Tyrosine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
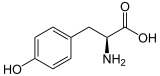
| Structure of L- tyrosine, the naturally occurring isomer | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Tyrosine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 9 H 11 NO 3 | |||||||||||||||||||||
| Brief description |
colorless, silky, shiny needles |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 181.19 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.46 g cm −3 (25 ° C) |
|||||||||||||||||||||
| Melting point |
342–344 ° C (decomposition) |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Tyrosine (abbreviated Tyr or Y ) is in its natural L form a non- essential proteinogenic α - amino acid that is found in most proteins . Tyrosine is the starting substance for the biosynthesis of DOPA , dopamine , catecholamines , melanin , thyroxine and tyramine . In many animals, biosynthesis takes place from the essential amino acid phenylalanine , and impairment of this pathway can trigger a variety of defects.
Isomers
Tyrosine has a stereocenter, so there are two enantiomers . Whenever "tyrosine" is mentioned in this text or in the scientific literature without any additional name ( prefix ), the naturally occurring L- tyrosine is meant.
| Enantiomers of Tyrosine | ||
| Surname | L -yrosine | D- tyrosine |
| other names | ( S ) - (-) - tyrosine | ( R ) - (+) - tyrosine |
| Structural formula |  |

|
| CAS number | 60-18-4 | 556-02-5 |
| 556-03-6 (unspec.) | ||
| EC number | 200-460-4 | 209-112-6 |
| 209-113-1 (unspec.) | ||
| ECHA info card | 100,000,419 | 100.008.285 |
| 100.008.286 (unspec.) | ||
| PubChem | 6057 | 71098 |
| 1153 (unspec.) | ||
| DrugBank | DB00135 | - |
| - (unspec.) | ||
| Wikidata | Q188017 | Q16082044 |
| Q27102882 (unspec.) | ||
Occurrence
L -Tyrosine was first characterized by Justus von Liebig in 1846 as a protein component of cheese ( ancient Greek τύρος týros , cheese '), hence the name. It is found in large quantities in casein .
The following examples give an overview of the tyrosine content and each relate to 100 g of the food; the percentage of tyrosine in the total protein is also given:
| Food per 100 g | protein | Tyrosine | proportion of |
|---|---|---|---|
| Pork, raw | 20.95 g | 797 mg | 3.8% |
| Chicken breast fillet, raw | 21.23 g | 765 mg | 3.6% |
| Salmon, raw | 20.42 g | 759 mg | 3.7% |
| Chicken egg | 12.56 g | 499 mg | 4.0% |
| Cow's milk, 3.7% fat | 3.28 g | 158 mg | 4.8% |
| Pumpkin seeds | 30.23 g | 1093 mg | 3.6% |
| Walnuts | 15.23 g | 406 mg | 2.7% |
| wheat flour | 10.33 g | 312 mg | 3.0% |
| Wholemeal corn flour | 6.93 g | 282 mg | 4.1% |
| Rice, unpeeled | 7.94 g | 298 mg | 3.8% |
| Soybeans, dried | 36.49 g | 1539 mg | 4.2% |
| Peas, dried | 24.55 g | 711 mg | 2.9% |
All of these foods contain almost exclusively chemically bound L- tyrosine as a protein component, but no free L- tyrosine.
properties
As a monomer
Depending on the pH value , tyrosine can be present as an “inner salt” or zwitterion . The proton of the carboxy group attaches itself to the free electron pair of the nitrogen atom of the amino group :
The zwitterions do not migrate in the electric field because they are uncharged to the outside. The isoelectric point is pH = 5.66 for tyrosine; at this pH value it has its lowest solubility in water.
Isolated L- tyrosine fluoresces - like many other aromatic compounds - when excited with UV light .
- Van der Waals volume : 141
- Degree of hydrophobicity : −1.3
With a suitable diazo component, tyrosine forms a red azo dye and can thus be qualitatively detected using the Pauly reaction .
In proteins
The L -tyrosine is a proteinogenic amino acid. It is required as a building block for the construction of numerous proteins during translation .
L- tyrosine is of particular importance in proteins that are involved in signal transduction processes. It functions here as a recipient of phosphate groups, which are transferred by protein kinases and change the activity of the target protein, a receptor (see receptor tyrosine kinases ).
L -yrosine also plays an important role in photosynthesis by reducing the oxidized chlorophyll as an electron donor in photosystem II . It initially loses the proton of its phenolic OH group, becomes a neutral radical , and is then reduced again by the quadricuclear manganese cluster in photosystem II .
metabolism
biogenesis

Plants and most microorganisms synthesize tyrosine in the shikimic acid pathway via chorismic acid . After the rearrangement of chorismate into prephenate, 4-hydroxyphenylpyruvate is produced by means of a prephenate dehydrogenase, from which tyrosine is then formed by transamination under the action of a transaminase .
In the animal organism, tyrosine is produced by biopterin- dependent 4- hydroxylation on the phenyl ring of L - phenylalanine . The enzyme that catalyzes this reaction is phenylalanine hydroxylase , a monooxygenase . An oxygen molecule (O 2 ) is required and this reaction creates a water molecule (H 2 O). The precursor, the essential amino acid L- phenylalanine, is taken in with food.
As a result of phenylketonuria (PKU) there may be a lack of L come tyrosine. L - phenylalanine ingested through food cannot be correctly hydroxylated in the para position, so that no L- tyrosine can be formed from phenylalanine. In this case must L -tyrosine body fed to be.

Precursor
Tyrosine serves as a starting material ( precursor ) for the biosynthesis of various other substances.
- The formation of the thyroid hormones L - triiodothyronine (T 3 ) and L - thyroxine (T 4 ) in the colloid of the thyroid is based on tyrosine subunits.
- The decarboxylation by the enzyme aromatic L-amino acid decarboxylase (AADC) gives the biogenic amine tyramine .
- A hydroxylation by the enzyme tyrosine hydroxylase - in melanocytes often the enzyme tyrosinase - leads to DOPA .
DOPA, in turn, is a precursor for various neurotransmitters as well as for melanin . In the adrenal medulla , decarboxylase enables the production of the catecholamines adrenaline and noradrenaline , which are released into the blood as messenger substances. The production of dopamine from DOPA takes place within the membrane in nerve cells. Melanin from DOPA is produced and released in particular by melanocytes in the skin and in the pigment cells of the eyes , where it is stored.
Pathophysiology
In the case of nitrosative stress , peroxynitrite and tyrosine are converted into nitrotyrosine by means of nucleophilic aromatic substitution . Nitrotyrosine is used in laboratory diagnostics as a biomarker for nitrosative stress or apoptosis (programmed cell death).
Dismantling
The degradation of L -yrosine ( para -hydroxyphenylalanine) begins with an α-ketoglutarate -dependent transamination by the L- tyrosine transaminase ( EC 2.6.1.5 ) to p -hydroxyphenyl pyruvate .

The next step is catalyzed by 4-hydroxyphenylpyruvate dioxygenase ( EC 1.13.11.27 ) with the incorporation of oxygen and the splitting off of CO 2 to form the homogenate (2,5-dihydroxyphenyl-1-acetate). To split the aromatic ring of the homogenate, another dioxygenase, the homogenate oxygenase ( EC 1.13.11.5 ), is required. The maleyl acetoacetate is formed by the incorporation of another O 2 molecule .
With the Maleylacetacetat- cis - trans - isomerase ( EC 5.2.1.2 ) is formed in this case Fumarylacetat by rotation of the resulting oxidation (from the hydroxyl group) carboxy group . This cis-trans isomerase contains glutathione as a coenzyme . Fumarylacetoacetate can finally be cleaved by the fumarylacetoacetate hydrolase through water retention.
Here be fumarate (also a metabolite of the Citric Acid Cycle ) and acetoacetate (butane (3) -one acid) free. Acetoacetate is a ketone body which is activated with succinyl-CoA and can then be converted into two molecules of acetyl-CoA (for the citric acid cycle and fatty acid synthesis ).
Applications
Tyrosine is a precursor to neurotransmitters , especially dopamine and norepinephrine . By increasing the intake of tyrosine, its synthesis can be temporarily increased significantly, for about half an hour. However, this has only a minor influence on the mood. The step that determines the rate of the conversion in the metabolism is catalyzed and limited by the tyrosine hydroxylase , which is why the effects are less than when adding L-DOPA . It is known from animal experiments that their enzyme activity decreases sharply at high doses of tyrosine due to substrate excess inhibition, so that the dopamine level drops.
Some studies found a benefit under stress , cold or fatigue. An increase in performance in endurance sports (one and a half hour cycling) through tyrosine intake could not be determined, but through carbohydrate intake.
The dietary intake of L- tyrosine serves as a substitution therapy or supplementation in the event of deficiency, e.g. B. in phenylketonuria , otherwise an underproduction of melanin ( albinism ) and L - thyroxine ( cretinism ) results. In addition, there can be problems in the production of catecholamines .
In addition, L- tyrosine has been used for years as an adjuvant depot carrier in specific subcutaneous immunotherapy (SCIT) due to its protein-adsorbing properties . Compared to other depot carriers such as aluminum hydroxide or calcium phosphate , L- tyrosine is characterized, among other things, by the advantage of being completely metabolizable and a shorter half-life of 48 hours at the injection site.
Manufacturing
The acid hydrolysis of keratin-containing proteins results in a protein hydrolyzate after neutralization, which consists of about 20 proteinogenic α-amino acids. A fraction rich in L - cystine and L- tyrosine can be obtained from this simply by separating the readily water-soluble amino acids, since L- cystine and L- tyrosine are only slightly soluble in water. L- tyrosine is obtained commercially using this simple separation method.
literature
- Jeremy M.Berg, John L.Tymoczko, Lubert Stryer: Biochemie , 5th edition, Spektrum Akademischer Verlag, Heidelberg 2003, ISBN 3-8274-1303-6 .
Web links
Individual evidence
- ↑ a b c d Entry on l-tyrosine. In: Römpp Online . Georg Thieme Verlag, accessed on June 21, 2014.
- ↑ a b Data sheet (S) - (-) - Tyrosine (PDF) from Merck , accessed on March 13, 2010.
- ^ A b F. A. Carey: Organic Chemistry , 5th edition, The McGraw Companies 2001, p. 1059, Link
- ^ A b Hans-Dieter Jakubke and Hans Jeschkeit: Amino acids, peptides, proteins , Verlag Chemie, Weinheim 1982, ISBN 3-527-25892-2 , p. 40.
- ↑ a b Entry on tyrosine in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ^ Entry on tyrosine in the DrugBank of the University of Alberta .
- ^ A b Hans-Dieter Jakubke, Hans Jeschkeit: amino acids, peptides, proteins , Verlag Chemie, Weinheim, p. 19, 1982, ISBN 3-527-25892-2 .
- ↑ nutrient database of the US Department of Agriculture , 23rd edition.
- ↑ Student assignment: CHEMKON 3/2018 . In: CHEMKON . tape 25 , no. 3 , June 2018, p. 121–122 , doi : 10.1002 / ckon.201880371 .
- ↑ Entry in the ExPASy Proteomics Server: EC 1.14.16.1 .
- ↑ JM Berg, JL Tymoczko, L. Stryer: Biochemistry. 6th edition. Spectrum Academic Publishing House, Elsevier GmbH, Munich 2007; Pp. 747f, 773ff; ISBN 978-3-8274-1800-5 .
- ↑ AW Abu-Qare and MB Abou-Donia: Biomarkers of apoptosis: release of cytochrome c, activation of caspase-3, induction of 8-hydroxy-2'-deoxyguanosine, increased 3-nitrotyrosine, and alteration of p53 gene , in: J Toxicol Env Health Pt B-Crit Rev , 2001 , 4 , pp. 313-332; PMID 11503418 .
- ↑ Rasmussen DD, Ishizuka B, Quigley ME, Yen SS: Effects of tyrosine and tryptophan ingestion on plasma catecholamine and 3,4-dihydroxyphenylacetic acid concentrations . In: J. Clin. Endocrinol. Metab. . 57, No. 4, 1983, pp. 760-763. doi : 10.1210 / jcem-57-4-760 . PMID 6885965 .
- ↑ Leathwood PD, Pollet P: Diet-induced mood changes in normal populations . In: Journal of Psychiatric Research . 17, No. 2, 1982, pp. 147-54. doi : 10.1016 / 0022-3956 (82) 90016-4 . PMID 6764931 .
- ↑ a b Deijen JB, Orlebeke JF: Effect of tyrosine on cognitive function and blood pressure under stress . In: Brain Res Bull.. . 33, No. 3, 1994, pp. 319-323. doi : 10.1016 / 0361-9230 (94) 90200-3 . PMID 8293316 .
- ↑ Lieberman HR, Corkin S, Spring BJ, Wurtman RJ, Growdon JH: The effects of dietary neurotransmitter precursors on human behavior . In: Am J Clin Nutr. . 42, No. 2, 1985, pp. 366-370. PMID 4025206 .
- ↑ a b T. D. Chinevere, RD Sawyer, AR Creer, RK Conlee, AC Parcell: Effects of L-tyrosine and carbohydrate ingestion on endurance exercise performance. In: Journal of applied physiology. Volume 93, Number 5, November 2002, pp. 1590-1597, doi : 10.1152 / japplphysiol.00625.2001 , PMID 12381742 .
- ↑ Strüder HK, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K: Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans . In: Horm. Metab. Res. . 30, No. 4, 1998, pp. 188-194. doi : 10.1055 / s-2007-978864 . PMID 9623632 .
- ↑ Thomas JR, Lockwood PA, Singh A, Deuster PA: Tyrosine improves working memory in a multitasking environment . In: Pharmacol. Biochem. Behav. . 64, No. 3, 1999, pp. 495-500. doi : 10.1016 / S0091-3057 (99) 00094-5 . PMID 10548261 .
- ↑ Abdulla A.-B. Badawy, David L. Williams: Enhancement of rat brain catecholamine synthesis by administration of small doses of tyrosine and evidence for substrate inhibition of tyrosine hydroxylase activity by large doses of the amino acid. In: Biochemical Journal. Volume 206, No. 1, July 1982, pp. 165-168; doi: 10.1042 / bj2060165 .
- ↑ Noelene S. Quinsey, Anh Q. Luong, Phillip W. Dickson: Mutational Analysis of substrates inhibition in tyrosine hydroxylase. In: Journal of Neurochemistry. Volume 70, No. 5, November 1998, pp. 2132-2138; doi: 10.1046 / j.1471-4159.1998.71052132.x .
- ^ A b Hao S, Avraham Y, Bonne O, Berry EM: Separation-induced body weight loss, impairment in alternation behavior, and autonomic tone: effects of tyrosine . In: Pharmacol. Biochem. Behav. . 68, No. 2, 2001, pp. 273-281. doi : 10.1016 / S0091-3057 (00) 00448-2 . PMID 11267632 .
- ↑ Magill RA, Waters WF, Bray GA, Volaufova J, Smith SR, Lieberman HR, McNevin N, Ryan DH: Effects of tyrosine, phentermine, caffeine D-amphetamine, and placebo on cognitive and motor performance deficits during sleep deprivation . In: Nutritional Neuroscience . 6, No. 4, 2003, pp. 237-246. doi : 10.1080 / 1028415031000120552 . PMID 12887140 .
- ^ Neri DF, Wiegmann D, Stanny RR, Shappell SA, McCardie A, McKay DL: The effects of tyrosine on cognitive performance during extended wakefulness . In: Aviation, space, and environmental medicine . 66, No. 4, 1995, pp. 313-319. PMID 7794222 .
- ↑ Reinstein DK, Lehnert H, Wurtman RJ: Dietary tyrosine suppresses the rise in plasma corticosterone following acute stress in rats . In: Life Sci. . 37, No. 23, 1985, pp. 2157-2163. doi : 10.1016 / 0024-3205 (85) 90566-1 . PMID 4068899 .
- ↑ Deijen JB, Wientjes CJ, Vullinghs HF, Cloin PA, Langefeld JJ: Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course . In: Brain Res Bull.. . 48, No. 2, 1999, pp. 203-209. doi : 10.1016 / S0361-9230 (98) 00163-4 . PMID 10230711 .
- ↑ Mahoney CR, Castellani J, Kramer FM, Young A, Lieberman HR: Tyrosine supplementation mitigates working memory decrements during cold exposure . In: Physiology and Behavior . IN PRESS, No. 4, 2007, pp. 575-582. doi : 10.1016 / j.physbeh.2007.05.003 . PMID 17585971 .
- ↑ P. Baldrick, D. Richardson, AW Wheeler: Review of L-tyrosine confirming its safe human use as an adjuvant. in J. Appl. Toxicol. 22 (2002) 333-344, doi: 10.1002 / jat.869 .
- ↑ Yoshiharu Izumi, Ichiro Chibata and Tamio Itoh: Production and Use of Amino Acids , in: Angewandte Chemie , 1978 , 90 , pp. 187-194, doi: 10.1002 / anie.19780900307 .


