Influenza virus
| Influenza viruses | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Influenza virus A / Hong Kong / 1/68 at 70,000 times magnification |
||||||||||||||||||
| Systematics | ||||||||||||||||||
|
||||||||||||||||||
| Taxonomic characteristics | ||||||||||||||||||
|
||||||||||||||||||
| Scientific name | ||||||||||||||||||
| Alpha fluenza virus to delta fluenza virus | ||||||||||||||||||
| Left | ||||||||||||||||||
|
The genera alpha- , beta , gamma and delta fluenza virus from the family Orthomyxoviridae are enveloped viruses with a single-stranded, segmented RNA of negative polarity as the genome . The species that cause influenza or “real” flu are also found among the genera . For medical aspects of influenza viruses and influenza, see Influenza . According to the International Committee on Taxonomy of Viruses (ICTV, as of November 2018), each of the four genera has only one species, in order of Influenza A virus (FLUAV) to Influenza D virus (FLUDV).
construction
Virion

The ribonucleoprotein of the virus particle (virion), which is located within the lipid envelope (more precisely: virus membrane ), has an approximately helical symmetry . The ribonucleoprotein is a complex of the genome of the virus, the structural (M1 and NP) and the replication-relevant proteins (PA, PB1, PB2).
In the transmission electron microscope one sees all genera of this virus as spherical or spherical ellipsoid (round to ovoid), occasionally also filamentous (thread-like), enveloped virus particles with a diameter of 80 to 120 nm, in their lipid envelope a varying number of the three membrane proteins HA, NA and M2 in influenza A viruses or the two membrane proteins HA and NA in influenza B viruses (IBV) or the hemagglutinin esterase factor HEF and the matrix protein CM2 in influenza C. The glycoproteins HA and NA protrude above the virus surface of influenza A viruses (IAV) as 10 to 14 nm long spikes or peplomers . In contrast, the M2 of influenza A only protrudes with 24 amino acids from the lipid bilayer and is hardly recognized by antibodies in the course of an immune reaction under the coverage of the HA and NA . In the case of influenza A and influenza B viruses (FLUAV and FLUBV), therefore, exactly two types of these spikes are of particular serological interest: hemagglutinin (HA) and neuraminidase (NA), against antibodies after an illness and to a lesser extent Extent also arise after vaccination with an influenza vaccine against influenza A and B. These antibodies can be used for the serological classification of the 18 HA and 11 NA subtypes of influenza A viruses (according to the current status 2017, see also A / H18N11 ).
Genome
The genome of almost all influenza viruses consists of eight RNA sections (segments) of negative polarity , in influenza C there are only seven. These eight RNA molecules contain the genetic information required for the replication and assembly of the virus particles and are preferably single-stranded in the virion. Likewise, only one copy of the genome occurs in a virion. The segmentation of the genome is also responsible for the considerable increase in the genetic variability ( variability ) of the influenza viruses via the ability to genetic reassortment (also antigen shift ), since the segments can be exchanged when a cell is superinfected (with another influenza strain) . The RNA-based single-stranded genome often causes point mutations (so-called antigen drift ) because the RNA polymerases of RNA viruses do not have an exonuclease function to correct copying errors. Both mechanisms cause escape mutations to bypass the immune response, while the functions of the virion are to be preserved. The genome receives the strongest adaptation through the selection pressure generated by the immune system of its reservoir hosts, whereby the maintenance of the functions of the proteins is necessary for a high rate of reproduction and infection.
The eight different RNA segments (HA, NA, M, NP, PA, PB1, PB2 and NS) usually code ten, occasionally eleven, viral proteins in influenza A : hemagglutinin (HA), neuraminidase (NA), nucleoprotein ( NP), the matrix proteins (M1) and ( M2 ), the RNA polymerase (PA), the polymerase-binding proteins (PB1, PB2 and occasionally also PB1-F2) and the non-structural proteins (NS1 and NS2). The genome of Influenza C has only seven segments, neuraminidase is missing, as its function is integrated with the hemagglutinin function in HEF. In influenza A, alternative splicing of the RNA segments M, NS and in some strains also PB1 results in two proteins, M1 and M2 or NS1 and NS2 or PB1 and PB1-F2. A polymerase complex composed of PA, PB1 and PB2 is located in the virion at the 5 'and 3' ends of each segment.
Envelope proteins
The hemagglutinin (HA) is a lectin and causes the agglutination of erythrocytes and, in the event of infection, mediates the attachment of the virus particle (virion) to a host cell. The coupling of the hemagglutinin to a cell occurs through an attachment of part of the hemagglutinin molecule to sialic acids (SA) on proteins of the host cell envelope, which function as receptors (SA receptors). These sialic acids are more often α2,3-linked in birds and more often α2,6-linked in mammals. In addition, the distributions of these links in the lungs differ in mammals and birds. Each hemagglutin invariant fits a certain host cell receptor according to the lock and key principle , whereby each host only has a part of all receptors used by influenza viruses. This fact is also the reason that certain subtypes or virus variants with their special hemagglutinin type can easily infect certain hosts and thereby trigger a disease, and other hosts that are possible in principle cannot or only to a very limited extent. After a proteolytic activation of the hemagglutinin taken up by a cell by cellular serine peptidases and an acidification of the endosome , the hemagglutinin in its second function as a fusogenic protein causes a fusion with the endosomal membrane via its fusion domain to release the ribonucleoprotein into the cytosol . The hemagglutinin is made up of three identical proteins (a homotrimer ). By means of proteolysis , each of these three parts (monomers) is split into two polypeptide chains, which, however , remain connected to one another via a disulfide bridge . This cleavage is absolutely necessary for the fusion of the virus membrane with the endosome membrane when the viruses are unpacked, but not for the receptor binding. By mutations , especially in view of possible changes in the hemagglutinin, the risk of infection for one or the other potential host may change significantly. However, the viruses cannot change the HA binding site at will, since if there is a loss of function, they can no longer re-enter cells and the chain of infection is thus interrupted. Above all, neutralizing antibodies are formed against hemagglutinin , which prevent re-infection with the same virus strain. Therefore, newly emerging epidemics are usually accompanied by changes in hemagglutinin.
The neuraminidase (NA) in the process of infection, many features, including an enzymatic function for cleavage (hydrolysis) of the N-acetylneuraminic acid (a sialic acid) to cellular receptors . This releases the viruses newly created by the replication (daughter viruses from the infected cells) and thus when the infection spreads both within the same organism and to other organisms. In addition, the neuraminidase prevents hemagglutinin-mediated attachment of the daughter viruses to cells that are already infected, because the infected cells hardly have any N-acetylneuraminic acid on their cell surface due to the neuraminidase on their cell surface. As a side effect, the mucus in the lungs is liquefied. In addition, the neuraminidase prevents a cell death program from starting in an infected cell during replication . Oseltamivir , zanamivir and peramivir inhibit neuraminidase in non-resistant Influenza A and Influenza B strains.
The matrix protein 2 (M2) is the smallest of the three membrane proteins of influenza A viruses. In influenza A, M2 consists of around 97 amino acids, 24 of which protrude from the membrane. In influenza A viruses, the M2 matrix protein is a proton channel for acidifying the interior of the virion after endocytosis , so that the fusion domain of the hemagglutinin is triggered and the virus and endosome membrane can fuse to release the ribonucleoprotein into the cytosol . At the same time, the acidification inside the virion causes a dissociation of the matrix protein 1 from the ribonucleoprotein. In influenza B viruses, matrix protein 2 (BM2) is not an envelope protein, but a soluble protein of around 109 amino acids. In Influenza C, the corresponding matrix protein CM2 is, as in Influenza A, an ion channel. Amantadine and rimantadine inhibit the matrix protein M2 in non-resistant influenza A strains.
Internal proteins
As a further located in the virion proteins ( structural proteins , engl. Structural protein ) in addition to the coat proteins, the internal proteins exist (engl. Core protein ). The area between the virus membrane and the ribonucleoprotein is called the matrix or virus lumen (lat. Lumen for 'light') because it appears brighter than the ribonucleoprotein in the transmission electron microscope due to a lower electron density. On the inside of the virus membrane are the cytosolic parts of the three membrane proteins HA, NA and M2, as well as the matrix protein M1 , which connects the virus membrane with the ribonucleoprotein, but releases the ribonucleoprotein when acidified. The ribonucleoprotein consists of the nucleoprotein NP , the viral RNA segments and the proteins of the polymerase complex ( PA , PB1 and PB2 , with A for acidic or B for basic , depending on their isoelectric points ) necessary for replication and transcription . In addition to the matrix protein M1, the nucleoprotein NP binds to the viral RNA and mediates the transport into the cell nucleus via its nuclear localization signal . NS2 is also occasionally found in the virion in small amounts.
Non-structural proteins
The regulatory proteins NS1 , NS2 and PB1-F2, which occurs in some strains, do not occur in the virion and are therefore referred to as non-structural proteins. The NS1 reduces by binding to PDZ domains , the interferon reaction of the host and thus the immune response. In addition, NS1 prevents the host's own mRNA from being released from the cell nucleus by binding its cap structure , which means that the viral RNA is increasingly translated into proteins . NS2 mediates the export of viral mRNA from the nucleus. PB1-F2 promotes the correct localization of PB1 in the cell nucleus and binds to the polymerase PA and promotes the initiation of apoptosis via the mitochondrial path in order to improve the release of the daughter viruses.
Host restriction
The coevolution of humans and viruses (in this case of RNA viruses ) has produced antiviral mechanisms in humans, which are referred to as host restriction factors . In the case of influenza viruses, these include the myxovirus resistance factor Mx1 , NOD-2 , the toll-like receptors 3, 7 and 8, RIG-I , the dsRNA-activated inhibitor of translation DAI, MDA5 , the oligoadenylate synthase OAS1 , the Nod-like receptor protein 3 (NLRP-3) and the protein kinase R.
Replication cycle
For the replication cycle of the influenza A virus at least 219 proteins of the host are necessary.
import
The influenza viruses are replicated in humans in the respiratory tract (respiratory tract) of an infected individual . Human flu viruses prefer cells of the ciliated epithelium . In contrast, the flu virus in birds reproduces mainly in the intestinal epithelial cells.
The viruses migrate through the mucin (mucus) of their host into the epithelial cells, which serve as host cells. Neuraminidase, which liquefies the mucus, ensures that they do not stick to the mucus. After the hemagglutinin binds to an N-acetyl-neuraminic acid on a cell surface, the virion is invaded by endocytosis . In the endosome, cellular serine proteases cut the hemagglutinin into its activated form. In addition, the pH value in the endosome drops, as a result of which the inside of the virion is acidified via the ion channel protein M2. The acidification triggers the fusion domain of the hemagglutinin, whereby the virus membrane fuses with the endosome membrane and the ribonucleoprotein is released into the cytosol, at the same time M1 is detached from the ribonucleoprotein, whereby the nuclear localization signals of the NP in the ribonucleoprotein are exposed. The ribonucleoprotein is also freed from the matrix protein by a cellular breakdown mechanism, the aggregosome, presumably using the cell's own motor proteins . The ribonucleoprotein is then imported into the cell nucleus via the nuclear localization sequence of the nucleoprotein.
Replication
In IAV and IBV, the viral RNA is copied through the viral polymerase complex - a heterotrimer made up of the three proteins PA, PB1 and PB2 - using the host's ribonucleotides. The transcription for the generation of the viral mRNA takes place by the rarely occurring mechanism of cap snatching . The cellular mRNA modified with 5'-methylated cap structures are bound by PB2 to the 7-methylguanosine group and then cut by PA 10 to 13 nucleotides according to the cap structure . The short cap-bearing fragments are bound by PB1 and used in the RNA polymerase complex as primers for the transcription of viral RNA. The polymerase complex binds briefly to the cellular RNA polymerase II . In addition, this breaks down the host's mRNA, leaving the ribosome free to synthesize the viral mRNA. While the viral mRNA has a cap structure and a polyadenylation , the replication viral RNA does not have either.
export
The NS2 protein transports the replicated viral RNA from the cell nucleus into the cytosol, where proteins are produced from the RNA template on the ribosome. The structural proteins bind to one another, the polymerase complex of PA, PB1 and PB2 binds to the 5 'and 3' ends of the viral RNA, and the NP protein binds to the rest of the viral RNA, thus assembling the ribonucleoprotein. The viral envelope proteins HA and NA collect on the cell surface at the lipid rafts , but not M2. The ribonucleoprotein binds to the inside of the lipid raft. The mechanism of budding is not yet clear. Influenza viruses are lytic viruses. They induce programmed cell death in the host cell.
In a single infected host cell can be up to about 20,000 new influenza viruses form ( English burst size , Berstgröße ') before it then dies and then the released viruses more neighboring cells to infect . Virus strain-dependent values between 1,000 and 18,755 daughter viruses per cell were determined in infected cell cultures or embryonated chicken eggs . These then also each produce many thousands of new viruses. This also explains the speed with which this viral infection usually spreads in the body of an affected person.
Systematics
There are four different genera of influenza viruses (alpha to delta), which together with the genera Isavirus , Quaranjavirus and Thogotovirus all belong to the Orthomyxovirus family ( Orthomyxoviridae ).
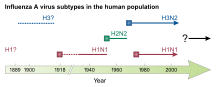
In professional circles, each virus strain is named with the identifier type, location of the initial isolation (virus cultivation), number of the isolate, isolation year (example: Influenza B / Shanghai / 361/2002) and only with the A viruses also with the identifier of the surface antigen [Example: Influenza A / California / 7/2004 (H3N2)]. Influenza viruses are also named after their natural host (bird flu viruses, swine flu viruses). However, the flu that is caused is only referred to as bird flu or swine flu if the infection occurs in that host species, but not in humans. If humans become more frequently infected host species due to a change in an influenza virus, the notation of the serotypes with a trailing v (from English variant ) is used, e.g. B. H1N1v, H3N2v.
Alpha influenza virus with species influenza virus A
- The linear, single-stranded RNA of their genome has eight segments and they are characterized in particular by large differences in the antigenic properties, which are based on particularly high mutation frequencies and regrouping compared to the other genera . These sub-types usually only attack certain hosts and within them certain types of cells (see tropism ). These include humans and various types of mammals such as pigs (see under swine influenza ), horses (see under equine influenza ), mink , seals and whales , domestic dogs , some types of cats and numerous species of birds . The primary reservoir of all influenza A viruses is in the water fowl . All of the first 16 HA and 9 NA serotypes of influenza A can infect birds. The subtypes A / H17N10 and A / H18N11 , which were only discovered in 2012 and 2013, have only been isolated from fruit bats so far.
Influenza A subtypes
In general, the subtypes of the influenza A virus (FLUA) species are primarily classified serologically. This happens according to the pattern A / HxNx or A / Isolationort / Isolat / Year (HxNx). So far, at least 18 H subtypes and 11 N subtypes have been recognized.
The most important surface antigens in the influenza A virus for infection in humans are the hemagglutinin serotypes H1, H2, H3, H5, more rarely H7 and H9 and the neuraminidase serotypes N1, N2, less often N7, which is why the following subtypes for humans of particular importance are:
- Influenza A virus H1N1
- A variant of A / H1N1 was found to be the trigger for the so-called Spanish flu of 1918/1920 in the lung tissue of victims. In 2005 Jeffery Taubenberger succeeded in reconstructing the pathogen causing the Spanish flu from gene fragments. Another global outbreak - the so-called Russian flu - occurred in 1977. In April 2009, an epidemic-like outbreak of a previously unknown variant of the H1N1 subtype occurred in Mexico, from which numerous people fell ill (see: Pandemic H1N1 2009/10 ). Since A / H1N1 was first detected in pigs in 1930, infections in pigs caused by this subtype are referred to as swine influenza .
- Influenza A virus H2N2
- A global outbreak of this subtype of human influenza in 1957 caused a pandemic known as Asian flu .
- Influenza A virus H3N2
- A worldwide outbreak of this subtype (A / Hong Kong / 1/1968 H3N2) caused a pandemic known as the Hong Kong flu in 1968 .
- Influenza A virus H5N1
- So far, this subtype can only be transmitted from person to person in very rare individual cases, although there have been several hundred deaths reported by the WHO since 2003 (→ bird flu H5N1 ).
- Influenza A virus H7N9
- Presumably after contact with infected poultry, human infections with the influenza A virus H7N9 occurred for the first time in February 2013 and, as a result, deaths from the so-called avian flu H7N9 due to severe pneumonia caused by a previously unknown, reassorted variant of virus A / H7N9. In rare cases, human-to-human transmission is possible. However, the pathogen can only be transmitted to a limited extent and there has not yet been any sustained human-to-human transmission.
For information on influenza A subtypes in birds, see the article Avian influenza .
Betainfluenza virus with species influenza virus B
- Their genome also has an eight-fold segmented linear, single-stranded RNA and they only affect humans and seals.
Influenza B subtypes
The species influenza B virus (FLUB) is divided into several strain lines according to the place of occurrence, e.g. B .:
- B / Victoria Line
- B / Yamagata line
- B / Yamaguchi line
- B / Yokohama line
- B / Yunnan Line
- B / Zhuhai line
Gamma influenza virus with species influenza virus C
- In contrast to the influenza A and B viruses, the linear, single-stranded RNA of the genome of the influenza C viruses has only seven segments and they have no neuraminidase (NA). In addition, these viruses contain a surface glycoprotein haemagglutinin esterase fusion protein (HEF) , which is responsible for the receptor binding of the virus to the host cell, the subsequent penetration (fusion) and the subsequent release of the newly formed viruses from the cell takes over. This type C virus affects humans and pigs (see also under swine influenza ), but it does not play a relevant role in human diseases, since it only leads to mild diseases, if at all.
Influenza C subtypes
The differences between individual strains of the influenza C virus (FLUC) species are small. A subdivision into subtypes can be found at NCBI.
Delta fluenza virus with species influenza virus D
- Another variant confirmed by ICTV as of 2018.
Influenza D subtypes
The first representatives of the influenza D virus (FLUD) species were isolated in 2011. This genus seems to be very closely related to influenza C, the split apparently only took place a few hundred years ago, so the genome also has seven segments. There are currently at least two subtypes. Mainly cattle are infected, but also pigs. A subdivision into subtypes can be found at NCBI.
variability
Antigen drift
An accumulation of point mutations in the nucleotides leads to a change in the genetic information ( genetic drift ). In influenza viruses, point mutations arise mainly from the imprecision of the polymerase complex. If such changes affect the two glycoproteins HA and NA (or the area coding for them), this causes a change in the surface antigens of the flu virus ( antigen drift ). Human antibodies and cytotoxic T cells can only recognize one such variant at a time; a virus that is now undetected is called an escape mutant . These rather small changes are the reason that a person can be infected several times in his life with another, only slightly changed virus variant (drift variant) and that both epidemics and regionally limited outbreaks recur regularly.
Therefore, one goal of influenza vaccine design is to elicit broadly neutralizing antibodies. After surviving several infections with different strains, there is a long-term increase in the titre of broadly neutralizing anti-influenza antibodies, possibly due to the high genetic variability of the influenza virus strains. Attempts are also made to direct the cellular immune response to conserved areas of the viral proteins where fewer escape mutations can occur. The mutation rate of influenza virus A is 0.000015 mutations per nucleotide and replication cycle. However, the repertoire of antibodies and cytotoxic T cells formed during an immune reaction has a decisive influence on their respective immunodominance , which influences the adaptation of the immune reaction in later infections with modified influenza viruses ( antigenic sin ).
Antigen shift
If an organism is infected by two virus variants at the same time ( double infection ) or if two viruses of the same origin exchange different mutated gene segments (in the latter case the changes are smaller), the eight genome segments of the influenza viruses involved can be rearranged in the single or multiple RNA molecules are exchanged between the influenza viruses in a doubly infected cell. This process is called genetic reassortment , and it can take place in humans, but also in other hosts such as birds and pigs. The larger changes in the viral surface antigens caused in this way, known as antigen shifts, are observed only in the influenza A viruses (shift variants), but they only occur rarely. Such changes can then be the origin of pandemics , of which there were in the 20th century those from 1918 to 1919 with the subtype H1N1, 1957 with H2N2, 1968 with H3N2 and those of 1977 with the reappearance of H1N1.
Environmental stability
Depending on the temperature, the environmental stability (synonymous tenacity ) of the influenza viruses is very different. At a normal summer daytime temperature of around 20 ° C, viruses that have dried on surfaces can usually last two to eight hours. At a temperature of 0 ° C for more than 30 days and in the ice, they can survive almost indefinitely. At 22 ° C they survive for at least four days in the excrement as well as in the tissues of deceased animals and in water. The lipid envelope of the influenza virus changes at lower temperatures, which stabilizes the virus and remains pathogenic for longer. Above 22 ° C, however, the environmental stability of the influenza virus is significantly reduced. At 56 ° C they are inactivated within 3 hours and at 60 ° C within 30 minutes. From 70 ° C, the virus finally loses its infectivity .
As an enveloped virus, the influenza A virus is sensitive to detergents and organic solvents such as alcohols (e.g. ethanol , isopropanol ). The metastable fusion domain of hemagglutinin can be irreversibly triggered by acids . In addition, chemical or physical denaturation can lead to a loss of function of the viral proteins. Due to the large number of effective disinfection mechanisms, influenza viruses are relatively unstable compared to other viruses (e.g. the poliovirus or the hepatitis B virus ).
Occurrence
In humans, the influenza viruses and the diseases they cause exist worldwide, but in contrast to the other virus types, the influenza C viruses only occur occasionally. While the infections in temperate climates manifest with two main waves in October / November and February / March, the frequency of infections in tropical regions is constant with one or two peaks in the rainy season.
Reporting requirement
In Germany, only direct evidence of influenza viruses is notifiable by name in accordance with Section 7 of the Infection Protection Act , if the evidence indicates an acute infection.
In Switzerland, is a positive laboratory analysis findings to influenza viruses (seasonal, non-pandemic types and subtypes) notifiable and that after the Epidemics Act (EpG) in connection with the epidemic Regulation and Annex 3 of the Regulation of EDI on the reporting of observations of communicable diseases of man . In the case of an influenza A virus of the HxNy type (new subtype with pandemic potential) , both positive and negative laboratory analytical findings must be reported in accordance with the standards mentioned.
Web links
- Robert Koch Institute : Influenza information portal
- WHO : FluNet Influenza Epidemiology Worldwide
- Influenza Research Database: FluDB Influenza Research Database
Individual evidence
- ↑ ICTV Master Species List 2018b.v2 . MSL # 34, March 2019
- ↑ a b ICTV: ICTV Taxonomy history: Akabane orthobunyavirus , EC 51, Berlin, Germany, July 2019; Email ratification March 2020 (MSL # 35)
- ^ Kurt Tobler, Mathias Ackermann, Cornel Fraefel: General Virology . Ed .: utb. 1st edition. Haupt Verlag, Bern 2016, ISBN 978-3-8252-4516-0 , p. 50 .
- ↑ Negative Sense RNA Viruses: Orthomyxoviridae , in: ICTV 9th Report (2011)
- ^ A b R. A. Lamb, PW Choppin: The gene structure and replication of influenza virus. In: Annu. Rev. Biochem. No. 52, 1983, pp. 467-506, doi: 10.1146 / annurev.bi.52.070183.002343 , PMID 6351727 .
- ↑ a b c d e f J. S. Rossman, RA Lamb: Influenza virus assembly and budding. In: Virology. Volume 411, number 2, March 2011, pp. 229-236, doi: 10.1016 / j.virol.2010.12.003 , PMID 21237476 , PMC 3086653 (free full text).
- ↑ Renate König et al .: Human host factors required for influenza virus replication. In: Nature . Volume 463, 2010, pp. 813-817, doi: 10.1038 / nature08699
- ↑ AJ Te Velthuis, E. Fodor: Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis. In: Nature reviews. Microbiology. Volume 14, No. 8, 08 2016, pp. 479-493, doi: 10.1038 / nrmicro.2016.87 , PMID 27396566 , PMC 4966622 (free full text).
- ↑ L. Byrd-Leotis, RD Cummings, DA Steinhauer: The Interplay between the Host Receptor and Influenza Virus Hemagglutinin and Neuraminidase. In: International journal of molecular sciences. Volume 18, No. 7, July 2017, doi: 10.3390 / ijms18071541 , PMID 28714909 , PMC 5536029 (free full text).
- ↑ a b Zhi-Yong Yang, Chih-Jen Wei, Wing-Pui Kong, Lan Wu, Ling Xu, David F. Smith, Gary J. Nabel: Immunization by Avian H5 Influenza Hemagglutinin Mutants with Altered Receptor Binding Specificity . In: Science . tape 317 , no. 5837 , August 10, 2007, ISSN 0036-8075 , p. 825-828 , doi : 10.1126 / science.1135165 .
- ↑ L. Yao, C. Korteweg, W. Hsueh, J. Gu: Avian influenza receptor expression in H5N1-infected and noninfected human tissues. In: FASEB J . 2008, Vol. 22, No. 3, pp. 733-740, PMID 17925493 .
- ^ RG Webster , WG Laver: The origin of pandemic influenza. In: Bull World Health Organ. 1972, Vol. 47, No. 4, pp. 449-452, PMID 4540994 , PMC 2480853 (free full text).
- ↑ Y. Suzuki: Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. In: Biol Pharm Bull . Volume 28, 2005, No. 3, pp. 399-408, doi: 10.1248 / bpb.28.399 , PMID 15744059 .
- ↑ A. Iwai, T. Shiozaki, T. Miyazaki: Relevance of signaling molecules for apoptosis induction on influenza A virus replication. In: Biochemical and biophysical research communications. Volume 441, Number 3, November 2013, pp. 531-537, doi: 10.1016 / j.bbrc.2013.10.100 , PMID 24177013 .
- ↑ P. Gaur, P. Ranjan, S. Sharma, JR Patel, JB Bowzard, SK Rahman, R. Kumari, S. Gangappa, JM Katz, NJ Cox, RB Lal, S. Sambhara, SK Lal: Influenza A virus neuraminidase protein enhances cell survival through interaction with carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) protein. In: The Journal of biological chemistry. Volume 287, Number 18, April 2012, pp. 15109-15117, doi: 10.1074 / jbc.M111.328070 , PMID 22396546 , PMC 3340274 (free full text).
- ^ SE Lindstrom, Y. Hiromoto, H. Nishimura, T. Saito, R. Nerome, K. Nerome: Comparative analysis of evolutionary mechanisms of the hemagglutinin and three internal protein genes of influenza B virus: multiple cocirculating lineages and frequent reassortment of the NP, M, and NS genes. In: Journal of virology. (J. Virol.) 1999, Vol. 73, No. 5, pp. 4413-4426, PMID 10196339 , PMC 104222 (free full text).
- ↑ MR Hilleman: Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. In: Vaccine Volume 20, 2002, No. 25-26, pp. 3068-3087, PMID 12163258 .
- ↑ K. Martin, A. Helenius: Transport of incoming Influenza Virus Nucleocapsids into the Nucleus. In: J. Virol. 1991, Vol. 56, No. 1, p. 232, PMID 1985199 .
- ↑ DM Knipe, Peter M. Howley (Ed.): Fields Virology . 4th edition, Philadelphia 2001, ISBN 0-7817-6060-7 .
- ↑ K. Haye, S. Bourmakina, T. Moran, A. García-Sastre, A. Fernandez-Sesma: The NS1 protein of a human influenza virus inhibits type I interferon production and the induction of antiviral responses in primary human dendritic and respiratory epithelial cells. In: J Virol. 2009, Vol. 83, No. 13, pp. 6849-6862, PMID 19403682 .
- ↑ BG Hale, RE Randall, J. Ortín, D. Jackson: The multifunctional NS1 protein of influenza A viruses. In: J Gen Virol. 2008, Vol. 89, No. 10, pp. 2359-2376, PMID 18796704 .
- ^ NC Robb, M. Smith, FT Vreede, E. Fodor: NS2 / NEP protein regulates transcription and replication of the influenza virus RNA genome . In: J Gen Virol. 2009, Volume 90, Part 6, pp. 1398-1407, PMID 19264657 .
- ↑ I. Mazur, D. Anhlan, D. Mitzner, L. Wixler, U. Schubert, S. Ludwig: The proapoptotic influenza A virus protein PB1-F2 Regulates viral polymerase activity by interaction with the PB1 protein. In: Cell Microbiol. 2008, Vol. 10, No. 5, pp. 1140-1152, PMID 18182088 .
- ↑ O. Haller, P. Staeheli, M. Schwemmle, G. Kochs: Mx GTPases: dynamin-like antiviral machines of innate immunity. In: Trends in microbiology. Volume 23, No. 3, March 2015, pp. 154-163, doi: 10.1016 / j.tim.2014.12.003 , PMID 25572883 .
- ↑ M. Le Bel, J. Gosselin: Leukotriene B4 Enhances NOD2-Dependent Innate Response against Influenza Virus Infection. In: PloS one. Volume 10, number 10, 2015, p. E0139856, doi: 10.1371 / journal.pone.0139856 , PMID 26444420 , PMC 4596707 (free full text).
- ↑ a b W. Wu, W. Zhang, ES Duggan, JL Booth, MH Zou, JP Metcalf: RIG-I and TLR3 are both required for maximum interferon induction by influenza virus in human lung alveolar epithelial cells. In: Virology. Volume 482, August 2015, pp. 181-188, doi: 10.1016 / j.virol.2015.03.048 , PMID 25880109 , PMC 4461467 (free full text).
- ↑ IK Pang, PS Pillai, A. Iwasaki: Efficient influenza A virus replication in the respiratory tract requires signals from TLR7 and RIG-I. In: Proceedings of the National Academy of Sciences . Volume 110, number 34, August 2013, pp. 13910-13915, doi: 10.1073 / pnas.1303275110 , PMID 23918369 , PMC 3752242 (free full text).
- ↑ MG Torcia, L. Nencioni, AM Clemente, L. Civitelli, I. Celestino, D. Limongi, G. Fadigati, E. Perissi, F. Cozzolino, E. Garaci, AT Palamara: Sex differences in the response to viral infections : TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. In: PloS one. Volume 7, number 6, 2012, p. E39853, doi: 10.1371 / journal.pone.0039853 , PMID 22768144 , PMC 3387221 (free full text).
- ↑ RJ Thapa, JP Ingram, KB Ragan, S. Nogusa, DF Boyd, AA Benitez, H. Sridharan, R. Kosoff, M. Shubina, VJ Landsteiner, M. Andrake, P. Vogel, LJ Sigal, BR tenOever, PG Thomas, JW Upton, S. Balachandran: DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. In: Cell host & microbe. Volume 20, number 5, November 2016, pp. 674-681, doi: 10.1016 / j.chom.2016.09.014 , PMID 27746097 .
- ↑ AA Benitez, M. Panis, J. Xue, A. Varble, JV Shim, AL Frick, CB López, D. Sachs, BR tenOever: In Vivo RNAi Screening Identifies MDA5 as a Significant Contributor to the Cellular Defense against Influenza A Virus . In: Cell reports. Volume 11, Number 11, June 2015, pp. 1714–1726, doi: 10.1016 / j.celrep.2015.05.032 , PMID 26074083 , PMC 4586153 (free full text).
- ↑ S. Kim, MJ Kim, DY Park, HJ Chung, CH Kim, JH Yoon, HJ Kim: Mitochondrial reactive oxygen species modulate innate immune response to influenza A virus in human nasal epithelium. In: Antiviral research. Volume 119, July 2015, pp. 78-83, doi: 10.1016 / j.antiviral.2015.04.011 , PMID 25930096 .
- ↑ JD Ong, A. Mansell, MD Tate: Hero turned villain: NLRP3 inflammasome-induced inflammation during influenza A virus infection. In: Journal of leukocyte biology. [Electronic publication before printing] October 2016, doi: 10.1189 / jlb.4MR0616-288R , PMID 27707881 .
- ↑ AM Pham, FG Santa Maria, T. Lahiri, E. Friedman, IJ Marié, DE Levy: PKR Transduces MDA5-Dependent Signals for Type I IFN Induction. In: PLoS pathogens. Volume 12, number 3, March 2016, p. E1005489, doi: 10.1371 / journal.ppat.1005489 , PMID 26939124 , PMC 4777437 (free full text).
- ↑ R. König, S. Stertz, Y. Zhou, A. Inoue, HH Hoffmann, S. Bhattacharyya, JG Alamares, DM Tscherne, MB Ortigoza, Y. Liang, Q. Gao, SE Andrews, S. Bandyopadhyay, P. De Jesus, BP Tu, L. Pache, C. Shih, A. Orth, G. Bonamy, L. Miraglia, T. Ideker, A. García-Sastre, JA Young, P. Palese, ML Shaw, SK Chanda: Human host factors required for influenza virus replication. In: Nature. Volume 463, number 7282, February 2010, pp. 813-817, doi: 10.1038 / nature08699 , PMID 20027183 , PMC 2862546 (free full text).
- ^ Franz X. Heinz: On the trail of the secret of the influenza pandemic. Retrieved January 9, 2013
- ^ Maria Jose Alonso: Nanostructured Biomaterials for Overcoming Biological Barriers. Royal Society of Chemistry, 2012, ISBN 978-1-84973-529-2 , p. 161.
- ^ I. Banerjee, Y. Miyake, SP Nobs, C. Schneider, P. Horvath, M. Kopf, P. Matthias, A. Helenius, Y. Yamauchi: Influenza A virus uses the aggresome processing machinery for host cell entry. In: Science. Volume 346, Number 6208, October 2014, pp. 473-477, ISSN 1095-9203 . doi: 10.1126 / science.1257037 . PMID 25342804 .
- ↑ A. Stevaert, L. Naesens: The Influenza Virus Polymerase Complex: An Update on Its Structure, Functions, and Significance for Antiviral Drug Design. In: Medicinal research reviews. Volume 36, number 6, 11 2016, pp. 1127–1173, doi: 10.1002 / med. 21401 , PMID 27569399 , PMC 5108440 (free full text).
- ↑ a b c A. Pflug, M. Lukarska, P. Resa-Infante, S. Reich, S. Cusack: Structural insights into RNA synthesis by the influenza virus transcription-replication machine. In: Virus research. Volume 234, April 2017, pp. 103-117, doi: 10.1016 / j.virusres.2017.01.013 , PMID 28115197 .
- ^ E. Fodor: The RNA polymerase of influenza a virus: mechanisms of viral transcription and replication. In: Acta virologica. Volume 57, Number 2, 2013, pp. 113-122, PMID 23600869 .
- ^ J. Reguera, P. Gerlach, S. Cusack: Towards a structural understanding of RNA synthesis by negative strand RNA viral polymerases. In: Current opinion in structural biology. Volume 36, February 2016, pp. 75-84, doi: 10.1016 / j.sbi.2016.01.002 , PMID 26826467 .
- ^ EW Brydon, SJ Morris, C. Sweet: Role of apoptosis and cytokines in influenza virus morbidity. (PDF) In: FEMS microbiology reviews. Volume 29, Number 4, September 2005, pp. 837-850, doi: 10.1016 / j.femsre.2004.12.003 , PMID 16102605 .
- ↑ SJ Stray, GM Air: Apoptosis by influenza viruses correlates with efficiency of viral mRNA synthesis. In: Virus research. Volume 77, Number 1, September 2001, ISSN 0168-1702 , pp. 3-17, PMID 11451482 .
- ↑ L. Möhler, D. Flockerzi, H. Sann, U. Reichl: Mathematical model of influenza A virus production in large-scale microcarrier culture. In: Biotechnology and bioengineering. Volume 90, Number 1, April 2005, ISSN 0006-3592 , pp. 46-58, doi: 10.1002 / bit.20363 , PMID 15736163 .
- ^ HJ Cairns, M. Edney, S. Fazekas de St Groth: Quantitative aspects of influenza virus multiplication. In: Journal of Immunology (Baltimore, Md .: 1950). Volume 69, Number 2, August 1952, ISSN 0022-1767 , pp. 155-181, PMID 14946411 .
- ^ WHO : A revision of the system of nomenclature for influenza viruses: a WHO memorandum. In: Bulletin of the World Health Organization. Volume 58, No. 4, 1980, pp. 585-591, PMID 6969132 , PMC 2395936 (free full text).
- ↑ a b WHO: Influenza virus infections in humans (February 2014) (PDF) Retrieved on March 2, 2018.
- ^ WHO: Standardization of terminology for the influenza virus variants infecting humans: Update. Joint announcement of FAO, OIE and WHO . January 30, 2014. Retrieved March 2, 2018.
- ↑ MJ Pantin-Jackwood, DE Swayne: Pathogenesis and pathobiology of avian influenza virus infection in birds. In: Rev Sci Tech. Volume 28, 2009, No. 1, pp. 113-136, PMID 19618622 .
- ↑ TW Vahlenkamp, TC Harder: Influenza virus infections in mammals. In: Berl Munch Tierarztl Wochenschr. Volume 119, 2006, No. 3-4, pp. 123-131, PMID 16573202 .
- ↑ HM Yassine, CW Lee, R. Gourapura, YM Saif: Interspecies and intraspecies transmission of influenza A viruses: viral, host and environmental factors. In: Anim Health Res Rev. Volume 11, 2010, No. 1, pp. 53-72, PMID 20591213 .
- ↑ NCBI: Influenza A virus (species)
- ↑ Information on bird flu ( memento of March 30, 2009 in the Internet Archive )
- ↑ Richard E. Shope: Swine Influenza: III. Filtration and Ion Experiments and Etiology. In: Journal of Experimental Medicine . Volume 54, 1931, pp. 373-385.
- ^ Suburban Emergency Management Project ( Memento of March 4, 2010 in the Internet Archive ), Biot Report 162 of January 9, 2005: What Is Swine Flu?
- ↑ H3N2 in the Influenza Research Database.
- ↑ World Organization for Animal Health of April 4, 2013: Low pathogenic avian influenza (poultry), China (People's Rep. Of). Information received on 04/04/2013 from Dr Zhang Zhongqui, Director General, China Animal Disease Control Center, Veterinary Bureau, Ministry of Agriculture, Beijing, China (People's Rep. Of).
- ↑ Xian Qi, Yan-Hua Qian, Chang-Jun Bao et al. : Probable person to person transmission of novel avian influenza A (H7N9) virus in Eastern China, 2013: epidemiological investigation. In: British Medical Journal. (BMJ) August 6, 2013, Volume 347, p. 4752, doi: 10.1136 / bmj.f4752
- ↑ The RKI on human cases of avian influenza A (H7N9). On: rki.de , as of May 24, 2018; accessed on April 19, 2019.
- ↑ RJ Webby, RG Webster, JA Richt: Influenza viruses in animal wildlife populations. In: Curr Top Microbiol Immunol. No. 315, 2007, pp. 67-83, PMID 17848061 .
- ↑ AD Osterhaus, Rimmelzwaan GF, Martina BE, TM Bestebroer, Fouchier RA: Influenza B virus in seals . In: Science . Volume 288, No. 5468, 2000, pp. 1051-1053. doi : 10.1126 / science.288.5468.1051 . PMID 10807575 .
- ↑ R. Bodewes, D. Morick, G. de Mutsert, N. Osinga, T. Bestebroer, S. van der Vliet, SL Smits, T. Kuiken, GF Rimmelzwaan, RA Fouchier, AD Osterhaus: Recurring influenza B virus infections in seals . In: Emerg Infect Dis. . Volume 19, No. 3, 2013, pp. 511-512. doi : 10.3201 / eid1903.120965 . PMID 23750359 .
- ↑ NCBI: Influenza B virus (species)
- ↑ NCBI: Influenza B virus, Victoria strains
- ^ NCBI: Influenza B virus, Yamagata strains
- ↑ NCBI: Influenza B virus, Yamaguchi strains
- ↑ NCBI: Influenza B virus, Yokohama strains
- ↑ NCBI: Influenza B virus, Yunnan strains
- ↑ NCBI: Influenza B virus, Zhuhai strains
- ↑ NCBI: Influenza C virus (species)
- ↑ BM home, M. Ducatez, EA Collin, Z. Ran et al. : Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses . In: PLOS Pathogens (PLoS Pathog) . Volume 9, No. 2, 2013. doi : 10.1371 / journal.ppat.1003176 .
- ↑ Z. Sheng, Z. Ran, D. Wang et al. : Genomic and evolutionary characterization of a novel influenza-C-like virus from swine . In: Archives of Virology (Arch Virol) . Volume 159, No. 2, 2014, pp. 249-255. doi : 10.1007 / s00705-013-1815-3 .
- ↑ Delta fluenza virus , on: ViralZone
- ^ EA Collin, Z. Sheng, Y. Lang, W. Ma, BM Haus, F. Li: Cocirculation of two distinct genetic and antigenic lineages of proposed influenza D virus in cattle . In: Journal of Virology (J Virol) . Volume 89, No. 2, Jan 2015, pp. 1036-1042. doi : 10.1128 / JVI.02718-14 .
- ↑ NCBI: Influenza D virus (species)
- ↑ MS Miller, TJ Gardner, F. Krammer, LC Aguado, D. Tortorella, CF Basler, P. Palese: Neutralizing Antibodies Against Previously Encountered Influenza Virus Strains Increase over Time: A Longitudinal Analysis. In: Science Translational Medicine. 5, 2013, pp. 198ra107-198ra107, doi: 10.1126 / scitranslmed.3006637 .
- ↑ JD Parvin, A. Moscona, WT Pan, JM Leider, P. Palese: Measurement of the mutation rates of animal viruses: influenza A virus and poliovirus type 1. In: Journal of virology. Volume 59, Number 2, August 1986, ISSN 0022-538X , pp. 377-383, PMID 3016304 , PMC 253087 (free full text).
- ↑ Ivan V. Polozov, Ludmila Bezrukov, Klaus Gawrisch, Joshua Zimmerberg: Progressive ordering with decreasing temperature of the phospholipids of influenza virus. In: Nature Chemical Biology . Volume 4, 2008, pp. 248-255, PMID 18311130 .
- ^ Academy for Public Health Düsseldorf (Ed.): Focus on public health. No. 04/2001.
- ↑ a b M. L. Grayson, S. Melvani, J. Druce, IG Barr, SA Ballard, PD Johnson, T. Mastorakos, C. Birch: Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. In: Clin Infect Dis . 2009, Vol. 48, No. 3, pp. 285-291, PMID 19115974 .
- ↑ a b E. K. Jeong, JE Bae, IS Kim: Inactivation of influenza A virus H1N1 by disinfection process. In: Am J Infect Control. 2010, Vol. 38, No. 5, pp. 354-360, PMID 20430477 .
- ↑ M. Nishide, K. Tsujimoto, M. Uozaki, K. Ikeda, H. Yamasaki, AH Koyama, T. Arakawa: Effects of electrolytes on virus inactivation by acidic solutions. In: Int J Mol Med. 2011, Volume 27, No. 6, pp. 803-809, doi: 10.3892 / ijmm.2011.668 , PMID 21468540 .
- ↑ M. Abe, K. Kaneko, A. Ueda, H. Otsuka, K. Shiosaki, C. Nozaki, S. Goto: Effects of several virucidal agents on inactivation of influenza, Newcastle disease, and avian infectious bronchitis viruses in the allantoic fluid of chicken eggs. In: Jpn J Infect Dis. 2007, Vol. 60, No. 6, pp. 342-346, PMID 18032831 .