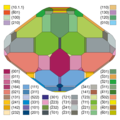
Colemanite
| Colemanite | |
|---|---|
| Colorless to white Colemanite crystals from the Baker Mine near Boron, Kramer District, Kern County, California, USA. Size: 19.9 × 17.9 × 5.6 cm. | |
| General and classification | |
| other names |
|
| chemical formula |
|
|
Mineral class (and possibly department) |
Borates (inoborate) (formerly carbonates, nitrates and borates) |
|
System no. to Strunz and to Dana |
6.CB.10 ( 8th edition : V / J.03) 03/26/05/01 |
| Similar minerals | Datolith |
| Crystallographic Data | |
| Crystal system | monoclinic |
| Crystal class ; symbol | monoclinic prismatic; 2 / m |
| Space group | P 2 1 / a (No. 14, position 3) |
| Lattice parameters |
a = 8.71 Å ; b = 11.25 Å; c = 6.09 Å β = 110.1 ° |
| Formula units | Z = 4 |
| Frequent crystal faces | {110}, { 3 01}, { 2 21}, {011} |
| Physical Properties | |
| Mohs hardness | 4.5 |
| Density (g / cm 3 ) | measured: 2.423 (5); calculated: 2.42 |
| Cleavage | very perfect after {010}, clearly after {001} |
| Break ; Tenacity | uneven to half-mussel; brittle |
| colour | colorless, milk white, pale yellow, yellow, gray, cinnamon brown, brown, black; Colorless in transmitted light |
| Line color | White |
| transparency | translucent to translucent |
| shine | Glass to diamond gloss |
| Crystal optics | |
| Refractive indices |
n α = 1.586 n β = 1.592 n γ = 1.614 |
| Birefringence | δ = 0.028 |
| Optical character | biaxial positive |
| Axis angle | 2V = 55 to 56 ° (measured), 56 ° (calculated) |
| Other properties | |
| Chemical behavior | soluble in hot HCl, boric acid is released on cooling; slightly soluble in water (1 part in 1100 parts H 2 O at 20-25 ° C) |
| Special features | Light pale yellow fluorescence, pale green phosphorescence. Pyro- and piezoelectric at very low temperatures. |
Colemanite is a rarely occurring mineral from the mineral class of " borates ". It crystallizes in the monoclinic crystal system with the chemical composition Ca [B 3 O 4 (OH) 3 ] · H 2 O and is therefore chemically seen a hydrous calcium - borate with three additional hydroxyl groups .
Colemanite forms isometric to short prismatic as well as pseudorhombohedral and pseudoctahedral crystals up to 30 cm in size . It also occurs in the form of cryptocrystalline massive, fissile, granular and bulbous aggregates . In its pure form, colemanite is colorless and transparent with a glass-like to diamond-like sheen on the surfaces. However, due to multiple light refraction due to lattice construction defects or polycrystalline training, it can also be translucent white and, due to foreign admixtures, take on a gray to light yellow or cinnamon brown to black color. His line color , however, is always white.
The type locality of the mineral is the Furnace Creek Borate District (Death Valley Area Borate Deposits), Inyo County , California , USA , which belongs to the mining area discovered in 1882 by R. Neuschwander, in which boron minerals have been mined.
Etymology and history
R. Neuschwander is considered the discoverer of colemanite, who is said to have found the first stages of the mineral in October 1882 at the type locality. The California "state mineralogist" Henry Garber Hanks then described the mineral from Death Valley in 1883 as a crystalline variety of the chalky priceit known from Oregon .
"As this mineral possesses certain physical properties differing from price, the name colemanite has been given to it to distinguish it from the soft chalky mineral found both in southern Oregon and San Bernardino County, California."
"Since, however, the new deposit presents certain differences in price, a special name should be recommended to distinguish it from the soft chalk-like minerals from South Oregon and S. Bernadino Co."
Earl Pemberton points out that the name “R. Neuschwander ”is only mentioned in Evans' work, but not in the type publication. Hanks is therefore both a finder and a first-time writer.
The new mineral was named after the American mine owner, founder and pioneer of the borax industry in California, William Tell Coleman (1824–1893), who was also the owner of the "Harmony Borax Works", where the mineral was first found. Coleman himself had proposed the name "Smithite" in honor of his business partner Francis Marion Smith . Although a chemical analysis and a number of properties of colemanite have already been compiled in the "Report on the Borax deposits of California and Nevada" by Hanks, the work of JT Evans in the American science magazine "Bulletin of the California Academy of Sciences" is considered to be first scientific description of colemanite. The colemanite crystals, several centimeters in size, also caused a sensation on an international scale, so that the publication of several works in German on chemical and crystallographic investigations on this mineral as early as 1885 is not surprising.
As Anna Hedlik showed, Adolf Kenngott came to the assumption on the basis of the chemical analyzes by Benjamin Silliman , AW Chase and Félix Pisani that there was a uniform CaO: B 2 O 3 ratio for both Priceit and Pandermit and Colemanite , which is why he did not make a sharp separation of these minerals. In the case of Priceit and Pandermit, this was justified, as both names stand for the same mineral and today Pandermit is just a synonym for Priceit. Henri Buttgenbach (quoted in Hermann Steinmetz ) even assumed the identity of pandermite and colemanite on the basis of optical investigations. Esper Larsen did prove the identity of Priceit and Pandermit, but because of the different optical properties, he considered Priceit / Pandermit on the one hand and Colemanite on the other to be different minerals. It was not until 1948 that Anna Hedlik was able to completely rule out the identity of pandermite with colemanite, which was concluded from the above-mentioned investigations, by means of X-ray structure analyzes .
A mineral that was found by Arthur Starr Eakle in 1911 and is chemically identical to colemanite, but which should differ optically and crystallographically from him and therefore was called neocolemanite, later turned out to be identical to colemanite in investigations by Arthur Hutchinson .
Type material for colemanite is not defined.
classification
In the now outdated, but still in use 8th edition of the mineral classification according to Strunz , the colemanite belonged to the common mineral class of "carbonates, nitrates and borates" and there to the division of "chain borates [B 2 O 4 ] 2− to [B 6 O 10 ] 2− ", where together with hydroboracite he forms the" colemanite hydroboracite group "with the system no. V / J.03 and the other member Jarandolith formed.
The 9th edition of Strunz's mineral systematics, which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), assigns colemanite to the now independent class of "borates" and there in the department of "triborates". This is also further divided by the crystal structure, so that the mineral according to its construction in the subdivision "chain and belt-Triborate (Ino-Triborate)" is to find where it is the only member of the unnamed group 6.CB.10 forms .
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns the hydroboracite - like the outdated Strunz system - to the common class of "carbonates, nitrates and borates" and there in the department and subdivision of the same name of "hydrous borates with hydroxyl or halogen ”. Here he can be found as the only member of the unnamed group 03/26/05 .
Chemism
A chemical analysis of a colemanite from Death Valley showed 27.31% CaO, 0.10% MgO, 50.70% B 2 O 3 and 21.87% H 2 O. Published chemical analyzes of colemanite are mostly relatively old mentioned values come from a determination from the year 1887! Thirteen microprobe analyzes on colemanite showed mean values of 25.38% CaO; 0.26% SiO 2 ; 0.16% Na 2 O; 0.07% Al 2 O 3 ; 0.01% K 2 O; 0.01% FeO; 0.01% MgO and 0.01% TiO 2 (boron contents assumed via stoichiometry and mass balance). From them, the empirical formula Ca 0.99 Na 0.01 B 3.00 O 4 (OH) 3 · H 2 O is calculated on the basis of one oxygen atom , which leads to Ca [B 3 O 4 (OH) 3 ] · H 2 O was idealized and required levels of 27.28% CaO, 50.81% B 2 O 3 and 21.91% H 2 O.
Turkish colemanite crystals in particular have contents of arsenic in the form of As 3+ and / or As 5+ , which can be up to 125 ppm and which are said to be responsible for the fluorescence of these crystals.
Colemanite is chemically similar to hydroboracite , CaMg [B 3 O 4 (OH) 3 ] 2 · 3H 2 O, but has no formula-effective magnesium content and is also significantly poorer in water of crystallization. Inyoite , Ca [B 3 O 3 (OH) 5 ] · 4H 2 O, Meyerhofferite , Ca [B 3 O 3 (OH) 5 ] · H 2 O, Nifontovite , Ca 2 [B 3 O 3 (OH) 6 ] 2 · 2H 2 O, Tertschite , Ca 2 [B 5 O 7 (OH) 5 ] · 7H 2 O, and ginorite , Ca 2 [B 14 O 20 (OH) 6 ] · 5H 2 O, are all significantly richer than Colemanite and also have a different structure.
Crystal structure
Colemanite crystallizes in the monoclinic crystal system in the space group P 2 1 / a (space group no. 14, position 3) with the lattice parameters a = 8.71 Å ; b = 11.25 Å; c = 6.09 Å; and β = 110.1 ° and four formula units per unit cell .
In colemanite, corner-linked [B [3] B 2 [4] O5 (OH) 3 ] rings, each consisting of two [BO 3 OH] tetrahedra and a planar [BO 2 OH] group, form wavy borate chains parallel to the a-axis [100]. Chains made from CaO 3 (OH) 4 H 2 O polyhedra also run parallel [100]. Both types of chains are connected by common corners and edges and form stable layers in parallel (010). The connection between these layers is weak and consists - together with a network of hydrogen bonds - from a small number of B-Φ-Ca linkages. This weak connection is responsible for the very perfect cleavage of colemanite according to {010}. Each Ca atom is dodecahedral surrounded by eight anions. Both the Raman and the infrared spectrum of colemanite are characterized by multiple stretching oscillating water bands, which indicate that water plays an important role in the structure of this mineral.
Colemanite is isotypic (isostructural) to calciborite and hydroboracite.
properties
morphology
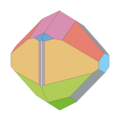
Abraham Wendell Jackson had already established in 1884 that all colemanite crystals of the type locality have a highly complex crystal costume and one of the crystals has no fewer than 24 different crystal forms. Jackson was the first to study colemanite crystals from California. Here he made drawings of ten different crystals, to which those in the adjacent table belong. He assigned the medium to short columnar crystals to three different habitus variants. However, it was only later that he learned that the material he was investigating was only slightly dependent on the type locality, but mainly from deposits in the Calico District, five miles from Daggett, California, in the Calico Mountains in the Mojave Desert , San Bernardino County , California.
Artur S. Eakle, who also examined material from the Calico District and found 47 different surface shapes on the crystals, distinguished four typical habitus variants among the colemanite crystals. Habitus 1 represents long prismatic crystals for which the prism {110} is decisive. It is also characterized by the dominance of {001} and the pinacoid { 2 01}. The habitus 2 with the prism parallel to the a-axis {021} as well as the prism {311} is found most frequently on the colemanite crystals, which makes the crystals appear characteristically pointed. Habit 3 is characterized by the predominance of {110} and { 2 01}, which results in a flattened appearance. Habitus 4 is rather poor in area and has a wedge-shaped appearance due to the formation of {110} and the pinacoid parallel to the b-axis { 3 01} in equilibrium. The surface of { 3 01} is clearly rounded and may merge into { 4 01} or { 6 01}.
- Costume and habitus of colemanite crystals (the same colors mean the same surface shapes)
Colemanite generally develops up to 30 cm in size, isometric to [001] short prismatic crystals with large prisms parallel to the c-axis {110} and partly large, partly less large basic pinacoids {001} as well as various, partly extremely complex terminating forms. Also in pseudorhombohedral crystals with large {110} and { 3 01} (which corresponds to Habitus 4 of Eakle) as well as pseudo-octahedral crystals with large { 2 21} and prisms {011}. Steinmetz lists 62 different surface shapes. In general, alongside calcite, colemannite is one of the minerals with the most shape.
Colemanite also occurs in aggregates that have been described in particular from Turkish deposits. In addition to clear, transparent split pieces ("Borspat"), approximately spherical colemanite concretions up to 5 cm in diameter are mentioned, which have radial stems on the inside and genetically correspond to similar gypsum concretions. Also in the form of "Colemanitrosen" up to the size of a fist, which are similar to the well-known "Gypsum Roses" and are also to be understood as concretions. Another form of development is called ribbon colemanite, with colemanite layers up to 6 cm thick being sharply separated from one another by tape. The Colemanite shows pronounced stem growth, the stems are up to 2 mm thick and are always perpendicular to the layers of the claystone.
Pseudomorphoses
From the "Biddy McCarty Mine" of the Pacific Coast Borax Company in Death Valley, Inyo Co., California, pseudomorphoses of tabular inyoite crystals according to {001} are described, which arose as a result of the dehydration of inyoite. Snow-white and brightly shiny, pseudorhombohedral crystal aggregates up to 2 cm in diameter from the "Aceb" pit near Iskeleköy not far from Bigadiç in the Turkish province of Balıkesir also represent pseudomorphoses according to Inyoit, consisting of innumerable, water-clear, columnar columns measuring a maximum of 0.7 × 3 mm , large-area crystals can be built up as a confused, bulky pile with plenty of cavities.
From the “Corkscrew Canyon Mine” in Death Valley, pseudomorphoses have been described that arose in a somewhat more complicated way. In a first step, free-standing inyoite crystals formed by dehydration Meyerhofferite. In a second step, tiny colemanite crystals are deposited on the surface of these newly formed Meyerhofferite crystals. In a third step, the Meyerhofferite was extracted - what remained were perimorphoses from colemanite after pseudomorphoses from Meyerhofferite to Inyoite.
Colemanite replaces fibrous ulexite and hydroboracite, but can also be pseudomorphosed by hydroboracite and howlite . Furthermore, pseudomorphoses from calcite to colemanite are known. As a curiosity, colemanite has been observed together with bitumen in a fossil egg in the gravel of the Gila River in Arizona .
physical and chemical properties
The crystals of the colemanite are characterized by a large variety of colors for a borate mineral. Colorless, milky white, pale yellow, yellow, gray, cinnamon brown, light brown, brown and black varieties were described. The cause of the brownish and black hues are microscopic crystals of manganese minerals such as birnessite and todorokite . The color of the colemanite streak is always white. According to the values for the refraction of light (n α = 1.586, n γ = 1.614), the surfaces of the translucent to transparent crystals have a glass-like, occasionally a diamond-like sheen . Colemanite is colorless under the microscope in transmitted light.
The mineral has a very perfect cleavage according to {010} and a clear cleavage according to {001}, but due to its brittleness it breaks like quartz , with the fracture surfaces being uneven to hemispherical. With a Mohs hardness of 4.5, colemanite is one of the medium-hard minerals, which means it is between the reference minerals fluorite (hardness 4) and apatite (hardness 5) and, like these, can be easily scratched with a pocket knife to a greater extent (fluorite) or less (apatite). The measured density for colemanite is 2.423 g / cm³, the calculated density is 2.42 g / cm³.
Colemanite melts incompletely in front of the soldering pipe, inflating and decripitating. When heated with fluorite or potassium disulfate , it colors the flame green. It is soluble in hot hydrochloric acid (HCl) or nitric acid (HNO 3 ), whereby flakes of boric acid H 3 BO 3 form on cooling . The filtrate gives a white precipitate with ammonia and ammonium oxalate . In contrast, colemanite is only very slightly soluble in water (1 part in 1100 parts H 2 O at 20-25 ° C).
- Colors of colemanite
Fluorescence and phosphorescence
The first mention of the fluorescence and phosphorescence of colemanite dates back to 1903. Colemanite shows light pale yellow fluorescence in UV light and can have pale green phosphorescence . According to other information, the mineral has a bluish-white, sometimes yellowish-white or greenish-white fluorescence in long-wave UV light (365 nm) as well as in short-wave UV light (254 nm). When excited with short-wave UV light (254 nm), a greenish-white phosphorescence appears.
According to recent studies on Turkish borates, the majority of fluorescent borate minerals react more intensely to long-wave UV light (> 350 nm) than to light with shorter wavelengths. It was also found that different generations of the same mineral show marked differences in the response to UV light. These investigations show that the white fluorescence of colemanite with a bluish or greenish tinge is intrinsic and has two causes: on the one hand, inorganic radicals such as BO 2− , BO 2 2− , BO 3 2− and BO 4 4− and, on the other hand, typical low-temperature inclusions of organic luminophores . In addition, the white and yellowish to greenish-white fluorescence is mainly limited to idiomorphic colemanite crystals, while less well-crystallized formations, xenomorphic grains and crystals with cracks and cavities filled with silt and clay minerals show a light orange to reddish fluorescence. This is attributed to a calcium humate complex, whereby the As 3+ and / or As 5+ ions contained should also contribute to the fluorescence. Organic luminophores such as B. Humic acids cause the greenish component in the fluorescence and the greenish phosphorescence which lasts up to ten seconds.
At very low temperatures, colemanite is pyro- and piezoelectric.
Dehydration
When the heating starts, colemanite loses a total of 1.8% of its weight up to a temperature of 350 ° C. The actual thermal decomposition of the colemanite takes place in the range from 300 ° C to 450 ° C. It begins with the loss of parts of its crystal water . Then the bonds between the molecular water and the borate rings are broken, which is indicated by an endothermic reaction at 389 ° C. Finally, the remaining water is released - the process continues up to a temperature of 600 ° C and is then ended. Between 350 ° C and 600 ° C, colemanite loses a total of 35.7% of its weight.
Education and Locations
Colemanite is found in borax lakes and the resulting deposits . It is a typical and frequent component of borate deposits that are low in sodium and carbonate and have formed under arid, alkaline lacustrian conditions. Calcium and boron are supplied from different sources. The former comes from geothermal waters, the latter from warm to very hot geothermal brines (brines). For a long time, colemanite was regarded as a secondary mineral, which is formed from the primary ulexite through "penetrating warm borax solutions". However, when the large Turkish deposits were examined in the mid-1970s and a decade later when the newly discovered Argentine deposits were explored, there was no evidence of a possible formation of colemanite through transformation from other boron minerals. The theory of primary colemanite formation is therefore gaining ground more and more.
As paragenesis minerals for colemanite are other borates as Howlith, Ulexit, Searlesit , Priceit, nobleite , Ginorit , gowerite , Lüneburgit , kernite and carbonates and sulfates, such as gypsum , calcite and celestite , but in addition also sulfides such as realgar and orpiment stated.
As a rather rare mineral formation, Colemanite could only be described from about 150 sites so far (as of 2017). The type locality is the Death Valley, from where it was first described in 1883. The first colemanite-mining deposit was the "Lila C. Mine" at the foot of the eastern slope of the Greenwater Range. When the Lila C. Mine was wiped out, mining shifted to the Furnace Creek District at the foot of the western slope of the Greenwater Range.
The best colemanite crystals come from sites in Turkey and the United States. The “Famous Mineral Findings” compiled by Paul Ramdohr and Hugo Strunz also include the huge boron deposit of Boron in Kern Co., California, and Bandırma (“Panderma” is the type locality for the Priceit variety Pandermit) on the Sea of Marmara in Turkey's Asia Minor .
Of the countless deposits and discovery sites in California, only the most important can be mentioned here. In Inyo County these include the Gower Gulch Mine at Zabriskie Point , Black Mts., Amargosa Range; the "Billie Mine", the "Boraxo Mine", the "Thompson Mine" (Kern Borate Mine, Borax Pit No. 1) and the "Corkscrew Canyon Mine" (Corkscrew Mine), all at Ryan in the Furnace Creek Borate District. Furthermore from the deposit "Kramer Borate Deposit" at Boron in the Kramer Borate District, Kern County , the "Neocolemanite" delivering "Lang Mine" (Sterling Borax Mine or Tick Canyon Borax Mine) in Tick Canyon near Lang, Los Angeles County , and the "Pacific Mine" near Calico in the district of the same name in the Calico Hills, San Bernardino County , all in California, USA. Finally from the "Anniversary Mine" (Callville Wash) in the White Basin, Muddy Mountains District, Muddy Mts., Clark County , Nevada .
From the vaporite deposits of Penobsquis and Salt Springs near Sussex , New Brunswick , Canada . In Mexico from the large El Torreon and La Tinaja del Oso deposits near Magdalena , Sonora . In Argentina from the “Salinas Grandes Playa” and the “Loma Blanca” deposit located 8 km southwest of Coranzuli in the Susques department , Jujuy province , and from various deposits in the Sijes district, Los Andes department in the Salta province .
In Turkey from huge deposits with reserves of around one billion tons. These include the two pits "Emet Eti Bor Mine" and "Hisarcik Mine" near Emet , province of Kütahya , Aegean region , belonging to the Emet boron deposit ; the deposits “Göcenoluk” and “Sarıkaya B” near Kırka , Eskişehir Province , Central Anatolia and “Sebepliköy” (Sebepli) near Gönen on the Biga peninsula. All in the province of Balıkesir , Marmara region , are the "Bigadiç Mine" near Bigadiç , the deposits "Çakmakderesi Domuzderesi" and "Kireçlik" (Büyükkireçlik) near Çamköy not far from Bigadiç, the deposit "Kurtpınarıç" near Faraşköy “Near Iskeleköy (Iskele) not far from Bigadiç and the“ Sultançayırı Mine ”near Sultançayırı not far from Susurluk . Particularly large crystals come from the “Kestelek” deposit near Mustafakemalpaşa , Bursa province , Marmara region.
Finally from the “Inder” borate deposit near Atyrau in the area of the same name in Kazakhstan . In Europe from various deposits and deposits at Bella Stena in the Jarandol Basin near Raška in the administrative district of the same name , Serbia , as well as from a salt lake in the Karlovasi Basin on the island of Samos , North Aegean region , Greece .
use
Colemanite is an important ore in sedimentary boron deposits due to its abundance and its content of up to 50.80% B 2 O 3 for the extraction of this element for the chemical industry . Boron itself is the most important element worldwide, both from a strategic and an industrial point of view, since boron compounds have an unusually wide range of applications in all areas of production with the exception of food. Boron is an integral part of modern technology. Boron nitride , boron carbide and borosilicide are hard materials that are used instead of diamond for abrasives and as cutting material for machining steel. They are also used as alloy additives for fine-grain structural steels and nickel-based alloys. Metal borides such as ferroboron are heat-resistant materials, with even small amounts of boron improving the creep behavior and hot formability of steels. Neodymium-iron-boron compounds are used to produce the strongest magnets for e.g. B. Magnetic resonance tomographs , micromotors and hard drives are used. Crystalline boron and boron fibers are required for applications with extremely high strength and stiffness such as e.g. B. Components for helicopter rotors. Because of the very high cross-section for thermal neutrons in the nuclear reaction 10 B (n, α) 7 Li, boron has a very high neutron absorption. Boron compounds are therefore added to radiation protective clothing and walls, steels for storage vessels for nuclear fuels and the radiation protection used concrete shell for shielding purposes. Control rods used to regulate and switch off the chain reaction in nuclear reactors contain boron carbide or strontium boride . Amorphous boron is used as an additive for rocket propellants (heterogeneous solid propellants, composites). After all, boron is required as an additive for the production of extremely chemical and temperature-resistant borosilicate glasses and for the production of borax and perborates for detergents, cleaning agents and disinfectants.
Regardless of its temperature sensitivity, the low hardness and the very seldom pronounced colors, colemanite has also been polished, but mostly only as a curiosity. Stones weighing 50–100 carats can be ground from large crystals or fissile masses. The largest crystal in the Smithsonian Institution ( Washington, DC ) collection weighs 14.9 ct.
See also
literature
- Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 725 .
- Friedrich Klockmann : Klockmann's textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 590 (first edition: 1891).
- Helmut Schrätze , Karl-Ludwig Weiner : Mineralogy. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 565-566 .
- Martin Okrusch , Siegfried Matthes : Mineralogy. An introduction to special mineralogy, petrology and geology . 7th fully revised and updated edition. Springer Verlag, Berlin et al. 2005, ISBN 3-540-23812-3 , pp. 103-104 (first edition: 1983).
- Colemanite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 67 kB ; accessed on August 23, 2017]).
Web links
- Mineral Atlas: Colemanite (Wiki)
- Mindat - Colemanite (English)
- Webmineral - Colemanite (English)
- Colemanite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed March 24, 2019 .
- American-Mineralogist-Crystal-Structure-Database - Colemanite. In: rruff.geo.arizona.edu. Retrieved March 24, 2019 .
Individual evidence
- ↑ a b c d e f g h i Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 343-344 .
- ↑ a b c d e f g h i j k l m n o p q r s t Colemanite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( available online at handbookofmineralogy.org [PDF; 69 kB ; accessed on March 24, 2019]).
- ↑ a b Gerhard vom Rath : About Colemanite . In: New Yearbook for Mineralogy, Geology and Palaeontology Vol. 1 . tape 1885 , 1885, p. 77-78 .
- ↑ a b Friedrich Klockmann : Klockmanns textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 590 (first edition: 1891).
- ↑ a b c d e f Mindat - Colemanite
- ↑ a b c d Charles Palache , Harry Berman , Clifford Frondel : Colemanite . In: The System of Mineralogy . of James Dwight Dana and Edward Salisbury Dana Yale University 1837-1892. 7th edition. II (Halides Nitrates, Borates, Carbonates, Sulfates, Phosphates, Arsenates, Tungstates, Molybdates etc.). John Wiley & Sons, New York 1951, ISBN 0-471-19272-4 , pp. 351–352 (English, first edition: 1892).
- ↑ a b c JT Evans: Colemanite . In: Bulletin of the California Academy of Sciences . tape 1 , no. 1 , 1884, p. 57-59 .
- ^ A b c Henry Garber Hanks: Report on the Borax Deposits of California and Nevada (California State Mining Bureau Part 2. Third Annual report of the State Mineralogist) . In: California journal of mines and geology . tape 3 , 1883, p. 86–87 ( available online at rruff.info [PDF; 818 kB ; accessed on March 24, 2019]).
- ↑ a b c d Hermann Steinmetz : Colemanite. Ca 2 B 6 O 11 · 5H 2 O . In: Gottlob Linck (Ed.): Handbuch der Mineralogie von Dr. Carl Hintze . Borates aluminates and ferrates. Phosphates, arsenates, antimonates, vanadates, niobates and tantalates 1st part. 1st edition. tape 1 , fourth division. 1st half. Walter de Gruyter & Co., Berlin and Leipzig 1933, p. 170-181 .
- ↑ H. Earl Pemberton: Colemanite: type locality and describer . In: The Mineralogical Record . tape 4 , no. 6 , 1973, p. 272-274 .
- ↑ George Herbert Hildebrand: Borax Pioneer: Frances Marion Smith . 1st edition. Howell-North Books, San Diego 1982, ISBN 978-0-8310-7148-6 , pp. 318 .
- ^ A b Gustav Adolf Kenngott : About Priceit, Colemanit and Pandermit . In: New Yearbook for Mineralogy, Geology and Palaeontology Vol. 1 . tape 1885 , 1885, p. 241 .
- ↑ Thorstein Hiortdahl: Colemanite, a crystallized kakborate from California . In: Journal for Crystallography and Mineralogy . tape 10 , no. 1 , 1885, p. 25-31 .
- ^ Carl Bodewig, Gerhard vom Rath: Colemanite from California . In: Journal for Crystallography and Mineralogy . tape 10 , no. 1 , 1885, p. 179-186 .
- ^ Benjamin Silliman: Mineralogical notes on Utah, California, and Nevada, with a description of priceite, a new borate of lime . In: The American Journal of Science . tape 6 , 1873, p. 126–133 , doi : 10.2475 / ajs.s3-6.32.126 ( available online at rruff.info [PDF; 495 kB ; accessed on March 24, 2019]).
- ^ AW Chase: On the Oregon borate of lime (cryptomorphite?) . In: The American Journal of Science . tape 5 , 1873, p. 287-290 , doi : 10.2475 / ajs.s3-5.28.287 .
- ^ Félix Pisani : Traité élémentaire de minéralogie . 1st edition. Masson, Paris 1875, p. 216 , doi : 10.3931 / e-rara-17795 .
- ↑ Hermann Steinmetz : Pandermit. Ca 8 B 20 O 38 · 15H 2 O . In: Gottlob Linck (Ed.): Handbuch der Mineralogie von Dr. Carl Hintze . Borates aluminates and ferrates. Phosphates, arsenates, antimonates, vanadates, niobates and tantalates 1st part. 1st edition. tape 1 , fourth division. 1st half. Walter de Gruyter & Co., Berlin and Leipzig 1933, p. 168 .
- ↑ Esper S. Larsen: Proof that price is a distinct mineral species . In: The American Mineralogist . tape 2 , no. 1 , 1917, p. 1–3 ( available online at minsocam.org [PDF; 181 kB ; accessed on March 24, 2019]).
- ↑ Anna Hedlik: On the knowledge of pandermite and colemanite . In: Tschermaks mineralogical and petrographic communications . tape 1 , no. 4 , 1950, p. 419-421 , doi : 10.1007 / BF01145397 .
- ^ Arthur Hutchinson: On the identity of Neocolemanite with Colemanite . In: Mineralogical Magazine . tape 16 , no. 75 , 1912, pp. 239–246 , doi : 10.1180 / minmag.1912.016.75.10 ( available online at rruff.info [PDF; 341 kB ; accessed on March 24, 2019]).
- ↑ Catalog of Type Mineral Specimens - C. (PDF 131 kB) In: docs.wixstatic.com. Commission on Museums (IMA), December 12, 2018, accessed August 29, 2019 .
- ↑ James Edward Whitfield: Analyzes of some natural borates and borosilicates . In: American Journal of Science . tape 34 , 1887, pp. 281-287 , doi : 10.2475 / ajs.s3-34.202.281 .
- ^ Colemanite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed March 24, 2019 .
- ↑ Jinru LIN, Yuanming PAN, Ning CHEN, Mao MAO, Rong LI, Renfei FENG: Arsenic incorporation in colemanite from borate deposits: Data from ICP-MS, μ-SXRF, XAFS and EPR analyzes . In: The Canadian Mineralogist . tape 49 , no. 3 , 2011, p. 809–822 , doi : 10.3749 / canmin.49.3.809 ( available online at rruff.info [PDF; 6.7 MB ; accessed on March 24, 2019]).
- ↑ a b c Cahit Helvacı, Yeşim Yücel Öztürk, Axel Emmermann: Fluorescence survey of Turkish borate minerals: comparative measurements of fluorescence spectra of the most important borate mineral species, Turkey . In: New Yearbook for Mineralogy Treatises (Journal of Mineralogy and Geochemistry) . tape 194 , no. 1 , 2017, p. 1–17 , doi : 10.1127 / njma / 2016/0016 .
- ^ Peter C. Burns, Frank C. Hawthorne : Hydrogen bonding in colemanite: an X-ray and structure-energy study . In: The Canadian Mineralogist . tape 31 , no. 2 , 1993, p. 297–304 ( available online at researchgate.net [PDF; 773 kB ; accessed on March 24, 2019]).
- ↑ Frank C. Hawthorne, Peter C. Burns, Joel D. Grice: The crystal chemistry of Boron. In: Edward S. Grew & Lawrence M. Anovitz (Eds.), Boron: mineralogy, petrology and geochemistry . In: Reviews in Mineralogy and Geochemistry . tape 33 , 2003, ISBN 978-0-939950-41-6 , pp. 41-116 .
- ↑ Ray L. Frost, Yunfei Xi, Ricardo Scholz, Fernanda Maria Belotti, Mauro Cândido Filho: Infrared and Raman spectroscopic characterization of the borate mineral colemanite - CaB 3 O 4 (OH) 3 H 2 O - implications for the molecular structure . In: Journal of Molecular Structure . tape 1037 , 2013, p. 23–28 , doi : 10.1016 / j.molstruc.2012.11.047 ( PDF 711 ( memento of October 19, 2017 in the Internet Archive )).
- ↑ Abraham Wendell Jackson: Colemanite, a new borate of lime . In: The American Journal of Science . tape 28 , 1884, p. 447–448 , doi : 10.2475 / ajs.s3-28.168.447 ( available online at rruff.info [PDF; 795 kB ; accessed on March 24, 2019]).
- ↑ Abraham Wendell Jackson: On the morphology of colemanite . In: Bulletin of the California Academy of Sciences . tape 1 , no. 2 , 1885, p. 3-36 .
- ↑ Abraham Wendell Jackson: Mineralogical contributions: Colemanite . In: Bulletin of the California Academy of Sciences . tape 1 , no. 4 , 1886, p. 358-365 .
- ↑ a b c d e f g h Arthur S. Eakle: Colemanite from southern California: A description of the crystals and of the method of measurement with the two-circle goniometer . In: University of California Bulletin of the Department of Geology . tape 3 , no. 2 , 1902, pp. 31-50 .
- ↑ Arthur S. Eakle: Neocolemanite, a variety of colemanite, and howlite from Lang, Los Angeles County, California . In: University of California Bulletin of the Department of Geology . tape 6 , no. 9 , 1911, pp. 179-189 .
- ↑ a b Heinz Meixner : Some borate minerals (colemanite and terschite, a new mineral) from Turkey . In: Advances in Mineralogy . tape 31 , 1953, pp. 39-42 .
- ↑ a b c d e Heinz Meixner : Mineralogical observations on colemanite, inyoite, meyerhofferite, tertschite and ulexite from new Turkish borate deposits . In: Heidelberg contributions to mineralogy and petrography . tape 3 , no. 6 , 1953, pp. 445-455 , doi : 10.1007 / BF01129197 .
- ^ Austin F. Rogers: Colemanite pseudomorphous after inyoite from Death Valley, California . In: The American Mineralogist . tape 4 , no. 11 , 1917, pp. 134–139 ( available online at minsocam.org [PDF; 379 kB ; accessed on March 24, 2019]).
- ↑ a b Gerhard Mühle: Colemanite pseudomorphs . In: The Mineralogical Record . tape 5 , no. 4 , 1974, p. 174-177 .
- ^ Cahit Helvacı, Federico Ortí: Sedimentology and diagenesis of Miocene colemanite-ulexite deposits (western Anatolia, Turkey) . In: Journal of Sedimentary Research . tape 68 , no. 5 , 1998, pp. 1021-1033 , doi : 10.2110 / jsr.68.1021 .
- ^ A b c d John K. Warren: Evaporites: Sediments, Resources and Hydrocarbons . 1st edition. Springer, Berlin 2006, ISBN 978-3-540-26011-0 , pp. 1-1035 .
- ↑ a b Rupert high Leitner, Stefan White: Characteristics colemanite Ca [B 3 O 4 (OH) 3 ] · H 2 O . In: Lapis . tape 26 , no. 11 , 2001, p. 9-11 .
- ^ William Conger Morgan, MC Tallmon: A peculiar occurrence of bitumen and evidence as to its origin . In: American Journal of Science . tape 18 , 1904, pp. 363-377 , doi : 10.2475 / ajs.s4-18.107.363 .
- ^ FH Brown, Adolf Pabst, DL Sawyer: Birnessite on colemanite at Boron, California . In: The American Mineralogist . tape 56 , no. 1 , 1971, p. 1057-1064 ( available online at minsocam.org [PDF; 557 kB ; accessed on March 24, 2019]).
- ^ JT Evans: The chemical properties and relations of colemanite . In: Bulletin of the California Academy of Sciences . tape 1 , no. 2 , 1885, p. 37-42 .
- ↑ George Frederick Kunz , Charles Baskerville : The action of radium, Roentgen rays and ultra-violet light on minerals and gems . In: Science . tape XVIII , no. 468 , 1903, pp. 769-783 , doi : 10.1126 / science.18.468.769 .
- ^ Colemanite mineral atlas
- ↑ JW Davisson: The pyroelectric behavior of colemanite . In: Acta Crystallographica . tape 10 , no. 7 , 1956, pp. 511-514 , doi : 10.1107 / S0365110X5700184X .
- ↑ AG Chynoweth: The pyroelectric behavior of colemanite . In: Acta Crystallographica . tape 10 , no. 7 , 1957, pp. 511-514 , doi : 10.1107 / S0365110X5700184X .
- ^ Atila G. Celik, Gaye O. Cakal: Characterization of Espey colemanite and variation of its physical properties with temperature . In: Physicochemical Problems of Mineral Processing . tape 52 , no. 1 , 2016, p. 66–76 , doi : 10.5277 / ppmp160106 ( available online at minproc.pwr.wroc.pl [PDF; 769 kB ; accessed on March 24, 2019]).
- ↑ Irena Waclawska, Leszek Stoch, J. Paulik, F. Paulik: Thermal decomposition of colemnanite . In: Thermochimica Acta . tape 126 , no. 11 , 1988, pp. 307-318 , doi : 10.1016 / 0040-6031 (88) 87276-9 .
- ^ A b Donald E. Garrett: Borates: Handbook of Deposits, Processing, Properties, and Use . 16th edition. Academic Press, San Diego 1998, ISBN 978-0-12-276060-0 , pp. 483 .
- ^ William Frederick Foshag: The origin of the colemanite deposits of California . In: Economic Geology . tape 16 , no. 3 , 1921, pp. 199-214 , doi : 10.2113 / gsecongeo.16.3.199 .
- ^ John K. Warren: Evaporites: A Geological Compendium . 2nd Edition. Springer, Berlin 2016, ISBN 978-3-319-13511-3 , pp. 1–1813 (first edition: 2006).
- ↑ a b c Cahit Helvacı, Ricardo N. Alonso: Borate deposits of Turkey and Argentina; a summary and geological comparison . In: Turkish Journal of Earth Sciences . tape 9 , no. 1 , 2000, pp. 1–27 ( available online at journals.tubitak.gov.tr [PDF; 401 kB ; accessed on March 24, 2019]).
- ↑ Mindat - Number of localities for Colemanite
- ↑ a b c List of places where colemanite was found in the Mineralienatlas and Mindat
- ↑ H. Earl Pemberton: Minerals of California . 1st edition. Van Nostrand Reinhold, New York 1983, ISBN 0-442-27488-2 , pp. 246 .
- ↑ James W. Minette, Gerhard Mühle: Colemanite from the Thompson Mine . In: The Mineralogical Record . tape 5 , no. 2 , 1974, p. 67-73 .
- ↑ Ricardo N. Alonso, Cahit Helvacı, Ricardo Jose Sureda, JG Viramonte: A new Tertiary borax deposit in the Andes . In: Mineralium Deposita . tape 23 , no. 4 , 1988, pp. 299-305 , doi : 10.1007 / BF00206411 .
- ^ Ricardo N. Alonso: On the origin of La Puna Borates . In: Acta geológica hispánica . tape 34 , no. 2–3 , 1999, pp. 141-166 .
- ↑ E. Çetin, İ. Eroğlu, S. Özkar: Kinetics of gypsum formation and growth during the dissolution of colemanite in sulfuric acid . In: Journal of Crystal Growth . tape 231 , no. 4 , 2001, p. 559-567 , doi : 10.1016 / S0022-0248 (01) 01525-1 .
- ↑ Lucien F. Trueb: The chemical elements: A foray through the periodic table . 1st edition. S.-Hirzel-Verlag, Stuttgart 1996, ISBN 3-7776-0674-X , p. 229-236 .
- ↑ International Gem Society IGS - Colemanite