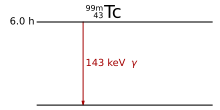radioactivity

Radioactivity (from French radioactivité ; from Latin radiare “radiate” and activus “active”, “effective”; put together “radiation activity”) is the property of unstable atomic nuclei to spontaneously emit ionizing radiation . The nucleus transforms itself into another nucleus by emitting particles or changes its state while releasing energy . The ionizing radiation emitted by the process is also known colloquially as "radioactive radiation".
The term radioactivity was first coined in 1898 by the married couple Marie Curie and Pierre Curie for the phenomenon discovered two years earlier by Antoine Henri Becquerel . The conversion process is also known as radioactive decay or nuclear decay . Types of atoms with unstable nuclei are called radionuclides .
The energy released during the conversion process is given off as kinetic energy of emitted particles (mostly alpha or beta particles) or as radiation energy from gamma radiation . The type and energy spectrum of the radiation are typical for the respective radionuclide. These types of radiation - like altitude and X-ray radiation - are not directly perceptible to humans and can be harmful (see radiation damage , radiation effects ) or useful (see e.g. radiation sterilization , radionuclide therapy , brachytherapy ) depending on the circumstances .
After a period of time that is characteristic of each radioactive nuclide , the half-life , its amount has halved, thus its activity as well . Half-lives can range from fractions of a second to quadrillion years.
Radionuclides occur in nature. But they also arise z. B. in nuclear reactors or through nuclear weapons explosions. They can be specifically produced in particle accelerators . Radioactive substances are used u. a. in radionuclide batteries and heating elements for energy supply in space travel as well as in nuclear medicine and radiation therapy . In archeology , radioactive decay is used to determine the age, for example with the radiocarbon method .
Uses of terms
Radioactive decay
The term “radioactive decay” originally refers to the decrease in radiation intensity observed on a radionuclide over time (provided that it is not constantly being regenerated by other processes). It is also used to decrease the amount of radionuclide.
In technical terms, the spontaneous transformation of the individual atomic nucleus - and sometimes any spontaneous change in state of a system described in quantum mechanics - is also referred to as decay, e.g. B. "Gamma decay" for the emission of a single gamma quantum. In the literal sense of the word, it is less a matter of a decay than a transformation of the atomic nucleus or the system.
Radioactive substances and radiation
In everyday speech and in public discussions, no distinction is made between radioactive substances and their radiation. This is how we speak of radioactive radiation . Strictly speaking, this combination of words is wrong, because it is not the radiation itself that is radioactive, but the substances ( emitters ) from which it emerges; what is meant is ionizing radiation from radioactive substances . Earlier this the term was Becquerel ( Engl. : Becquerel rays ) use.
Leaked radiation is often referred to in reports of nuclear incidents , even though it is about unintentionally released radioactive substances such as cesium-137 and iodine-131. Even in the worst case, the emitted radiation would only have a range of a few kilometers.
history
In 1896, while trying to explain the X-ray radiation he had just found as a fluorescent phenomenon, Antoine Henri Becquerel discovered that uranium salts blackened photographic plates even without prior exposure . This ruled out fluorescence as a cause. As he later discovered, this new radiation could penetrate opaque materials and ionize air without being affected by temperature changes or chemical treatments of the sample. In 1898, Marie and Pierre Curie discovered the radioactivity of thorium oxide and isolated two previously unknown, far more strongly radiating substances, which they named radium and polonium .
In 1898 Ernest Rutherford succeeded in examining the penetration ability to distinguish between two radiation components, which he called α- (alpha) - and β- (beta) radiation. A year later Stefan Meyer and Egon Schweidler as well as Friedrich Giesel were able to show that these are deflected in opposite directions in magnetic fields . In 1900 Paul Villard discovered a third component that could not be distracted by magnetic fields and that was particularly penetrating. In 1903, Rutherford coined the designation γ (gamma) radiation for this third type of radiation . By 1909 it had been shown that alpha radiation consists of helium nuclei and beta radiation consists of electrons . The assumption that gamma radiation is electromagnetic radiation could only be confirmed in 1914 by Rutherford and Edward Andrade .
As early as 1903 - six years before the evidence of atomic nuclei - Rutherford and Frederick Soddy developed a hypothesis according to which radioactivity is associated with the conversion of elements ( transmutation ). Based on this, Kasimir Fajans and Frederick Soddy formulated the radioactive displacement theorems in 1913 . These describe the change in mass and atomic number during alpha and beta decay , which means that the natural decay series could be explained as a step-by-step sequence of these decay processes.
In 1933 Irène and Frédéric Joliot-Curie succeeded for the first time in producing new radioactive elements. By bombarding samples with α-particles, they were able to produce nuclides that do not occur in nature due to their short half-lives . In 1934, during their experiments, they discovered a new type of beta decay in which positrons were emitted instead of electrons. Since then, a distinction has been made between β + and β - radiation.
In 1980, Aureliu Săndulescu , Dorin N. Poenaru and Walter Greiner predicted a new kind of radioactivity based on theoretical considerations, in which nuclei that are heavier than α-particles are emitted. The first experimental evidence of such a cluster disintegration came from HJ Rose and George Arnold Jones in 1983 at the University of Oxford . They observed that 223 Ra , normally an α emitter, very rarely decays to 209 Pb with the emission of a 14 C core .
Physical basics
stability
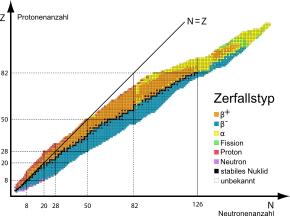
According to the current state of knowledge, there are 255 stable nuclides and around 100 unstable nuclides in nature . A total of about 3000 radioactive nuclides (radionuclides) are known. The vast majority of all known nuclides have thus been proven to be radioactive.
If radioactivity has not been observed in a nuclide, there are two possibilities:
- the nuclide is stable in the absolute sense, i.e. That is, according to the state of the art in physics, there is no lower energy state into which it could pass (decay);
- the nuclide could theoretically decay, but no decay event or clear decay product has been reliably detected so far ( observationally stable nuclide ).
An example of the first type is helium-4. An example of the second type is lead-208, the heaviest nuclide with no demonstrated decay. Its alpha decay 208 Pb → 204 Hg + α would release about 0.5 MeV of energy. Estimates of the half-life according to various variants of the Geiger-Nuttall rule result in more than 10 100 years, i.e. at least 10 90 times the age of the universe. Hence, this decay is unlikely to ever be observed. There are other nuclides with possible, but not observed, decay. The total number of stable nuclides has therefore not yet been determined today (2020).
All elements up to lead, except technetium and promethium , have one or more stable isotopes; the number of stable isotopes goes up to ten (tin). All elements heavier than lead are unstable (radioactive).
Influence of nuclear mass and neutron-proton ratio
Only two very light nuclides, the normal hydrogen 1 H and the rare helium isotope 3 He, are stable with fewer neutrons than protons. All other nuclides “need” at least as many for stability ( 6 Li, 10 B, 12 C, 14 N, 16 O, 20 Ne, 24 Mg, 28 Si, 32 S, 36 Ar and 40 Ca), but mostly even more Neutrons as protons. The average ratio of the number of neutrons to the number of protons increases with an increasing atomic number from 1: 1 for very light nuclides to 1.54: 1 for the heaviest stable nuclides (see also neutron excess ). All nuclides with too many or too few neutrons are unstable and therefore radioactive. Nuclei with more than 208 particles are always unstable.
The most stable nuclides - i.e. those with the highest binding energy per nucleon - are 62 Ni, 58 Fe and 56 Fe. Immediate neighbors such as B. 63 Ni or 60 Co are already radioactive. In addition to a balanced ratio of neutrons to protons, it is crucial whether the number of neutrons and protons is even (paired and cheap) or odd (unpaired and unfavorable). The binding energy can be calculated approximately with the Bethe-Weizsäcker formula .
For unstable nuclides, one can estimate the type of decay (described below):
- too many neutrons: beta-minus decay; with a large excess also direct neutron emission
- too severe: alpha decay; sometimes also cluster disintegration or spontaneous splitting (fission)
- too many protons: beta plus decay or electron capture ; with a large excess also direct proton emission
A gamma decay usually occurs as a follow-up process after a previous decay of another type.
In general, the further the nuclide is from stability (black fields on the nuclide map), the shorter the half-life .
Temporal decrease due to decay
Radioactive decay is not a deterministic process . The time of decay of each individual atomic nucleus is random . However, there is a certain probability of decay per unit of time for every radionuclide ; In the case of macroscopic amounts of substance, this means that the amount of nuclide decreases exponentially to a good approximation , as described by the law of decay . The probability of decay can be indicated indirectly, but clearly by the half-life , i.e. H. the period after which half of the atomic nuclei of an initial set have decayed. Radioactive half-lives range from tiny fractions of a second to quadrillion years. The shorter the half-life, the greater the activity of this nuclide for a given amount of substance .
The total activity of an original quantity can increase many times over if no stable or long-lived nuclide is formed during decay. The substance is enriched with radionuclides of the decay series, each of which has the same activity as the original process. A secular equilibrium is established . This is done at z. B. 137 Cs after a few minutes, at 232 Th it takes several years.
| isotope | Half-life | specific activity of the nuclide | specific activity of the decay series | Types of decay |
|---|---|---|---|---|
| 131 I. | 8 days | 4,600,000,000,000 Bq / mg | 4,600,000,000,000 Bq / mg | β - |
| 137 Cs | 30 years | 3,200,000,000 Bq / mg | 6,230,000,000 Bq / mg | β - |
| 239 Pu | 24,110 years | 2,300,000 Bq / mg | 2,300,000 Bq / mg | α |
| 235 U | 704,000,000 years | 80 Bq / mg | 160 Bq / mg | α, β - |
| 238 U | 4,468,000,000 years | 12 Bq / mg | 37 Bq / mg | α, β - |
| 232 Th | 14,050,000,000 years | 4 Bq / mg | 41 Bq / mg | α, β - |
Statistical fluctuations
The activity A of an amount of substance is the expected value of the number of decays N per unit of time. The actual number of decays observed in a certain time interval T fluctuates randomly around the expected value N T = A · T ; the frequency with which a certain number k occurs follows a Poisson distribution . This process is z. B. behind the irregularity of the cracking of a contamination detection device (colloquially "Geiger counter").
The Poisson distribution can be approximately described by the Gaussian distribution given a sufficiently large mean number . The standard deviation for decay events in the selected time interval is .
Types of decay

The most common, most important and longest known types of decay , also known as decay mode (ZM) or decay channel, are alpha, beta and gamma decays. Since the nature of these processes was unknown at the time of their discovery, the three types of radiation were designated in the order of increasing penetration with the first three (lower) letters of the Greek alphabet: α, β and γ.
- During alpha decay, the atomic nucleus emits an alpha particle that consists of two protons and two neutrons. This reduces the mass number by 4 and the ordinal number by 2.
- During beta decay, the atomic nucleus emits either an electron or a positron ; this is created in the atomic nucleus when a neutron is converted into a proton or a proton into a neutron. The mass number remains the same, the ordinal number changes by +1 or −1.
- During gamma decay, the atomic nucleus emits a high-energy photon . The mass and atomic number remain the same, only the excitation state of the nucleus is reduced. Gamma decay usually occurs as a direct consequence of a previous alpha or beta decay.
In addition to these three types of transformation, others were discovered later. Most of them are rare and of interest only to physical research itself; Besides alpha, beta and gamma decay, spontaneous fission has a certain practical significance.
Some nuclides can decay in several ways, i.e. have more than one decay channel. A nuclide map is a graphic overview of all stable and unstable nuclides including their observed types of decay and half-lives .
The large number of existing types of decay can be divided into different categories:
- Decays with emission of nucleons
- Many radioactive nuclei change with the emission of nucleons, i. H. of protons, neutrons or light nuclei. The most prominent example is alpha decay . Here, the mother core splits off a helium core. Individual neutrons or protons or whole carbon or other light nuclei are emitted (emitted) less frequently. All decays with emission of nucleons are mediated by the strong interaction together with the electromagnetic interaction .
- Beta decays
- If electrons (or their antiparticles) are involved in a decay, it is called beta decay. There are several such processes. An electron does not always have to be created as a product; an electron can also be converted, as in electron capture. All beta decays are processes of weak interaction .
- Transitions between states of the same nucleus
- In this case no matter particles are emitted. Correspondingly, the nucleus does not change into another; it just gives off excess energy. This can be released as gamma radiation or given off to an electron in the atomic shell (internal conversion). These are processes of electromagnetic interaction.
Overview
| △ | Decay mode | participating particles | Daughter core | emitted particles |
|
|---|---|---|---|---|---|
| Decays with emission of nucleons | |||||
| α | Alpha decay | The core emits a 4 He core ( A = 4, Z = 2), also called alpha particles . | ( A −4, Z −2) | 4 He | |
| SF | Spontaneous split | With the emission of mostly two to three neutrons, the nucleus disintegrates into two medium-weight nuclei, rarely into additional (mostly light) nuclei. | 2+ cores | 2… 3 after | |
| p | Proton emission | The nucleus emits a proton. | ( A −1, Z −1) | p | |
| n | Neutron emission | The nucleus emits a neutron. | ( A −1, Z ) | n | |
| 2p | Double proton emission | The nucleus emits two protons at the same time. | ( A −2, Z −2) | 2 p | |
| 2n | Double neutron emission | The nucleus emits two neutrons at the same time. | ( A −2, Z ) | 2 n | |
| A c Z c | Cluster disintegration | The core emits a smaller core ( 14 C to 28 Si) with A c , Z c . A heavy nucleus between 204 Hg, 212 Pb and 211 Bi remains . The alpha decay (see above) is i. General not counted among the cluster disintegrations. |
( A - A c , Z - Z c ) | ( A c , Z c ) | |
| Beta decays | |||||
| β - | Beta-minus decay | The nucleus emits an electron and an electron antineutrino. | ( A , Z +1) | ν̅ e , e - | |
| β + | Beta plus decay | The nucleus emits a positron and an electron neutrino . | ( A , Z −1) | ν e , e + | |
| K (ε) | Electron capture | The nucleus absorbs an electron from the atomic shell and emits an electron neutrino. | ( A , Z −1) | ν e | |
| ββ (2β - ) | Double beta-minus decay | The nucleus emits two electrons and two electron antineutrinos. | ( A , Z +2) | 2 ν̅ e , 2 e - | |
| (2β + ) | Double beta plus decay | The nucleus emits two positrons and two electron neutrinos. | ( A , Z −2) | 2 ν e , 2 e + | |
| (εβ + ) | Electron capture with positron emission | The nucleus absorbs an electron from the atomic shell and emits a positron and two electron neutrinos. | ( A , Z −2) | 2 ν e , e + | |
| KEC (2ε) | Double electron capture | The nucleus absorbs two electrons from the atomic shell and emits two electron neutrinos. | ( A , Z −2) | 2 ν e | |
| Transitions between states of the same nucleus | |||||
| IT | Gamma decay | The excited nucleus emits a (mostly) high-energy photon (gamma quantum). | ( A , Z ) | γ | |
| (IC) | Inner conversion | The excited nucleus transfers energy to a shell electron, which leaves the atom. | ( A , Z ) | e - | |
- The abbreviations without brackets are used in the isotope listing of the German language Wikipedia, those in brackets are often used on other websites.
- K / β + denotes the occurrence of electron capture as well as beta plus decay.
Decays with emission of nucleons
Alpha decay (α)
Alpha decay occurs mainly in heavier and relatively low neutron nuclides. A helium- 4 nucleus, called an alpha particle in this case, leaves the mother nucleus at a speed of 3 to 8 percent of the speed of light . This is possible despite the high Coulomb barrier due to the tunnel effect . The residual nucleus, also known as the recoil nucleus or daughter nucleus, has after the process a nucleon number reduced by 4 and a nuclear charge number reduced by 2 .
The general formula for alpha decay is
- The parent nucleus X with nucleon number A and proton number Z decays with emission of an alpha particle into the daughter nucleus Y with a nucleon number reduced by 4 and a number of protons reduced by 2.
Example : The decay of uranium-238 into thorium-234:
Further decays with emission of nucleons follow here .
Beta decays
Beta decay occurs when there is an imbalance in the ratio of neutrons to protons in the nucleus. The resulting beta radiation consists either of electrons (β - ) or positrons (β + ), which leave the nucleus at up to 99.9 percent of the speed of light, depending on the nuclide.
Beta-minus decay (β - )
During beta-minus decay , a neutron is converted into a proton in the nucleus. An electron and an electron antineutrino are emitted. The nucleon number of the nucleus does not change, its atomic number increases by 1.
The general formula is
- The mother nucleus X with nucleon number A and proton number Z decays with emission of an electron and an electron antineutrino into the daughter nucleus Y with the same number of nucleons and one increased number of protons.
Example: The decay of carbon-14 into the stable isotope nitrogen-14:
Beta-minus radiation can be transmitted through a few meters of air or z. B. completely shield with a plexiglass plate .
Neutrino and antineutrino are only subject to weak interaction . Because of this extremely rare interaction with matter, they are difficult to detect and harmless to living things. Sun neutrinos cross parts of the sun and the whole earth almost without being weakened.
Beta plus decay (β + )
In beta-plus decay , a proton is converted into a neutron in the nucleus ; a positron and an electron neutrino are emitted. The nucleon number of the nucleus does not change, its atomic number is reduced by 1.
The general formula is
- The parent nucleus X with nucleon number A and proton number Z decays with emission of a positron and an electron neutrino into the daughter nucleus Y with the same number of nucleons and one less number of protons.
Example: The decay of nitrogen-13 into the stable isotope carbon-13:
(Simple) electron capture (ε)
Another way of converting a proton into a neutron is electron capture , also known as ε-decay or sometimes inverse β-decay . An electron is "pulled" from the atomic shell into the nucleus. After the typically affected electron shell , the K shell, electron capture is also referred to as K capture . A proton in the nucleus is converted into a neutron and an electron neutrino is emitted. The change in the nucleus is the same as in the β + decay: the number of nucleons remains unchanged, the atomic number is reduced by one. Electron capture therefore competes with β + decay. Since the β + decay has to generate the energy for the emitted positron, the β + decay is energetically not an option for every nuclide that decays with electron capture . A space is freed up in the shell affected by the electron capture and electrons from the outer shells move up, with characteristic X-rays being emitted.
In general, the formula for electron capture is
- The mother nucleus X captures an electron from the atomic shell and, with the emission of an electron neutrino, transforms itself into the daughter nucleus with the same number of nucleons and one less number of protons.
Example: The decay of nickel-59 to cobalt-59:
Double electron capture (2ε)
For some nuclei, simple electron capture is not energetically possible, but they can decay by capturing two electrons at the same time. Since such decays require two weak interactions at the same time, they have extremely long half-lives. They were first detected directly in 1986.
Example: The decay of Xenon-124 into Tellurium-124:
Double beta-minus decay (2β - )
For some nuclei, a simple beta decay is not energetically possible, but they can decay while emitting two electrons. Since such decays require two weak interactions at the same time, they have extremely long half-lives. They were first detected directly in 1987.
Example: The decay of zirconium-96 into molybdenum-96:
Whether two neutrinos are always emitted during double beta decay or whether a neutrino-free double beta decay also occurs has not yet been answered (2016). If the neutrino-less case could be proven, the neutrinos would have annihilated each other , which would mean that neutrinos are their own antiparticles. That would make them so-called Majorana particles .
Transitions between states of the same nucleus
Gamma decay (γ)
A gamma decay generally occurs when an atomic nucleus remains in an excited state after another previous decay . The atomic nucleus emits energy through the emission of high-energy electromagnetic radiation (γ radiation) and changes to a state of lower energy. The number of neutrons and protons in the nucleus does not change. The term gamma “decay” is somewhat misleading in this respect, but it is still a common nomenclature. With a few exceptions, the gamma decay occurs within a very short time (10 −18 to 10 −12 seconds) after a previous decay.
The general formula is
- The excited nucleus X is excited by emitting a gamma quantum.
A well-known example is the emission of gamma radiation through a nickel-60 core, which is (mostly) created by beta decay of a cobalt-60 core:
The decay scheme of this process is shown in the graphic on the right. 60 Co , a nuclide with many practical applications, is a beta-minus emitter with a half-life of 5.26 years. It decays into an excited state of 60 Ni * , which almost immediately changes to the ground state with a half-life of a little less than 1 ps through the emission of (mostly) a cascade of two gamma quanta.
In the practical applications of 60 Co and many other radionuclides, it is very often only about this gamma radiation; the alpha or beta radiation is shielded in these cases by the housing of the radioactive preparation and only the gamma radiation penetrates to the outside.
Although the gamma radiation comes from the daughter nuclide of the alpha or beta decay, it is always linguistically assigned to its parent nuclide. One speaks of the "gamma emitter" Cobalt-60 etc., because the only practically usable source of this gamma radiation is a 60 co-preparation.
Only when the excited state is an isomer i.e. H. has a sufficiently long half-life, the actual gamma radiation source can be used separately from its generation, as in the case of technetium -99:
This technetium isotope with a half-life of six hours is used in medical diagnostics.
In order to shield from γ radiation, concrete or lead plates that are decimeter thick may be necessary, because it has no specific range in matter, but is only attenuated exponentially . There is therefore a half-value thickness that depends on the gamma energy for each shielding material . Like light, gamma radiation is electromagnetic radiation, but its quantum is much more energetic and therefore lies far outside the spectrum that is visible to the human eye.
Inner Conversion (IC)
The energy released during the transition of an atomic nucleus to an energetically lower state can also be given to an electron in the atomic shell. This process is called internal conversion. The conversion electrons accordingly have very characteristic energies, i.e., in contrast to β electrons, they show a line spectrum.
- The excited nucleus X is de-excited. The energy released is transferred as kinetic energy to an electron in the atomic shell.
In the case of internal conversion, a negative elementary charge is missing in the shell after the disintegration and a positive ion remains .
Other types of decay with emission of nucleons
Spontaneous split (SF)
In the case of particularly heavy nuclei beyond the atomic number 90 ( thorium ), spontaneous fission is another radioactive conversion process. The atomic nucleus decays into two (rarely more) medium-weight daughter nuclei and releases two or three neutrons. Different pairs of daughter nuclei are possible, but the sum of the atomic numbers and the sum of the mass numbers of all fission products are each equal to that of the original nucleus :
Naturally occurring uranium isotopes decay to a tiny extent through spontaneous fission:
In addition to the mostly binary nuclear fission, a ternary nuclear fission also rarely occurs, in which a third (light) particle occurs. Usually this particle is a 4 He or 3 H nucleus.
Quaternary nuclear fission occurs even more rarely, in which two further light particles (also here mostly 4 He) are formed.
Spontaneous nucleon emission (p, n, 2p, 2n)
In the case of nuclei with a particularly high or particularly low number of neutrons, spontaneous nucleon emission can occur . H. lead to proton or neutron emission . Atomic nuclei with a very high excess of protons can emit a proton, and atomic nuclei with a high excess of neutrons can release neutrons.
Example: Boron-9 splits off a proton to compensate for the excess:
Example: Helium-5, on the other hand, spontaneously emits a neutron:
Two proton decay (2p)
In the case of an extreme excess of protons, two-proton decay can occur, in which even two protons are emitted at the same time.
Example: The decay of sulfur -26 into silicon -24:
Two-neutron decay (2n)
If there is an extreme excess of neutrons, two-neutron decay can occur, in which even two neutrons are emitted at the same time.
Example: The decay of beryllium -16 into beryllium-14:
Both two-nucleon processes occur near the theoretical stability limit, the "edge of the nuclide map". Outside of this there can be no bound atomic nuclei.
Cluster disintegration (A c Z c )
Instead of individual nucleons or helium-4 nuclei, larger atomic nuclei are also emitted in very rare cases. This form of decay was predicted in 1980 and experimentally confirmed in 1983.
Examples:
Decay series
The product of a decay can be stable or, in turn, radioactive. In the latter case, a sequence of radioactive decays will take place until a stable nuclide is finally formed as the end product. This sequence of radioactive decays is called a decay series or decay chain .
The isotope uranium- 238 decays with the emission of an alpha particle in thorium- 234, this then converts through beta-decay into protactinium- 234, which is again unstable and so on. After a total of 14 or 15 decays, this series of decays ends with the stable core of lead -206. Since some nuclei can decay in different ways (see decay channel ), several branches of the same decay series can originate from a mother nucleus (which can also meet again). For example, about 64% of the atoms in a bismuth- 212 sample are converted into polonium- 212 through beta decay , and the remaining 36% through alpha decay into thallium- 208.
In this way, an originally pure sample of a radionuclide can turn into a mixture of different radionuclides over time. Long-lived nuclides accumulate more than short-lived ones.
Shielding and range
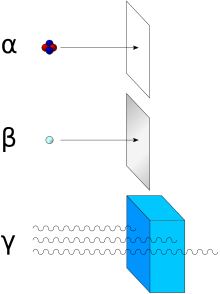
Alpha radiation can be shielded with a sheet of paper, thin cardboard or air. Thin layers of plexiglass or sheet metal are used to shield from β - radiation (electrons), materials with a lower atomic number being better suited due to the lower bremsstrahlung. Materials with high atomic numbers are used to shield β + and γ radiation at the same time (see annihilation ), e.g. B. lead . In general, the range of ionizing radiation increases with its energy and decreases with the density of the shielding material. α-radiation with the kinetic energy of 5 MeV has a range of 3.6 cm in air, but only 0.04 mm in tissue. Mainly, ionizing radiation emits energy through collisions with the atoms of the shielding material, atoms are ionized or excited , which in turn creates secondary electrons and X-rays within the shielding material.
Radioactivity in the environment
Radioactivity occurs partly naturally in our environment (without human intervention), partly it was or is generated by human activities (“anthropogenic”). The causes of natural radioactive radiation are primordial radionuclides with their secondary products as well as nuclides that are generated by cosmic radiation in the earth's atmosphere . Human-made radioactivity usually has an isotopic composition that differs from the natural one , because it also contains short-lived radionuclides that do not arise in series of decays or spallation processes .
Naturally occurring radioactivity
The primordial radionuclides come from the material of the primordial earth and are still present today because of their long half-life. These include potassium -40, which is always contained in the human body, and the isotopes of uranium, which are important as nuclear fuel . Other radionuclides arise indirectly as the decay products of the radioactive decay series of these primordial nuclides, which are constantly being reproduced , such as the radon gas that escapes everywhere from the earth . These nuclides are called radiogenic . Further, cosmogenic radionuclides are continuously generated in the atmosphere through nuclear reactions with cosmic rays. One of them is carbon -14, which, like potassium-40, enters all organisms through the metabolism .
The radiation of the ubiquitous natural radionuclides is called terrestrial radiation .
Radioactivity generated or released by humans
Long before radioactivity was discovered, human activities such as mining and burning coal had released radioactive substances. Paracelsus described Schneeberger disease in 1567 . Metal ores and coal contain more radionuclides than the average biosphere; Mine systems transport radon from the interior of the earth to the surface.
With the mining of uranium, the construction of nuclear power plants, and most importantly the construction and above-ground testing of nuclear weapons, radioactivity was released into the biosphere, which had a global impact.
Large amounts of radioactive substances were released (in addition to the nuclear tests until 1963) through accidents in nuclear facilities. The most famous are the Chernobyl nuclear disaster and the Fukushima nuclear disaster . After 1990, the Kyshtym accident in 1957 and the Ostural Trail that emerged in the process became known.
Medical applications or material studies with ionizing radiation do not contribute to human-induced radioactivity. If radioactive substances are used at all, these are short-lived nuclides in small quantities, such as B. in positron emission tomography .
Certain long-lived nuclides from the radioactive waste from nuclear fission could in future be transformed into shorter-lived nuclides that are less expensive to store by transmutation .
Sizes and units of measure
activity
When activity is defined as the number of decay events per unit of time that in a sample of radioactive or radioactive contaminated occurring substance. The activity is usually given in the SI unit Becquerel (Bq). 1 Becquerel corresponds to one decay per second.
Radiation dose
The quantities and units of measurement that relate to the effects of ionizing radiation (from radioactive or other sources) include
- the absorbed dose with the unit Gray , which describes the absorbed energy per mass in joules / kilogram (J / kg),
- the equivalent dose with the unit Sievert , corresponds to the absorbed dose, corrected by defined weighting factors for different types of radiation and
- the ion dose with the unit of measurement coulomb / kilogram (C / kg), which describes the amount of ionization processes caused.
Measuring devices for radiation from radioactivity
There are many types of detectors for the detection and quantitative measurement of radiation, each of which is suitable for certain types of radiation. A well-known example is the Geiger counter . Ionization chambers and cloud chambers can be used to detect alpha, beta and gamma radiation, scintillation counters and semiconductor detectors are used to detect beta and gamma rays.
For radiation protection for measuring different types of dosimeters and dose rate meters used. They each contain one or more of the detectors mentioned above.
The very first measurement that gave a quantitative statement about the radiation was carried out by Pierre Curie and Marie Curie with the help of an electroscope . This measured the decrease in an electrical charge due to the conductivity of the air caused by the ionization. The same measuring principle is still used today (2016) in the fountain pen dosimeter .
Applications

Technical applications
Radionuclide batteries are used in space travel for power supply and radionuclide heating elements for heating. Beyond Jupiter's orbit, the radiation from the distant sun is no longer sufficient to cover the energy requirements of the probes with solar cells of practicable size. Strong radiation belts , such as those used e.g. B. Surrounding Jupiter, make the use of solar cells impossible. In the USSR , very powerful radionuclide batteries filled with strontium -90 were used to operate lighthouses and radio beacons in the Arctic Circle.
Important applications that exploit the radioactivity of substances are the age determination of objects and material testing.
In archeology , art history , geology and paleoclimatology measurements of the concentration of radioactive isotopes are used to determine the age, e.g. B. radiocarbon dating (radiocarbon dating ).
A technical application is thickness measurement and material testing by means of radiation. Here, a material is irradiated with gamma rays and a counter determines the average density with a known layer thickness or, conversely, the layer thickness with a known density based on the penetrating rays and the law of absorption . The radiation can also create an image on an X-ray film behind the material layer. Radiographic testing is used in this form for materials.
Also radiometric level measurement in large containers with bulk or granules are performed with gamma irradiation from one to another vessel wall.
In geophysics and biology, radioactive substances are suitable as tracers to determine the flow behavior of z. B. to examine groundwater in the soil or blood in a tissue. For this purpose, a known amount of the substance is introduced at a certain point and the temporal and spatial distribution of the activity is measured.
Material investigations
In solid-state physics and solid-state chemistry , radioactive isotopes are used to study materials, such as B. Metals and alloys , semiconductors , insulators and functional ceramics . The focus here is on local defects and diffusion , which often determine the functionality of the materials. These are used today in many electronic applications such as electronics , batteries , computer chips , hard disk drives , lighting, etc. Without a deeper understanding of these materials, a specific application would be inconceivable.
One application is element analysis with gamma spectroscopy . Precision measurements in chemical analysis and investigations of the local structure in solids are carried out e.g. B. performed with Mössbauer spectroscopy or the disturbed gamma-gamma angle correlation . These methods of nuclear solid state physics use special radioactive isotopes that are found in special facilities such as B. ISOLDE at CERN or in nuclear reactors .
Radioactive probes have the great advantage that only very small amounts of substance are required and they are usually only used in traces. In tracer diffusion , a few kBq are usually sufficient to determine diffusion coefficients in solids. If the gamma-gamma angle correlation is disturbed , only about 10 10 to 10 12 atoms are required per measurement. With the method z. B. the binding of toxic metals such as cadmium , mercury or lead can be examined in-situ in biological cells . With beta-NMR only approx. 10 8 atoms are required per measurement .
Medical applications
The use of unsealed radioactive substances on humans is the subject of nuclear medicine .
Scintigraphy is mostly used in nuclear medicine diagnostics . Small amounts of a γ-emitting substance ( tracer ) are applied (“applied”) to the patient, for example injected into a vein or inhaled. The radiation emanating from the tracer is recorded outside the body by a gamma camera based on scintillation detectors and produces a two-dimensional image. Modern further developments of the method allow three-dimensional representations by means of computed tomography ( Single Photon Emission Computed Tomography , SPECT); Another imaging method in nuclear medicine that also provides three-dimensional images is positron emission tomography (PET). Certain laboratory tests can also be carried out with radioactive substances, for example the radioimmunoassay .
Pure or predominantly β-emitters are used in nuclear medicine therapy. The most common areas of application are radioiodine therapy for benign and malignant diseases of the thyroid gland , radiosynoviorthesis for certain joint diseases, and radionuclide treatment for pain relief in bone metastases .
In the radiation radionuclides were formerly often in the form of enclosed gamma emitters used in which escape any radioactive substance and can be absorbed by the body. Due to the risk potential for medical personnel, these are increasingly being replaced by hard X-rays for irradiating the body from the outside , which are generated with electron linear accelerators . The enclosed gamma emitters are still used, for example, in brachytherapy or radiosurgery .
Dangerousness
With regard to the dangerousness of radioactivity, various risks must be distinguished:
- Radiation exposure as a long-range effect ( see also dose conversion factor )
- Contamination (contamination) with radioactive material, which under certain circumstances can lead to long-term irradiation, e.g. B. with contamination of the skin
- Incorporation (uptake) of radioactive substances into the body through inhalation ( inhalation ) or eating / drinking ( ingestion ).
These terms are sometimes confused in reporting and the public. Correspondingly, for example, the expression “irradiated” is often used incorrectly today (2016) instead of contaminated ; Radiation originally means - analogous to the combustion - one caused by irradiation significant damage or injury.
It is not the radioactivity per se that is responsible for the sometimes dangerous biological effect , but the ionizing radiation emitted by it.
The consequences of the effects of low-dose radiation ( low radiation ) on the environment and living beings are widely discussed. They are difficult to prove. The definition of permissible limit values is also controversial.
Warning symbols
Since the radiation warning sign (Trefoil symbol: ☢ ) used so far was often not recognized as a warning of strong radioactive emitters and people removed a strongly radiating nuclide from its shielding (for example the Goiânia accident ), fatalities have already occurred, especially in developing countries Accidents. On February 15, 2007, the IAEA therefore announced that a new, more conspicuous warning sign should be attached directly to radiators of radiation categories 1, 2 and 3. This warns of the deadly danger of ionizing radiation with the help of more meaningful symbols and prompts people to flee. Only the old symbol should still be attached to the container itself, as it shields the radiation to such an extent that it does not pose any immediate danger. Through the standardization as ISO - Norm 21482 , the new warning label for dangerous radiation sources to be introduced as quickly as possible and internationally binding. In Germany, the warning sign has neither been adopted in a national standard nor included in the accident prevention regulations. It is also not included in the draft of the new version of DIN 4844-2, which regulates warning signs. In Austria it is standardized in OENORM ISO 21482.
The labeling should not be changed for weak radiation sources. The development of symbols to warn posterity about radioactive dangers is the subject of atomic semiotics .
literature
- Werner Stolz: Radioactivity. Basics, measurement, applications. 5th edition. Teubner, Wiesbaden 2005, ISBN 3-519-53022-8 .
- Bogdan Povh , K. Rith, C. Scholz, Zetsche: Particles and Cores. An introduction to the physical concepts. 7th edition. Springer, Berlin / Heidelberg 2006, ISBN 978-3-540-36685-0 .
- Klaus Bethge , Gertrud Walter, Bernhard Wiedemann: Nuclear Physics. 2nd Edition. Springer, Berlin / Heidelberg 2001, ISBN 3-540-41444-4 .
- Hanno Krieger: Fundamentals of radiation physics and radiation protection. 2nd Edition. Teubner, Wiesbaden 2007, ISBN 978-3-8351-0199-9
- IAEA Safety Glossary. Terminology Used in Nuclear Safety and Radiation Protection. IAEA Publications, Vienna 2007, ISBN 92-0-100707-8 .
- Michael G. Stabin: Radiation Protection and Dosimetry. An Introduction to Health Physics. Springer, 2007, ISBN 978-0-387-49982-6 .
- Glenn Knoll: Radiation Detection and Measurement. 3. Edition. Wiley & Sons, New York 2007, ISBN 978-0-471-07338-3 .
Web links
- What is radioactivity? from the alpha-Centauri television series(approx. 15 minutes). First broadcast on Nov 24, 2002.
- The "Radiation Protection Glossary" of Forschungszentrum Jülich explains many terms relating to radioactivity (units, dosimeters, dose terms, alpha, beta, gamma radiation, radiation protection, etc.)
- Mineralienatlas radioactivity
- Remote Lab for radioactivity (see there under "RCLs")
- Radioactivity monitoring network of the Federal Office for Radiation Protection
- Radioactivity (introduction at student level, LEIFIphysics )
Individual evidence
- ↑ Pierre Curie, Marie Curie, G. Bémont: Sur une nouvelle substance fortement radio-active contenue dans la pechblende . In: Comptes rendus hebdomadaires des séances de l'Académie des sciences . tape 127 , 1898, pp. 1215-1217 ( online ).
- ^ Johannes Friedrich Diehl: Radioactivity in food . John Wiley & Sons, 2008, ISBN 978-3-527-62374-7 , pp. 2 ( limited preview in Google Book search).
- ↑ Example of incorrect use: what is radioactivity and how does it work? from Greenpeace
- ↑ Radioactive radiation: Tokyo is spared for the time being .
-
↑ See for example:
* Becquerel rays . In: Brockhaus' Kleines Konversations-Lexikon . 5th edition. Volume 1, F. A. Brockhaus, Leipzig 1911, p. 171 .
* Becquerel rays . In: Meyers Großes Konversations-Lexikon . 6th edition. Volume 2, Bibliographisches Institut, Leipzig / Vienna 1905, pp. 541–542 .
* Robert Strutt : The Becquerel rays and the properties of Radium. Edward Arnold, 1904. - ↑ How dangerous is the radiation that has leaked to date for the population?
- ↑ Fukushima: «Much clearly exaggerated» .
- ^ Ernest Rutherford: Uranium Radiation and the Electrical Conduction Produced by It . In: Philosophical Magazine . 5th episode, volume 47, number 284, 1899, p. 116, doi: 10.1080 / 14786449908621245 .
- ^ Ernest Rutherford: The Magnetic and Electric Deviation of the Easily Absorbed Rays from Radium . In: Philosophical Magazine . 6th episode, volume 5, number 25, 1903, p. 177 doi: 10.1080 / 14786440309462912 .
- ↑ Aureliu Săndulescu, Dorin N. Poenaru, Walter Greiner: New type of decay of heavy nuclei intermediate between fission and α decay . In: Soviet Journal of Particles and Nuclei . Volume 11, number 6, 1980, p. 528 (= Fizika Elementarnykh Chastits i Atomnoya Yadra . Volume 11, 1980, p. 1334).
- ^ HJ Rose, GA Jones: A new kind of natural radioactivity . In: Nature . Volume 307, Number 5948, January 19, 1984, pp. 245-247 doi: 10.1038 / 307245a0 .
- ↑ NUBASE2016. (txt) Atomic Mass Data Center, Nuclear Data Section of the IAEA , 2017, accessed on August 10, 2018 (based on G. Audi, FG Kondev, Meng Wang, WJ Huang, S. Naimi: The NUBASE2016 evaluation of nuclear properties . In : Chinese Physics C . band 41 , no. 3 , March 10, 2017, doi : 10.1088 / 1674-1137 / 41/3/030001 ( iaea.org [PDF; 1.9 MB ; accessed on August 10, 2018]). ).
- ↑ Hanno Krieger: Fundamentals of radiation physics and radiation protection. 4th edition, Vieweg + Teubner, Wiesbaden 2012, ISBN 978-3-8348-1815-7 , pp. 150-160.
- ↑ a b G. Audi, O. Bersillon, J. Blachot and AH Wapstra: The NUBASE evaluation of nuclear and decay properties. (PDF; 1.0 MB) In: Nuclear Physics . Vol. A 729, 2003, pp. 3–128.
- ↑ Radioactive decays can therefore be used in random generators to generate real random numbers , see e.g. B. Ammar Alkassar, Thomas Nicolay, Markus Rohe: Obtaining True-Random Binary Numbers from a Weak Radioactive Source . In: Computational Science and Its Applications - ICCSA 2005 . tape 3481 . Springer Berlin Heidelberg, 2005, ISBN 978-3-540-25861-2 , p. 634-646 , doi : 10.1007 / 11424826_67 .
- ↑ journals.aps.org .
- ↑ Ternary and quaternary fission
- ↑ D. Eidemüller: At the borders of the nuclide map .
- ↑ Achim Rahn: Radiation Protection - Technology: Specialist course for radiation protection officers in accordance with the technical guidelines for radiation protection regulation (StrlSchV) and X-ray regulation (RöV) . Hüthig Jehle Rehm, ISBN 978-3-609-68452-9 , p. 58 ff . ( limited preview in Google Book search).
- ^ Hans Albrecht Bethe, Julius Ashkin: Passage of radiations through matter . In: Emilio Segré (Ed.): Experimental Nuclear Physics . Volume 1, Part II. John Wiley & Sons, New York 1953.
- ↑ MJ Berger, JS Coursey, MA Zucker, J. Chang: ESTAR, PSTAR, and ASTAR: computer programs for calculating stopping-power and range tables for electrons, protons, and helium ions (version 1.2.3). National Institute of Standards and Technology, Gaithersburg 2005.
- ↑ Bernd Leitenberger: The radioisotope elements on board space probes. Retrieved March 24, 2011 .
- ↑ British study - How weak radioactivity affects the body . In: Deutschlandfunk . ( deutschlandfunk.de [accessed November 26, 2017]).
- ^ New Symbol Launched to Warn Public About Radiation Dangers
- ↑ Flash video from the IAEA .