Magnetite
| Magnetite | |
|---|---|
| Magnetitoctahedron (silver) on chalcopyrite (golden) from Aggeneys, North Cape, South Africa (size: 7 cm × 6 cm × 4 cm) | |
| General and classification | |
| other names |
|
| chemical formula | Fe 3 O 4 more precisely: Fe 2+ (Fe 3+ ) 2 O 4 |
|
Mineral class (and possibly department) |
Oxides and hydroxides |
|
System no. to Strunz and to Dana |
4.BB.05 ( 8th edition : IV / B.02) 02/07/02/03 |
| Crystallographic Data | |
| Crystal system | cubic |
| Crystal class ; symbol | cubic hexakisoctahedral; 4 / m 3 2 / m |
| Space group | Fd 3 m (No. 227) |
| Lattice parameters | a = 8.3985 (5) Å |
| Formula units | Z = 8 |
| Frequent crystal faces | {111}, more rarely {110} or {100} |
| Twinning | often according to the spinel law: cross twins according to (111) |
| Physical Properties | |
| Mohs hardness | 5.5 to 6.5 |
| Density (g / cm 3 ) | measured: 5.175; calculated: 5.20 |
| Cleavage | indistinct after (111) |
| Break ; Tenacity | shell-like, brittle |
| colour | black |
| Line color | black |
| transparency | opaque, weakly translucent on thin edges |
| shine | weak metallic luster |
| magnetism | ferrimagnetic |
| Crystal optics | |
| Refractive index | n = 2.42 |
| Birefringence | none, as it is optically isotropic |
| Other properties | |
| Chemical behavior | acid and base stable |
Magnetite , out of date also known as magnet iron , magnet iron stone or iron oxy-suloxide and its chemical name iron (II, III) oxide , is a mineral from the mineral class of " oxides and hydroxides " and the most stable connection between iron and oxygen . It crystallizes in the cubic crystal system with the general chemical composition Fe 3 O 4 , which can be formulated more precisely as Fe 2+ (Fe 3+ ) 2 O 4 .
When naturally formed, magnetite usually develops centimeter-sized, octahedral crystals , but also granular to massive aggregates of gray-brown to black, metallic shimmering color. Due to its high iron content of up to 72.4% and its strong magnetism , magnetite is one of the most important iron ores and raw materials for the electrical industry . The mineral is rarely found anywhere in the world, but when accumulated locally it forms large ore deposits .
Magnetite forms a mixed crystal row with Ulvöspinell (Fe 2 TiO 4 ) , the links of which are referred to as titanomagnetite .
Etymology and history

From the Latin stem magnet- (with the nominative magnes - magnet) the designations magnet (from Middle High German magnete ) emerged, as a medieval mineral name Magneteisenstein (also "magnetenstain") and the name magnetite introduced in 1845 by Wilhelm Haidinger .
Already since the 11th century BC The Chinese used the mineral's magnetic properties.
According to reports by Theophrastus , a stone magnetis was known to the Greeks. The reference to a stone called magnes , which is said to be named after a shepherd of the same name, can be found in the Roman writer Pliny the Elder . This shepherd found the stone on Mount Ida when the shoe nails and the tip of his stick stuck to the ground. Pliny distinguished several types of magnes, but above all a “male” and a “female”, of which only the male had the power to attract iron and thus corresponded to the actual magnetite. The “female” magnesite was probably manganese ore, similar to the “male” in appearance, or a mineral of white color that was later referred to as magnesite MgCO 3 .
More likely, however, is the interpretation that the mineral was named after Magnesia , a landscape in Thessaly or the city of Magnesia on the meander . It is also possible to name magnetite after other places in Greece or Asia Minor of the same name, in which iron ore chunks with magnetic properties were found over 2500 years ago .
classification
The mineral systematics by Strunz and Dana classify magnetite into the mineral class of oxides and the department of molar ratio metal: oxygen = 3: 4 due to its crystal-chemical structure . In the new systematics of minerals according to Strunz (9th edition) , the minerals in this department are also sorted according to the size of the cations involved , with the positively charged iron ion counting among the medium-sized cations.
The systematics of minerals according to Dana , on the other hand , sorts according to the metal ion (Fe) involved and the crystal symmetry, so that the magnetite here in the ferrous subgroup with the common point group 4 / m 3 2 / m within the division of " multiple oxides with the general formula ( A + B 2+ ) 2 X 4 , spinel group “can be found.
Crystal structure
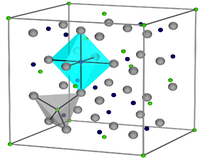 Unit cell of magnetite |
|
| Crystal system | cubic |
| Space group | Fd 3 m (No. 227) |
|
Lattice parameter (unit cell ) |
a = 8.3985 (5) Å |
| Number (Z) of the formula units |
Z = 8 |
From a crystallographic point of view, magnetite belongs to the spinel group and, as a naturally grown crystal, shows octahedron surfaces {111} and more rarely rhombic dodecahedron surfaces {110}. Twins are often formed according to the spinel law (cross twins according to (111)), but only rarely with ingrown crystals.
The crystal structure of magnetite (chemical formula Fe 3 O 4 ) can be written as Fe 3+ [Fe 3+ Fe 2+ ] O 4 according to the general formula for spinels AB 2 O 4 . The term inverse spinel structure for magnetite takes into account the fact that 1/3 of the iron ions (Fe 3+ ions) are tetrahedral and 2/3 of the iron ions (Fe 2+ and Fe 3+ ions in a ratio of 1: 1) are octahedrally coordinated by oxygen, which is exactly the inverse of normal spinel. The symmetry of the high temperature phase (T> 120 K ) of magnetite was clarified very early in 1915, it is cubic. More precisely, it is the space group Fd 3 m or O 7 h with a lattice parameter a = 8.394 Å. This results in eight formula units per unit cell with a total of 56 atoms.
The structure of the cubic high-temperature phase (T> 120 K) is shown schematically in the picture on the left. The cubic closest packing of oxide ions (gray), the octahedron (turquoise) and tetrahedral gaps (gray) are shown here. The Fe 3+ ions in the tetrahedral gaps are highlighted in green and the Fe 2+ / Fe 3+ ions in the octahedral gaps are highlighted in dark blue. The A sublattice, which is built up by the tetrahedrally coordinated Fe 3+ ions, forms a diamond lattice , while the B sublattice of the Fe 2+ or Fe 3+ ions of the octahedral oxygen environment forms a pyrochloride lattice that is geometrically frustrated . Geometric frustration means that a local order that is stabilized by local interactions cannot continue freely through the crystal. These special geometric properties enable a large number of different interactions with long or short range and very similar energy, often with a multiple degenerate ground state . One of the possibilities to reverse the degeneration is a long-range charge or spin order , which can lead to extremely complex crystal structures, of which only a few have been clarified to date.
The exact space group of the low-temperature phase (T <120 K) was not clearly determined until 1982 and is even discussed controversially to this day. Only through a carefully carried out neutron diffraction analysis on synthetic single crystals , which were measured with simultaneous application of pressure along the [111] direction and cooling in the magnetic field, could the crystalline order below T = 120 K be elucidated. It is a distortion of the monoclinic space group Cc with pseudo- orthorhombic symmetry (space group Pmca ; a c / √2 ⊗ a c / √ 2 ⊗ 2a c ), where a c corresponds to the length of an axis of the undisturbed cubic unit cell.
properties
Magnetite is highly resistant to acids and alkalis. Its Mohs hardness varies between 5.5 and 6.5 and its density between 5.1 and 5.2 g / cm³ , depending on the purity . His stroke color is black.
magnetism
Magnetite is one of the most powerful (ferri) magnetic minerals. If the temperature falls below the Curie temperature of 578 ° C, the magnetization aligns itself largely in the direction of the earth's magnetic field , so that a remanent magnetic polarization of the order of magnitude of up to 500 nT results. In this way, magnetite crystals can preserve the direction of the earth's magnetic field at the time of their formation. The investigation of the direction of magnetization of lava rock ( basalt ) led geologists to the observation that in the distant past the magnetic polarity of the earth must in fact have reversed from time to time.
The magnetic properties of magnetite, which have been known and used for a long time, can be explained very well by considering the local crystal structure. Fe 3 O 4 is a ferrimagnet, archetypal for the ferrites of spinels . The magnetic order in magnetite can be well understood in the context of the Néel model of two sublattices . In the model it is assumed that the exchange interaction between the octahedral and tetrahedral oxygen- coordinated iron ion sites is strongly negative, and the exchange interaction between the ions on the same sublattices is also negative, but less in magnitude. It follows from this that the ions of the same sublattice would adopt an antiferromagnetic spin position with respect to one another if this tendency were not counteracted by a stronger exchange interaction between the ions of the different sublattices. The relative strength of the exchange interaction between the ions of different sublattices is due to the differences in the distances between the ions of the same sublattice and ions of different sublattices. This constellation prefers an anti-parallel arrangement of the magnetic moments of the sublattices, the sublattice ions of which have a parallel spin arrangement to one another. In magnetite, the effective moments of the A / B sublattices couple antiferromagnetically via super exchange . The Fe 2+ ion has the spin S = 2 (4µ B ) and the Fe 3+ ion the spin S = 5/2 (5µ B ), so that in the antiparallel arrangement of the Fe 3+ ions explained above on the a-sublattice or the Fe 2 + / 3 + ions on the B-sublattice an effective saturation moment of (5-5 + 4) μ B = 4μ B results. The Neel or Curie temperature of magnetite is unusually high and is T N = 850 K.
Verwey transition
In the conductivity curve of magnetite, in which the conductivity is plotted against the temperature, the most striking feature is an abrupt change in conductivity of two orders of magnitude at T = 120K. Magnetite changes its properties from a poor conductor in the high temperature phase (approx. 0.2 mΩm at T> 120 K) to an insulator in the low temperature phase (40 mΩm at T <120 K). This behavior was systematically investigated by Evert Verwey in 1939 and a first theoretical explanation for the effect was published. In his honor, this transition and all physically similar transitions are referred to as Verwey transitions. The first indications of a phase transition in a temperature range around 120 K were provided by early heat capacity measurements on synthetically produced samples. The phase transition can be characterized as an isolator-isolator transition.
Modifications and varieties
As Titanomagnetit (English also Titaniferous magnetite which are mixed crystals of the series of magnetite (Fe 3 O 4 ) and Ulvöspinell (Fe 2 TiO 4 ), respectively. The mixed crystal formula is generally with Fe 2+ (Fe 3+ , Ti) 2 O 4 (more specifically, also with xFe 2 TiO 4 · (1-x) Fe 3 O 4 ). This mixed series is only complete above about 600 ° C. When the temperature drops, the mixed crystals disintegrate and segregation lamellae of Ulvöspinell and magnetite are formed. The most common variant of titanomagnetites is the so-called TM60 with an Ulvöspinell content of around 60%.
Titanomagnetite is of great importance when researching palaeomagnetism , as it becomes ferrimagnetic when it cools below the respective Curie temperature and then aligns itself permanently (remanently) with the earth's magnetic field in the surrounding rock. For example, on the basalts on both sides of the Juan de Fuca Ridge, it was not only possible to generally determine the increasing age of the rock depending on its distance from the ridge, but also the multiple changing polarity of the earth's magnetic field based on the contained titanomagnetite.
Education and Locations
Natural origin

Magnetite can be formed in igneous rocks as well as in metamorphic rocks and sedimentary rocks . In mafic igneous rocks such as basalts or gabbros, it is often an important secondary component that often crystallizes out early and therefore often forms well-defined crystals. However , it can also form dendrites in rapidly solidified rocks ( Limburgites ). It can also be found as an accessory in numerous other volcanic and plutonic rocks.
Remarkable are rocks consisting mainly of magmatite and apatite, which represent important commercial deposits (e.g. Kiruna in northern Sweden) and which are assumed to be of liquid-magmatic origin: Magmatic differentiation has resulted in a partial melt that is oxidic in character , d. i.e. it contains practically no more silicate components. In Kiruna this partial melt formed an intrusion body; however, lava flows from such rocks are also known (for example near El Laco in Chile ).
In conjunction with volcanic activity , magnetite can also be formed by pneumatolysis , if iron-containing volcanic gases (which carry volatile iron compounds such as iron (III) chloride ) can react with carbonate neighboring rocks. This mechanism can also form deposits ( skarn ore deposits ) with magnetite.
In metamorphic rocks, magnetite is a common mineral that can arise from numerous ferrous precursor minerals, especially under the conditions of contact metamorphism . An example of metamorphic rocks, which often have a high magnetite content , are the emery rocks formed from bauxites . Examples of regionally metamorphic magnetite rocks are the quartz-banded iron stones ( Itabirite ), which are also important as iron deposits.
Magnetite can also arise from the iron content of various precursor minerals through hydrothermal alteration processes . A well-known example is the magnetite content in serpentinites , which is often so high that the rock is visibly attracted by a magnet.
Since magnetite is very weather-resistant, it can be found accessory in numerous clastic sedimentary rocks. Here, too, it is sometimes enriched up to commercially relevant concentrations (magnetite sands). It also occurs very rarely as a primary mineral formation in sediments, for example in the Minette of Lorraine.
Depending on the formation conditions, magnetite occurs in paragenesis with other minerals, for example with chromite , ilmenite , Ulvöspinell , rutile and apatites in igneous rocks; with pyrrhotite , pyrite , chalcopyrite , pentlandite , sphalerite , hematite in hydrothermal or metamorphic rocks and with hematite and quartz in sedimentary rocks.
Synthetic manufacture
For the production of Fe 3 O 4 , a method which was first used by VAM Brabers for the production of single-crystal magnetite has proven to be the most suitable. With the help of the zone melting process, crystals are pulled in a mirror furnace . By heating a rod made of α-Fe 2 O 3 with 99.9% purity in the mirror furnace, a vertical melting zone is achieved between the supply and the crystal, which is held solely by the surface tension . B. prevented by the crucible material. The thus obtained crystals cm between the 2 and 5 are long and a diameter of about 5 have mm, are following the crystallization h in the mirror furnace 70 at 1130 ° C in an atmosphere of CO 2 and H 2 annealed to lattice defects heal and set the correct stoichiometry for magnetite. The orientation of the crystals along the rod axis roughly corresponds to the [100], [111] and [110] directions . The crystals are characterized by their excellent quality, measured by the characteristic of the transition temperature and the sharpness of the transition as expressed in the line of the conductivity curve (see Verwey transition ).
Occurrence

Magnetite occurs in solid or granular form and also as crystals, which are often octahedral in shape, so each have eight triangular boundary surfaces. It is a very common mineral, but it is rarely the main component of a rock. Magnetite is found in numerous igneous rocks such as basalt , diabase and gabbro , in metamorphic rocks and, due to its hardness, largely intact as magnetite sand is spent in river sediments due to weathering processes . From these it is partly washed out by hand today.
Magnetite can be found in large quantities on sandy beaches, where it leads to the typical black color of the sand. Such black beaches can be found e.g. B. in California , on the west coast of New Zealand and on the coasts of Fuerteventura and Iceland .
So far (as of 2010) magnetite has been detected at over 9600 sites worldwide. Very large deposits of magnetite can be found in Kiruna ( Sweden ), in the Pilbara Region in ( Western Australia ) and in the Adirondack Region of New York State (USA). Larger deposits of magnetite have been found in Norway , Germany , Italy , Switzerland , South Africa , India , Mexico and in Oregon , New Jersey , Pennsylvania , North Carolina , Virginia , New Mexico , Utah and Colorado in the USA .
Magnetite could also be detected in rock samples from the Mid-Atlantic Ridge and the East Pacific Ridge .
use
As a raw material
With an iron content of 72%, magnetite is one of the most important iron ores alongside hematite (70%) .
Magnetite is an important raw material for the production of ferrofluid . In the first step, magnetite nanoparticles (size approx. 10 nm) are produced, which are then colloidally suspended in a carrier liquid. To prevent agglomeration of the crystals, the nanoparticles are long-chain surfactants such. B. added oleic acid , which are grouped around the magnetite particles and prevent renewed sedimentation. The liquid obtained in this way retains the property of magnetite to react to magnetic fields.
As a building material
Magnetite is used in the construction industry as a naturally granular aggregate with a high bulk density (4.65 to 4.80 kg / dm 3 ) for sand-lime bricks and heavy concrete and for structural radiation protection .
As a pigment
Due to its excellent magnetic properties, magnetite is used as a magnetic pigment for data storage and is still used today in the construction of compasses . Fine-particle synthetic magnetite is known as iron oxide black (Pigment Black 11) (see also iron oxide pigment ) as a pigment , e.g. B. used for paints .
In semiconductor electronics
Due to the 100% spin polarization of the charge carriers predicted by the theory , magnetite is also traded as a hot candidate for spin valves in spin electronics .
In living beings
Various animal species rely on magnetite for orientation in the earth's magnetic field . These include bees and mollusks (Mollusca) . Particularly noteworthy are domestic pigeons , which is smaller by incorporating eindomäniger magnetite in the beak field strength determine the earth's magnetic field and can be oriented so (see also magnetic sense ).
Some bacteria , called magnetotactic bacteria, such as B. Magnetobacterium bavaricum , Magnetospirillum gryphiswaldense or Magnetospirillum magnetotacticum , form 40 to 100 nm large magnetite single crystals inside their cells, which are surrounded by a membrane . These particles are called magnetosomes and are arranged in the form of linear chains. The chains are, so to speak, compass needles and allow the bacteria to swim in a straight line along the earth's magnetic field lines.
Most regions of the human brain also contain around five million magnetite crystals per gram and the meninges , more precisely the outer and inner meninges ( dura and pia ), contain more than 100 million magnetite crystals with a size of around 50 nm.
In cancer therapy
Magnetite can be used to support cancer treatment. For this purpose, magnetite nanoparticles are modified in such a way that they are dispersed in a suspension in the body and are preferably taken up by tumor cells. This leads to the accumulation of the particles in the relevant areas. The particles are then made to vibrate by an external magnetic field. The resulting heat creates an artificial fever (so-called hyperthermia ), which makes the cell in question more susceptible to other treatment methods. In 2010, this therapy method received European approval.
Life on mars?
In 1996 scientists published an article in the recognized journal Science about the possible detection of life in the form of bacteria on Mars using a meteorite ( ALH 84001 ) that originated there. The meteorite contains small, single-domain magnetite particles, which are typically found in magnetotactic bacteria on earth. However, the debate about the interpretation of the measurement results continues to this day.
Esoteric
The properties that are ascribed to the stone magnetite were described by Hildegard von Bingen in the 12th century . According to esoteric doctrine, they are: activation (mental and physical), increasing the ability to react and stimulating the flow of energy and glandular activity. Magnetite is therefore a stone that emits rays in meditation and creates a particularly relaxing aura. He should z. B. help against feelings of hunger, body odor and heavy sweating, tension and cramps. Furthermore, it is said to be anti-inflammatory, helps with poisoning and cell renewal. It brings harmony, warmth, releases blockages and makes you happier and more carefree.
See also
literature
- Hans Berckhemer: Fundamentals of geophysics . 2nd Edition. Institute for Meteorology and Geophysics, Frankfurt am Main 2005, ISBN 3-534-13696-9 .
- Martin Okrusch, Siegfried Matthes: Mineralogy. An introduction to special mineralogy, petrology and geology . 7th, completely revised and updated edition. Springer, Berlin [a. a.] 2005, ISBN 3-540-23812-3 , pp. 83-84 .
- Albert Radl: The magnetic stone in antiquity. Sources and connections . Franz Steiner Verlag, Wiesbaden, Stuttgart 1988, ISBN 3-515-05232-1 .
Web links
- Mineralienatlas: Magnetit und Mineralienatlas: Mineralienportrait / Magnetit (Wiki)
- Magnetites. In: mindat.org. Hudson Institute of Mineralogy, accessed September 20, 2019 .
- Magnetite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed on September 20, 2019 .
- American-Mineralogist-Crystal-Structure-Database - Magnetite. In: rruff.geo.arizona.edu. Retrieved September 20, 2019 .
- Bacteria solving the mystery of the magnetic sense: Researchers decipher the origin of magnetosome chains. November 21, 2005, accessed September 20, 2019 .
Individual evidence
- ↑ a b Michael E. Fleet: The structure of magnetite: Symmetry of cubic spinels . In: Journal of Solid State Chemistry . tape 62 , no. 1 , March 15, 1986, p. 75-82 , doi : 10.1016 / 0022-4596 (86) 90218-5 .
- ↑ a b c d Magnetites . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 147 kB ; accessed on September 20, 2019]).
- ^ Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 363 .
- ↑ Malcolm Back, William D. Birch, Michel Blondieau and others: The New IMA List of Minerals - A Work in Progress - Updated: September 2019. (PDF 2672 kB) In: cnmnc.main.jp. IMA / CNMNC, Marco Pasero, September 2019, accessed September 20, 2019 .
- ↑ a b c Lexicon of Geosciences - Titanomagnetite. In: Wissenschaft-online.de. Spektrum.de , December 4, 2014, accessed on September 20, 2019 .
- ↑ cf. for example Jürgen Martin: The 'Ulmer Wundarznei'. Introduction - Text - Glossary on a monument to German specialist prose from the 15th century. Königshausen & Neumann, Würzburg 1991 (= Würzburg medical-historical research. Volume 52), ISBN 3-88479-801-4 (also medical dissertation Würzburg 1990), p. 149.
- ↑ Theophrast von Eresus: About the stones.
- ↑ Gaius Pliny Secundus: Historia Naturalis.
- ^ WH Bragg, FRS Cavendish: The Structure of the Spinel Group of Crystals . In: The philosophical magazine . tape 30 , no. 176 , 1915, ISSN 1478-6435 , pp. 305-315 , doi : 10.1080 / 14786440808635400 .
- ^ GA Samara, AA Giardini: Effect of Pressure on the Neel Temperature of Magnetite . In: The physical review . tape 186 , no. 2 , 1969, ISSN 0031-899X , p. 577-580 , doi : 10.1103 / PhysRev.186.577 ( documents.htracyhall.org [PDF; 2.1 MB ; accessed on September 20, 2019]).
- ↑ EJW Verwey: Electronic Conduction of Magnetite (Fe 3 O 4 ) and its Transition Point at Low Temperatures . In: Nature . tape 144 , August 1939, ISSN 0028-0836 , p. 327-328 , doi : 10.1038 / 144327b0 .
- ^ Russell W. Millar: The heat capacities at low temperatures of "Ferrous Oxide" magnetite and cuprous and cupric oxides . In: Journal of the American Chemical Society . tape 51 , no. 1 , 1929, ISSN 0002-7863 , pp. 215–224 , doi : 10.1021 / ja01376a026 .
- ↑ D. Schrupp, M. Sing, M. Tsunekawa, H. Fujiwara, S. Kasai, A. Sekiyama, S. Suga, T. Muro, VAM Brabers, R. Claessen: High-energy photoemission on Fe 3 O 4 : Small polaron physics and the Verwey transition . In: epl, a letters journal exploring the frontiers of physics . tape 70 , no. 6 , 2005, ISSN 0302-072X , p. 789-795 , doi : 10.1209 / epl / i2005-10045-y .
- ↑ a b Titaniferous Magnetites. In: mindat.org. Hudson Institute of Mineralogy, accessed September 20, 2019 .
- ↑ Mineral Atlas: Titanomagnetite
- ^ Victor Vacquier : Geomagnetism in Marine Geology . Elsevier Science Ltd, 1972, ISBN 978-0-444-41001-6 , pp. 40 . In: Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 363 .
- ^ Paul Ramdohr, Hugo Strunz: Klockmanns textbook of mineralogy . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 503-505 .
- ^ Walter Pohl: Mineral and energy raw materials . 5th edition. Schweizerbart, Stuttgart 2005, ISBN 3-510-65212-6 , p. 12-13 .
- ↑ Roland Vinx: Rock determination in the field . 3. Edition. Spectrum, Heidelberg 2011, ISBN 978-3-8274-2748-9 , p. 428-429 .
- ↑ VAM Brabers: The preparation of tetragonal single crystals in the Mn x Fe 3-x O 4 system . In: Journal of crystal growth . tape 8 , no. January 1 , 1971, ISSN 0022-0248 , pp. 26-28 , doi : 10.1016 / 0022-0248 (71) 90017-0 .
- ↑ a b List of locations for magnetite in the Mineralienatlas and Mindat (English)
- ↑ David Barthelmy: Mineral Species sorted by the element Fe (Iron). In: webmineral.com. Retrieved September 20, 2019 .
- ↑ Entry on iron oxide pigments. In: Römpp Online . Georg Thieme Verlag, accessed on June 12, 2014.
- ↑ Akira Yanase, Kiiti Siratori: Band Structure in the High Temperature Phase of Fe 3 O 4 . In: Journal of the Physical Society of Japan . tape 53 , no. 1 , 1984, ISSN 0031-9015 , pp. 312-317 , doi : 10.1143 / JPSJ.53.312 .
- ↑ W. Eerenstein, TTM Palstra, SS Saxena, T. Hibma: Spin-Polarized Transport across Sharp Antiferromagnetic Boundaries . In: Physical review letters (PRL) . tape 88 , no. 24 , 2002, ISSN 0031-9007 , doi : 10.1103 / PhysRevLett.88.247204 .
- ↑ AM Haghiri-Gosnet, T. Arnal, R. Soulimane, M. Koubaa, JP Renard: Spintronics, perspectives for the half-metallic oxides . In: Physica status solidi. A: Applications and materials science . tape 201 , no. 7 , 2004, ISSN 0031-8965 , p. 1392-1397 , doi : 10.1002 / pssa.200304403 .
- ↑ Michael Winklhofer: From magnetic bacteria to carrier pigeon . In: Physics of Our Time . tape 35 , no. 3 , 2004, ISSN 0031-9252 , p. 120–127 , doi : 10.1002 / piuz.200401039 ( From magnetic bacteria to carrier pigeon ( Memento from December 31, 2017 in the Internet Archive ) [PDF; 633 kB ; accessed on September 20, 2019]).
- ↑ Marianne Hanzlik: Electron microscopic and magnetomineralogical investigations on magnetotactic bacteria of the Chiemsee and on bacterial magnetite iron-reducing bacteria . Herbert Utz Verlag, Munich 1999, ISBN 978-3-89675-632-9 ( limited preview in the Google book search - dissertation. Ludwig Maximilians University).
- ^ André Scheffel, Manuela Gruska, Damien Faivre, Alexandros Linaroudis, Jürgen M. Plitzko, Dirk Schüler: An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria . In: Nature . tape 440 , 2005, pp. 110–114 , doi : 10.1038 / nature04382 .
- ↑ Joseph L. Kirschvink, Barbara J. Woodford: Superparamagnetism in the human brain . In: Thirteenth Annual Meeting of the Bioelectromagnetics Society . tape 80 , 1991.
- ↑ Joseph L. Kirschvink, Atsuko Kobayashi-Kirschvink, Barbara J. Woodford: Magnetite biomineralization in the human brain . In: Proc. Natl. Acad. Sci. USA . tape 89 , no. 16 , 1992, pp. 7683-7687 , doi : 10.1073 / pnas.89.16.7683 ( authors.library.caltech.edu [PDF; 1.7 MB ; accessed on September 20, 2019]).
- ↑ David S. McKay, Everet K. Gibson Jr., Kathi L. Thomas-Keprta, Hojatolla Vali, Christopher S. Romanek, Simon J. Clemett, Xavier DF Chillier, Claude R. Maechling, Richar N. Zare: Search for past life on Mars. Possible relic biogenic activity in martian meteorite ALH 84001 . In: Science . tape 273 , 1996, ISSN 0036-8075 , pp. 924–930 , doi : 10.1126 / science.273.5277.924 ( websites.pmc.ucsc.edu [PDF; 2.9 MB ; accessed on September 20, 2019]).



