Acidity (wine)

The acids in wine play an important role both in winemaking and in the finished product, wine . They have a direct influence on the color of the wine as well as on the sensory evaluation through the contrast to sugar and alcohol. They play a crucial role in the energy metabolism of the yeast during alcoholic fermentation. Investigations into the acids in wine therefore touch upon such different areas as the sensory system , the biochemistry and the microbiology of fermentation, viticulture practices, treatment methods for the aged wine as well as the stabilization and preservation of the wine. In particular, high-performance liquid chromatography brought important insights into the pH values of the individual acids as well as acids that are only present in tiny traces.
Certain acids are already present in the berry. The concentration of total acid in the grapes increases to a maximum as the berries begin to grow in the berries. After reaching the maximum, the synthesis of fructose begins and the concentration of acids decreases. The pH value of a wine is between 2.9 and 4.0, depending on the grape variety and growing area. The pH value is a measure of the strength of the acidic or basic effect of an aqueous solution, the neutral value being 7.0. Lower pH values in wine are caused by stronger acids and / or higher concentrations of the acids it contains. The three most important acids in the ripe berry are tartaric acid , malic acid and citric acid , all of which are non-volatile acids. During the alcoholic fermentation and when the wine is later matured in containers such as wooden barrels or stainless steel containers, further acids are produced. The most prominent representatives include acetic acid , butyric acid , lactic acid and succinic acid .
In terms of sensor technology, the acid gives the wine freshness and structure and promotes a balanced ratio of the most important taste components of the wine. However, this does not apply to all acids. The known as vinegar Note, vinegar stitch or volatile acid wine error caused by a too high proportion of acetic acid. During normal alcoholic fermentation approx. 0.2–0.4 g / l volatile acid is produced. The value can rise to 0.6 g / l in contact with air. These concentrations represent the usual range and should not be exceeded for reasons of taste. In addition to acetic acid, formic acid and, to a lesser extent, propionic acid and hexanoic acid are also responsible for this error.
Depending on the country of origin, ascorbic acid , sorbic acid and sulphurous acid can be added to stabilize the wine within the framework of local wine legislation .
Gustatory perception
At present, at least five basic qualities of gustatory perception are assumed:
- sweet - triggered by sugar , also by some amino acids , peptides , alcohols , see also: sweeteners
- salty - triggered by table salt , also by some other mineral salts
- acidic - triggered by acidic solutions and organic acids
- bitter - triggered by a variety of different substances, see also: bitter substances
- umami ( Japanese : meaty, hearty) - triggered by glutamic acid and aspartic acid .
It has been known since the beginning of the 20th century that the different taste qualities are perceived by all taste-sensitive parts of the tongue . The differences between the tongue areas with regard to the sensitivity for individual qualities are only slight in humans. Nevertheless, in many textbooks, the tongue is still divided into “taste zones”.
Determination of the total acidity as well as national characteristics
When acid is mentioned in wine analyzes, one speaks of titratable total acid . The value determined by titration does not, however, correspond to the total acid calculated as the sum of the individual acids. The titratable total acidity also does not correlate with the pH value of the wine. When the total acid is determined by chromatography, on the other hand, the concentration of all acids is measured regardless of the extent of the buffering. Both the total acid determined with the chromatograph and the pH value are thus a significantly poorer measure of acid perception than the total acid that can be titrated. Tartaric acid and citric acid lower the pH value at the same concentration significantly more than malic acid, lactic acid or succinic acid.
The calculation of total titratable acidity is not carried out using the same method in all countries! While in Germany the total acidity is calculated as tartaric acid, in France the acidity is given in g / l sulfuric acid . The conversion factor between the two values is 1.53. If a wine according to the German method has an acidity of 6 g / l, the French method results in a value of 3.9 g / l (= 6 g / l divided by 1.53) for the same wine. A mere indication of the acidity without information about the reference acid is therefore of little help.
Volatile and non-volatile acids
In contrast to the non-volatile acids, the volatile acids in wine evaporate during distillation or evaporate over time. The volatile acids contained in wine are mainly acetic acid (0.15 to 0.6 g / l in healthy wine) with their chemical compounds, as well as formic acid , succinic acid , butyric acid and propionic acid . The easily observable volatile carbon dioxide is not counted among the group of volatile acids in wine.
The three main non-volatile acids are tartaric acid, malic acid, and citric acid, which make up about two thirds. Lactic acid, galacturonic acid , gluconic acid , glucuronic acid , glycolic acid , oxalic acid and mucic acid as well as a large number of other acids that are only present in traces in wine are present in smaller quantities .
The maximum values of the volatile acidity depend on the type of wine and the quality level and must not be exceeded at any point in the winemaking process.
-
White wine and rosé wine :
- Table wine , country wine , QbA , Kabinett , Spätlese , Auslese 1.08 g / l
- Beerenauslese , ice wine 1.80 g / l
- Trockenbeerenauslese 2.10 g / l
-
Red wine :
- Table wine, country wine, QbA, Kabinett, Spätlese, Auslese 1.20 g / l
- Beerenauslese, ice wine 1.80 g / l
- Trockenbeerenauslese 2.10 g / l
The role of acids in winemaking

In addition to the must weight, the total acid content is an important indicator for the winemaker as to when the harvest can begin. The proportion of total acidity is highest shortly before the berries begin to ripen and then decreases steadily. While the malic acid is inhaled, the production of tartaric acid stops in the no longer green tissue of the berries. Especially for the production of sparkling wine, the berries are deliberately harvested before they are fully ripe in order to give the base wine a strong acid structure. In the hot 2003 vintage, the high temperatures caused intense inhalation of the malic acid. This often leads to flat wines with only a low aging potential, especially with white wine.
In winemaking, the acid plays an important role in stabilizing the wine in addition to sulfur dioxide . In the acidic milieu almost no bacteria survive longer. Important exceptions are acetic acid bacteria and lactic acid bacteria. Butyric acid bacteria can also be found to a small extent after malolactic fermentation, which are responsible for the acetone odor (eagle owl sound) in faulty wine.
In red wines , the acid helps with the long-term stabilization of the color and is also a determining element of the coloring. Anthocyanins absorb light in the wavelength range between 270 and 290 nanometers ( ultraviolet radiation ) and in the visible range between 465 and 560 nanometers ( blue to green ). The wavelength range is influenced not only by the molecular structure but also by the pH of the environment. The color spectrum ranges from blue to red, with all colors except green. In the acidic environment, the red color predominates, in the alkaline environment, blue and violet tones are to be found. Wines with a high pH value (such as Syrah wines ) have more blue color pigments, but they are not very stable. These wines can then age faster in terms of color and then have an unsightly grayish or brownish tone.
Acidity in the wine sensory system
The total acidity plays a major role in sensory terms. It gives the wine structure and ideally a fresh, mostly fruity taste impression. This impression is reinforced by a slight tingling sensation on the tongue. Wines with too low an acid content are usually perceived as flat and uninteresting.
In particular in the wine language of cooler wine-growing regions such as in Germany or in northern France, there is a wealth of descriptive words that describe the taste impression determined by the acid:
biting, green, lively, crisp, acidic, steely, acidic, tangy, angular or sour.
Tartaric acid
The tartaric acid , also known as 2,3-dihydroxysuccinic acid or 2,3-dihydroxybutanedioic acid (according to IUPAC nomenclature hereinafter) is an α- hydroxy carboxylic acid . The salts and esters of tartaric acid are called tartrates . In grapes occurring L - (+) - tartaric acid is in the EU as a food additive E 334 admitted. In Germany, the total acidity of wines - calculated as tartaric acid - is given. L - (+) - tartaric acid and its calcium, potassium and magnesium salts are particularly abundant in the vines, grapes and leaves of the vine.
The following applies to tartaric acid: the more ripe the grapes, the higher the proportion of tartaric acid in the total acidity. The tartaric acid plays an important role in wine. On the one hand, the chemical resistance of the wine is positively influenced by a high proportion of acid, i.e. H. the wine has a better aging potential. On the other hand, a high acid content supports the color of the wine. In terms of taste, tartaric acid has no different influence on the taste than malic acid. Both acids cannot be distinguished in terms of taste.
As the berries ripen, the concentration of both malic and tartaric acid decreases. While the malic acid is metabolized by cell respiration and the acid breakdown is also temperature-dependent (and therefore dependent on the year ), the tartaric acid is no longer produced in the no longer green fruit tissue of the berry. As the berries grow in size, the consistently high proportion of tartaric acid is increasingly diluted.
During the winemaking process, a large part of the tartaric acid precipitates in the form of tartar . During fermentation, the acid crystallizes from the lees, mash residues, tannins and pigments. The tartar in the bottle, which was often observed in the past, the crystallized form of tartaric acid, which is located on the cork or on the bottom of the bottle depending on the type of storage, can hardly be observed. At most, the crystals affect the clarity of a drink and can thus reduce drinking enjoyment. White wine is decanted , among other things , to separate tartar from the wine. Weinstein is tasteless and feels like sand in the mouth. Since this harmless deposit often led to (unfounded) complaints, many of the wines intended for the mass market now undergo a so-called tartar stabilization. The wine is cooled to 4 ° C or lower for two weeks and inoculated with very small tartrate crystals. At these temperatures, the tartaric acid is brought to supersaturation in order to accelerate the crystallization.
In contrast to malic acid, tartaric acid can be determined quite easily with the Rebelein rapid test. The tartaric acid in wine is converted into an orange-yellow color complex with an ammonium derivative, ammonium monovanadate. The intensity of the color is measured optically by means of photometry at a wavelength of 540 nm . The tartaric acid content is read from a conversion table .
Malic acid

Malic acid (2-hydroxy succinic acid) is a dicarboxylic acid , as the dextrorotatory D -malic acid, and as levorotatory L occurs -malic acid. The L -form is an intermediate product in the citric acid cycle . In nature, L malic acid in unripe apples , quince , grapes , barberries , rowan berries and gooseberries contain. In the wine description in particular, the taste note of green, unripe apples is wrongly attributed to a high proportion of malic acid. Malic acid cannot be distinguished from tartaric acid in terms of taste and serves more as an indicator that the fruit has not yet reached maturity.
Together with tartaric acid, malic acid is the most important of the organic acids produced within the grape. It is formed in the berry's chlorophyll-containing tissue through the metabolism of sugar and acts as an energy vector in the vine. As the berries begin to ripen, the proportion of tissue containing chlorophyll decreases (the berries change color from green to yellow, white, red or bluish-black) and acid production stops. Shortly after production has stopped, the concentration of malic acid in the berries is a very high 20 g / l. The amount of acid depends in part on the grape variety . Varieties such as Barbera , Carignan , Colombard , Riesling and Silvaner naturally have a high proportion of malic acid. When the berries ripen, acid is broken down through cell respiration. The rate of degradation is highly temperature-dependent and can be higher than the rate of physiological ripening, especially at high night temperatures. In the very warm 2003 vintage in Germany, this led to very low-acid musts. Low-acid musts produce flat wines and are not microbially stable enough. This phenomenon has long been known in warm growing areas and acidification of the must with tartaric acid or citric acid is common practice. In Germany, citric acid is not allowed for acidification.
In the cool wine-growing climate, when the berries are fully ripe, a high proportion of malic acid usually remains. In order to counteract the high acidity with a taste component, the aim in Germany was to achieve the highest possible must weight in the grapes up until the beginning of the 21st century. A high must weight is usually a guarantee for a high alcohol content in the wine and gives room to create a residual sweetness that can disguise an acid that is too sharp.
The amount of malic acid remaining at maturity can be between 1 and 9 g / l, depending on the vintage, growing area and microclimate, and is therefore an important cause of differences in quality depending on the vintage or location.
The malolactic acid content can be influenced with the method of malolactic fermentation (see the article on malolactic fermentation ). In malic acid degradation (also often called BSA), the more aggressive malic acid is metabolized into the less strong lactic acid by lactic acid bacteria. If the grapes are healthy, only small amounts of lactic acid bacteria can be found on the berries. Malolactic fermentation, which used to occur sporadically and spontaneously, is now carried out in a targeted manner, particularly in the case of red wines. The fermented wine is inoculated with lactic acid bacteria in the wooden barrel.
Lactic acid
Lactic acid ( Latin acidum lacticum ) is a chemical compound that is an important intermediate in metabolism . For example, lactic acid is a product of the breakdown of sugars through anaerobic glycolysis . Lactic acid is added to many foods in the form of the salts calcium lactate or calcium lactate gluconate for calcium fortification. As a food additive , it bears the designation E 270. Lactic acid is used in the luxury food industry (brewery, bakery, as an acidifier in confectionery , occasionally in lemonade). Yoghurt and sauerkraut are foods that are produced by the action of lactic acid bacteria.
The monocarboxylic acid does not occur naturally in the berries. The mild lactic acid is only produced through the action of bacteria of the genera Oenococcus , Pediococcus and Lactobacillus . The lactic acid bacteria convert sugar and malic acid into lactic acid during malolactic fermentation. Carbon dioxide is also produced as a by-product. In viticulture terminology, malolactic fermentation is called malolactic fermentation . However, the name fermentation is misleading and is only due to the production of carbonic acid. The mechanisms of malolactic fermentation have only been understood since the 1960s and have been used specifically in viticulture ever since. The malolactic fermentation gives the wines a little more complexity and converts the more aggressive malic acid into the gentler lactic acid. However, white wines lose some of their fruitiness, so going through acid degradation is not always welcome. The wines of the grape varieties Chenin Blanc or Riesling usually lose quality. If the acid degradation is not carried out in a controlled manner, the wine can also become cloudy .
When going through the malolactic fermentation, the biogenic amines histamine (→ histamine intolerance ), tyramine , putrescine and phenylethylamine are also formed . The biogenic amines have a reputation for causing headaches. The addition of sulfur dioxide can stabilize the wine and prevent the spread of lactic acid bacteria. This can prevent the malolactic fermentation. Lactic acid bacteria can survive deep in the walls of a wooden barrel. Aging in the barrel is therefore problematic if the wine is not to go through malolactic fermentation.
Citric acid
The colorless, sour-tasting citric acid is found in citrus fruits, mushrooms and milk. It is found in wine in a proportion of 0.1 to 0.4 g / l. However, the acid is only produced in traces within the berries; it is a by-product of alcoholic fermentation. During malolactic fermentation, the citric acid (also called citric acid) is broken down by the lactic acid bacteria. It has a stabilizing effect against iron and copper cloudiness (→ wine defects) in wine. According to EU law, the use of acid is permitted with regard to the development of the wine (in English spelling for wine stabilization purposes ).
Smaller increases in total acidity of up to 0.5 g / l can also be made with citric acid. With these small additions, the acid cannot be sensed and helps low-acid wines to have more structure and freshness. Citric acid is only added after alcoholic fermentation in the microbiologically stable wine, as the yeast metabolizes citric acid into the undesired acetic acid diacetyl (butter clay) during fermentation . The addition of citric acid for acidification is not allowed in Germany, only for metal stabilization. The limit is 1 g / l.
Other acids
acetic acid
Acetic acid is a carboxylic acid and one of the volatile acids. At a higher concentration, the vinegar tinge is one of the wine defects that can no longer be hidden in the aged wine. According to the ordinance, white wines with more than 1.08 g / l and red wines with more than 1.2 g / l due to the stronger tannins are considered spoiled. A real vinegar tinge is only noticeable in the nose with these wines from 1.5 g / l volatile acidity. Sensitive tasters can spot a vinegar tinge from a concentration of 0.5 g / l. The noble sweet wines are an exception, in which the concentrated aromas are able to conceal a slight vinegar note. In this case the legal upper limit is 1.8 g / l. Acetic acid can already be present in the must, for example if the grapes are damaged by hail or bird damage and the bacteria on the berry skin come into contact with the sugar in the berries. In extreme cases, a slight vinegar note can already be heard in the vineyard.
Vinegar (lat. Acetum ) is a strongly sour-tasting seasoning and preservative that is produced by fermenting liquids containing alcohol (including wine, apple cider , beer or rice wine ) with acetic acid bacteria ( mother vinegar ).
Ascorbic acid
As a natural ingredient, the acid in the berries hardly plays a role. At less than 20 mg / l, their content is ten times lower than in citrus fruits. The stabilizing effect of the acid as an antioxidant was hardly used in Germany. It was only when researchers at the Bavarian State Institute for Viticulture and Horticulture in Veitshöchheim discovered that ascorbic acid can prevent or at least delay the UTA (atypical aging grade) wine error , known since the early 1990s , that aroused interest. Since the 2006 vintage, the addition of ascorbic acid to grapes, mash and must has been allowed. Up to 250 mg / l could already be added to the wine shortly before bottling.
Wines with the UTA error have little or no bouquet typical of the grape variety and appear overlaid and heavily aged after a short ripening period.
Butyric acid
The smell of butyric acid can man and some animals are perceived in small tracks. Humans rate the smell negatively. The smell of rancid fat, camembert or parmesan cheese is known. Butyric acid can arise especially if malolactic fermentation is not carried out correctly.
Sorbic acid

Sorbic acid is permitted as a preservative in wine up to an amount of 200 mg / l. The acid is often processed in the form of potassium sorbate (max. 275 mg / l). Sorbic acid is mostly used in residual sweet wines, as it suppresses the growth of fungi, bacteria and yeast. However, this does not apply to lactic acid bacteria, which continue to multiply in the presence of sorbic acid. If the malolactic fermentation starts unintentionally after the addition of the acid, the wine error described with the word geranium tone can arise. Even the smallest traces of the substance 2-ethoxy-hexa-3,5-diene can be determined by sensors. The perception threshold is 0.001 mg / l! It is often claimed that sorbic acid has not been used in Germany for decades. In 2007, Rainer Amann from the State Viticulture Institute in Freiburg published a study on 85 German wines and found the preservative in 4 residual sweet wines.
Succinic acid
Succinic acid is primarily a by-product of alcoholic fermentation, but is found in tiny amounts in the grapes of the vine. It is approved in the EU as a food additive with the number E 363 and as such serves, for example, as a flavor enhancer . The acid is created particularly during the carbonic acid maceration. While the succinic acid tastes slightly bitter and salty, the esterification monomethyl succinate brings a mild, fruity component to the wine.
The following table gives an overview of the organic acids that can be detected in wine .
| designation | Designation according to IUAPC | Structural formula | Molar mass | Acid constant | origin |
|---|---|---|---|---|---|
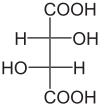
| Tartaric acid | 2,3-dihydroxybutanedioic acid |
|
150 g • mol −1 | 3.01 / 4.37 | healthy berry |
| Malic acid | 2-hydroxybutanedioic acid |

|
134.09 g • mol −1 | 3.46 / 5.13 | healthy berry |
| Citric acid | 2-hydroxypropane-1,2,3-tricarboxylic acid |
|
192.43 g • mol −1 | 3.14 / 5.74 | healthy berry |
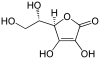
| Ascorbic acid | ( R ) -5 - [( S ) -1,2-dihydroxyethyl] -3,4-dihydroxy-5 H -furan-2-one |
|
176.13 g • mol −1 | 4.10 | healthy berry |
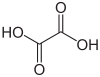
| Oxalic acid | Ethanedioic acid |
|
90.04 g • mol −1 | 1.27 / 4.28 | healthy berry |
| Glycolic acid | Hydroxyacetic acid |

|
76.05 g • mol −1 | 3.83 | healthy berry |
| Fumaric acid | trans -butenedioic acid |

|
116.07 g • mol −1 | 3.03 / 4.44 | healthy berry |
| Glucuronic acid | 3,4,5,6-tetrahydroxytetrahydropyran-2-carboxylic acid |

|
194.14 g • mol −1 | healthy berry + botrytis | |
| Galacturonic acid |

|
194.14 g • mol −1 | healthy berry + botrytis | ||
| Gluconic acid | 2,3,4,5,6-pentahydroxyhexanoic acid |

|
196.16 g • mol −1 | 3.86 | healthy berry + botrytis |
| Mucic acid | 2,3,4,5-tetrahydroxyadipic acid |

|
210.14 g • mol −1 | healthy berry + botrytis |
literature
- Jancis Robinson : The Oxford Wine Lexicon . 1st edition. Gräfe and Unzer Verlag, Munich 2003, ISBN 3-7742-0914-6 .
- Michael Broadbent : Check out wines, know them, enjoy them . 3. Edition. Raeber Verlag, Lucerne and Stuttgart 1986, ISBN 3-7239-0040-2 .
- Pascal Ribéreau-Gayon , Denis Dubourdieu, Bernard Donèche, Aline Lonvaud: Traité d'oenologie, Microbiologie du vin. Vinifications . 5th edition. Dunod, Éditions La Vigne, 2004, ISBN 2-10-007301-X .
- Pascal Ribéreau-Gayon, Denis Dubourdieu, Yves Glories, Alain Maujean: Traité d'oenologie, Chimie du vin. Stabilization et traitements . 5th edition. Dunod, Éditions La Vigne, 2004, ISBN 2-10-007302-8 .
Individual evidence
- ^ B. Lindemann: Receptors and transduction in taste . In: Nature . No. 413, 2001, ISSN 0028-0836 , pp. 219-25 PMID 11557991 .
- ↑ Determination of tartaric acid (according to Rebelein) University of Hohenheim.
- ↑ Jörg Gaffner: Biogenic Amines in Food ( Memento of the original from April 2, 2015 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , Institute Agroscope Changins - Wädenswil.
- ^ Rainer Amann: Acids in must and wine (PDF). In: The German Wine Magazine, September 15, 2007; State Viticulture Institute Freiburg.
- ^ Walter Kettet: Untypical aging notes in wine (PDF). In: Beverage Industry , 10/2000.
- ↑ International Sommelier from October 2003 ( Memento of the original from April 25, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , Page 10. Last access on November 29, 2008 ( English ).
- ↑ The determination of galacturonic acid in wine ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. .


