 21, 2014 .
- ^ WHO Ebola Response Team: Ebola Virus Disease in West Africa - The First 9 Months of the Epidemic and Forward Projections In: New England Journal of Medicine. September 9, 2014, pp. 1481–1495, ISSN 0028-4793 . doi: 10.1056 / NEJMoa1411100 .
- ^ A b Charles N. Haas: On the Quarantine Period for Ebola Virus . In: PLOS Currents Outbreaks . tape 1 , October 14, 2014 ( online [accessed October 25, 2014]).
- ↑ Ebola: 21 days of quarantine too short? Deutsches Ärzteblatt , October 15, 2014, accessed on October 25, 2014 .
- ^ DR Franz, PB Jahrling et al .: Clinical recognition and management of patients exposed to biological warfare agents. In: JAMA. Volume 278, No. 5, August 1997, pp. 399-411, ISSN 0098-7484 . PMID 9244332 . (Review).
- ↑ a b TRBA (Technical Rules for Biological Agents) 462: Classification of viruses in risk groups. Federal Institute for Occupational Safety and Health (BAuA), April 25, 2012, pp. 4, 27 , accessed on August 9, 2014 .
- ↑ a b c JH Kuhn, Y. Bao et al .: Virus nomenclature below the species level: a standardized nomenclature for laboratory animal-adapted strains and variants of viruses assigned to the family Filoviridae. In: Archives of Virology. Volume 158, No. 6, June 2013, pp. 1425-1432, ISSN 1432-8798 . doi: 10.1007 / s00705-012-1594-2 . PMID 23358612 . PMC 3669655 (free full text).
- ↑ Centers for Disease Control and Prevention (CDC): Bioterrorism Agents / Diseases ( Memento of July 22, 2014 in the Internet Archive ). In: cdc.gov , accessed on August 24, 2014.
- ↑ Why Ebola worries the Defense Department . From washingtonpost.com , accessed August 18, 2014.
- ↑ Jonathan S. Towner, Tara K. Sealy et al .: Newly Discovered Ebola Virus Associated with Hemorrhagic Fever Outbreak in Uganda. In: PLoS Pathogens. , Volume 4, No. 11, November 2008, p. E1000212, ISSN 1553-7374 . doi: 10.1371 / journal.ppat.1000212 .
- ↑ a b JH Kuhn, S. Becker et al .: Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. In: Archives of Virology. Volume 155, No. 12, December 2010, pp. 2083-2103, ISSN 1432-8798 . doi: 10.1007 / s00705-010-0814-x . PMID 21046175 . PMC 3074192 (free full text).
- ↑ JH Kuhn, S. Becker u. a .: Family Filoviridae . In: AMQ King, MJ Adams, EB Carstens, EJ Lefkowitz (Eds.): Virus Taxonomy - Ninth Report of the International Committee on Taxonomy of Viruses . Elsevier / Academic Press, London, UK 2011, ISBN 978-0-12-384684-6 , pp. 665-671 ( online ).
- ^ End of Ebola outbreak in the Democratic Republic of the Congo . WHO, February 17, 2009
- ^ WHO: Ebola Response Roadmap Situation Report. (PDF; 1.6 MB) World Health Organization (WHO): Situation reports: Ebola response roadmap , September 18, 2014, accessed on September 19, 2014 .
- ↑ RW Barrette, SAMetwally, JM Rowland et al. :: Discovery of swine as a host for the Reston ebolavirus . In: Science . Volume 325, No. 5937, July 2009, pp. 204-6. doi : 10.1126 / science.1172705 . PMID 19590002 .
- ↑ Outbreaks Chronology: Ebola Hemorrhagic Fever - Known Cases and Outbreaks of Ebola Hemorrhagic Fever, in Chronological Order. Centers for Disease Control and Prevention (CDC), August 22, 2014, accessed August 23, 2014 .
- ↑ Possible vaccine against Ebola (Tagesschau, July 31, 2015)
- ↑ HW Doerr, WH Gerlich: Medical Virology. Stuttgart 2002, p. 577 f.
- ↑ Waste Manager Medicine: Prepared for a Crisis - Disposal of Ebola Waste , October 28, 2016.
