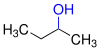
Alcohols
Alcohols ( Arabic الكحول, DMG al-kuḥūl , actually: fine antimony powder ) are organic chemical compounds that have one or more hydroxyl groups (–O – H) attached to different aliphatic carbon atoms .
The difference between alcohols and other compounds with OH groups (e.g. enols , hemiacetals or carboxylic acids ) as part of the functional group is that in alcohols every carbon atom that carries an OH group must be sp 3 -hybridized and except the hydroxyl group may only be bound to carbon or hydrogen atoms. Only this bond state corresponds to the oxidation state of a normal alkanol .
If the hydroxyl group is bonded to a non-sp 3 hybridized carbon atom that is part of an aromatic ring , then these compounds are referred to as phenols and do not count among the alcohols.
While alcohols are less acidic than water and with a pKa value of approx. 16 are among the “very weak acids”, normal phenols with a pKa value of 10 are already among the “weak acids”.
Nomenclature and classification
The name of simple alcohols is a combination of the name of the original alkane and the ending "-ol" . In addition, the position of the OH group is indicated by a preceding number, for example propan-2-ol . An outdated name for alcohols that was valid until 1957 is - according to a suggestion by Hermann Kolbe - carbinols .
The substance group of the alcohols is classified according to various criteria (number of non-hydrogen neighbors, valence, presence of double / triple bonds and chain length).

Number of non-hydrogen neighbors
A distinction is made between alcohols according to the number of C and H atoms on the C atom of the functional group to which the hydroxyl group is also bound. In the case of primary alcohols, two H atoms are bonded to this carbon atom in addition to one C atom, in secondary alcohols in addition to two C atoms one H atom and in tertiary alcohols in addition to three C atoms no hydrogen atom. A special case is alcohol with only one carbon atom, methanol, which in addition to the hydroxyl group has only three hydrogen atoms on the carbon atom of the functional group.
Valence of alcohols
If there is more than one hydroxyl group on different carbon atoms in an alcohol molecule , their number is indicated by adding a Greek syllable (-di, -tri- etc.) before the ending -ol , which corresponds to the number of hydroxyl groups , and one speaks of polyhydric alcohols. An alkane di ol is the ethane-1,2-diol (trivial name ethylene glycol ), an alkane tri ol , the propane-1,2,3-triol (common name glycerol ). The number before the ending -ol indicates the position of the functional group (s). This also applies to monohydric alcohols, for example propan-2-ol (common name isopropanol ).
Structural formula of ethanol
Structural formula propane-1,2-diol
Structural formula propane-1,3-diol
Double or triple bonds
With regard to the presence of double or triple bonds in the chain of carbon atoms, a distinction is made between alkanols (derived from alkanes ), alkenols (derived from alkenes ) and alkynols (derived from alkynes ). In the event that the OH group is bound to an sp 2 -hybridized carbon atom, you are dealing with a different oxidation state and thus with a different group of substances, namely with the mostly unstable enols .
Structural formula of allyl alcohol ( 2-propen-1-ol )
Structural formula of butynediol
Structural formula of ascorbic acid , an enediol
Chain length
Alcohols are also differentiated based on the chain length. The term fatty alcohols is used for alcohols with a terminal primary -OH group with a straight chain and a length of six ( hexanol ) up to 22 ( behenyl alcohol ) carbon atoms. They are mostly obtained from fatty acids by reducing the –COOH group . The higher primary alcohols with 24 to 36 carbon atoms are called wax alcohols .
Physical Properties
Low molecular weight alcohols are liquids that have a characteristic odor and a burning taste. Higher alcohols are mostly solid compounds with only a weak odor. Due to intermolecular hydrogen bonds , the alcohols have relatively high melting and boiling points compared to hydrocarbons of the same molecular mass . The most important common characteristic of the alcohols is their hydrophilicity . This property decreases with increasing length of the alkyl radical and increases with the number of hydroxyl groups. The short-chain alcohols in particular are often used as solvents due to their amphiphilic character .
High boiling points

Oxygen is more electronegative than hydrogen and carbon; i.e., it attracts electrons more strongly than these. This leads to an unsymmetrical distribution of the electrons along the C – O – H bond ; one speaks of a polar bond, a molecular dipole is formed. These dipoles can form hydrogen bonds with one another , which drastically increase the attraction between the individual molecules. For alcohols, this leads to relatively high boiling points compared to the homologues of their parent compounds, which are lengthened by one methylene unit and have approximately the same molar mass . For example, the non-polar ethane (C 2 H 6 ) (M = 30) has a boiling point of −89 ° C, while methanol (CH 3 OH) (M = 32) only reaches this at 65 ° C.
In summary:
- Compared to alkanes with a comparable molar mass, alcohols have a higher melting and boiling point because the hydroxyl group (OH group) forms hydrogen bonds.
- The more hydroxyl groups a molecule has, the more hydrogen bonds can be formed and the higher the boiling point.
- Van der Waals forces also develop between the alkyl radicals . Therefore the boiling point increases with the length of the alkyl radical
- Since the strength of the Van der Waals interactions depends not only on the size of the alkyl group but also on its surface area, strongly branched, rather spherical molecules with a central hydroxyl group have a lower boiling point than straight, elongated, primary alcohols.
Hydrophilicity
The OH group is also able to form hydrogen bonds with water. It increases the hydrophilicity , the water solubility, of the connection. Organic alkyl radicals themselves are not water-soluble, so they are hydrophobic . The solubility in water therefore decreases with the size of the organic fraction and increases with the number of hydroxyl groups. The propanols and tert- butanol can still be mixed with water in any ratio at room temperature; all longer-chain alcohols only dissolve in increasingly small amounts. Larger amounts of dissolved inorganic salts can also cause the short-chain alcohols to phase separate (“salt load”).
In summary:
- The hydroxyl group of an alcohol is polar due to the uneven charge distribution. Thus, the ability of these to form hydrogen bonds with polar water molecules is responsible for the good solubility of short-chain alcohols in particular.
- The more hydroxyl groups an alcohol has, the more hydrogen bonds it can form with the water. Therefore, the greater the number of hydrophilic hydroxyl groups, the greater the water solubility.
- The hydrophobic, i.e. water-repellent, non-polar alkyl radical counteracts this effect: the longer it is, the lower the water solubility of the alcohol.
Acidity and deprotonation
With a pK s value (acid strength) of about 16 alcohols are less acidic than water and thus react in aqueous solution is approximately neutral. The acidity of alcohols decreases in the series from methanol through primary, secondary and tertiary alcohols. It is possible to use alcohols with strong bases such as. B. to deprotonate hydride anions or by reaction with sodium with evolution of hydrogen. The resulting alcoholates can then be used as strongly nucleophilic anions for further reactions.
It is also possible to protonate alcohols to some extent with strong acids:
Spectroscopy
In the IR spectrum of alcohols, the broad band of the O – H stretching vibration in the range of 3200–3650 cm −1 can be clearly seen. The width of the peak is caused by hydrogen bonds with water molecules and is found in spectra of anhydrous alcohols in a narrower range of 3620–3650 cm −1 .
Chemical properties
Reaction with concentrated sulfuric acid
Below 140 ° C, the ester forming sulfuric acid .
The condensation reaction to form an ether takes place at about 140 ° C.
Above 170 ° C, primary alcohols are dehydrated to alkenes . ( Elimination )
Selenium Oxide Elimination
The selenium-elimination is a mild variant of elimination.
Esterification
Alcohols react with carboxylic acids to form esters , releasing water . This reaction is also called esterification . This reaction is catalyzed by acids.
oxidation
Primary alcohols can be oxidized to aldehydes and carboxylic acids , secondary alcohols to ketones . Tertiary alcohols cannot be further oxidized unless the carbon structure is destroyed.
| Oxidation of alcohols | ||||
| alcohol | primary | secondary | tertiary | |
| Oxidation product I | aldehyde | Ketone | no reaction | |
| Oxidation product II | Carboxylic acid | no reaction | - | |
| Example: Oxidation products of the structurally isomeric butanols | ||||
| Butanol | ||||
| Surname | Butan-1-ol | Butan-2-ol | 2-methylpropan-2-ol | |
| Oxidation product I | no reaction | |||
| Surname | Butanal (butyraldehyde) | Butanone (methyl ethyl ketone) | - | |
| Oxidation product II | no reaction | - | ||
| Surname | Butanoic acid (butyric acid) | - | - | |
| The functional groups are marked in blue . | ||||
For the oxidation of primary alcohols to carboxylic acid, chromium (VI) -containing oxidizing agents can be used, such as are used, for. B. in the Jones oxidation application. Aqueous ruthenium tetroxide is available as a chromium-free, less toxic reagent .
The oxidation of a primary alcohol can also only take place up to the level of the aldehyde using certain chromium (VI) compounds such as the Collins reagent . It is crucial that anhydrous solvents are used. If no water is present, hydration to the geminal diol of the aldehyde ( aldehyde hydrates ) cannot take place.
Since soluble chromates are very toxic and have carcinogenic and mutagenic properties, alternative methods for the oxidation of alcohols have been developed. A frequently used method is the Swern oxidation with activated dimethyl sulfoxide . Almost all methods are also suitable for the oxidation of secondary alcohols to ketones. The following list provides an overview of the most important methods.
Oxidation to carboxylic acid / ketone:
- Jones oxidation ( chromium (VI) oxide in sulfuric acid in the presence of acetone )
- Potassium dichromate in sulfuric acid
- Ruthenium tetroxide
Oxidation to aldehyde / ketone:
- Collins reagent (CrO 3 py 2 in dichloromethane )
- Corey reagent (pyridinium chlorochromate (PCC))
- Cornforth reagent (pyridinium dichromate (PDC))
- Anelli oxidation (cat. TEMPO , stoich. NaOCl )
- Dess-Martin-Oxidation ( Dess-Martin-Periodinan )
- Ley oxidation ( cat. TPAP , stoich. NMO )
- Pfitzner-Moffatt oxidation (DMSO, DCC )
- Swern oxidation ( DMSO , oxalyl chloride , NEt 3 )
Acetal formation
In the presence of acidic catalysts, alcohols react with aldehydes to form hemiacetals or acetals .
use
Many alcohols are important solvents that are used in both industry and households; the most important in terms of quantity are methanol , ethanol , 2-propanol and n- butanol . In 2011, around 6.4 million tons of these alcoholic solvents were in demand worldwide.
proof
Alcotest
The conversion of alcohols with dichromates in sulfuric acid solution is suitable for the quantitative detection of alcohols and was previously used in the Alcotest tubes:
The detection principle is based on the color change from yellow-orange (acidic dichromate solution) to green (chromium (III) ions) and can be measured spectrophotometrically.
Certest
Another possibility is the reaction with ammonium cerium (IV) nitrate . Here a concentrated solution of ammonium cerium (IV) nitrate is mixed with a dilute solution of the unknown substance. If the unknown substance contains alcohol groups, the mixture turns red (sometimes also green). If the substance contains phenols , a brown precipitate forms . The reason for this color reaction is a complex formation , more precisely a ligand substitution in which an alcohol / phenol coordinates with the oxygen atom on cerium (IV). By changing the ligand sphere, the color of the cerium (IV) changes from light yellow to red / green / brown. Easily oxidizable alcohols / phenols can result in a negative detection by the cerium (IV) to cerium (III) reduce .
Lucas sample
The degree of substitution of an alcohol, i.e. whether it is a primary, secondary or tertiary alcohol, is verified by nucleophilic substitution of the OH group for chloride by the Lucas sample . The substitution has the consequence that the resulting substance no longer dissolves in water and thus forms its own phase . The speed of this phase formation is decisive:
- Tertiary alcohols react immediately at room temperature.
- Secondary alcohols react after about five minutes.
- Primary alcohols only react when heated.
The prerequisite for this test is that the original alcohol dissolves in water. There must also be no other group which can be substituted under the reaction conditions.
Spectroscopy and Derivatization
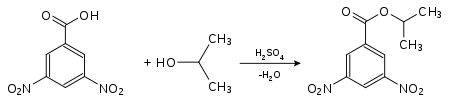
The unambiguous identification of an unknown alcohol is carried out either by spectroscopy or by synthesis of a characteristic derivative that has a melting point that can be easily distinguished from the melting points of the same derivatives of similar alcohols. They are often identified via esters of 4-nitrobenzoic acid or 3,5-dinitrobenzoic acid . For this purpose, the substance to be analyzed is converted in the presence of small amounts of sulfuric acid. The melting points of these derivatives are usually sharp.
 |
|
| Detection of propan-2-ol (isopropanol) as a derivative of 4-nitrobenzoic acid: 4-nitrobenzoic acid-2-propyl ester (melting point: 100.5 ° C) |
Detection of propan-2-ol (isopropanol) as a derivative of 3,5-dinitrobenzoic acid: 3,5-dinitrobenzoic acid-2-propyl ester (melting point: 123 ° C) |
The derivatives of 3,5-dinitrobenzoic acid generally have higher melting points than those of 4-nitrobenzoic acid . They are preferred when the melting point of 4-nitrobenzoic acid is too low and an exact determination is no longer possible.
List of important alcohols with melting and boiling points
| Homologous series of monohydric, primary, linear, unbranched alcohols | ||||||
|---|---|---|---|---|---|---|
| C atoms |
Molar mass g mol −1 |
Systematic name | Common name |
Melting point ° C |
Boiling temperature ° C |
Solubility g · l −1 |
| 1 | 32.0 | Methanol | Wood spirit, methyl alcohol | −97.8 | 64.7 | ∞ |
| 2 | 46.1 | Ethanol | Alcohol, ethyl alcohol, alcohol | −114.1 | 78.3 | ∞ |
| 3 | 60.1 | Propan-1-ol | n -propyl alcohol | −126.2 | 97.2 | ∞ |
| 4th | 74.1 | Butan-1-ol | n -butyl alcohol | −89.3 | 117.3 | 79 |
| 5 | 88.2 | Pentan-1-ol | n -amyl alcohol | −78.2 | 138 | 23 |
| 6th | 102.2 | Hexan-1-ol | n -hexyl alcohol | −48.6 | 157.5 | 6th |
| 7th | 116.2 | Heptan-1-ol | −34.0 | 176 | 2 | |
| 8th | 130.2 | Octan-1-ol | −14.9 | 194.5 | 0.5 | |
| 9 | 144.3 | Nonan-1-ol | −6 | 214 | 0 | |
| 10 | 158.3 | Decan-1-ol | 7th | 230 | 0 | |
| 11 | 172.3 | Undecan-1-ol | 15.9 | 243 | 0 | |
| 12 | 186.3 | Dodecan-1-ol | Lauryl alcohol | 24 | 259 | 0 |
| 13 | 200.4 | Tridecan-1-ol | 31.7 | 274 | 0 | |
| 14th | 214.4 | Tetradecan-1-ol | Myristyl alcohol | 39-40 | 289 | 0 |
| 15th | 228.4 | Pentadecan-1-ol | 44 | 270 | 0 | |
| 16 | 242.4 | Hexadecan-1-ol | Cetyl alcohol | 50 | 344 | 0 |
| ... | ||||||
| 18th | 270.5 | Octadecan-1-ol | Stearyl alcohol | 56-59 | 336 | 0 |
| ... | ||||||
| 26th | 382.7 | Hexacosan-1-ol | Ceryl alcohol | 79-81 | 240 (13 Pa) | 0 |
| ... | ||||||
| 30th | 438.8 | Triacontan-1-ol | Myricyl alcohol | 88 | 0 | |
| Monohydric alcohols: secondary and tertiary, primarily with branched chains | ||||||
| C atoms | Molar mass g mol −1 |
Systematic name | Common name | Melting point ° C |
Boiling temperature ° C |
Solubility g · l −1 |
| 3 | 60.1 | Propan-2-ol | Isopropyl alcohol, isopropanol | −88.5 | 82.3 | ∞ |
| 4th | 74.1 | Butan-2-ol | Secondary butyl alcohol | −114.7 | 99.5 | 125 |
| 4th | 74.1 | 2-methylpropan-1-ol | Isobutyl alcohol | −108 | 108 | 100 |
| 4th | 74.1 | 2-methylpropan-2-ol | Tertiary butyl alcohol, trimethyl carbinol | 25.5 | 82.3 | ∞ |
| 5 | 88.2 | Pentan-2-ol | sec - n -amyl alcohol | −50 | 118.9 | 166 |
| 5 | 88.2 | Pentan-3-ol | Diethyl carbinol | −8 | 116.1 | 55 (30 ° C) |
| 5 | 88.2 | 2-methylbutan-1-ol | −70 | 129 | 36 | |
| 5 | 88.2 | 3-methylbutan-1-ol | Isoamyl alcohol | −117 | 130.8 | 20th |
| 5 | 88.2 | 2-methylbutan-2-ol | −8.4 | 102 | ||
| 5 | 88.2 | 3-methylbutan-2-ol | 112.9 | |||
| 5 | 83.2 | 2,2-dimethylpropan-1-ol | neo- pentyl alcohol, tertiary amyl alcohol | −12 | 102 | 125 |
| Polyhydric alcohols | ||||||
| C atoms | Molar mass g mol −1 |
Systematic name | Common name | Melting point ° C |
Boiling temperature ° C |
Solubility g · l −1 |
| 2 | 62.1 | Ethane-1,2-diol | Ethylene glycol, 1,2-glycol | −15.6 | 197.2 | ∞ |
| 3 | 76 | Propane-1,2-diol | Propylene glycol | −68 | 188 | ∞ |
| 3 | 76 | Propane-1,3-diol | Trimethylene glycol | −32 | 215 | ∞ |
| 4th | 90 | Butane-1,2-diol | 1,2-butylene glycol | −114 | 192 | ∞ |
| 4th | 90 | Butane-1,3-diol | 1,3-butylene glycol | <−50 | 207.5 | ∞ |
| 4th | 90 | Butane-1,4-diol | Tetramethylene glycol | 16 | 230 | ∞ |
| 4th | 90 | Butane-2,3-diol | 2,3-butylene glycol | 34 ( meso ) | 183 ( meso ) | ∞ |
| 5 | 104 | Pentane-1,5-diol | Pentamethylene glycol | −16 | 241 | ∞ |
| 6th | 118 | Hexane-1,6-diol | Hexamethylene glycol | 39-42 | 253-260 | 5000 |
| 8th | 146 | Octane-1,8-diol | Octamethylene glycol | 58-61 | 171–173 (27 hPa) | |
| 9 | 160 | Nonane-1,9-diol | Nonamethylene glycol | 45-46 | 288 | 9 |
| 10 | 174 | Decane-1,10-diol | Decamethylene glycol | 72 | 297 | 0.7 |
| 3 | 104 | Propane-1,2,3-triol | Glycerin , glycerol | 18th | 290 | ∞ |
| Other alcohols | ||||||
| C atoms | Molar mass g mol −1 |
Systematic name | Common name | Melting point ° C |
Boiling temperature ° C |
Solubility g · l −1 |
| 5 | 86.13 | Cyclopentanol | −19 | 141 | 13 | |
| 6th | 100.2 | Cyclohexanol | 25.2 | 161.5 | 36 | |
| 3 = | 58 | Prop-2-en-1-ol | Allyl alcohol | −129 | 97 | ∞ |
| 4 = | 71 | But-2-en-1-ol | Crotyl alcohol | −30 | 118 | 166 |
| 4th | 70.09 | 3-butyn-1-ol | −63 | 128.9 | ||
| 6th | 98.14 | 2-hexyn-1-ol | 66-67 | |||
| 6th | 98.14 | 3-hexyn-1-ol | 63-64 | |||
| 6th | 98.14 | 3-hexyn-2-ol | 79-80 | |||
| 6th | 98.14 | 5-hexyn-1-ol | 73-75 | |||
| 6th | 98.14 | 5-hexyn-3-ol | ||||
| 7th | 108.14 | Phenylmethanol, (hydroxymethyl) benzene | Benzyl alcohol | −15 | 205.4 | 39 |
| 7th | 114.19 | Cyclohexylmethanol | 19th | 187-188 | ||
| 8th | 126.20 | 3-octyn-1-ol | 880 | |||
| 8th | 126.20 | 7-octyn-1-ol | 70 | |||
| 8th | 122.14 | 1-phenylethan-1-ol , (1-hydroxyethyl) benzene (C 6 H 5 CH (OH) CH 3 ) | α-phenylethyl alcohol | 21st | 205 | 0 |
| 8th | 122.14 | 2-phenylethan-1-ol , (2-hydroxyethyl) benzene (C 6 H 5 CH 2 CH 2 OH) | β-phenylethyl alcohol | −27 | 221 | 16 |
| 10 | 158.29 | 3-decanol | 213 | |||
| 10 | 158.29 | 4-decanol | −11 | 210-211 | ||
| 11 | 164.25 | 4-tert-butylbenzyl alcohol | 139-140 | |||
| 13 | 184.23 | Diphenylmethanol (C 6 H 5 ) 2 CHOH | Diphenyltricarbinol, benzhydrol | 69 | 298 | 0 |
| 19th | 260.33 | Triphenylmethanol (C 6 H 5 ) 3 COH | Triphenyl carbinol | 162.5 | > 360 | 0 |
| For comparison the phenol and alcohols with similar molar masses | ||||||
| C atoms | Molar mass g mol −1 |
Systematic name | Common name | Melting point ° C |
Boiling temperature ° C |
Solubility g · l −1 |
| 6th | 94.1 | phenol | Carbolic acid, benzene | 41 | 181.7 | 84 |
| 5 | 88.2 | Pentan-1-ol | n -amyl alcohol | −78.2 | 128.0 | 23 |
| 6th | 102.2 | Hexan-1-ol | n -hexyl alcohol | −48.6 | 157.1 | 6th |
| 5 | 86.13 | Cyclopentanol | −19 | 141 | 13 | |
| 6th | 100.2 | Cyclohexanol | 25.2 | 161.5 | 36 | |
Annotation:
- = Double bonds
- * at boiling point: the substance decomposes before it reaches the boiling point. Values in brackets indicate the boiling point at 20 hPa pressure.
- ∞ Solubility: infinitely miscible with water.
There may be deviations in the literature for individual values.
See also
Individual evidence
- ↑ Entry on alcohols . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00204 Version: 2.3.1.
- ^ Clayden, Greeves Warren, Wothers: Organic Chemistry . Oxford University Press Inc, New York 2001, ISBN 978-0-19-850346-0 , pp. 35-36 .
- ↑ Entry on phenols . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.P04539 Version: 2.3.1.
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag, Leipzig 1965, p. 44.
- ↑ Entry on alcohols. In: Römpp Online . Georg Thieme Verlag, accessed on May 23, 2014.
- ↑ Science Vision, Vol. 3, No. 25, March 2000 ( limited preview in the Google book search).
- ↑ Entry on fatty alcohols. In: Römpp Online . Georg Thieme Verlag, accessed on January 10, 2013.
- ↑ Entry on wax alcohols. In: Römpp Online . Georg Thieme Verlag, accessed on September 8, 2014.
- ↑ Wissenschaft-Online-Lexika: Entry on alkanols / alcohols in the Lexikon der Chemie , accessed on July 1, 2008.
- ↑ Paula Yurkanis Bruice: Organic Chemistry , Pearson Education Inc., 2007, 5th edition, p 829, ISBN 978-3-8273-7190-4 .
- ↑ Paula Yurkanis Bruice: Organic Chemistry , Pearson Education Inc., 2007, 5th Edition, pp 830-831, ISBN 978-3-8273-7190-4
- ↑ Ceresana: Market Study Solvents, 2nd edition (UC-3505), April 2012.
- ↑ Paula Yurkanis Bruice: Organic Chemistry , Pearson Education Inc., 2007, 5th edition, p 412, ISBN 978-3-8273-7190-4 .
- ↑ a b c CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ a b c d e f g h i j Siegfried Hauptmann : Organische Chemie , 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 312.
- ↑ Hans Beyer and Wolfgang Walter : Organische Chemie , S. Hirzel Verlag, Stuttgart, 22nd edition, 1991, p. 120, ISBN 3-7776-0485-2 .
- ↑ a b Hans Beyer and Wolfgang Walter : Organische Chemie , S. Hirzel Verlag, Stuttgart, 22nd edition, 1991, p. 124, ISBN 3-7776-0485-2 .
- ^ A b Hans Rudolf Christensen: Fundamentals of Organic Chemistry , Verlag Sauerländer Aarau, 1st edition, 1970, p. 166.
- ↑ a b Entry for CAS no. 143-08-8 in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b Entry for CAS no. 112-30-1 in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-517.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-496.
- ↑ Robert C. Weast (ed.): CRC Handbook of Chemistry and Physics , 1st Student Edition, 1988, CRC Press Baton Rouge, Florida, S. C-507, ISBN 0-8493-0740-6 .
- ↑ a b Robert C. Weast (ed.): CRC Handbook of Chemistry and Physics , 1st Student Edition, 1988, CRC Press Baton Rouge, Florida, S. C-301, ISBN 0-8493-0740-6 .
- ↑ Robert C. Weast (ed.): CRC Handbook of Chemistry and Physics , 1st Student Edition, 1988, CRC Press Baton Rouge, Florida, S. C-496, ISBN 0-8493-0740-6 .
- ↑ Robert C. Weast (ed.): CRC Handbook of Chemistry and Physics , 1st Student Edition, 1988, CRC Press Baton Rouge, Florida, S. C-355, ISBN 0-8493-0740-6 .
- ^ A b c Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics , 1st Student Edition, 1988, CRC Press Baton Rouge, Florida, pp. C-169, ISBN 0-8493-0740-6 .
- ↑ Robert C. Weast (ed.): CRC Handbook of Chemistry and Physics , 1st Student Edition, 1988, CRC Press Baton Rouge, Florida, S. C-446, ISBN 0-8493-0740-6 .
- ↑ a b Robert C. Weast (ed.): CRC Handbook of Chemistry and Physics , 1st Student Edition, 1988, CRC Press Baton Rouge, Florida, S. C-447, ISBN 0-8493-0740-6 .
- ↑ Robert C. Weast (ed.): CRC Handbook of Chemistry and Physics , 1st Student Edition, 1988, CRC Press Baton Rouge, Florida, S. C-396, ISBN 0-8493-0740-6 .
- ↑ Robert C. Weast (ed.): CRC Handbook of Chemistry and Physics , 1st Student Edition, 1988, CRC Press Baton Rouge, Florida, S. C-397, ISBN 0-8493-0740-6 .
- ↑ Robert C. Weast (ed.): CRC Handbook of Chemistry and Physics , 1st Student Edition, 1988, CRC Press Baton Rouge, Florida, S. C-168, ISBN 0-8493-0740-6 .
- ↑ Entry on cyclopentanol in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018 or earlier.
Web links
- School experiments on the subject of alcohols
- Learning circle alcohols. (No longer available online.) In: ZUM-Wiki. Headquarters for teaching media on the Internet e. V. [TO the Internet e. V.], December 28, 2009, archived from the original on December 28, 2009 ; Retrieved February 7, 2010 .