Hyperparathyroidism
| Classification according to ICD-10 | |
|---|---|
| E21.0 | Primary hyperparathyroidism |
| E21.1 | Secondary hyperparathyroidism |
| E21.2 | Tertiary, quaternary, or quadratic hyperparathyroidism |
| ICD-10 online (WHO version 2019) | |
Hyperparathyroidism ( HPT ) is a regulatory disorder of the epithelial cells ( parathyroid glands ). Hyperparathyroidism is characterized by an increased formation of parathyroid hormone (parathyroid hormone) , which regulates the calcium level in the blood .
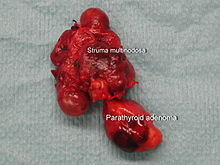
If the increased formation of parathyroid hormone is based on a benign tumor ( adenoma ) of the parathyroid gland, one speaks of a primary overactive parathyroid gland ( primary hyperparathyroidism ). Primary hyperparathyroidism is characterized by an increased parathyroid hormone level and an increased serum calcium.
If the increased formation of parathyroid hormone is the adequate response of the parathyroid glands to a reduced serum calcium ( hypocalcemia , e.g. in the case of vitamin D deficiency), one speaks of secondary hyperparathyroidism . Secondary hyperparathyroidism is characterized by an increased parathyroid hormone level with low serum calcium. An important cause of secondary hyperparathyroidism is the decreased activation of vitamin D due to chronic kidney disease .
Long-term secondary hyperparathyroidism can lead to an inadequate increase in parathyroid hormone due to chronic overstimulation of the parathyroid glands. Here too, parathyroid hormone levels and serum calcium are increased, but one speaks of tertiary hyperparathyroidism . Tertiary hyperparathyroidism can be differentiated from primary hyperparathyroidism by the medical history .
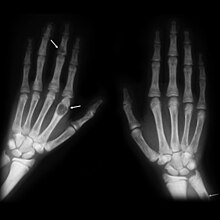
Consequences of hyperparathyroidism, which form the clinical picture of ostitis fibrosa generalisata , are degradation of bone substance due to an increased release of calcium from the bones , kidney stones due to increased calcium excretion in the urine , calcification of the blood vessels due to the deposition of calcium and phosphate and a large number of them further, partly unspecific symptoms. Since the serum calcium is often determined as part of a routine blood test , the diagnosis is usually made at an early stage when there are no or only unspecific symptoms.
Primary hyperparathyroidism is treated by surgical removal of the parathyroid adenoma. If an operation is not possible or if it is not desired, the course of the disease can be observed by regular checks of parathyroid hormone and calcium if the serum calcium is slightly increased. If the calcium is very high, the formation of parathyroid hormone can be inhibited by the drug Cinacalcet .
Secondary hyperparathyroidism is treated with vitamin D , cinacalcet and phosphate binders , the latter lowering increased phosphate levels in chronic kidney disease.
Tertiary hyperparathyroidism is treated by surgical removal of the parathyroid glands. To ensure sufficient formation of parathyroid hormone, either part of an epithelial body is left in place (subtotal parathyroidectomy) or part of an epithelial body is implanted in a muscle elsewhere (autologous retransplantation).
The parathyroid glands (epithelial cells)
The epithelial bodies are roughly the size of a lens. They have a diameter of 5 to 8 mm and a weight of around 20 to 50 mg. They are usually located at the back of the upper and lower poles of the thyroid gland . However, parathyroid glands can rarely be found in the thorax area. The reason can be found in the embryological development of the parathyroid glands. Most people have four epithelial cells. They form the parathyroid hormone , a hormone that regulates the calcium level in the body.
Parathyroid hormone and calcium balance
Parathyroid hormone and calcitriol are the two main hormones that regulate the calcium-phosphate balance. Parathyroid hormone keeps the serum level of calcium in a narrow range, in the short term by increasing the reabsorption of calcium in the tubular system of the kidneys and promoting the release of calcium by breaking down bone substance . In the long term, parathyroid hormone promotes the conversion of calcidiol into calcitriol and thus stimulates calcium absorption in the intestine .
The secretion of parathyroid hormone is by the calcium sensing receptor regulated which on the surface of parathyroid - cells expressed is. An increase in the concentration of ionized calcium leads, via activation of the calcium-sensitive receptor, to an inhibition of parathyroid hormone secretion; a decrease in ionized calcium leads to an increased secretion of parathyroid hormone.
Primary hyperparathyroidism
Pathogenesis
In patients with primary hyperparathyroidism, the parathyroid hormone secretion is inappropriately high in relation to the calcium concentration in the serum, triggered by an adenoma , adenocarcinoma or hyperplasia of one or more parathyroid glands (epithelial cells). The cause of the increased parathyroid hormone secretion is a reduced sensitivity (sensitivity) of the calcium-sensitive receptor due to a reduced number of calcium-sensitive receptors per parathyroid cell and / or an increased mass of parathyroid tissue due to an increased number of parathyroid cells. The regulation of the parathyroid hormone secretion is not completely eliminated, but a higher calcium concentration is necessary to inhibit the parathyroid hormone secretion - the target value of the calcium concentration is shifted to higher ranges.
etiology
Only in rare cases can a cause of primary hyperparathyroidism, ionizing radiation or genetic changes , be found.
Ionizing radiation
An increased incidence of primary hyperparathyroidism was found after irradiation of the cervical region; the risk of developing primary hyperparathyroidism increases with the radiation dose . An increased incidence of primary hyperparathyroidism was also found in survivors of the atomic bombing on Hiroshima . In patients with radiation-induced hyperparathyroidism, the incidence of thyroid cancer is increased, but otherwise the course does not differ from that of patients with idiopathic hyperparathyroidism (i.e. hyperparathyroidism with no demonstrable cause). The data on the occurrence of primary hyperparathyroidism after radioiodine therapy are contradicting, one study found an increased occurrence, in another study a connection between radioiodine therapy and hyperparathyroidism could not be confirmed.
A recent study has observed that liquidators who were busy decontaminating the Chernobyl nuclear reactor in 1986 had a significantly increased risk of developing primary hyperparathyroidism.
Genetic changes
In primary hyperparathyroidism there is usually a monoclonal proliferation of atypical parathyroid cells, i.e. H. the changed cells go back to a single changed mother cell. In the altered parathyroid tissue, mutations have been detected in a whole series of genes : in genes that control cell growth , in proto-oncogenes and in tumor suppressor genes .
So far, changes in the following genes have been described in detail:
- CYCLIN D1 has multiple functions in cell growth , cell differentiation and cell metabolism . An increased activity of cyclin D1 plays an important role in the development of tumors, including adenomas of the parathyroid gland.
- Multiple endocrine neoplasia type I; MEN1 : Inactivating mutations in MEN1, a tumor suppressor gene, lead to autosomal dominant inherited neoplasms of the parathyroid glands , the pituitary gland and the islet cells in the pancreas as well as an increased incidence of gastric ulcers .
- HRPT2 encodes for the protein Parafibromin , which in a complex with other proteins ( PAF1 and CTR9 ) of RNA polymerase II and histone - methyltransferase binds. Mutations in HRPT2 lead to adenoma of the parathyroid glands in conjunction with jaw - tumors , as well as parathyroid carcinoma .
- RET codes for a receptor tyrosine kinase . Mutations lead, among other things, to multiple endocrine neoplasia type IIA . Affected patients develop C-cell carcinomas of the thyroid gland , pheochromocytomas, and primary hyperparathyroidism in 15 to 20% of patients.
clinic
The increased parathyroid hormone level leads to increased bone loss and thus to an increased calcium concentration in the blood . The bone is demineralized. Bone pain can occur. In the kidneys, the parathyroid hormone ensures a reduced excretion of calcium with the urine, so that the amount of calcium in the blood also increases. The solubility product can be exceeded due to the reabsorption of calcium from the urine. As a result, kidney stones develop . In addition, gallstones and inflammation of the pancreas occur. The symptoms of hyperparathyroidism are described with the three words "stone, leg and stomach pain".
Secondary hyperparathyroidism
The cause here is increased hormone production in response to an increased loss of calcium (calcium deficiency, hypocalcemia ) in the body. Due to the compensatory increased hormone production, the calcium concentration in the blood can be in the lower normal range.
Underlying diseases of secondary hyperparathyroidism:
- Chronic kidney failure (typical: calcium level low, phosphate level too high)
- Malassimilation syndrome: disruption of calcium absorption in the intestine.
- Liver cirrhosis : Impaired conversion of vitamin D 3 (cholecalciferol) into 25-hydroxycholecalciferol in the liver. The lack of active vitamin D 3 means that less calcium is absorbed in the intestine and less calcium is reabsorbed in the kidneys.
- Cholestasis : Due to the lack of bile acids , the absorption of vitamin D 3 from food is disturbed.
- Lack of exposure to sunlight: Vitamin D 3 can no longer be formed in the skin from cholesterol (or 7-dehydro-cholesterol).
- rickets
Tertiary hyperparathyroidism
If secondary hyperparathyroidism persists over a longer period of time, in addition to the function of the parathyroid glands, their growth is also stimulated, so that an autonomous overproduction in the epithelial cells can ultimately occur. As with primary hyperparathyroidism, the control loop is separated at the site of the parathyroid glands.
Quaternary and quinary hyperparathyroidism
Quaternary and quadratic forms of hyperparathyroidism are rare. Quaternary hyperparathyroidism is a secondary hyperparathyroidism based on kidney damage if the kidney damage itself was caused by primary hyperparathyroidism. From a pathophysiological point of view, the control loop is separated, as in secondary hyperparathyroidism in the kidney. A hyperparathyroidism is referred to as quinary if the decoupling of the parathyroid hormone secretion from one or more remaining epithelial bodies is based on a long-standing quaternary hyperparathyroidism. In terms of differential diagnosis, the possibility of a sleeping parathyroid adenoma must be considered in both situations.
Symptoms of hyperparathyroidism
When diagnosing primary hyperparathyroidism, the following disease manifestations may exist:
- Accidentally discovered increase in calcium levels in the blood without clinical signs of disease ( asymptomatic hypercalcaemia )
- Increase in calcium levels in the blood with clinical signs ( symptomatic hypercalcaemia),
- Secondary diseases of hyperparathyroidism: decreased bone density (osteopenia) , bone loss (osteoporosis) , kidney stones (nephrolithiasis) ,
- rarely typical bone changes ( ostitis fibrosa cystica ) or hyperparathyroid crisis.
In earlier years, the classic symptoms of the advanced disease were in the foreground when they first appeared: stone - leg - stomach pain from kidney stones , pathological fractures and gastric ulcers . Since the increase in blood tests and bone density measurements , symptom-free (asymptomatic) and low-symptom (oligosymptomatic) courses predominate .
For the generalized demineralization with brown tumors in the advanced stage of the disease, the term Engel-von Recklinghausen syndrome or Recklinghausen's disease was used .
Asymptomatic hyperparathyroidism
At least 80% of patients with primary hyperparathyroidism be the incidental finding of elevated serum - calcium diagnosed. These patients mostly have mild (mean 2.8 mmol / l, norm 2.1–2.6 mmol / l) and intermittent hypercalcaemia. Occasionally calcium levels are normal and primary hyperparathyroidism is noted due to decreased bone density . With a more detailed history , some patients report unspecific symptoms such as loss of appetite (anorexia) , mild depressive mood , mild memory or neuromuscular disorders.
In primary hyperparathyroidism, there is often a vitamin D deficiency, which can obscure the diagnosis. After administration of vitamin D, the calcium level can then rise, which indicates the diagnosis. Vitamin D deficiency and hyperparathyroidism can also lead to more pronounced clinical pictures with larger parathyroid adenomas, higher concentrations of parathyroid hormone, increased bone remodeling and increased bone fractures.
Symptomatic hyperparathyroidism
Calcium is broken down from the bones due to the increased parathyroid hormone level . The bone density decreases, calcium levels in the blood and calcium excretion in the urine increase.
The increased calcium level in the blood ( hypercalcemia ) leads to unspecific complaints, loss of appetite (anorexia) , nausea (nausea), constipation (constipation) , increased thirst (polydipsia) and increased urine production (polyuria) .
At the bones occurs Osteoporosis and pathological fractures , especially in the area of the spine .
Long-term secondary renal hyperparathyroidism can cause so-called brown tumors, which consist of bone-degrading cells (osteoclasts) and connective tissue , in the bones . The term brown tumor refers to the brown color that is caused by bleeding.
In the kidneys , the increased calcium excretion leads to the precipitation of kidney stones (nephrolithiais) , soft tissue calcifications (nephrocalcinosis) , renal colic , chronic kidney failure and disorders of the tubular function . The kidney stones consist mainly of calcium oxalate , less often calcium phosphate . Kidney stones are present in around 15–20% of patients with primary hyperparathyroidism, whereas in around 5% of those who have kidney stones, primary hyperparathyroidism is the cause of the stone disease.
Patients often complain of neuromuscular complaints such as weakness, exhaustion and tiredness.
In addition, patients with primary hyperparathyroidism are more likely to suffer from psychiatric illnesses than in the normal population, such as indifference (lethargy) , depression , loss of contact with reality (psychoses) , reduced social interaction skills and impaired cognitive abilities (dementia) . However, exact figures are not available.
There is an increased prevalence of high blood pressure , obesity and impaired glucose tolerance as well as rheumatological diseases such as increased uric acid (hyperuricemia) , gout and pseudogout . There is evidence that the prevalence of cancer is increased in primary hyperparathyroidism, and that the increased risk of cancer persists even after surgical removal of the parathyroid adenoma. Based on these findings, common predisposing factors for hyperparathyroidism and cancer have been speculated.
In patients with primary hyperparathyroidism, there is a high incidence of cardiovascular diseases , especially left ventricular hypertrophy and calcifications of the heart muscle (myocardium) , aortic and mitral valves . The risk of dying from heart disease may be increased.
Even with mild primary hyperparathyroidism, the blood vessels stiffen .
In particular, secondary renal hyperparathyroidism can lead to severe calcification of the vessels , including the coronary arteries . The consequences are a significantly increased morbidity and mortality from cardiovascular diseases .
The close connection between bone and cardiovascular disease in secondary hyperparathyroidism has led to the fact that in the latest guidelines the term renal osteopathy has been replaced by the term renal mineral and bone disease (chronic kidney disease-mineral and bone disorder), which includes both bone metabolism and bone disorder Vascular calcifications and their consequences.
A particularly serious complication of secondary hyperparathylaxis is ( calciphylaxis ), in which calcium deposits lead to thrombosis in the arterioles of the skin . These can cause severe circulatory disorders with extensive skin necrosis , which often lead to death.
In addition, gastrin production is stimulated, which can lead to inflammation of the stomach lining (gastritis) and duodenal ulcer (ulcus duodeni) .
Epidemiology
The incidence of primary hyperparathyroidism has decreased in recent years. In an American registry, the incidence was 21.6 cases per 100,000 person-years from 1993 to 2001, 29.1 cases from 1983 to 1992 and 82.5 cases from 1974 to 1982. The reasons for this decrease in the frequency is not known, possible causes include decreased exposure to ionizing radiation and improved supply of vitamin D .
Primary hyperparathyroidism can occur at any age, but most diseases do not manifest until after the age of 45. Women are affected about twice as often as men, possibly because bone loss occurring after menopause can unmask latent hyperparathyroidism.
histology
Parathyroid
In primary hyperparathyroidism, the tissue examination of the parathyroid glands describes the following changes:
- A single benign tumor (adenoma) of the parathyroid gland is found in 89% of cases of primary hyperparathyroidism, and two adenomas are found in about 5% of cases. Most adenomas are formed by the main cells of the parathyroid and are surrounded by a capsule. Occasionally, adenomas made up of oxyphilic cells are found, these adenomas are usually larger. There are also parathyroid hormone-producing adenomas in the thymus .
- Glandular hyperplasia is found in 6% of cases . H. a diffuse enlargement of all four parathyroid bodies due to the multiplication of the main cells. Very rarely, glandular hyperplasia is caused by an increase in clear cells.
- Parathyroid carcinoma is found in approx. 2% of cases and is characterized by invasion of the capsule and vessels , lymph nodes and distant metastases .
bone
A histological examination of the bone reveals changes that are described as ostitis fibrosa . The increased parathyroid hormone level activates osteoclasts . The increased osteoclast activity leads to a breakdown of bone substance. The tissue examination reveals microfractures and bleeding. Cavities are formed that are filled with connective tissue , osteoclasts and hemosiderin- laden macrophages . Increasing dissolution (resorption) of bone tissue and connective tissue remodeling (fibrosis) leads to the formation of bone cysts that are visible to the naked eye. As the disease progresses, the bone cysts fuse to form brown tumors ; the brown color is the result of bleeding and hemosiderin deposits (case studies and fig. Below)
diagnosis
Diagnosis of primary hyperparathyroidism
The first indication of primary hyperparathyroidism is an increased calcium level ( hypercalcemia ). Other causes of hypercalcaemia can be differentiated by determining the parathyroid hormone concentration. The diagnosis of primary hyperparathyroidism is made when the parathyroid hormone is increased, or when the parathyroid hormone is in the normal range of 10 to 60 pg / ml, but is relatively increased, based on a simultaneously increased calcium level. About 80–90% of the patients with primary hyperparathyroidism have an elevated parathyroid hormone, in 10–20% of the patients the parathyroid hormone is in the normal range.
In primary hyperparathyroidism, osteolytic changes in the mouth, jaw and face area, in contrast to the rest of the skeletal system, e.g. B. acroosteolysis , rarely observed, but may be the first clinical indication of endocrine disorder.
Differential diagnosis of primary hyperparathyroidism
Besides hyperparathyroidism, malignant cancers are the most common causes of hypercalcaemia . Most of the time, when hypercalcaemia occurs, the cancer is known and has progressed. In addition, the calcium levels in cancer are higher and the associated symptoms are more severe. The parathyroid hormone is usually very low in hypercalcaemia due to cancer.
Other causes of hypercalcaemia such as milk-alkali syndrome , sarcoidosis and vitamin D overdose can also be differentiated from hyperparathyroidism by a reduced parathyroid hormone.
In familial hypocalzuric hypercalcemia (FHH) , the excretion of calcium in the urine is reduced, whereas in patients with primary hyperparathyroidism, calcium excretion is normal or increased.
Thiazide diuretics reduce the excretion of calcium by the kidneys and lead to mild hypercalcaemia. In patients with mild hyperparathyroidism, thiazide diuretics can unmask the underlying disease by causing a significant increase in calcium in the serum. If an increased calcium level does not drop after discontinuation of thiazide diuretics, this indicates the presence of primary hyperparathyroidism.
Lithium increases the secretion of parathyroid hormones and reduces the excretion of calcium via the kidneys, hypercalcaemia and hypocalzuria occur, and some patients have increased parathyroid hormone levels. The hypercalcaemia may normalize after discontinuation of lithium; after prolonged treatment with lithium (more than 10 years), normalization of the calcium level after the end of therapy is less likely.
Diagnosis of secondary hyperparathyroidism
Secondary hyperparathyroidism is characterized by a decreased calcium level with an adequate increase in parathyroid hormone.
The most common cause of secondary hyperparathyroidism is a reduced production of activated vitamin D ( calcitriol ) due to chronic kidney impairment .
Other causes of secondary hyperparathyroidism are:
- decreased calcium intake with food
- decreased calcium absorption through the intestine (calcium malabsorption )
- increased phosphate ( hyperphosphataemia )
- Vitamin D deficiency
- Bariatric Surgery
- Celiac disease
- Exocrine pancreatic insufficiency
- Loss of calcium through the kidneys
- Idiopathic hypercalciuria
- Loop diuretics
- Decreased bone remodeling, e.g. B. due to bisphosphonates
- Hungry bone syndrome - "hungry bone" syndrome
Differential diagnosis of secondary hyperparathyroidism
Some patients with primary hyperparathyroidism have normal serum calcium (normocalcemic primary hyperparathyroidism). In these cases, it is difficult to differentiate between primary and secondary hyperparathyroidism. Often, decreased bone density is the first manifestation of the disease.
Causes of normoccalemic primary hyperparathyroidism are:
- Early stage or incomplete expression (“forme fruste”) of the disease; Over the course of 3 years, about 40% of those affected develop further disease manifestations (in half of the cases hypercalcemia, otherwise kidney stones, broken bones, hypercalciuria, osteoporosis). Follow-up checks are therefore indicated.
- Primary hyperparathyroidism with simultaneous vitamin D deficiency
Clinical Chemistry
The diagnosis is made through laboratory tests .
- An increased parathyroid hormone or in the high normal range indicates primary hyperparathyroidism with a simultaneous increase in serum calcium .
- The calcium excretion in the 24-hour urine collection is increased in approx. 40% of patients with primary hyperparathyroidism and normal in approx. 60%. If the calcium excretion exceeds 400 mg / 24 h, the risk of kidney stones increases. A reduced calcium excretion is found in familial hypocalzuric hypercalcemia or in primary hyperparathyroidism with a simultaneous vitamin D deficiency.
- Due to a reduced blood level of 25 (OH) vitamin D3 , primary hyperparathyroidism with a simultaneous vitamin D deficiency can be differentiated from familial hypocalzuric hypercalcemia.
- In primary hyperparathyroidism, the serum calcitriol levels are in the highly normal range or increased; in secondary hyperparathyroidism due to chronic kidney failure, the calcitriol levels are reduced.
- In primary hyperparathyroidism, the phosphate excretion via the kidneys is increased, the phosphate level in the serum is reduced ( hypophosphataemia ) or is in the low normal range. In secondary renal hyperparathyroidism, the phosphate level is typically increased.
- An increased serum creatinine indicates impaired kidney function. This can be the result of hypercalcemia in primary hyperparathyroidism, but it can also be the cause of secondary hyperparathyroidism.
- Biochemical markers of bone turnover (e.g. alkaline phosphatase ) are in the high normal or elevated range in primary and secondary hyperparathyroidism.
Osteodensitometry
In patients with primary hyperparathyroidism, the mineral salt content of the bone is reduced. The bone density measurement ( bone densitometry ) is not required for diagnosis, but to assess the severity of the disease to progress monitoring and for determining whether a conservative or surgical treatment is indicated.
There is no close relationship between fractures and bone density in patients with secondary renal hyperparathyroidism. According to the current state of knowledge, routine bone density measurement is not useful in renal hyperparathyroidism.
Localization diagnostics
Before a planned operation, the location and size of the affected parathyroid bodies can be visualized by sonography , scintigraphy with technetium -99m- sestamibi , computed tomography or magnetic resonance imaging .
Therapy of hyperparathyroidism
Therapy of primary hyperparathyroidism
Surgical therapy
The surgical removal of (or all) the enlarged epithelial body leads to an improvement in bone density and quality of life as well as to a reduction in bone fractures , in particular femoral neck fractures , compared to monitoring the progression . In the majority of asymptomatic patients, however, there is no evidence of progression of the disease, such as a decrease in bone density, an increase in calcium and parathyroid hormone or the occurrence of kidney stones, even after long-term observation.
For this reason, guidelines have been developed with the aim of referring those patients to surgical therapy who are at greatest risk of organ damage or disease progression.
Criteria of the 2002 NIH Workshop on Asymptomatic Primary Hyperparathyroidism for Surgical Therapy:
- Serum calcium 0.25 mmol / l (1.0 mg / dl) above the upper limit of normal.
- Calcium excretion in the urine over 10 mmol / day (400 mg / day) with normal diet.
- Creatinine clearance 30% of the average age or less.
- Bone density at the hip, lumbar spine or radius more than 2.5 standard deviations below the mean (T value <−2.5).
- Age under 50 years.
- Regular follow-up checks are not guaranteed.
Non-surgical therapy
Although the surgical therapy leads to a definitive cure of the primary hyperparathyroidism, not all patients are operated on, especially if there are no symptoms, only a slight increase in calcium, if they are over 50 years old, if they have severe comorbidities or if an operation is not desired. In these cases, the following non-surgical procedures are available:
- Bisphosphonates inhibit the breakdown of bone by osteoclasts . Alendronate leads to an increase in bone mass in asymptomatic primary hyperparathyroidism. Risedronate lowers high calcium levels.
- Raloxifene is a selective estrogen receptor modulator. Raloxifene decreases serum calcium in postmenopausal women with primary hyperparathyroidism. After careful risk-benefit assessment, an alternative estrogen replacement therapy can be considered.
- Calcimimetics (e.g. cinacalcet ) that attack the calcium-sensitive receptor of the parathyroid cell and thus directly inhibit the excretion of parathyroid hormones have been available since 2004 .
- Percutaneous alcohol ablation: Injecting alcohol into the enlarged parathyroid gland can destroy it. Side effects are damage to the recurrent laryngeal nerve and an excessive drop in serum calcium ( hypocalcemia )
- In addition, a low-calcium diet with sufficient vitamin D content and regular physical activity are recommended
In patients who do not undergo surgery, calcium is checked every six months, creatinine and bone density checked annually. If there is any indication that the disease is progressing, surgery is performed.
Therapy of secondary hyperparathyroidism
Secondary hyperparathyroidism is generally treated by eliminating the underlying disease. For secondary hyperparathyroidism as a result of chronic kidney failure, there is a graduated drug therapy established by guidelines.
Secondary hyperparathyroidism in chronic kidney failure
Secondary hyperparathyroidism in chronic kidney failure is treated with a low-phosphate diet, vitamin D metabolites, phosphate binders, and cinacalcet.
If the parathyroid hormone level in stages 2-3 of chronic kidney failure is above 70 pg / ml (or> 110 pg / ml in stage 4 or> 300 pg / ml in stage 5), a low-phosphate diet is started. If a low-phosphate diet fails to reduce the phosphate level sufficiently, drugs are used that bind the phosphate supplied with food in the intestine (oral phosphate binders): calcium carbonate , calcium acetate , aluminum hydroxide , sevelamer or lanthanum carbonate . Calcium-containing phosphate binders are not used in the case of increased calcium values because of the risk of soft tissue calcification; the amount is limited to 2.5 g calcium per day. Phosphate binders containing aluminum are only used at very high phosphate levels and for a limited period of time because of the risk of aluminum being deposited in bones and brain.
A reduced vitamin D level in the serum, (25 (OH) vitamin D 3 <30 ng / ml) is compensated for by administration of vitamin D. If the parathyroid hormone level does not fall within the target range despite these measures, active vitamin D ( alfacalcidol or calcitriol ) is also administered. The side effect of therapy with active vitamin D is an increase in calcium and phosphate levels with the risk of soft tissue calcification. This side effect is less when using pharmacologically modified vitamin D metabolites such as paricalcitol .
If a low-phosphate diet, phosphate binders and vitamin D metabolites are unsuccessful, cinacalcet , an antagonist of the calcium-sensitive receptor, is used.
In the absence of therapy, a transition to tertiary hyperparathyroidism is likely.
Therapy of tertiary hyperparathyroidism
If the parathyroid hormone level rises to values above 1000 pg / ml despite drug therapy and bone changes and therapy-refractory hypercalcemia and / or hyperphosphataemia occur , all 4 (to 8) epithelial cells must be removed ( parathyroidectomy ) and then part of an epithelial body must be transplanted into the forearm or the sternocleidomastoid muscle , as otherwise no more parathyroid hormone formation could take place. Alternatively, a subtotal parathyroidectomy can be performed. H. a parathyroid body is not completely removed.
See also
Guidelines
- John P. Bilezikian et al .: Summary Statement from a Workshop on Asymptomatic Primary Hyperparathyroidism: A Perspective for the 21st Century . In: J Clin Endocrinol Metab . No. 12 , 2002, p. 5353-5361 ( Article ).
- KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease ( Memento of February 14, 2015 in the Internet Archive ).
literature
- Ludwig Weissbecker : Hyperparathyroidism (Ostitis fibrosa generalisata, Recklinghausen's disease). In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition, ibid. 1961, pp. 1057-1060.
Web links
- Hyperparathyroidism Pathology - Pathopic image database of the University of Basel ( PathoPic instructions (PDF; 2.2 MB))
Individual evidence
- ↑ EM Brown: Four-parameter model of the sigmoidal relationship between parathyroid hormone release and extracellular calcium concentration in normal and abnormal parathyroid tissue . In: The Journal of Clinical Endocrinology and Metabolism . tape 56 , no. 3 , March 1983, ISSN 0021-972X , p. 572-581 , PMID 6822654 .
- ↑ JH Brossard, S. Whittom, R. Lepage, P. D'Amour: Carboxyl-terminal fragments of parathyroid hormone are not secreted preferentially in primary hyperparathyroidism as they are in other hypercalcemic conditions . In: The Journal of Clinical Endocrinology and Metabolism . tape 77 , no. 2 , August 1993, ISSN 0021-972X , p. 413-419 , PMID 8345045 .
- ↑ LE Tisell, S. Carlsson, M. Fjälling, G. Hansson, S. Lindberg, LM Lundberg, A. Odén: hyperparathyroidism Subsequent to neck irradiation. Risk factors . In: Cancer . tape 56 , no. 7 , October 1, 1985, ISSN 0008-543X , pp. 1529-1533 , PMID 4027889 .
- ↑ AB Schneider, TC Gierlowski, E. Shore Freedman, M. Stovall, E. Ron, J. Lubin: Dose-response relationships for radiation-induced hyperparathyroidism . In: The Journal of Clinical Endocrinology and Metabolism . tape 80 , no. 1 , January 1995, ISSN 0021-972X , p. 254-257 , PMID 7829622 .
- Jump up ↑ S. Fujiwara, R. Sposto, H. Ezaki, S. Akiba, K. Neriishi, K. Kodama, Y. Hosoda, K. Shimaoka: Hyperparathyroidism among atomic bomb survivors in Hiroshima . In: Radiation Research . tape 130 , no. 3 , June 1992, ISSN 0033-7587 , pp. 372-378 , PMID 1594765 .
- ↑ S. Tezelman, JM Rodriguez, W. Shen, AE Siperstein, QY Duh, OH Clark: Primary hyperparathyroidism in patients who have received radiation therapy and in patients who have not received radiation therapy . In: Journal of the American College of Surgeons . tape 180 , no. 1 , January 1995, ISSN 1072-7515 , pp. 81-87 , PMID 8000660 .
- ↑ Shanthi M Colaço, Ming Si, Emily Reiff, Orlo H Clark: hyperparathyroidism after radioactive iodine therapy . In: American Journal of Surgery . tape 194 , no. 3 , September 2007, ISSN 0002-9610 , p. 323-327 , doi : 10.1016 / j.amjsurg.2007.04.005 , PMID 17693276 .
- ↑ M. Fjälling, A. Dackenberg, I. Hedman, LE Tisell: An evaluation of the risk of developing hyperparathyroidism after 131I treatment for thyrotoxicosis . In: Acta Chirurgica Scandinavica . tape 149 , no. 7 , 1983, ISSN 0001-5482 , pp. 681-686 , PMID 6650083 .
- ^ Bernhard O. Boehm, Silke Rosinger, David Belyi, Johannes W. Dietrich: The Parathyroid as a Target for Radiation Damage . In: New England Journal of Medicine . tape 365 , no. 7 , August 2011, p. 676-678 , doi : 10.1056 / NEJMc1104982 , PMID 21848480 .
- ^ S. Yavuz, WF Simonds, LS Weinstein, MT Collins, E. Kebebew, N. Nilubol, GQ Phan, SK Libutti, AT Remaley, M. Van Deventer, SJ Marx: Sleeping parathyroid tumor: rapid hyperfunction after removal of the dominant tumor. In: J Clin Endocrinol Metab. 97 (6), Jun 2012, pp. 1834-1841, doi: 10.1210 / jc.2011-3030 , Epub April 16, 2012. PMID 22508712
- ^ A b John P Bilezikian, John T Potts: Asymptomatic primary hyperparathyroidism: new issues and new questions-bridging the past with the future . In: Journal of Bone and Mineral Research . 17 Suppl 2, November 2002, ISSN 0884-0431 , p. N57-N67 , PMID 12412779 .
- ↑ Who named it
- ^ A b S. J. Silverberg, JP Bilezikian: Evaluation and management of primary hyperparathyroidism . In: The Journal of Clinical Endocrinology and Metabolism . tape 81 , no. 6 , June 1996, ISSN 0021-972X , p. 2036-2040 , PMID 8964825 .
- ↑ Yuko Chiba, Katsuhiko Satoh, Satoshi Ueda, Nobuo Kanazawa, Yoshiaki Tamura, Toshiyuki Horiuchi: Marked improvement of psychiatric symptoms after parathyroidectomy in elderly primary hyperparathyroidism . In: Endocrine Journal . tape 54 , no. 3 , June 2007, ISSN 0918-8959 , p. 379-383 , doi : 10.1507 / endocrj.K06-152 , PMID 17420608 .
- ^ E. Lundgren, E. Szabo, S. Ljunghall, R. Bergström, L. Holmberg, J. Rastad: Population based case-control study of sick leave in postmenopausal women before diagnosis of hyperparathyroidism . In: BMJ (Clinical Research Ed.) . tape 317 , no. 7162 , September 26, 1998, ISSN 0959-8138 , p. 848-851 , PMID 9748176 , PMC 31094 (free full text).
- ↑ P. Boudou, F. Ibrahim, C. Cormier, E. Sarfati, JC Souberbielle: A very high incidence of low 25 hydroxy-vitamin D serum concentration in a French population of patients with primary hyperparathyroidism . In: Journal of Endocrinological Investigation . tape 29 , no. 6 , June 2006, ISSN 0391-4097 , p. 511-515 , PMID 16840828 .
- ^ SJ Silverberg, E. Shane, DW Dempster, JP Bilezikian: The effects of vitamin D insufficiency in patients with primary hyperparathyroidism . In: The American Journal of Medicine . tape 107 , no. 6 , December 1999, ISSN 0002-9343 , p. 561-567 , PMID 10625024 .
- ^ Sundeep Khosla, Joseph Melton: Fracture risk in primary hyperparathyroidism . In: Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research . 17 Suppl 2, November 2002, ISSN 0884-0431 , p. N103-N107 , PMID 12412786 .
- ↑ Wanjun Ren et al.: Quiz page September 2008: progressive paraplegia in a long-term hemodialysis patient. Brown tumor compressing the thoracic spinal column . In: American Journal of Kidney Diseases . tape 52 , no. 3 , September 2008, ISSN 1523-6838 , p. A37 – A39 , doi : 10.1053 / j.ajkd.2007.12.029 ( ajkd.org [accessed November 7, 2008]).
- ↑ Jane M Suh et al .: Primary hyperparathyroidism: is there an increased prevalence of renal stone disease? In: AJR. American Journal of Roentgenology . tape 191 , no. 3 , September 2008, ISSN 1546-3141 , p. 908-911 , doi : 10.2214 / AJR.07.3160 , PMID 18716127 .
- ^ E. Lundgren et al .: Case-control study on symptoms and signs of "asymptomatic" primary hyperparathyroidism . In: Surgery . tape 124 , no. 6 , December 1998, ISSN 0039-6060 , p. 980-985; discussion 985-986 , PMID 9854572 .
- ^ Theresia Weber et al .: Effect of parathyroidectomy on quality of life and neuropsychological symptoms in primary hyperparathyroidism . In: World Journal of Surgery . tape 31 , no. 6 , June 2007, ISSN 0364-2313 , p. 1202-1209 , doi : 10.1007 / s00268-007-9006-6 , PMID 17460812 .
- ^ L. Lind, S. Jacobsson, M. Palmér, H. Lithell, B. Wengle, S. Ljunghall: Cardiovascular risk factors in primary hyperparathyroidism: a 15-year follow-up of operated and unoperated cases . In: Journal of Internal Medicine . tape 230 , no. 1 , July 1991, ISSN 0954-6820 , p. 29-35 , PMID 2066709 .
- ↑ Mark J Bolland, Andrew B Gray, Greg D Gamble, Ian R Reid: Association between primary hyperparathyroidism and increased body weight: a meta-analysis . In: The Journal of Clinical Endocrinology and Metabolism . tape 90 , no. 3 , March 2005, ISSN 0021-972X , p. 1525-1530 , doi : 10.1210 / jc.2004-1891 , PMID 15613408 .
- ↑ M. Procopio, G. Magro, F. Cesario, A. Piovesan, A. Pia, N. Molineri, G. Borretta: The oral glucose tolerance test reveals a high frequency of both impaired glucose tolerance and undiagnosed Type 2 diabetes mellitus in primary hyperparathyroidism . In: Diabetic Medicine: A Journal of the British Diabetic Association . tape 19 , no. 11 , November 2002, ISSN 0742-3071 , p. 958-961 , PMID 12421435 .
- ↑ Mishaela R Rubin, Shonni J Silverberg: Rheumatic manifestations of primary hyperparathyroidism and parathyroid hormone therapy . In: Current Rheumatology Reports . tape 4 , no. 2 , April 2002, ISSN 1523-3774 , p. 179-185 , PMID 11890884 .
- ↑ M. Palmér HO Adami, UB Krusemo, S. Ljunghall: Increased risk of malignant diseases after surgery for primary hyperparathyroidism. A nationwide cohort study . In: American Journal of Epidemiology . tape 127 , no. 5 , May 1988, ISSN 0002-9262 , pp. 1031-1040 , PMID 3358404 .
- ^ Inga-Lena Nilsson, Jan Zedenius, Li Yin, Anders Ekbom: The association between primary hyperparathyroidism and malignancy: nationwide cohort analysis on cancer incidence after parathyroidectomy . In: Endocrine-Related Cancer . tape 14 , no. 1 , March 2007, ISSN 1351-0088 , p. 135-140 , doi : 10.1677 / erc.1.01261 , PMID 17395982 .
- ↑ Karin B Michels, Fei Xue, Lena Brandt, Anders Ekbom: Hyperparathyroidism and subsequent incidence of breast cancer . In: International Journal of Cancer . tape 110 , no. 3 , June 20, 2004, ISSN 0020-7136 , p. 449-451 , PMID 15095313 .
- ↑ T. Stefenelli et al .: Cardiac abnormalities in patients with primary hyperparathyroidism: implications for follow-up . In: The Journal of Clinical Endocrinology and Metabolism . tape 82 , no. 1 , January 1997, ISSN 0021-972X , p. 106-112 , PMID 8989242 .
- ↑ Patrik Andersson, Erik Rydberg, Ronnie Willenheimer: Primary hyperparathyroidism and heart disease - a review . In: European Heart Journal . tape 25 , no. October 20 , 2004, ISSN 0195-668X , p. 1776–1787 , doi : 10.1016 / y.ehj 2004.07.010 , PMID 15474692 .
- ↑ JC Smith et al .: Augmentation of central arterial pressure in mild primary hyperparathyroidism . In: The Journal of Clinical Endocrinology and Metabolism . tape 85 , no. October 10 , 2000, ISSN 0021-972X , p. 3515-3519 , PMID 11061493 .
- ↑ Mishaela R Rubin include: Arterial stiffness in mild primary hyperparathyroidism . In: The Journal of Clinical Endocrinology and Metabolism . tape 90 , no. 6 , June 2005, ISSN 0021-972X , p. 3326-3330 , doi : 10.1210 / jc.2004-1400 , PMID 15769995 .
- ^ Giorgio Coen: Calcimimetics, parathyroid hormone, and vascular calcification in chronic kidney disease . In: Kidney International . tape 74 , no. November 10 , 2008, ISSN 1523-1755 , pp. 1229-1231 , doi : 10.1038 / ki.2008.417 , PMID 18974757 .
- ↑ Goce B. Spasovski: Bone health and vascular calcification in chronic kidney disease relationships . In: International Urology and Nephrology . tape 39 , no. 4 , 2007, ISSN 0301-1623 , p. 1209-1216 , doi : 10.1007 / s11255-007-9276-9 , PMID 17899431 .
- ↑ Hirotaka Komaba et al: Treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) . In: Internal Medicine (Tokyo, Japan) . tape 47 , no. 11 , 2008, ISSN 1349-7235 , p. 989-994 , doi : 10.2169 / internalmedicine.47.1051 , PMID 18520108 .
- ^ MD Arenas et al: Management of calcific uremic arteriolopathy (calciphylaxis) with a combination of treatments, including hyperbaric oxygen therapy . In: Clinical Nephrology . tape 70 , no. 3 , September 2008, ISSN 0301-0430 , p. 261-264 , PMID 18793571 .
- ↑ Hiroshi Sato et al.: Primary hyperparathyroidism with duodenal ulcer and H. pylori infection . In: Internal Medicine (Tokyo, Japan) . tape 41 , no. 5 , May 2002, ISSN 0918-2918 , p. 377-380 , PMID 12058887 .
- ^ RA Wermers et al .: Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993-2001: an update on the changing epidemiology of the disease . In: J Bone Miner Res. Band 21 , no. 1 , 2006, p. 171-177 , doi : 10.1359 / JBMR.050910 , PMID 16355286 .
- ↑ GI Salti, I. Fedorak, T. Yashiro, N. Fulton, H. Hara, D. Yousefzadeh, EL Kaplan: Continuing evolution in the surgical management of primary hyperparathyroidism . In: Archives of Surgery . tape 127 , no. 7 , July 1992, ISSN 0004-0010 , pp. 831-836; discussion 836-837 , PMID 1524484 .
- ↑ D. Bartsch, C. Nies, C. Hasse, J. Willuhn, M. Rothmund: Clinical and surgical aspects of double adenoma in patients with primary hyperparathyroidism . In: British Journal of Surgery . tape 82 , no. 7 , July 1995, ISSN 0007-1323 , pp. 926-929 , PMID 7648110 .
- ↑ Alexander Maret, Isabelle Bourdeau, Changlin Ding, Shrihari S Kadkol, William H Westra, Michael A Levine: Expression of GCMB by intrathymic parathyroid hormone-secreting adenomas indicates their parathyroid cell origin . In: The Journal of Clinical Endocrinology and Metabolism . tape 89 , no. 1 , January 2004, ISSN 0021-972X , p. 8-12 , PMID 14715818 .
- ↑ James M Ruda, Christopher S Hollenbeak, Brendan C Stack: A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003 . In: Otolaryngology - Head and Neck Surgery . tape 132 , no. 3 , March 2005, ISSN 0194-5998 , p. 359-372 , doi : 10.1016 / j.otohns.2004.10.005 , PMID 15746845 .
- ↑ AG Wynne, J. van Heerden, JA Carney, LA Fitzpatrick: Parathyroid carcinoma: clinical and pathologic features in 43 patients . In: Medicine . tape 71 , no. 4 , July 1992, ISSN 0025-7974 , pp. 197-205 , PMID 1518393 .
- ↑ Wanjun Ren, Xiaoping Wang, Bin Zhu, Zidong Liu: Quiz page September 2008: progressive paraplegia in a long-term hemodialysis patient. Brown tumor compressing the thoracic spinal column . In: American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation . tape 52 , no. 3 , September 2008, ISSN 1523-6838 , p. A37 – A39 , doi : 10.1053 / j.ajkd.2007.12.029 ( ajkd.org [accessed November 7, 2008]).
- ↑ George M. Nassar, Juan Carlos Ayus: Brown Tumor in End-Stage Renal Disease . In: N Engl J Med . tape 341 , no. 22 , November 25, 1999, pp. 1652 , doi : 10.1056 / NEJM199911253412204 .
- ^ AN Hollenberg, A. Arnold: Hypercalcemia with low-normal serum intact PTH: a novel presentation of primary hyperparathyroidism . In: Am J Med . tape 91 , no. 5 , November 1991, pp. 547-548 , PMID 1951417 .
- ↑ SR Nussbaum et al .: Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia . In: Clin Chem . tape 33 , no. 8 , August 1987, p. 1364-1367 , PMID 3608153 ( clinchem.org ).
- ^ W. Millesi, B. Niederle et al.: Osteolyses in the jaw area as the first indication of primary hyperparathyroidism. In: Acta Chirurgica Austriaca. Volume 26, Issue 6, 1994, pp. 410-414. doi: 10.1007 / BF02620046
- ↑ gel H Fuleihan: Familial benign hypocalciuric hypercalcemia . In: J Bone Miner Res . 17 Suppl 2, 2002, p. 51-56 , PMID 12412778 .
- ↑ H. Lowe: Normocalcemic primary hyperparathyroidism: Further characterization of a new clinical phenotype . In: J Clin Endocrinol Metab . No. 92 , 2007, p. 3001 , PMID 17536001 ( endojournals.org ).
- ↑ P. Ureña et al .: Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients . In: Nephrol Dial Transplant . No. 18 , 2003, p. 2325-2331 , PMID 14551361 ( oxfordjournals.org ).
- ↑ SA Jamal et al .: Low bone mineral density and fractures in long-term hemodialysis patients: a meta-analysis . In: Am J Kidney Dis . tape 49 , no. 5 , May 2007, pp. 674-681 , PMID 17472850 .
- ↑ Elena Ambrogini et al: Surgery or Surveillance for Mild Asymptomatic Primary Hyperparathyroidism: A Prospective, Randomized Clinical Trial . In: J Clin Endocrinol Metab . No. 92 , 2007, p. 3114-3121 ( abstract ).
- ^ Lindi H. VanderWalde et al .: The Effect of Parathyroidectomy on Bone Fracture Risk in Patients With Primary Hyperparathyroidism . In: Arch Surg . No. 141 , 2006, pp. 885-891 ( abstract ).
- ^ John P. Bilezikian et al .: Summary Statement from a Workshop on Asymptomatic Primary Hyperparathyroidism: A Perspective for the 21st Century . In: J. Clin. Endocrinol. Metab. No. 12 , 2002, p. 5353-5361 ( Article ).
- ↑ B. Farford, RJ Presutti, TJ Moraghan: Nonsurgical Management of Primary hyperparathyroidism . In: Mayo Clin Proc . tape 82 , no. 3 , 2007, p. 351-355 .
- ↑ Eleanor Lederer, Rosemary Ouseph: Chronic Kidney Disease . In: American Journal of Kidney Diseases . tape 49 , no. 1 , 2007, p. 162-171 ( Article ).
- ↑ K / DOQI: Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease - Guideline 14 . In: American Journal of Kidney Diseases . 42, Supplement 3, 2003, p. 1–202 ( Article ( Memento of February 21, 2013 in the Internet Archive )). Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease - Guideline 14 ( Memento of the original dated February 21, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ K / DOQI: Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease Guideline 15 . In: American Journal of Kidney Diseases . tape 42 , Supplement 3, October 2003, p. 1–202 ( Article ( Memento from June 20, 2012 in the Internet Archive )). Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease Guideline 15 ( Memento of the original from June 20, 2012 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.