Penicillins
The penicillins or penicillins (singular penicillin , from Latin penicillium , ' brush mold ') are a group of antibiotic substances that are structurally derived from 6-aminopenicillanic acid . In addition to naturally occurring penicillins, which are formed as secondary metabolic products by various Penicillium , Aspergillus , Trichophyton and Streptomyces species, this also includes biosynthetic and partly syntheticmanufactured penicillins. The naturally occurring penicillin that was first discovered was isolated from the brush mold Penicillium notatum .
Penicillin G , a natural penicillin that is still used therapeutically today, is one of the oldest antibiotics used , which, in addition to its great medical benefit, is also assigned the pioneering role for the scientific use of this group of active ingredients. After the rediscovery of the antibiotic effectiveness of penicillins, recognized and published by Theodor Billroth in 1874, by Alexander Fleming in 1928, the enormous importance of antibiotics for medicine was recognized, which has significantly influenced and revolutionized the modern understanding of the importance of bacterial pathogens. In the decades after its discovery, penicillin G helped save countless lives. Although today there are numerous strains of bacteria that are resistant to this antibiotic, it can still be used successfully worldwide.
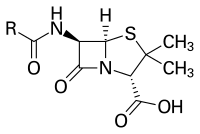
Penicillins belong to the group of β-lactam antibiotics . The empirical formula is RC 9 H 11 N 2 O 4 S, where “R” stands for a variable side chain.
Classification
Naturally occurring penicillins
The parent substance of penicillins, 6-aminopenicillanic acid, arises biologically from L-α-amino adipic acid and the amino acids L- cysteine and L- valine , whereby isopenicillin N is initially formed. The replacement of the L-α-aminoadipyl residue by other organic acid residues, catalyzed by the acyl transferase , results in various penicillins. Of the natural penicillins, only penicillin G ( benzylpenicillin ), which is obtained by fermentation from Penicillium chrysogenum , is therapeutically significant . It is not broken down as quickly as the more effective penicillin K. Using a suitable precursor , phenylacetic acid , it can also be specifically produced by fermentation. In addition to Penicillium species as biological producers, penicillins are also formed by other molds such as Acremonium chrysogenum (formerly Cephalosporium acremonium ) and Aspergillus nidulans , as well as by certain bacteria belonging to the order Actinomycetales , for example Streptomyces clavuligerus produces penicillin N.
| Surname | Other names | activity | -R | Molecular formula | Molar mass | CAS number | PubChem |
|---|---|---|---|---|---|---|---|
| Penicillin F | Penicillin I, penten (2) yl-penicillin | 1500 IU / ml | -CH 2 -CH = CH-CH 2 -CH 3 | C 14 H 20 N 2 O 4 S | 312.38 g · mol -1 | 118-53-6 | 6438232 |
| Penicillin G | Penicillin II, benzyl penicillin | 1670 IU / ml ( sodium salt ) | -CH 2 -C 6 H 5 | C 16 H 18 N 2 O 4 S | 334.39 g · mol -1 | 61-33-6 | 5904 |
| Penicillin X | Penicillin III, p-hydroxybenzyl penicillin | 850-900 IU / ml | -CH 2 -C 6 H 4 -OH | C 16 H 18 N 2 O 5 S | 350.39 g · mol -1 | 525-91-7 | 120720 |
| Penicillin K | Penicillin IV, heptylpenicillin | 2200 IU / ml | -CH 2 - (CH 2 ) 5 -CH 3 | C 16 H 25 N 2 O 4 S | 341.45 g · mol -1 | 525-97-3 | 44123577 |
| Penicillin DF | Dihydropenicillin F, penicillin H 2 F, amylpenicillin | 1610 IU / ml | -CH 2 - (CH 2 ) 3 -CH 3 | C 14 H 22 N 2 O 4 S | 314.40 g · mol -1 | 4493-18-9 | 107556 |
| Penicillin N | Adicillin | - (CH 2 ) 3 -CH (NH 2 ) -COOH | C 14 H 21 N 3 O 6 S | 359,40 g · mol -1 | 71724 | ||
| Penicillin M | Isopenicillin N | - (CH 2 ) 3 -CH (NH 2 ) -COOH | C 14 H 21 N 3 O 6 S | 359,40 g · mol -1 | 440723 | ||
Bio- and partially synthetic penicillins
The orally effective penicillin V ( phenoxymethylpenicillin ) is also produced fermentatively, but not spontaneously, but through the addition of a synthetic precursor , phenoxyacetic acid ("biosynthesis").
Partially synthetic further developments of the natural penicillins result from the reaction of 6-aminopenicillic acid with carboxylic acid halides . Compared to penicillin G, they have certain advantages such as acid and penicillinase stability or an extended spectrum of activity.
Acid-stable (orally effective) penicillins with or without penicillinase stability
- Phenoxymethylpenicillin (penicillin V), propicillin , pheneticillin
Due to the reduced nucleophilicity of the carbonyl oxygen , the attack by hydronium ions is made more difficult, which inhibits the conversion to the ineffective penillic acid. Acid-stable penicillins are not destroyed by stomach acid and can therefore be administered orally . They have the same spectrum of activity as penicillin G.
Penicillinase-stable penicillins
By shielding the beta-lactam ring with isoxazolyl structures from the penicillinase formed by some pathogens , such penicillins are also effective against penicillinase-forming staphylococci (“staphylococcal penicillin”). Compared to penicillin G, their potency is significantly lower and isoxazolylpenicillins are completely ineffective against gram-negative pathogens.
Broad spectrum penicillins
- Aminopenicillins : Amoxicillin , Ampicillin , Bacampicillin , Pivampicillin , Sultamicillin
- Acylaminopenicillins: Azlocillin , Mezlocillin , Piperacillin
- Carboxypenicillins: carbenicillin , ticarcillin
- Ureidopenicillins : Azlocillin , Piperacillin , Mezlocillin
By introducing polar substituents, such hydrophilic penicillins are able to pass through the cell walls of gram-negative pathogens, thereby expanding their spectrum of activity. Some of the broad-spectrum penicillins are acid- and penicillinase-sensitive, and their effectiveness against some gram-positive bacteria is also reduced.
Pivmecillinam is not an acylation product of 6-aminopenicillanic acid, but it has the bicyclic lactam backbone of penicillins.
pharmacology
Mechanism of action
Penicillins have a bacteriolytic effect on the cell division of the bacteria , namely when the cell wall is rebuilt by intervening in the synthesis of the cell wall, preventing the internal crosslinking there and thus weakening the cell wall so that it bursts when exposed to stress. In particular, gram-positive bacteria that divide die under the influence of penicillin. The basic structure of penicillins consists of 6-aminopenicillanic acid, a bicyclic dipeptide made from cysteine (L-configured) and valine (D-configured, after configuration reversal in the biosynthetic pathway). This so-called beta-lactam ring is bound (when it is open) by the bacterial enzyme D-alanine transpeptidase , which is responsible for cross-linking the peptidoglycans in the bacterial cell walls of gram-positive bacteria. The enzyme is required above all in dividing bacteria, since in these the rigid cell wall has to be opened and at least partially re-synthesized. Since the binding to the D-alanine transpeptidase is irreversible, the cell wall can no longer be synthesized, and the gram-positive bacterium loses its most important protective covering. In addition, the constant build-up and breakdown of the defective cell wall leads to toxic breakdown products.
The effect of the penicillins only affects bacteria that multiply, but not those that do not divide: This no longer affects the antibiotic because no new cell wall synthesis has to take place - it is already completely completed and therefore no longer forms a point of attack for penicillin. However, bacteria that do not multiply pose no threat to the host organism and are rendered harmless relatively quickly by the patient's own immune system. If, on the other hand, they enter a cycle of reproduction again, the cell wall is again partially broken down and has to be synthesized again; such bacteria can therefore be attacked again by penicillins. For this reason, penicillins must continue to be administered for a certain period after the symptoms have subsided.
Penicillins are only effective if the bacteria are otherwise unhindered in their growth; So penicillins should not be administered together with drugs that prevent the bacteria from multiplying, otherwise the starting point of the penicillin mode of action is blocked therapeutically.
Penicillins not only act on bacteria, including cyanobacteria , but also block the division of cyanelles, the photosynthetically active organelles of the Glaucocystaceae (a family of algae) and the chloroplasts of bladder moss . However , they have no effect on the division of the plastids in more highly developed vascular plants such as tomatoes . This is an indication that in higher plants due to evolutionary changes in plastid division, β-lactam antibiotics generally no longer have any effect on chloroplasts.
Penicillin G and V are not effective against gram-negative bacteria (with the exception of gram-negative cocci such as Neisseria ), which have an additional outer membrane over their cell membrane. This makes it impossible for the penicillin to attack, as it has to intervene in the formation of the peptidoglycan layer below. Therefore, the use of penicillin G and V only makes sense with gram-positive bacteria. Structural variants such as aminopenicillins are used against gram-negative bacteria .
Resistances
Numerous clinically occurring bacteria are now already to penicillin G resistant , which has led to a number of advanced β-lactam antibiotics. The problem of cross-resistance remains critical, which means that germs that have once developed resistance to penicillins also become insensitive to other β-lactam antibiotics (e.g. cephalosporins ).
Resistant mutants would actually do no harm, as they only occur to a small extent. However, if the penicillin acts on the other, non-resistant bacteria and eliminates them, a resistant bacterium can reproduce much better and thus becomes a danger, as it passes on its resistance to the subsequent generations. By exchanging resistance genes between different types of bacteria, antibiotic resistance is also passed on to other types. Methicillin resistance is particularly feared in Staphylococcus aureus .
The process of resistance development is a very clear example of Darwin's theory of evolution ( natural selection ); Due to the rapid division and succession of generations, the resistant bacteria adapted to their environment are selected and form the basis for future generations. The formation of penicillin-resistant strains is considered to be one of the earliest experimental evidence of observed microevolution . The biological basis of the active ingredient group is the competition between the two organism strains fungi and bacteria, which rely on the same resources, whereby the fungi protect themselves against the bacteria with antibacterial growth-inhibiting substances.
Side effects
In addition to the risk of resistance to penicillins, another disadvantage is the relatively frequent allergy of patients to these drugs (around one in 7000 patients). Allergic reactions can range from slight reddening of the skin to anaphylactic shock .
Penicillins can kill beneficial bacteria such as those in the intestinal flora , especially broad-spectrum penicillins , which are also effective against gram-negative bacteria. In the worst case, harmful microorganisms can spread in the intestine and lead to antibiotic-associated colitis .
Another rare (with a probability of about three per thousand) undesirable effect is the triggering of epileptic seizures .
history
discovery
As early as 1874, the surgeon Theodor Billroth in Vienna had unequivocally recognized the bacterial growth-inhibiting effect of the fungus Penicillium. In 1923, in San José, Clodomiro Picado Twight , a former scientist at the Pasteur Institute , researched the growth-inhibiting effects on staphylococci and streptococci . His research results were published in 1927 by the Société de Biologie in Paris. The (re) discovery of penicillins, which was far more publicized, began with a moldy bacterial culture a year later: Alexander Fleming , who was studying staphylococci at St. Mary's Hospital in London , inoculated an agar plate with staphylococci before the summer vacation in 1928 and then set it aside. On his return on September 28, 1928, he discovered that a mold ( Penicillium notatum ) was growing on the nutrient medium and that the bacteria had not multiplied in the vicinity of the fungus. Fleming called the bactericidal substance that could be obtained from the nutrient medium penicillin and first described it to the public in 1929 in the British Journal of Experimental Pathology . He examined the effect of penicillin on different types of bacteria and animal cells ; He found that penicillin only killed gram-positive bacteria such as staphylococci, streptococci or pneumococci , but not gram-negative bacteria such as salmonella . It was also found to be non-toxic to white blood cells and human cells or to rabbits. Despite this knowledge, Fleming apparently never had the idea of using penicillin as a drug.
Almost ten years later, in 1938, Howard W. Florey , Ernst B. Chain, and Norman Heatley set out to systematically examine all substances produced by microorganisms that were known to damage bacteria. This is how they came across Fleming's penicillin. They cleaned it and examined its therapeutic effects first on mice and then also on humans. In 1939, René Dubos of the Rockefeller Institute for Medical Research isolated tyrothricin from soil samples and showed that it had the ability to cure certain bacterial infections. Florey and Chain undertook the first clinical test in Oxford in 1941, but it was limited to only a few people. Since penicillin was still very laborious to manufacture, they even recovered it from the urine of the treated people.
With the beginning of World War II , the Allies wanted to develop an effective drug for their wounded soldiers. Antibiotic research shifted to the USA and took off there at a rapid pace. It was found that it is cheaper to cultivate the fungus in suitable liquid nutrient media. In the United States, where small amounts of penicillin had already been produced in 1940 for the treatment of syphilis among draftees , new strains of Penicillium chrysogenum were bred , which produced more penicillin. This means that the substance was available as a drug in the required quantity. Penicillin was first used on the wounded in 1942 by the Allies. In 1943 it turned out that penicillin is not a chemically uniform substance: The crude penicillin obtained by the British researchers by means of superficial fermentation (emerse fermentation) consisted primarily of penicillin F, while the crude penicillin produced in the USA by submerged fermentation mainly consisted of penicillin G. A total of four variants were recognized early on, which were designated with Roman numerals as penicillin I, II, III and IV or with the letters F, G, X and K.
In 1945, Fleming, Chain and Florey jointly received the Nobel Prize for their discovery, which marked a turning point in the history of medicine. The active ingredient ended the medical problem that had existed since ancient times that surgical injuries due to simple wound infections can lead to the death of those affected long after the war, and was therefore still regarded by the population as a miracle medicine after the war. In Germany, the focus was on the further development of the sulfonamides , which are still in limited use today , since the Allied Control Council had not allowed the use of penicillin in research or medical applications. It was not until 1946 that a strain of penicillin came to the Stolberg-based company Chemie Grünenthal under ultimately unexplained circumstances , where production began. The previously imported penicillin only became generally available in Germany after 1949. Biochemie Kundl - now Sandoz / Novartis was also involved in the research and dissemination of penicillin (see the film The Third Man ).
Previous work
Even the Nubians used a beer with antibacterial agents. The ancient Egyptians treated inflammations with healing potions brewed from grain. In ancient times and in the Middle Ages , surgeons placed moldy rags on wounds to prevent infection. However, the active ingredients were not recognized as such, the term antibiotic was only introduced with the penicillin.
However, Fleming was not the first modern scientist to discover that molds can inhibit bacterial growth: As early as 1870, John Scott Burdon-Sanderson had recognized a connection between mold and bacterial growth. In 1884 treated Joseph Lister the abscess of a nurse with a Penicillium mold (more precisely, Penicillium glaucum ), results not published. Ernest Duchesne carried out a successful animal experiment with guinea pigs in 1896 . However, all of these findings remained without resonance in the scientific world and were completely misunderstood. First Fleming certainly used the Penicillium notatum (which penicillin Burdon-Sanderson and Duchesne used is unfortunately unknown).
Development to the breakthrough in medical practice
At first, Fleming's publications received little attention from colleagues. Penicillin only made its breakthrough in World War II . The fact that the sulfonamides , one of which was the first antibiotic to be used in practice under the trade name Prontosil , were manufactured in Germany and patented by German companies, so that they were no longer available in the same way to the opponents of the war after the outbreak of war played a role . Only in further research did the advantages of penicillin G over this class of active ingredients emerge. However, the Germans continued to use sulfonamides until the end of the war.
In 1939, Howard Walter Florey and Ernst Boris Chain became interested in penicillin. Norman Heatley managed to extract and purify the antibiotic from the culture fluid in which the molds were grown. On August 24, 1940, an animal experiment took place on 50 rats infected with a lethal dose of streptococci . Half of them received penicillin, and only one of this group died. The rats in the other group all died within a few hours. This animal experiment surprisingly revealed the powerful effect of penicillin, which was not expected in this aggressive bacterial strain.
On February 12, 1941, the first patient was treated with the penicillin obtained. It was a 43-year-old London policeman who was injured while cutting a rose and sustained blood poisoning from infection of the wound . After five days of treatment, the fever was gone. However, the penicillin supplies were exhausted and the treatment had to be discontinued. The man passed away a month later. In retrospect , this highlighted the need for antibiotics to be taken longer than the visible symptoms persist. A premature termination always carries the risk of a relapse of the disease, even today often only treatable through the use of alternative antibiotics.
It wasn't until Florey and Heatley flew to the US to advertise penicillin that general interest, especially among the American military, was aroused. The first step was to look for a strain of fungus that produced more penicillin. The American Air Force collected soil samples from as many airfields as possible around the world. The most productive strain, Penicillium chrysogenum , was discovered on a moldy melon in front of the research institute.
industrial production

After the "Oxford Circle" around EB Chain and HW Florey had published a process for the production and isolation of penicillin in " Lancet " in 1940 and 1941, the first industrial productions were started in 1942, above all by Glaxo and ICI in England, MSD Sharp & Dohme (MSD), Pfizer & Co. and Squibb & Sons in the USA and Schott Jena (promoted by Hans Knöll ) in Germany. From 1942 research on penicillin was also carried out at the Hoechst paintworks (production on a smaller scale began there in spring 1945). The researchers had to rely on Fleming's brief publications. Hoechst also did not have the abundant Chrysogenum strain available. It was not until 1950 that the US group MSD sent a sample of this strain to West Germany as part of a collaboration.
At the same time, Robert Thren was doing research on penicillin production at Madaus in Radebeul, Saxony , from which East German production at the Dresden pharmaceuticals plant emerged in collaboration with the chemist Alfred Kuhn . As the parent company of the Pharmaceutical Combine GERMED , the Eastern Bloc was supplied.
In 1945 the amount of penicillin produced in the US was 20 times greater than that produced in Europe. Ordinary corn , soaked in water, called corn steep liquor by the Americans , turned out to be the ideal nutrient medium for the fungus. In the process (1944 at Pfizer) the surface process (emersed culture) that was initially used was replaced by the submerged process (liquid culture in a stirred tank reactor ), with which higher productivities were achieved. In October 1944, the first injection preparations were made. In 1943, 22 companies offered penicillin. At first it was mainly reserved for wounded soldiers, because the production volume was not yet sufficient to treat all civilian patients with it. However, since 1944 the US has been able to meet all of its civil and military needs for penicillin. On the other hand, in Europe after the Second World War, demand was high and penicillin production was not sufficient for all patients. Smuggling and black trafficking in penicillin developed, which is also the subject of the film The Third Man .
In the decades after the Second World War, the Nederlandsche Gist-en Spiritusfabrik (NG & SF), later Gist Brocades, now DSM , developed into the world's largest penicillin manufacturer. Today DSM produces penicillins within the framework of joint ventures in China , while Sandoz GmbH in Kundl (Austria) today operates the largest production site for penicillins in the western world.
Human experiments
literature
- Charles R. Cramer: On cleaning and chemistry of penicillins , Zurich 1949, DNB 570057728 (dissertation ETH Zurich 1949, 106 pages).
- Christof Goddemeier: Alexander Fleming (1881–1955): Penicillin . In: Deutsches Ärzteblatt , Volume 103, No. 36, 2006, p. A2286.
- Peter Imming: How does the fungus make penicillin? Current research, trends in β-lactam antibiotics. Biosynthesis of penicillins and cephalosporins. In: Pharmazie in our time , Volume 18, No. 1, 1989, pp. 20-24, doi: 10.1002 / pauz.19890180104 .
- Christian Mähr : From alcohol to sugar - twelve substances that changed the world. DuMont, Cologne 2015, ISBN 978-3-8321-9549-6 .
- Ingrid Pieroth: Penicillin production: from the beginnings to large-scale production (= Heidelberger Schriften zur Pharmazie- und Naturwissenschaftsgeschichte , Volume 9), Wissenschaftliche Verlagsgesellschaft , Stuttgart 1992, ISBN 3-8047-1248-7 (Dissertation University of Regensburg 1991, 168 pages, under the Title: On the history of industrial penicillin production from the beginnings to the development of large-scale production ).
- Vladimir Pliška: Penicillin and suilfonamide in the fight against infections: between enthusiasm and skepticism = La penicilline et les sulfonamides dans la lutte contre les infections: benediction ou malédiction (= BioFokus , Volume 24, No. 87), Association Research for Life, Zurich 2014 , DNB 1049772873 (German and French, full text (PDF) PDF, free of charge, 9 pages, 1.6 MB).
- Barbara I. Tshisuaka: Penicillin. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 1120.
- Susanne Krejsa MacManus: Penicillin from urine. In: Deutsche Apotheker Zeitung , Volume 160, No. 21, May 21, 2020, pp. 71–74
Web links
- Biography of Fleming (English)
- Fleming Nobel Prize Speech (PDF; 336 kB)
Individual evidence
- ↑ 6-aminopenicillanic acid ; University of Hamburg, Department of Chemistry.
- ↑ Penicillins ( Memento from April 24, 2015 in the Internet Archive ): Building blocks and metabolic products, Urban & Fischer 2003 - Roche Lexicon Medicine, 5th ed.
- ↑ Michael T. Madigan, John M. Martinko, Jack Parker: Brock Microbiology. German translation edited by Werner Goebel, 1st edition. Spectrum Akademischer Verlag, Heidelberg / Berlin 2000, ISBN 3-8274-0566-1 , pp. 440–441.
- ↑ CE Higgens, RE Kastner: Streptomyces clavuligerus sp. nov., a β-Lactam Antibiotic Producer. In: International Journal of Systematic and Evolutionary Bacteriology. Volume 21, Number 4, October 1971, pp. 326-331, doi: 10.1099 / 00207713-21-4-326 .
- ↑ H. Cho, T. Uehara, TG Bernhardt: Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. In: Cell. Volume 159, number 6, December 2014, pp. 1300-1311, doi: 10.1016 / j.cell.2014.11.017 . PMID 25480295 .
- ↑ a b Britta Kasten, Ralf Reski : β-Lactam Antibiotics Inhibit Chloroplast Division in a Moss (Physcomitrella patens) but not in Tomato (Lycopersicon esculentum) . In: J. Plant Physiol. , 1997, 150, pp. 137-140 (English); Free full text access ( Memento from February 4, 2012 in the Internet Archive ; PDF)
- ↑ Epileptic seizures from antibiotics? ( Memento from April 15, 2018 in the Internet Archive ; PDF).
- ↑ Ernst Kern: Real and supposed progress in surgery. In: Würzburg medical history reports. Volume 9, 1991, pp. 417-429, here: p. 418.
- ↑ Dr. Clodomiro Picado Twight Honored with WIPO medal (PDF). In: WIPO Magazine , p. 11. Retrieved March 2, 2008.
- ↑ A. Fleming: On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzæ. Br J Exp Pathol 10 (3): pp. 226-236. (1929). PMC 2048009 (free full text)
- ^ HL Van Epps: René Dubos: unearthing antibiotics . In: Journal of Experimental Medicine (JEM) , Volume 203, 2006, S 259. doi: 10.1084 / jem.2032fta PMC 2118194 (free full text)
- ↑ Jonathan Wood: Penicillin: the Oxford story . Oxford Science Blog, July 16, 2010.
- ↑ Alfred Marchionini , Hans Götz: Penicillin treatment of skin diseases. Springer-Verlag, Heidelberg 1950, pp. 11 and 131.
- ↑ Florian G. Mildenberger : No salvation through arsenic? The salvarsand debate and its consequences. In: Specialized prose research - Border Crossing , 8/9, 2012/2013, pp. 327–390, here: p. 365.
- ↑ Ernst Kern : Seeing - Thinking - Acting of a surgeon in the 20th century. ecomed, Landsberg am Lech 2000. ISBN 3-609-20149-5 , p. 298.
- ^ Robert Bud: Penicillin - Triumph and Tragedy . Oxford University Press, 2007, p. 51 books.google.de
- ^ Nobel Prize for Medicine 1945. nobelprize.org
- ↑ Ernst Kern : Seeing - Thinking - Acting of a surgeon in the 20th century. ecomed, Landsberg am Lech 2000, ISBN 3-609-20149-5 , p. 198.
- ↑ Who discovered penicillin - Fleming or Lister? In: Doctors newspaper , November 9, 2004
- ↑ David Wootton : Bad Medicine. Doctors doing harm since Hippocrates. Oxford, p. 248.
- ↑ Florian G. Mildenberger (2012/13), p. 365.
- ^ I. Pieroth: Penicillin production. From the beginnings to large-scale production (Heidelberger Schriften zur Pharmazie- und Naturwissenschaftsgeschichte, Volume 9). Wissenschaftliche Verlagsgesellschaft, Stuttgart 1992, ISBN 978-3-8047-1248-5 .
- ↑ L. Marshall: In the shadow of chemical synthesis. Industrial biotechnology in Germany (1900–1970). Campus Verlag, Frankfurt 2000, ISBN 3-593-36585-5 .