ice
| ice | |
|---|---|
| Ice crystals in the Kungur ice cave with a clearly hexagonal structure | |
| General and classification | |
| chemical formula | H 2 O |
|
Mineral class (and possibly department) |
Oxides and hydroxides |
|
System no. to Strunz and to Dana |
4.AA.05 ( 8th edition : IV / A.01) 04.01.02.01 |
| Crystallographic Data | |
| Crystal system | hexagonal |
| Crystal class ; symbol | dihexagonal-dipyramidal; 6 / m 2 / m 2 / m |
| Space group | P 6 3 / mmc (No. 194) |
| Lattice parameters | a = 4.497 (5) Å ; c = 7.322 (4) Å |
| Formula units | Z = 4 |
| Twinning | {0001} and {000 1 } |
| Physical Properties | |
| Mohs hardness | 1.5 at 0 ° C, increasing at lower temperatures |
| Density (g / cm 3 ) | 0.917 |
| Cleavage | is missing |
| Break ; Tenacity | shell-like |
| colour | colorless, white, shimmering pale blue-green in thick layers |
| Line color | White |
| transparency | transparent to opaque |
| shine | Glass gloss |
| Crystal optics | |
| Refractive indices |
n ω = 1.309 n ε = 1.311 |
| Birefringence | δ = 1.001 |
| Optical character | uniaxial (direction not defined) |
| Pleochroism | unavailable |
Ice is the third state of aggregation of water and is formed at normal pressure , the presence of crystallization nuclei and a temperature lower than 0 ° C . As a naturally occurring crystalline solid with a defined chemical composition, ice is a mineral . Due to its chemical structure H 2 O, ice belongs to the group of oxides .
Ice crystallizes in the hexagonal crystal system and occurs in nature in various forms, from snowflakes to hailstones and the frozen surface of mostly stagnant water to glaciers . Its density of 0.918 g / cm³ (pure, air-free ice at 0 ° C ) is lower than that of water (1 g / cm³). Due to this density anomaly, ice floats on the surface of the water and forms ice sheets , ice floes and icebergs there . Around 90 percent by volume of the ice is under water (buoyancy of the water against the weight of the ice) and only around 10 percent by volume above the water surface.
In its pure form, ice consists of colorless, transparent crystals . However, ice blocks usually contain many fine air bubbles that are enclosed during the solidification of the ice crystals and therefore appear white due to multiple refraction . As a chemical substance , it is characterized by some special properties that are based on the anomalies of the water .
Ice plays an important role in numerous meteorological phenomena. The ice caps of the polar regions are of great importance for the global climate and especially for the global water cycle . Accordingly, it also has a decisive influence on our biosphere .
The science of the shapes, occurrences and properties of ice and snow is called glaciology .
Etymology and history
The origin of the word ( etymology ) of ice can be traced back via the Old High German , Middle High German and Low German 'îs' to the Germanic 'īsa'. By diphthonging (sound change from one to two vowels), this original word became, among other things, the German ice and the English ice.
However, ice did not appear as an independent type of mineral until the beginning of the 19th century . Previously, it was (including water, snow and hail) since antiquity according to the four-element theory, alongside fire, air and earth, as one of the four basic elements, and even in Abraham Gottlob Werner's systematics , ice was not used until the last edition in 1817 listed (1st edition 1787).
It was only Friedrich Hausmanns who described water or its various solid forms (varieties) in his Handbuch der Mineralogie from 1813 as a mineral, classified in the second class of "incombustibilien" and the second class of "oxydoids". According to Hausmann, ice and snow belong to “soft water”, which occurs in tabular form as ice floes, stalactitic as icicles, bark- shaped as black ice and spheroidal as hail.
classification
Already in the outdated 8th edition of the mineral classification by Strunz ice belonged to the class of "oxides and hydroxides" and then to the Department of "compounds with M 2 O and MO", where it acts as ice (I) together with ice (Ic) the unnamed group IV / A.01 formed. In the last revised and updated Lapis mineral directory by Stefan Weiß in 2018 , which, out of consideration for private collectors and institutional collections, is still based on this classic system of Karl Hugo Strunz , the mineral was given the system and mineral number. IV / A.01-10 . In the "Lapis system" this corresponds to the department "Oxides with the ratio metal: oxygen = 1: 1 and 2: 1 (M 2 O, MO)", where ice is the only member that forms an independent but unnamed group.
The 9th edition of Strunz's mineral systematics , valid since 2001 and updated by the International Mineralogical Association (IMA) until 2009, also classifies ice in the department of "Oxides with the molar ratio of metal: oxygen = 2: 1 and 1: 1". However, this is further subdivided according to the exact anion-cation ratio and the relative size of the cations, so that the mineral is classified according to its composition in the sub-section “Cation: Anion (M: O) = 2: 1 (and 1.8: 1 ) “ Can be found, where it forms the unnamed group 4.AA.05 as Eis-Ih together with Eis-Ic .
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns the ice to the class of "oxides and hydroxides" and there in the category of "oxides". Here it is to be found as the only member in the unnamed group 04.01.02 within the sub-section " Simple oxides with a cation charge of 1+ (A 2 O) ".
Crystal structure
In the solid state of aggregation of the water usually is a high as ice long-range order due to formation of a crystal lattice in the course of crystallization achieved. In the liquid state there is a mixture of order and chaos.
Natural ice crystallizes in the hexagonal crystal system in the space group P 6 3 / mmc (space group no. 194) with the lattice parameters a = 4.497 (5) Å and c = 7.322 (4) Å as well as four formula units per unit cell .
Six water molecules combine to form a ring via hydrogen bridges , with each molecule also being part of two neighboring rings. The hexagonal symmetry of the crystal structure is reflected in the macroscopic shape of the ice crystals . In this structure, each oxygen atom is tetrahedrally surrounded by four other O atoms.
Hexagonal ice is referred to as ice I h .
Modifications
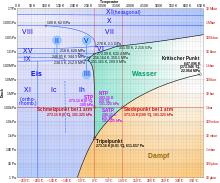
Below −22 ° C and above 207.5 MPa , other, for example, cubic ice shapes form, such as the metastable, cubic ice I c , in which the oxygen atoms have a diamond structure. So far 17 crystalline and 5 amorphous modifications are known (as of January 2010). The latter are forms without a crystal structure .
The 17 crystalline forms are referred to as ice I h , ice I c , and ice II to ice XVI .
Ice clouds in interstellar space have a temperature of approx. −260 ° C and are amorphous in structure ("flow").
properties
Solidification process
The melting or freezing point of ice under normal conditions is 0 ° C (the “ ice point ”), the specific heat of fusion is λ S = 332.8 kJ / kg .
Crystallization nuclei , i.e. impurities such as dust particles, bacteria, etc. are, however, a condition for ice crystal formation, since the crystallizing water molecules have to attach to them. In so-called “supercooled water”, not frozen water below 0 ° C, the molecules have a local order that differs from the normal case, and icosahedral structures are formed . B. clean, supercooled mineral water spontaneously freezes on the gas pearls produced when the bottles are opened. Without an external trigger, water freezes at −48 ° C. Very pure ( distilled ) water can be up to -70 ° C supercooled be.
The freezing point can be reduced by sprinkling with salt (road salt ). This is a colligative property , the lowering of the freezing point depends only on the amount of dissolved particles, not on their type. The same effect can also be achieved with sugar .
In addition, the heat of solution in a substance can melt ice. The decisive factor here is that the substance added is insoluble in the solid solvent. This effect is achieved by lowering the chemical potential of the liquid phase. This effect also increases the boiling point of the water.
Melting process
The transition from a solid to a liquid state is called melting . To melt ice, hydrogen bonds between the water molecules in the ice must be broken. To do this, energy must be supplied to the ice. When melting, it absorbs as much energy as would be required to heat an equivalent mass of water to 80 ° C. The temperature of the melting ice surface remains constant at 0 ° C during melting. The speed of the melting process therefore depends on the efficiency of the energy supply to the ice surface. An ice surface in fresh water melts solely by free convection at moderate water temperature at a rate that, like (T ∞ - 4 ° C) 4/3 , depends on the temperature of the fresh water, T ∞ .
sublimation
If the air is sufficiently cold and dry at atmospheric pressure, ice passes directly into gaseous form (water vapor) through sublimation . This effect is u. a. used in freeze drying on an industrial scale.
colour

Ice changes its color with the air content and can therefore also be divided into different groups. Ice that contains a lot of air is white; ice that contains little air is transparent and blue or green. A special case of "colored" ice are so-called green icebergs , which are old overturned icebergs whose algae-covered underside is now visible.
Ice and snow reflect the sunlight. Ice particles cause columns of light in the earth's atmosphere. (The related halos , on the other hand, are created by the refraction of light in ice crystals.) Astronomically and geophysically, ice and snow are often the cause of high reflections from an object.
Sound propagation
The speed of sound in ice at maximum density is 3250 m / s. In contrast to most solids, the dispersion for sound propagation in ice is negative. This effect can be observed on frozen lakes. If, for example, a crack develops in the ice surface at a sufficiently large distance from the observer (e.g. from solar radiation), a whistling noise can be heard in which the pitch drops from very high frequencies to very low frequencies in a fraction of a second. The sound is similar to that of a projectile flying past , which creates a falling pitch through the Doppler effect.
Heat absorption and conduction
At a temperature of 0 ° C, ice has a specific heat capacity of 2.12 kJ / (kg · K), which decreases slightly at lower temperatures. Its thermal conductivity at 0 ° C is 2.21 W / (m · K) and increases slightly as the temperature drops. Compared to liquid water at 20 ° C, ice near the melting point only has half the specific heat capacity, but three and a half times the thermal conductivity.
hardness
According to the Mohs hardness scale , ice at a few degrees below zero only has a low hardness of 1.5 and can be scratched with a fingernail. However, the Mohs hardness of ice increases at lower temperatures. At −30 ° C it exceeds that of limestone (hardness 3) with a hardness of 3.5 , until it finally reaches the hardness of heat-treated steel (Mohs hardness approx. 6) at −80 ° C.
Carrying capacity of ice sheets
Even at a few degrees below zero, ice is able to carry people and even heavy vehicles such as trucks . During the construction of the Trans-Siberian Railway at the beginning of the 20th century, rails were even laid on Lake Baikal. Hundreds of rail vehicles crossed it without any problems, only one locomotive broke through the ice sheet and sank. The prerequisite for this is a sufficient thickness of the ice cover for the respective load. The minimum thickness for a safe load capacity according to the requirement is based on empirical values or can be calculated using various methods. The resilience and the minimum thickness are significantly influenced by the nature of the ice, such as cracks and air pockets, as well as the state of swimming.
The following ice thicknesses (on liquid water) are considered sufficient:
- Individuals: 5 cm
- Groups of people: 8 cm
- Sleigh vehicles: 12 cm
- Cars, other vehicles: 18 cm
The load-bearing capacity of an ice sheet depends on the one hand on its swimming buoyancy on the carrying water and on the other hand on the load distribution capacity (deflection) in the case of point loads. In both cases, the thickness of the ice cover is the decisive parameter for the bearing capacity. The load capacity due to the buoyancy is proportional to the ice thickness, while the load distribution capacity is proportional to the square of the ice thickness.
If the load is evenly distributed over large areas without deflection, the load-bearing capacity is limited, as with a raft, by the floatability of the ice cover. Corresponding to the buoyancy of bubble-free ice with a density of 917 kg / m³, the load-bearing capacity (in kg / m²) for large areas of the thickness is: (in m).
So z. B. 8.3 kg / m² with an ice thickness of 10 cm.
By distributing the load in the surrounding area, limited areas of an ice sheet can be subjected to significantly higher loads. However, it must always be ensured that the permissible load on partial areas does not exceed the maximum load on the entire ice cover.
The load-bearing capacity of an ice road in relation to individual vehicles can also be estimated using the so-called "gold formula" (named after Lorne W. Gold ):
With
- = permissible total mass of a single vehicle
- = Thickness of blue ice
- = Thickness of the white ice
The Canadian province of Manitoba uses these formulas to determine the carrying capacity of an ice sheet for use as a winter road. The decision as to which load the ice road is released for is ultimately always made by an ice road expert.
There are temporary ice roads in Sweden, Finland, Estonia, Canada, the United States and Russia.
Entering ice surfaces is generally dangerous and should be avoided if in doubt. This is especially true because the thickness and texture of the ice often cannot be reliably determined. To determine the thickness of the ice, ice screws or drills with centimeter marks are suitable, as well as measuring holes made in the ice.
Breaking into the ice creates the risk of severe hypothermia , frostbite and drowning . When rescuing, rescue aids should be used if possible, which distribute the weight of the rescuer over a larger area. The hand of the person who has broken in should not be shaken, but aids that can be let go in an emergency. Ice claws can be carried with you to rescue yourself and make it easier to pull it out of the hole.
Anomalies
Water exhibits numerous anomalies : properties that deviate from the rules that can be applied to most substances. The following anomalies are important for its solid state as ice:
- Ice is less dense, so it is lighter than water, so it floats on the water. This density anomaly occurs because the water molecules in the hexagonal lattice of the ice have a greater distance from one another than in the liquid, disordered state. → See also: Numerical values for density anomaly and expansion coefficient of ice and water
- In the phase diagram , water 14 has crystalline and therefore a particularly large number of fixed modifications. There are also other metastable ones, five of which are crystalline and three are amorphous .
- As amorphous ice, a state is referred to, in the solid water do not like in a crystal having a regular structure but with an irregular structure, such as a liquid, but without mutual displacement of molecules . Three of these glass waters are known: one that has a lower density than liquid water (LDA), one with high density (HDA) and one with very high density (VHDA), which can exist in the low temperature range down to a maximum of −122 ° C.
- Increased pressure lowers the melting point of water instead of increasing it (see phase diagram). Per bar pressure increase, the melting point is lowered by approximately 0.0077 K (at pressures above about 500 bar, the melting point decrease behaves disproportionately). This is also known as pressure melting .
- With the help of coherent and strongly collimated X-rays , which are totally reflected on the surface of ice , it can be shown that above −38 ° Celsius it is liquid in the outermost molecular layer. Above −16 ° Celsius, a second molecular layer is added, and the layer thickness of this liquid film increases to 50 nanometers until the melting point.
- Magnetic fields can change the melting point slightly. It is believed that the magnetic field indirectly strengthens the hydrogen bonds of the water molecules. With a magnetic field of six Tesla, the melting point rises from normal water of 5.6 m K and at heavy water 21.8 mK.
Education and Locations


On earth
Ice forms around the world where the humidity is high enough and the temperature has dropped to or below freezing point .
Free ice crystals arise in the form of frost and hoarfrost through resublimation (direct transition from the gaseous to the crystalline state) of the atmospheric water vapor . Sleet and hail consist of roundish grains of ice. They are formed in thunderclouds from water droplets, which condense in deep cloud layers and are then transported by updrafts to higher and colder layers of air, where they then freeze. Larger hailstones are often agglomerations of smaller ice particles and go through the process of rising through winds and sinking through their weight several times in the history of their formation . Snow consists of more or less filigree ramified ice crystals. Snowflakes are formed by the slow accumulation and freezing of the finest water droplets on a crystallization nucleus (e.g. dust particles).
The sea frosts on Lake Constance are events of the century. The ice cover is then so stable that the entire lake can be crossed on foot. During the last sea frost in 1963, daredevils even drove a small car from Lindau across the ice to Switzerland.
Ice surfaces that are permanently connected to the mainland are called ice shelves . The ice shelves are mostly fed by flowing glaciers. Icebergs are ice masses broken off (calved) by glaciers.
So-called sea ice is formed when seawater crystallizes ; the salt is released into the sea or collects in brine (salt) inclusions (ice itself is always solid fresh water). Depending on the size and agglomeration of the ice, a distinction Nadeleis, Grieseis, pancake ice , ice floes and pack ice . A natural ice-free area that is completely surrounded by pack ice is called polynya . Artificial gullies and holes made in the ice are called Wuhnen .
Ice which is exceptionally because of its history at the bottom of a water body is Grundeis called. The formation of new ice on the sea is known as the Nilas .
The ice conditions in marine areas are identified with an international ice code :
- 0: No ice ; no ice, ice-free
- 1: slush or young ice ; Mud or new ice (young ice)
- 2: Fast ice ; Fast ice
- 3: drift ice ; Drift ice, ice rush
- 4: Packed slush or strips of hummocked ice ; packed muddy ice or hump ice strips (ice hump strips)
- 5: Open lead near shore ; open ice channel (continuous channel in the ice) near the coast
- 6: heavy fast ice ; strong fast ice
- 7: heavy drift ice ; strong drift ice
- 8: hummocked ice ; Ice cusp, ice cusps (ice pyramids rising above the smooth ice), pressed ice
- 9: ice jamming ; Ice blocking
Ice VII can also occur on Earth as an inclusion in diamonds. This has a cubic crystal structure.
In the solar system
Ice deposits have been detected in our solar system in comets , asteroids , on Mars and on some moons of the outer planets . In icy moons , the entire surface of ice is almost covered.
Numerous comets are known to consist largely of water ice, which is why they are sometimes referred to as "dirty snowballs". It is speculated that a large part of the earth's water resources can be traced back to a long-lasting bombardment of the still young earth by comets. Most of the water in the universe is in the form of ice.
So far, ice has also been found on Mars . In addition to the polar ice caps, which undoubtedly consist partly of frozen water, there may also be ice deposits in other regions, namely as permafrost in deeper soil layers.
In 1975, the Mariner 10 space probe provided evidence of the presence of ice in meteorite craters near the poles near Mercury , the planet closest to the sun . More detailed investigations by the MESSENGER space probe were able to confirm water on the North Pole, on which no sunlight falls, in November 2011.
Some moons of the outer planets are known or assumed to be covered by an ice crust. Examples are the Jupiter moons Europa , Ganymede and Callisto , the Saturn moons Enceladus and Titan , the Neptune moon Triton and the Pluto moon Charon . Some of these moons are under their surface layers of ice modifications have that occur only at high pressure.
Early radar images of the south pole of the moon from the 1990s with many small, strikingly bright spots gave many researchers hope that the moon had large water reserves, which, among other things, could have survived as relics of cometary impacts at the bottom of deep craters. Such deposits would be important sources of water and oxygen for future lunar bases. Investigations in 2006 with radio telescopes were negative. In 2009 the LCROSS mission was able to detect water ice. In 2010, the Chandrayaan-1 probe found evidence of at least 600 million tons of water at the Moon's North Pole.
Use and disability
- food and drinks
Even the Romans used expensive imported glacier ice to cool food and to make soft drinks .
Commercial use of winter ice began in North America in the 19th century , initially as a luxury item for people in tropical countries , and later as a bulk item for household use. The export of natural ice from Norway to England and France and, to a lesser extent, to Germany is also known from the 19th century . The ice cream man brought ice blocks with which perishable food, typically in a refrigerator , could be kept longer edible. With the electrification and introduction of the refrigerator , this trade came to an end. Today almost all ice cream used by humans for food purposes is made by refrigerators or in refrigerators.
In beer production, too, cooling with natural ice, which was stored in so-called ice cellars in summer , played a decisive role in shelf life. As early as the middle of the 19th century, refrigeration machines powered by steam were mostly used for this purpose.
Under Nutzeis flavors are called, which are produced in ice factories to a certain benefit. This includes B. classic stick ice , but also ice cubes and flake ice , which are consumed, but only because they are added to the drink or in the sausage production for cooling and inevitably dissolve in it.
Flake ice is used in the laboratory and in the production of food when mechanical processing such as B. the cutting in sausage production , temperatures arise that are detrimental to the consistency or color and taste of the food. Ice is ground in flake ice makers down to a grain size of a few millimeters. Flake ice plays an important role in the storage and freshness of fish and seafood. Ice cubes are often used for drinks .
Ice cream , on the other hand, is a mass of snow or ice slurry made from fruit juices or mixed milk beverages.
- Crop production
In plant cultivation, ice serves as frost protection by spraying water on the plants when it is frosty , whereby all parts are covered by a layer of ice. The freezing of the water creates heat of crystallization .
- Sports
The frictional heat generated by runners on firm ice creates a layer of water a few µm thick under an ice skate, on which the rear part of the runners then glides almost smoothly. Ice skating , but also skiing , sledding or sledding as a means of transport are therefore possible. The pressure under the narrow runners only lowers the freezing point of the water by a few tenths of a degree.
- traffic
Ice deposits have a particularly hindering effect on traffic in the form of pack ice for shipping (see also icebreaker ), as a smooth film of ice on roads (see also snow chains ), footpaths or on airplanes, and as snow drifts on all land modes of transport. Freezing rain (“lightning ice”) is a problem in traffic . Ice on overhead lines prevents electricity from being drawn. To reduce the risk of slipping, ice surfaces are blunted with sand or thawed away with salt .
Dangerous ice can, if for ships water on deck or fog or drizzle freezes on the superstructure and a thick layer of ice forms. This moves the center of the ship upwards, leading to shifts capsize can result in the ship. When colliding with ships, icebergs can damage them or, as with the Titanic , cause them to sink. Pack ice can crush ships trapped in it. Frozen bodies of water can, on the one hand, hinder shipping, but on the other hand, they can also shorten transport routes, as land transport can be carried directly over the water surface ( ice runways ).
- Construction
The ice load caused by freezing rain or frost is a problem for buildings (see snow load , also applies to ice). Overhead lines can tear due to the ice load.
Construction projects can also be hampered by the solidification of the ground by ice. On the other hand, the consolidation of the soil can be intentional and, for example, make tunneling work in loose soil possible in the first place. The icing is usually created artificially with large cooling units. In permafrost regions , the softening of the soil by the lack of frost a threat to buildings represent. Supports the Trans-Alaska Pipeline System (from 1975/1977) and parts of the route of the Qinghai-Tibet Railway (built in 2005), this via heat pipes (heatpipes) by Ambient air cooled.
Water pipes burst if they freeze in an uncontrolled manner - for example over a longer length or towards a barrier or a plug of ice. For protection, such lines are laid in the ground below the frost line or a minimum flow is ensured or emptied in good time. Water and sewage pipes, and occasionally rain pipes from roofs, may be equipped with electrical trace heating where they can be exposed to the cold. Conversely, ice can also be used for repairs: To replace a radiator or a piece of pipe, 2 small spots in the flow and return lines are plugged with ice by cooling the pipeline a few cm in length from the outside using carbon dioxide snow or a refrigeration machine .
Ice flowers on window panes obstruct the view, but are often aesthetically very attractive. However, they are considered to be signs of inadequate thermal insulation and are threatened with "extinction".
Entire houses made of ice are also possible. In the past, ice was used by Eskimos to build igloos , and there are also modern forms of construction.
Ice sculptures are made from blocks of ice .
simulation
As artificial ice an ice surface generated by technical cooling is called for ice skating and ice hockey.
In most cases, artificial ice surfaces are created using EPDM absorbers. This technology is very energy-efficient, cost-effective both to purchase and to operate. This is why this system is increasingly being used for large projects such as ice rinks, speed skating rings, etc. Furthermore, the flexible absorbers (ice mats) enable the production of mobile artificial ice rinks. The ice mats are rolled out next to each other, connected to a circuit and then filled with a water / glycol mixture. A refrigeration machine cools the mixture to approx. -10 ° C and pumps it through the ice mat surface, while the sprayed water freezes and then turns into an even surface of ice.
In a bouldering hall in Klagenfurt that opened on December 31, 2016 , ice climbing is simulated using plastic handle packages that also provide hold to piercing climbing axles.
See also
literature
- Astrid Döppenschmidt: The ice surface - investigations with the atomic force microscope . GCA-Verlag, Herdecke 2000, ISBN 3-934389-71-6 .
- Werner F. Kuhs: Physics and chemistry of ice . RSC Publ., London 2007, ISBN 978-0-85404-350-7 (English).
- Victor F. Petrenko, Robert W. Whitworth: Physics of ice . Oxford Univ. Press, Oxford 2006, ISBN 0-19-851894-3 (English).
- Miles McPhee: Air-ice-ocean interaction - turbulent ocean boundary layer exchange processes . Springer, New York 2008, ISBN 978-0-387-78334-5 (English).
- John D. Castello: Life in ancient ice . Princeton Univ. Press, Princeton 2005, ISBN 0-691-07475-5 (English).
- Pat Dasch: Icy worlds of the solar system . Cambridge Univ. Press, Cambridge 2004, ISBN 0-521-64048-2 (English).
- Guriĭ Nikolaevich I︠A︡kovlev: Studies in ice physics and ice engineering . Israel Program for Scientific Translations, Jerusalem 1973, ISBN 0-7065-1275-8 (English).
- LW Gold: Use of Ice Covers for Transportation . In: Canadian Geotechnical Journal . tape 8 , no. 2 , 1971, p. 170-181 , doi : 10.1139 / t71-018 .
- Kay D. Bidle, SangHoon Lee, David R. Marchant, Paul G. Falkowski: Fossil genes and microbes in the oldest ice on Earth . In: Proceedings of the National Academy of Sciences . tape 104 , no. 33 , 2007, p. 13455-13460 , doi : 10.1073 / pnas.0702196104 , PMID 17686983 (English).
- Don Hayley, Sam Proskin: Managing the safety of ice covers used for transportation in an environment of climate warming . In: 4th Canadian Conference on Geohazards . University Laval, Quebec January 2008, p. 1–7 (English, geohazard.ggl.ulaval.ca [PDF; 3.2 MB ; accessed on July 10, 2019]).
Web links
- Ice. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on July 10, 2019 .
- Christine Reinke-Kunze: (Almost) everything about ice and snow. In: folio.nzz.ch. NZZ Folio , accessed on July 10, 2019 ( full text of the article available in the web archive ( Memento from September 16, 2010 in the Internet Archive )).
- Ute Kehse: Ice cream in summer. In: Wissenschaft.de. Image of Science , August 19, 2005, accessed July 10, 2019 .
- Martin Chaplin: Water Structure and Science. In: lsbu.ac.uk. May 11, 2019, accessed July 10, 2019 .
Individual evidence
- ↑ a b c d David Barthelmy: Ice Mineral Data. In: webmineral.com. Retrieved July 12, 2019 .
- ↑ a b c American-Mineralogist-Crystal-Structure-Database - Ice. In: rruff.geo.arizona.edu. Accessed July 10, 2019 .
- ↑ Harvey, Allan H. (2017). "Properties of Ice and Supercooled Water". In Haynes, William M .; Lide, David R .; Bruno, Thomas J. (eds.). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton, FL: CRC Press. ISBN 978-1-4987-5429-3 .
- ↑ a b c Ice. In: mindat.org. Hudson Institute of Mineralogy, accessed July 10, 2019 .
- ↑ Water and its properties: water and density. In: Wissenschaft-technik-ethik.de. June 28, 2009, accessed on July 12, 2019 (density table of pure, air-free water at normal pressure (101300 Pa ("Pascal"), = 1013 mbar) between 0 and 100 ° C).
- ↑ a b Hans Lüschen: The names of the stones. The mineral kingdom in the mirror of language . 2nd Edition. Ott Verlag, Thun 1979, ISBN 3-7225-6265-1 , p. 208 .
- ↑ Boris Paraschkewow: Words and names of the same origin and structure: Lexicon of etymological duplicates in German . De Gruyter, Berlin 2004, ISBN 3-11-017470-7 ( limited preview in Google book search).
- ^ Johann Friedrich Ludwig Hausmann: Handbook of Mineralogy . Volume 3, from the class of the Incombustibile containing the orders of oxydoids, acids and salts. Vandenhoeck and Ruprecht, Göttingen 1813, p. 766 ( limited preview in Google Book search).
- ↑ Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ Ernest H. Nickel, Monte C. Nichols: IMA / CNMNC List of Minerals 2009. (PDF 1703 kB) In: cnmnc.main.jp. IMA / CNMNC, January 2009, accessed July 10, 2019 .
- ^ A b R. Steudel, Y. Drozdova: Chemistry of the non-metals: With atomic structure, molecular geometry and bond theory . 2nd, completely revised edition. de Gruyter, Berlin, New York 1998, ISBN 978-3-11-012322-7 , pp. 206-208 .
- ↑ Wolfgang W. Merkel: H 2 O - the eccentric. In: welt.de. Die Welt online, June 27, 2010, accessed on July 12, 2019 .
- ↑ Laura Sanders: A very special snowball. In: sciencenews.org. ScienceNews, September 11, 2009, accessed July 12, 2019 .
- ^ Christoph G. Salzmann, Paolo G. Radaelli, Erwin Mayer, John L. Finney: Ice XV: A New Thermodynamically Stable Phase of Ice. In: ucl.ac.uk. November 17, 2009, archived from the original on April 11, 2016 ; accessed on July 12, 2019 .
- ↑ Werner Kuhs: Eis XVI: Göttingen scientists produce new crystalline form of water. In: uni-goettingen.de. University of Göttingen, December 10, 2014, accessed on July 10, 2019 .
- ↑ alpha-Centauri: How is ice formed in the cosmos? In: www.br.de. Bayerischer Rundfunk Mediathek, accessed on July 10, 2019 .
- ↑ David F. Blake et al .: Life in Space . Ice cream - cradle of life? Spectrum of Science Publishing Company , Heidelberg 2002, ISBN 3-936278-14-8 , p. 25 22-27 .
- ↑ Heat of fusion and heat of vaporization. In: physik.uni-wuerzburg.de. University of Würzburg , December 14, 2004, accessed on July 12, 2019 .
- ↑ Josephina Maier: Just ask !: The core of the ice. In: badische-zeitung.de. Badische Zeitung , February 18, 2012, accessed on July 12, 2019 .
- ↑ Supercooled water. In: am.rlp.de. Rural Service Centers Rhineland-Palatinate, accessed on July 10, 2019 .
- ↑ Thomas Keitzl, Juan Pedro Mellado, Dirk Notz: Impact of Thermally Driven Turbulence on the Bottom Melting of Ice . In: Journal of Physical Oceanography . tape 46 , no. 4 , 2016, p. 1171–1187 , doi : 10.1175 / JPO-D-15-0126.1 (English, pure.mpg.de [PDF; 1.9 MB ; accessed on July 12, 2019]).
- ↑ ott: The unreal glowing icebergs of the Antarctic. In: welt.de. Die Welt online, February 26, 2015, accessed on July 12, 2019 .
- ↑ Mystery of Rare Emerald Icebergs Is Solved. In: nytimes.com. The New York Times online, May 4, 1993, accessed July 12, 2019 .
- ^ Martin Chaplin: Hexagonal Ice (ice Ih). June 3, 2019, accessed on July 12, 2019 (English, see footnote h).
- ↑ Michael Juhnke, Reiner Weichert: Generation of nanoparticles through fine comminution with high purity requirements. (PDF 4.2 MB) In: mvt.tu-clausthal.de. Institute for Mechanical Process Engineering, Clausthal University of Technology, January 18, 2006, p. 13 , accessed on July 12, 2019 .
- ↑ Bodo Thöns, Hans Engberding: Transsib manual. On the way with the Trans-Siberian Railway . 6th, revised and expanded edition. Trescher-Verlag, Berlin 2010, ISBN 978-3-89794-173-1 .
- ↑ Behavior at and on wintry waters. (PDF 779 KB) In: drk-berlin.de. DRK Wasserwacht Berlin, January 30, 2018, accessed on July 12, 2019 .
- ↑ Leaflet No. 2.0 / 3: Assessment of the bearing capacity of ice sheets. (PDF 109 kB) In: lfu.bayern.de. Bavarian State Office for the Environment, January 17, 2012, archived from the original on December 30, 2016 ; accessed on July 12, 2019 .
- ^ LW Gold: Use of Ice Covers for Transportation . In: Canadian Geotechnical Journal . tape 8 , no. 2 , 1971, p. 170-181 , doi : 10.1139 / t71-018 .
- ↑ Dangers in wintry waters. (PDF 1.09MB) In: lv-thueringen.drk.de. DRK Landesverband Thüringen eV, March 5, 2018, accessed on July 12, 2019 .
- ↑ Martin Chaplin: Explanation of the Density Anomalies of Water: D2. Water expands on freezing. In: www1.lsbu.ac.uk. London South Bank University , accessed July 12, 2019 .
- ↑ Bernd Müller: On the black ice . In: Physics Journal . 17th year, no. 1 , January 2018, p. 44-45 .
- ↑ Dörte Saße: Magnetic fields change the melting point of water. Die Welt , January 8, 2005, accessed July 12, 2019 .
- ↑ Deborah Netburn: What scientists found trapped in a diamond: a type of ice not known on Earth. In: latimes.com. Los Angeles Times , March 9, 2018, accessed July 12, 2019 .
- Jump up ↑ Humberto Campins, K. Hargrove, ES Howell, MS Kelley, J. Licandro, T. Mothé-Diniz, J. Zahl, Y. Fernandez, N. Pinilla-Alonso: Confirming Water Ice on the Surface of Asteroid 24 Themis . Ed .: American Astronomical Society. September 2009, bibcode : 2009DPS .... 41.3205C (English).
- ↑ Phoenix finds first Mars ice. In: scinexx.de. scinexx Das Wissensmagazin, June 2, 2008, accessed on July 12, 2019 .
- ↑ Water Ice in a Martian Crater - Astronomy Picture of the Day of July 20, 2005 (English).
- ↑ Tilmann Althaus: Planet moon Titan - a world with character. In: www.spektrum.de. Spectrum, February 24, 2012, accessed July 10, 2019 .
- ↑ Axel Orth: But no ice on the moon. In: Raumfahrer.net. October 22, 2006, accessed July 12, 2019 .
- ↑ Probe impact: Nasa finds water on the moon. In: spiegel.de. Spiegel Online , November 13, 2009, accessed July 12, 2019 .
- ↑ Alexis Madrigal: Lunar Impactor Finds Clear Evidence of Water Ice on Moon. In: wired.com. Wired Science, November 13, 2009, accessed July 12, 2019 .
- ^ LCROSS Impact Data Indicates Water on Moon. In: nasa.gov. NASA , November 13, 2009, accessed July 12, 2019 .
- ↑ NASA Radar Finds Ice Deposits at Moon's North Pole. In: nasa.gov. NASA , July 3, 2013, accessed July 10, 2019 .
- ↑ The ice cream cone. About glaciers, snow and ice cream . Birkhäuser Basel, Basel 1996, ISBN 978-3-0348-6110-6 , p. 230 , doi : 10.1007 / 978-3-0348-6110-6 .
- ↑ [1]
- ↑ [2]
- ↑ [3]
- ↑ Jürgen Vollmer, Ulrich Vetter: Ice skating: Why is ice so smooth? German Physical Society , February 22, 2008, accessed on July 12, 2019 .
- ↑ Ice climbing training in the new bouldering hall. In: kaernten.orf.at. ORF.at, December 30, 2016, accessed on July 12, 2019 .