semiconductor
Semiconductors are solids whose electrical conductivity is between that of electrical conductors (> 10 4 S / cm) and that of non-conductors (<10 −8 S / cm). Since the boundary areas of the three groups overlap, the negative temperature coefficient of the specific resistance is another important feature of semiconductors, that is, their conductivity increases with increasing temperature, they are so-called thermistors . The reason for this is the so-called band gap between the valence and conduction bands . Close to absolute temperature zero these are full or unoccupied, and semiconductors are therefore non-conductors. In contrast to metals, there are primarily no free charge carriers. B. arise from heating. The electrical conductivity of semiconductors increases steeply with temperature, so that at room temperature they are more or less conductive, depending on the material-specific distance between the conduction and valence bands. Furthermore, by introducing foreign atoms ( doping ) from another main chemical group, the conductivity and the conduction character (electron and hole conduction ) can be specifically influenced within wide limits.
Semiconductors are divided into crystalline and amorphous semiconductors based on their crystal structure ; see section classification . Furthermore, they can have different chemical structures. The best known are the element semiconductors silicon and germanium , which are composed of a single element, and compound semiconductors such as the III-V compound semiconductor gallium arsenide . In addition, organic semiconductors have gained in importance and popularity in the last few decades ; they are used, for example, in organic light-emitting diodes (OLEDs). However, there are also other substances with semiconductor properties, such as B. organometallic semiconductors as well as materials that acquire semiconductor properties through nanostructuring. Brand new are ternary hydride compounds such as lithium - barium - hydride (LiBaH 3 ).
Semiconductors are important for electrical engineering and especially for electronics , whereby the possibility of influencing their electrical conductivity through doping plays a decisive role. The combination of differently doped areas, e.g. B. the pn junction , enables both electronic components with a direction-dependent conductivity ( diode , rectifier ) or a switch function (z. B. transistor , thyristor , photodiode ), the z. B. can be controlled by applying an electrical voltage or a current (see. Working conditions in metal-insulator-semiconductor structure ). Other applications in addition to the transistor are: NTC thermistors , varistors , radiation sensors ( photoconductors , photoresistors , photodiodes or solar cells ), thermoelectric generators , Peltier elements and radiation or light sources ( laser diode , light emitting diode ). The majority of all semiconductor components manufactured are silicon-based . Silicon does not have the very best electrical properties (e.g. charge carrier mobility ), but in combination with its chemically stable oxide it has clear advantages in production (see also thermal oxidation of silicon ).
history
Stephen Gray discovered the difference between conductors and non-conductors in 1727. After Georg Simon Ohm established Ohm's law in 1821 , which describes the proportionality between current and voltage in an electrical conductor, the conductivity of an object could also be determined.
The Nobel laureate Ferdinand Braun discovered the rectifying effect of semiconductors in 1874. He wrote: “With a large number of natural and artificial sulfur metals [...] I found that the resistance of the same differed with the direction, intensity and duration of the current. The differences are up to 30 pCt. of all the worth. ”He described for the first time that resistance can be changeable.
Greenleaf Whittier Pickard received the first patent in 1906 for a silicon- based tip diode for demodulating the carrier signal in a detector receiver . Initially, in the same receiver ( " Pickard Crystal Radio Kit mostly") galena used as semiconductors, in the 1920s, more robust and more powerful diodes based on copper sulphide contacts emerged. The functioning of the rectifier effect based on a semiconductor-metal transition remained unexplained for decades despite its technical application. It was not until 1939 that Walter Schottky was able to lay the theoretical foundations for describing the Schottky diode named after him .
The first patent on the principle of the transistor was registered in 1925 by Julius Edgar Lilienfeld (US physicist of Austro-Hungarian descent). In his thesis, Lilienfeld described an electronic component which, in the broadest sense, can be compared with today's field effect transistors. At that time, he lacked the necessary technologies to implement field effect transistors in practice.
When scientists John Bardeen , William Bradford Shockley and Walter Houser Brattain at Bell Laboratories in 1947 put two metal wire tips on a germanium plate and were thus able to control the p-conductive zone with the second wire tip with an electrical voltage, they realized the tip transistor ( bipolar transistor ). . This earned them the 1956 Nobel Prize in Physics and founded microelectronics.
In 1954, Eberhard Spenke and his team at Siemens & Halske AG succeeded in producing high-purity silicon using the zone melting process . In the mid-1950s, this, together with the availability of an insulation material ( silicon dioxide ) with favorable properties (insoluble in water like germanium oxide , easy to manufacture, etc.) brought silicon to the breakthrough as a semiconductor material for the electronics industry and about 30 years later for the first microsystem technology products . For the production of integrated circuits today (2009) almost exclusively silicon which is produced more cheaply using the Czochralski process is used.
Alan Heeger , Alan MacDiarmid and Hideki Shirakawa showed in 1976 that when polyacetylene - a polymer that is an insulator in the undoped state - is doped with oxidizing agents, the specific electrical resistance is down to 10 −5 Ωm ( silver : ≈ 10 −8 Ω m) can decrease. In 2000 they received the Nobel Prize in Chemistry for this (see section organic semiconductors ).
Classification
The classic, i.e. crystalline electronic, semiconductors used in microelectronics can be divided into two groups, element semiconductors and compound semiconductors . Element semiconductors include elements with four valence electrons , for example silicon (Si) and germanium (Ge). The group of compound semiconductors includes chemical compounds that have an average of four valence electrons. These include compounds of elements of III. with the V. main group of the periodic table ( III-V compound semiconductors ) , such as gallium arsenide (GaAs) or indium antimonide (InSb), and the II. subsidiary with the VI. Main group (II-VI semiconductors) , such as zinc selenide (ZnSe) or cadmium sulfide (CdS).
In addition to these frequently used semiconductors, there are also I-VII semiconductors , such as copper (I) chloride . Materials that do not have four valence electrons on average can also be called semiconductors if they have a specific resistance in the range of greater than 10 −4 Ω · m and less than 10 6 Ω · m.
Organic semiconductors are another large class . They are called organic because they are mainly composed of carbon atoms. They are divided into semiconducting polymers (chains of individual monomers of different lengths) and small molecules (individual, closed units). Although fullerenes , carbon nanotubes and their derivatives are strictly speaking also small molecules, they are often perceived as a single subgroup. Classic examples of organic semiconductors are P3HT (poly-3-hexylthiophene, polymer), pentacene (small molecule) or PCBM ( phenyl-C61-butyric acid methyl ester , fullerene derivative). Organic semiconductors are used in light-emitting diodes (OLEDs), solar cells (OPVs) and field effect transistors.
Several semiconducting molecules or atoms combine to form a crystal or create a disordered (amorphous) solid. Most of the inorganic semiconductors can roughly be classified as crystalline, and most organic semiconductors as amorphous. However, whether a crystal or an amorphous solid is actually formed depends largely on the manufacturing process. For example, silicon can be crystalline (c-Si) or amorphous (a-Si), or it can also form a polycrystalline mixed form (poly-Si). There are also single crystals made of organic molecules.
| Element semiconductors | Compound semiconductor (without org. HL) | Organic semiconductors |
|---|---|---|
|
Ge , Si , α- Sn , C ( fullerenes ), B , Se , Te |
III-V : GaP , GaAs , InP , InSb , InAs , GaSb , GaN , AlN , InN , Al x Ga 1-x As , In x Ga 1-x N |
Tetracene , pentacene , phthalocyanine , polythiophene phene , PTCDA , MePTCDI , quinacridone , acridine don , indanthrone , flavanthrone , perinone , Alq3 |
| Under pressure: Bi , Ca , Sr , Ba , Yb , P , S , I |
II-VI : ZnO , ZnS , ZnSe , ZnTe , CdS , CdSe , CdTe , Hg (1-x) Cd (x) Te , BeSe , BeTe , HgS |
Mixed systems: polyvinyl carbazole , TCNQ complexes |
| III-VI: GaS , GaSe , GaTe , InS , InSe , InTe ... | ||
| I-III-VI: CuInSe 2 , CuInGaSe 2 , CuInS 2 , CuInGaS 2 … | ||
| IV-IV: SiC , SiGe | ||
| IV-VI: SnTe |
Crystalline semiconductors
Physical basics
The semiconductor properties of substances are based on their chemical bonds and thus their atomic structure. Semiconductors can crystallize in different structures , so silicon and germanium crystallize in the diamond structure (purely covalent bond ), III-V and II-VI compound semiconductors, on the other hand, usually in the zinc blende structure (mixed covalent-ionic bond).

The basic properties of crystalline semiconductors can be explained using the ribbon model : The electrons in solids interact with one another over a large number of atomic distances. In fact, this leads to an expansion of the possible energy values (which are still available in the individual atom as discrete levels) to form extended energy ranges, the so-called energy bands . Since the energy bands lie differently from one another depending on the expansion and atomic type, bands can overlap or be separated by energy ranges in which, according to quantum mechanics, no permitted states exist (energy or band gap ).
In semiconductors, the highest occupied energy band ( valence band ) and the next higher band ( conduction band ) are separated by a band gap. The Fermi level is exactly in the band gap. At a temperature close to absolute zero , the valence band is fully occupied and the conduction band is completely free of charge carriers. Since unoccupied bands do not conduct electrical current due to a lack of movable charge carriers and charge carriers in fully occupied bands cannot absorb energy due to a lack of free states that can be reached, semiconductors do not conduct electrical current at a temperature close to absolute zero.
Partially occupied strips are necessary for the conduction process, which can be found in metals by overlapping the outer strips at any temperature. As mentioned above, this is not the case with semiconductors and insulators. The band gap (called “forbidden band” or “forbidden zone”) in semiconductors is, in contrast to insulators (typically E G > 4 eV), relatively small ( InAs : ≈ 0.4 eV , Ge : ≈ 0.7 eV, Si : ≈ 1.1 eV, GaAs : ≈ 1.4 eV, SiC : ≈ 2.39 ... 3.33 eV, diamond : ≈ 5.45 eV), so that, for example, through the energy of the heat oscillations at room temperature or through the absorption of Light, many electrons can be excited from the fully occupied valence band into the conduction band. Semiconductors therefore have an intrinsic electrical conductivity that increases with temperature. That is why semiconductors are also counted among the thermistors . The transition from semiconductors to insulators is fluid. For example, gallium nitride (GaN; used in blue LEDs ) with a band gap energy of ≈ 3.2 eV is also counted among the semiconductors, but diamond with a band gap of ≈ 5.5 eV is no longer. Semiconductor with a bandgap significantly larger than 1 eV also be used as semiconductor with a large band gap ( English wide-bandgap semiconductor ), respectively.
If, as described above, an electron in a semiconductor is excited from the valence band into the conduction band, it leaves a defect electron , called a “hole”, in its original location . Bound valence electrons in the vicinity of such holes can “jump” into a hole by changing their place, and the hole moves. It can therefore be viewed as a mobile positive charge. Both the excited electrons and the defect electrons thus contribute to electrical conduction.
Electrons from the conduction band can recombine with the defect electrons (electron-hole recombination). This transition between the levels involved can take place with the emission of electromagnetic recombination radiation ( photon ) and / or with the emission of a pulse to the crystal lattice ( phonon ).
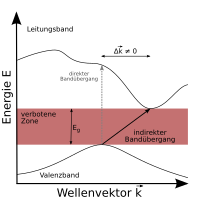
Direct and indirect semiconductors
Semiconductors are divided into two groups, direct and indirect semiconductors. Their different properties can only be understood by looking at the band structure in the so-called momentum space : The charge carriers in the semiconductor can be understood as matter waves with a quasi - momentum . Within a band, the energy depends on the quasi-momentum (often given as a wave vector).
The extreme values of the energy within the bands, i.e. the band edges, are at different wave vectors - where exactly depends on the material and the structure. When an electron is excited from the valence band into the conduction band, it is energetically most favorable (and therefore most likely) when it is excited from the maximum of the valence band to the minimum of the conduction band.
If these extremes are almost at the same quasi-impulse, excitation, for example by a photon, is easily possible, since the electron only has to change its energy, not its momentum. One speaks of a direct semiconductor . However, if the extremes are at different quasi-impulses, the electron must change its momentum in addition to its energy in order to be excited into the conduction band. This impulse cannot come from a photon (which has a very small impulse), but must be contributed by a lattice oscillation (also called a phonon ).
In principle, the same applies to the recombination of electron-hole pairs. In a direct semiconductor, a light quantum can be emitted during recombination. In the case of an indirect semiconductor, on the other hand, a phonon for the pulse would have to be generated (or absorbed) in addition to the photon for the energy, and the radiative recombination becomes less likely. Other, non-radiative recombination mechanisms then often dominate, e.g. B. About impurities. It follows from this that only direct semiconductors can be used for effective radiation generation. Direct and indirect semiconductors are distinguished from one another by means of an absorption test. As a rule, element semiconductors ( silicon , germanium ) and compound semiconductors from main group IV are indirect and compound semiconductors from various main groups (III / V: GaAs , InP, GaN) are direct.
In the case of a band structure in which different points in the momentum space are possible near the line or valence band edge, the so-called Gunn effect can occur.
Intrinsic semiconductors and impurity semiconductors
The density of free electrons and holes in pure, i.e. undoped, semiconductors is called intrinsic charge carrier density or intrinsic conduction density - an intrinsic semiconductor is therefore also called an intrinsic semiconductor, the dominant conduction mechanism is intrinsic conduction. The charge carrier density in the undoped semiconductor is strongly dependent on the temperature and increases with it. If, on the other hand, the concentration of charge carriers in the conduction band (electrons) or in the valence band (holes) is determined by the dopant, one speaks of an impurity semiconductor or extrinsic semiconductor - here the dominant conduction mechanism is the impurity conduction .
Doping and impurity conduction
Donors and Acceptors
| Doping strength | n-conductive | p-conducting |
|---|---|---|
| normal doping | one donor on 10 7 | one acceptor on 10 6 |
| heavy doping | one donor on 10 4 | one acceptor on 10 4 |
By introducing impurities into a semiconductor crystal, the electrical properties of the (pure) semiconductor can be influenced. Impurities are foreign atoms which, for example, differ in their valency from the atoms of the host material, examples are boron or phosphorus in a silicon crystal. The process is generally referred to as doping or “doping”. In addition, by combining differently doped areas, different components such. B. a bipolar transistor can be produced. In some semiconductors, even the smallest amounts of foreign atoms (e.g. one foreign atom for every 10 million semiconductor atoms) can lead to extreme changes in the electrical properties that far exceed the intrinsic semiconductor.
The introduction of impurities creates additional, locally bound energy levels in the band diagram of the crystal. The levels are generally in the energy gap ( band gap ) between the valence and conduction bands that would otherwise exist for the host material . Due to the lower energy differences between the “intermediate levels” and the valence or conduction band compared to undoped semiconductors, these levels can be more easily excited and thus make mobile charge carriers available. The chemical potential shifts from the middle of the band gap to the vicinity of the additional levels. There are therefore more charge carriers available for conducting the electrical current, which manifests itself in an increased conductivity compared to the pure semiconductor. This conduction mechanism is therefore also called impurity conduction . A distinction is made between two types of impurities: donors and acceptors.
Foreign atoms, which provide additional electrons in the conduction band, are called (electron) donors (Latin donare = to give); such areas are also called n-doped semiconductors. If such foreign atoms are introduced (substituted) into the semiconductor, each of these foreign atoms (in the case of silicon doped with phosphorus) brings with it an electron that is not required for the bond and can easily be detached. An impurity level forms near the lower energy of the conduction band.
Similarly, (electron) acceptors (Latin: accipere = to accept) are foreign atoms that have one electron less in the valence band. This electron is missing for the bond to the neighboring atom. They act as an additional defect electron (hole) with (p-doping), which can easily be occupied by valence band electrons - therefore the term hole donors is also used in some considerations. In the band scheme, such an impurity level is close to the valence band edge.
In an intrinsic semiconductor, the charge carrier concentrations of electrons and holes are the same (electron-hole pairs). Therefore, both types of charge carriers are approximately equally involved in charge transport. This equilibrium can be influenced in a targeted manner by introducing donors and acceptors.
When doping with donors, it is mainly the electrons in the conduction band that ensure electrical conductivity, when doping with acceptors the imaginary, positively charged holes in the valence band. In the first case one speaks of electron conduction or n-conduction (n → negative), in the other case of hole conduction or p-conduction (p → positive). Semiconductor areas with an excess of electrons are designated (as mentioned above) as n-doped , those with deficiencies, i.e. with “excess holes”, as p-doped . In the n-conductor, the electrons are called majority charge carriers (charge carriers present in the majority), the holes are called minority-charge carriers, in the p-conductor the corresponding reversal applies. By cleverly combining n- and p-doped areas (see pn junction ), it is possible to build individual, so-called discrete semiconductor components such as diodes and transistors and complex integrated circuits made up of many components in a single crystal . The intrinsic semiconductor in these electronic components is often even a disruptive factor (see e.g. leakage current ), so that they sometimes have to be explicitly cooled.
Conduction mechanisms in doped semiconductors
At absolute zero ( T = 0 K), doped and undoped semiconductors do not differ in terms of charge carrier density - there is not enough energy available to excite electrons into the conduction band or to the level of impurities. If the temperature is increased (this increases the available energy due to thermal excitation), the conditions change. Since the energetic distances between the impurities and the valence or conduction band are much smaller than the band gap, electrons can be excited from the donor level into the conduction band or holes from the acceptor level into the valence band. Depending on the temperature, free charge carriers are available and the conductivity of doped semiconductors increases. Since not all impurity levels are ionized or occupied, this area is called the impurity reserve . If the temperature is increased further until all impurity levels are ionized or occupied, one speaks of impurity depletion . The charge carrier density and thus the conductivity essentially only depends on the doping concentration in this area. Because of the decreasing mobility with increasing temperature, one has in this temperature range similar to metals i. A. a conductivity that decreases slightly with temperature. If the temperature is raised even further, enough energy is available to lift electrons directly from the valence band to the conduction band. Since typical doping concentrations are significantly lower than the number of semiconductor atoms (at least six orders of magnitude), the generation of charge carriers from electron-hole pairs predominates; this area is referred to as intrinsic or self-conduction of the semiconductor.
Interfaces
The combination of a p-doped and an n-doped semiconductor creates a pn junction at the interface . The combination of a doped semiconductor with a metal (e.g. Schottky diode ) or a dielectric is also of interest, and when two semiconductors, e.g. gallium arsenide and aluminum gallium arsenide , lie on top of one another, a heterojunction results . Not only pn-junctions are important, but also pp-junctions and nn-junctions, the so-called isotypic heterojunctions , which are used in a quantum well , for example.
Efforts have recently been made to combine semiconductors, superconductors, and silicon and III-V semiconductors on one chip. Since the crystal structures are not compatible, breaks and lattice defects occur in the interface if it is not possible to find suitable materials for an intermediate layer that is a few atomic layers thick and in which the lattice spacing can be adjusted.
Semimagnetic semiconductors
Semimagnetic semiconductor belong to the compound semiconductor ( English compound semiconductors ). These are compounds such as indium antimonide (InSb), which are doped with a few percent manganese (Mn) and which show semi-magnetic properties even at room temperature . Also, indium arsenide (InAs) and gallium arsenide (GaAs) show, at a high doping with manganese and then as InMnAs or GaMnAs designated semi magnetic properties. The Curie temperature for InMnAs is 50–100 K and for GaMnAs 100–200 K and thus well below room temperature. A characteristic property of these semi-magnetic semiconductors is the large Zeeman effect . In English, semimagnetic semiconductors are called diluted magnetic semiconductors because they are magnetically diluted.
Amorphous semiconductors
Amorphous semiconductors do not have a crystal structure. An example of its technical application is amorphous silicon in photovoltaics . Because of their high density of impurities, they have to be processed differently than crystalline semiconductors, e.g. B. to enable doping in the first place.
Organic semiconductors
In general, organic materials are electrically insulating. If molecules or polymers have a conjugated bond system consisting of double bonds, triple bonds and aromatic rings, these too can become electrically conductive and can be used as organic semiconductors. This was first observed in 1976 with polyacetylene . Polyacetylene is an unbranched polymer with an alternating double bond and a single bond (–C═C─C═C–). If this plastic is still an acceptor such. B. chlorine, bromine or iodine added (oxidative doping) , there are additional holes. By adding a donor such as B. sodium (reductive doping) the plastic receives additional electrons. This chemical change breaks the double bonds, and a continuous conduction band is created: the originally non-conductive polymer becomes electrically conductive. If molecules or polymers have semiconducting properties even in the undoped state, one speaks of intrinsic conductivity (intrinsic conductivity), as in the case of inorganic semiconductors, e.g. B. pentacene or poly (3-hexylthiophene) . If the plastic is produced in the form of a thin layer 5 to 1000 nm thick, it is ordered enough to form an electrically continuous layer.
Areas of application
Semiconductors are used in various forms in electronics. The associated sub-area is called semiconductor electronics . These include, above all, semiconductor-based integrated circuits (ICs, such as microprocessors , microcontrollers , etc.) and various components of power electronics (e.g. IGBTs ). Companies in this economic sector are also known as semiconductor manufacturers . Further areas of application with increasing importance are photovoltaics ( solar cells ) as well as detectors and radiation sources in optics and optoelectronics ( e.g. photodetectors and light emitting diodes ). In order to cover the wide spectral range of light-emitting diodes from infrared to ultraviolet, various wide-bandgap semiconductors are used, which are increasingly playing a role in high-frequency and power electronics.
The subject area that deals with the manufacture of semiconductor-based microelectronic components and assemblies is known as semiconductor technology . The prerequisite is knowledge of how the semiconductor must be processed in order to achieve the desired electrical behavior. This includes doping the semiconductor and designing the interface between the semiconductor and another material.
economy
The market for polysilicon is currently (2010) in transition. After polysilicon was in high demand in 2008/2009 due to the high demand from the solar market, the price rose sharply. This has led a number of companies to start setting up new production facilities. The established manufacturers also expanded their capacities. In addition, new providers - especially from Asia - are entering the market. It is uncertain which of these manufacturers will be able to commission their systems as announced and still operate profitably when prices have fallen sharply.
The world's largest manufacturer of wafers , including compound semiconductors, is the Japanese company Shin-Etsu Handotai (SEH) with wafer sales of 4 billion dollars in 2007. The world's second largest manufacturer, Sumitomo Mitsubishi Silicon Corp. , is also Japanese . (Sumco) had sales of $ 2.7 billion that same year. This is followed by the German Siltronic AG ( Wacker ) with 1.8 billion dollars and the American company MEMC Electronic Materials with 1.2 billion dollars. These four companies share about 79% of the total Si wafer market of $ 12.5 billion.
During the global financial crisis, sales almost halved, in 2009 only silicon for 6.7 billion dollars was sold. In 2010 sales had recovered to $ 9.7 billion.
See also
literature
- Peter Y. Yu, Manuel Cardona: Fundamentals of Semiconductors: Physics and Materials Properties. 3. Edition. Springer 2004, ISBN 3-540-41323-5 .
- Marius Grundmann: The Physics of Semiconductors. An Introduction Including Device and Nanophysics. Springer 2006, ISBN 3-540-25370-X .
- Simon M. Sze , Kwok K. Ng: Physics of Semiconductor Devices. 3. Edition. John Wiley & Sons 2006, ISBN 0-471-14323-5 .
- Michael Reisch: Semiconductor components . Springer 2004, ISBN 3-540-21384-8 .
- Ulrich Hilleringmann: Silicon semiconductor technology . Teubner 2004, ISBN 3-519-30149-0 .
- Bernhard Hoppe: Microelectronics 1st Vogel book Kamprath series, 1997, ISBN 3-8023-1518-9 .
- Werner Gans: The art of electrifying plastics. Nobel Prize in Chemistry 2000. In: Spectrum of Science. No. 12, 2000, pp. 16-19.
- Kai Handel: Beginnings of semiconductor research and development. Shown in the biographies of four German semiconductor pioneers . Aachen 1999 ( PDF - doctoral thesis).
Web links
- Ioffe Institute St.Petersburg - Physical data on semiconductor materials
- Flash animation for power conduction in semiconductors (dwu teaching materials)
- 3D animations on the topic ( Memento from May 2, 2008 in the Internet Archive )
Individual evidence
- ↑ Leonhard Stiny: Active electronic components: design, structure, mode of operation, properties and practical use of discrete and integrated semiconductor components . Springer-Verlag, 2016, ISBN 978-3-658-14387-9 , pp. 7 ( limited preview in Google Book Search [accessed December 23, 2016]).
- ↑ Ferdinand Braun: About the current line through sulfur metals . In: Annals of Physics and Chemistry . tape 153 , no. 4 , 1874, p. 556-563 ( digitized version ).
- ↑ Patent US836531 : Means For Receiving Intelligence Communicated By Electric Waves. Published November 20, 1905 , inventor: Greenleaf Whittier Pickard .
- ↑ Jed Margolin: The Road to the Transistor . 2004.
- ↑ Patent US1745175 : Method and Apparatus For Controlling Electric Currents. Inventor: Julius Edgar Lilienfeld (first registered on October 22, 1925 in Canada).
- ↑ Reinhold Paul: field effect transistors - physical principles and properties. Verlag Berliner Union et al., Stuttgart 1972, ISBN 3-408-53050-5 .
- ↑ Hideki Shirakawa, Edwin J. Louis, Alan G. MacDiarmid, Chwan K. Chiang, Alan J. Heeger: Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH) x . In: J. Chem. Soc., Chem. Commun. No. 16 , 1977, pp. 578-580 , doi : 10.1039 / C39770000578 .
- ^ CK Chiang, CR Fincher, YW Park, AJ Heeger, H. Shirakawa, EJ Louis, SC Gau, AG MacDiarmid: Electrical Conductivity in Doped Polyacetylene . In: Physical Review Letters . tape 39 , no. 17 , 1977, pp. 1098-1101 , doi : 10.1103 / PhysRevLett.39.1098 .
- ↑ Stefan Goßner: Basics of electronics. 9th edition. Shaker 2016, ISBN 978-3-8265-8825-9 , Chapter 1: "Semiconductors"
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 1312.
- ↑ World of technology: superconducting chips - a dream of the future?
- ^ Muons in Magnetic Semiconductors. Triumf.info, accessed September 19, 2010 .
- ↑ H. Ohno, A. Shen, F. Matsukura, A. Oiwa, A. Endo, S. Katsumoto, Y. Iye: (Ga, Mn) As: A new diluted magnetic semiconductor based on GaAs . In: Applied Physics Letters . tape 69 , no. 3 , July 15, 1996, p. 363–365 , doi : 10.1063 / 1.118061 , bibcode : 1996ApPhL..69..363O .
- ↑ CK Chiang et al.: Electrical Conductivity in Doped Polyacetylene. In: Physical Review Letters 39, 1977, pp. 1098-1101.
- ↑ Robert Schramm, Lauren Licuanan: Feedback form Solar Silicon Conference . April 28, 2010.
- ↑ Timothy Lam: Asia Solar View - May 2010 , May 3, 2010.
- ↑ Gartner Says Worldwide Silicon Wafer Revenue Reached $ 12.5 Trillion in 2007 . Gartner, Inc., June 11, 2008, accessed May 5, 2010 (press release).
- ↑ Silicon Wafer Shipments Reach Record Levels in 2010. In: Semi.org. February 8, 2011, archived from the original on May 9, 2018 ; accessed in 2018 .