Solar cell
A solar cell (also known as a photovoltaic cell in technical terms ) is an electrical component that converts radiant energy , usually sunlight , directly into electrical energy . The application of the solar cell is photovoltaics , where it serves as a power source . The physical basis of the conversion is the photovoltaic effect , which is a special case of the internal photoelectric effect .
There are many different cell types that can be differentiated both according to the semiconductor material used and according to the cell technology (wafer-based or thin-film). The most important semiconductor material is silicon , from which around 90% of all solar cells manufactured worldwide were made in 2013; the market share of thin-film cells was around 10%. The solar modules used to generate energy are created by connecting individual solar cells in series and then encapsulating them . In the case of thin-film modules, the series connection is integrated into the cell production process; in the case of the widespread crystalline modules, it is implemented by soldering connectors onto finished solar cells.
Sometimes elements of a solar collector are colloquially incorrectly referred to as solar cells. However, they do not generate electricity, but process heat and store their energy in a hot water tank ( boiler ).
Classification
Overview
Solar cells can be classified according to various criteria. The most common criterion is the material thickness. A distinction is made between thick-film and thin-film cells.
Another criterion is the semiconductor material used . The most commonly used is silicon . Other semiconductors such as cadmium telluride and gallium arsenide are also used . In so-called tandem solar cells , layers of different semiconductors are used, for example indium gallium arsenide in combination with indium gallium phosphide .
The crystal structure can be crystalline ( mono / polycrystalline ) or amorphous .
In addition to inorganic semiconductor materials, there are also organic solar cells and dye solar cells as well as inorganic-organic hybrids. The development is by no means over.
material
-
Silicon cells
- Thick film
- Monocrystalline silicon cells (c-Si) have an efficiency of over 25% and a power density of 20 to 50 W / kg in industrial use . The technique is considered to be well mastered.
- Polycrystalline cells, also called multicrystalline cells (poly-Si or mc-Si), have relatively short energy return times and have become the most common cells. They achieve efficiencies of almost 18% in large-scale use. Dispensing with the energy-consuming and time-consuming recrystallization of a single crystal is paid for with somewhat lower performance. Experimental cells achieve efficiencies over 20%.
- Thin film
- Amorphous silicon (a-Si) achieved the largest market share in thin-film cells from the 1980s. They are known from small applications such as pocket calculators. The module efficiencies are between 5 and 7% and have a power density of up to approx. 2000 W / kg. There are no material bottlenecks here, even when producing on a terawatt scale. With tandem and triple cells, some of which have different spectral sensitivity, it was possible to reduce the degradation problems in addition to increasing the efficiency by 10 to 20%.
- Crystalline silicon, e.g. B. microcrystalline silicon (µc-Si) is also used in combination with amorphous silicon as tandem cells and thus achieves higher efficiencies of up to the expected 15%. Similar to solar cells, they are made of amorphous silicon. By combining two solar cells with different spectral sensitivity (band gap), whereby the front one must of course be semitransparent, a higher overall efficiency can be achieved. However, with a series connection that is easy to implement, the required correspondence of the currents can only be achieved very imperfectly. Solar cell duos in a parallel connection that is more promising under practical conditions or with matching electronics are only known as laboratory experiments.
- Si Wire Array (laboratory stage): By equipping a surface with the thinnest wires, this new solar cell is flexible and requires only 1% of the amount of silicon compared to conventional solar cells.
- Thick film
-
III-V semiconductor solar cells
- Gallium arsenide cells (GaAs) are characterized by high efficiencies (experimentally up to 41.1% in 2009), very good temperature resistance, less power loss when heated than crystalline silicon cells and robustness against UV radiation. However, they are very expensive to manufacture. They are often used in space travel (gallium indium phosphide, (Ga, In) P / gallium arsenide, GaAs / germanium, Ge). Triple cells ( tandem solar cells with three monolithically stacked pn junctions) have the highest commercially available efficiency of almost 30%.
-
II-VI semiconductor solar cells
- CdTe cells can be manufactured very cheaply on an industrial scale by chemical bath deposition (CBD) or chemical vapor deposition (CVD) and are used in thin-film solar cells ; for a laboratory solar cell (19.6 ± 0.4)% has already been achieved, module efficiencies meanwhile (2007) at 10%, long-term behavior not yet known.
-
I-III-VI semiconductor solar cells
- CIS and CIGS solar cells ( Chalkopyrite ) consist of copper-indium-gallium-diselenide or copper-indium-disulfide . This material is used in thin-film solar cells - here CIGS is the most powerful material with laboratory efficiencies of now 22.6% (June 2016). The module efficiency is currently 17.4% (as of February 2012). In 1999 Siemens Solar was able to show the first modules. Different manufacturers have developed different manufacturing processes. So far, despite the excellent design, none of them have achieved any significant market share. Indium is expensive and a limited resource.
- Organic solar cells (OPV) : Organic chemistry provides materials that may allow the cost-effective manufacture of solar cells. The previous disadvantage is their currently still poor efficiency of a maximum of 12.0% and the very short service life (max. 5000 h) of the cells.
- Dye cells - Grätzel cells , DSC or DSSC (dye-sensitized (solar) cell) - use organic dyes to convert light into electrical energy; a process that is based on photosynthesis. They're mostly purple. With a conductive polymer such as polypyrrole at the cathode, these cells deliver the best efficiency of all organic solar cells of over 10%, but have a limited service life due to aggressive electrolytes.
- Semiconductor electrolyte cells : e.g. B. copper oxide / NaCl solution. Cell is very easy to manufacture, but its performance and reliability are limited.
Material availability
Silicon , the basic material for solar cells, is available in almost unlimited quantities. Silicon occurs naturally as silicon oxide (quartz) or silicate and is separated from oxygen at high temperatures. In addition, silicon cells require a contact layer, which in conventional cells mostly consists of silver ; a metal that is only available in limited quantities. Since silver is also expensive, alternatives to the use of silver were developed and introduced on the market, in particular based on aluminum and copper . These are available in large quantities and are not critical in terms of material availability even when used in the TW area. As of 2019, there are also high-efficiency cells that do not use silver as a dopant.
In the case of rare solar cell materials such as indium , gallium , tellurium and selenium , global consumption (indium around 850 tons, for gallium around 165 tons) exceeds the annual production volume. The sharp rise in the consumption of indium in the form of indium tin oxide in liquid crystal and OLED screen production as well as the use of gallium and indium in the production of light-emitting diodes for the production of energy-saving light sources and as background lighting for flat screens was striking.
In the case of indium, which is also important in the production of light-emitting diodes, resources are expected to dry up by 2035, since the theoretical indium reserves in 2006 amounted to only 6,000 tons and the economically exploitable reserves to only 2,800 tons. Other sources speak of 50,000 tons and feeding of consumption from recycling. Secondary production, i.e. recycling, exceeds primary production and was 800 tons in 2008.
The situation with selenium and with the even rarer tellurium (both semimetals are present in low concentrations in the anode sludge from the copper electrolysis ) appears at first glance to be less critical, since the copper producers currently only use part of the anode sludge from metal electrolysis for the production of selenium or tellurium. The economically accessible selenium reserves are estimated at 82,000 tons, the tellurium reserves at 43,000 tons. This is little, even in comparison to the reserves of the likewise not particularly common non-ferrous metal copper of 550 million tons.
The production processes in which gallium, indium, selenium and tellurium are used have an unfavorable material economy and must be improved.
Designs
In addition to the material, the construction is important. A distinction is made between solar cells according to their surface structures, the contacts on the front and back and their layer structures (partly due to material combinations of different absorption wavelengths, whereby the efficiency of the overall arrangement can be increased by making optimal use of the solar spectrum); More on this below under “Types of silicon solar cells” and “Other types of solar cells”.
At present, commercially available solar cells are made of semiconductor materials , predominantly of silicon. III / V semiconductor materials are also used (including on space probes). Because of their high cost, they are used for terrestrial applications in concentrator systems. Polymer solar cells are still being researched.
Semiconductor solar cells must be interconnected to form solar modules to generate energy . Crystalline cells are connected in series with conductor tracks on the front and back . The voltage of the individual cells of approx. 0.5 V under load and 0.64 V when idle add up. With the most common number of 60 cells today, a module is created with a typical working voltage of 30 V and an open circuit voltage of 38.4 V. Such a module made up of 6+ "cells delivers around 230–260 W of power under standard test conditions (STC These solar modules can easily be connected in series using special integrated connectors on cables to increase the output. Open circuit voltages of up to 1000 V at ambient temperatures below –10 ° C are reached as the maximum permissible limit value. As protection against an avalanche breakdown In the individual cells (for example in the case of partial shade from fallen leaves), additional bypass diodes must be provided in parallel to the cells , which electrically bypass the shaded cells. In generator junction boxes, a parallel connection via fuses can be made in large systems to further increase performance.
Working principle

In principle, solar cells made from semiconductor materials are constructed like large-area photodiodes . However, they are not operated as a radiation detector , but as a power source . The specialty of semiconductors is that the energy supplied (electromagnetic radiation) can generate free charge carriers in them ( electrons and holes , see generation ). In order to generate an electrical current from these charges , it is necessary to direct the generated free charge carriers in different directions; this happens very often through an internal electric field , which can be generated by a pn junction .
Depending on the design of the solar cell, different transport mechanisms are relevant for generating the photocurrent: There are semiconductor-based cells that rely exclusively on drift and others that also involve diffusion . In the case of organic solar cells, on the other hand, other mechanisms for generating, separating and decoupling free charge carriers are relevant (see organic solar cell # functional principle ).
In typical crystalline silicon solar cells with wafer thicknesses of around 200 µm, most of the light-absorbing material is field-free; it is called the base. The optically excited minority charge carriers (electrons in the case of a p-doped base or holes in the case of an n-doped base) freely diffuse around the base. (The majority charge carriers excited during light absorption play no role in the functioning of the solar cell.) As soon as minority charge carriers reach the space charge zone of the pn-junction, they are accelerated by the electric field to the other side of the pn-junction and thus by the majority charge carriers of the base Cut; the latter are held back by the electric field of the pn junction due to their opposite charge. These solar cells achieve a high photocurrent yield if the diffusion length of the minority charge carriers is large in relation to the thickness of the base and the rear side of the solar cell either has a so-called back surface field (BSF) or is dielectrically passivated, which reduces recombination losses.
In the case of solar cells made of a material with a short diffusion length, the space charge zone with the electric field extends as far into the material as possible. This is set by targeted doping of the material (see semiconductor technology ). To create the desired profile, a thin surface layer is usually heavily n-doped, the thicker layer underneath weakly p-doped. This results in a space charge zone with a large width. If photons now fall in this zone and generate electron-hole pairs ( internal photo effect ), the electric field accelerates the holes to the p-material below and, conversely, the electrons to the n-contact on the upper side (facing the sun).
Some of the minority charge carriers are recombined , their excitation energy is lost in heat. Further losses arise due to the inevitable series resistance. The photocurrent can be used directly by a consumer, temporarily stored in an accumulator or fed into the power grid with a grid-commutated solar inverter . The electrical voltage at maximum power ( maximum power point , power adjustment) is around 0.5 V for the most common cells (crystalline silicon cells).
The structure of solar cells is also adapted so that as much light as possible is captured and free charge carriers can be generated in the active layer (base or weakly doped area). For this purpose, the top electrode must be transparent, the contacts to this layer must be as narrow as possible, an anti-reflective layer is applied to the top (to reduce the degree of reflection ), and the rear side is mirrored if necessary. The anti-reflective layer ensures the typical bluish to black color of solar cells. Uncoated solar cells, on the other hand, have a silvery-gray appearance.
Sometimes the front is textured or roughened. Because of this advantage, wafers with errors in the grinding process or the like were originally used. sold as a raw material for solar cells. Black silicon has a roughened, needle-shaped surface that has very little reflections.
In modern solar cells, the anti-reflective layer is made of silicon nitride using a PE- CVD process. The layer thickness is approx. 70 nm (lambda quarter with a refractive index of 2.0). In addition, anti-reflective layers made of silicon dioxide and titanium dioxide , which are applied, for example, using the AP-CVD process, are used.
The color is also determined by the layer thickness ( interference color ). It is important that the coating thickness is as uniform as possible, since fluctuations of a few nanometers in the layer thickness increase the degree of reflection. Blue reflection results from the setting of the anti-reflective coating on the red part of the spectrum - the preferred absorption wavelength of silicon. In principle, however, red, yellow or green solar cells, for example, can also be produced in this way for special architectural applications, but they are less efficient.
In the case of silicon nitride and silicon dioxide, the anti-reflective layer still fulfills the function of a passivation layer which reduces the surface recombination speed. As a result, the charge carriers generated on the surface - to put it simply - cannot recombine as quickly and the generated charge can be diverted as electricity.
Silicon solar cells
The traditional basic material for semiconductor solar cells is silicon. Until 2005, mainly residual silicon from chip production was used; today, silicon is increasingly being produced specifically for solar applications. In general, silicon is almost ideal for semiconductor technology. It is inexpensive, can be produced in high purity and monocrystalline and doped as n- and p-semiconductors . Simple oxidation enables the production of thin insulation layers. However, the characteristic of its band gap as an indirect semiconductor is not very suitable for optical interaction. Silicon-based crystalline solar cells must have a layer thickness of at least 100 µm and more in order to absorb light sufficiently. In thin-film cells direct semiconductors , such as. B. gallium arsenide or silicon with a strongly disturbed crystal structure (see below) suffice 10 µm.
Depending on the crystal structure, a distinction is made between the following types of silicon:
- Monocrystalline cells are made from so-called wafers (monocrystalline silicon wafers), as they are also used for semiconductor production. They are relatively expensive.
- Polycrystalline cells consist of discs that do not have the same crystal orientation everywhere . You can e.g. B. by casting (see below) and are cheaper and most common in photovoltaic systems .
- Amorphous solar cells consist of a thin, non-crystalline (amorphous) silicon layer and are therefore also referred to as thin-film cells. They can be produced by vapor deposition, for example, and are inexpensive, have only a low degree of efficiency in sunlight, but offer advantages in low light, scattered light and at high operating temperatures. The amorphous cells can be found on pocket calculators or watches, for example.
- Microcrystalline cells are thin-film cells with a microcrystalline structure. They are more efficient than amorphous cells and are not as thick as common polycrystalline cells. Some of them are used for photovoltaic systems, but are not yet very widespread.
- Tandem solar cells are stacked solar cells, usually a combination of polycrystalline and amorphous cells. The individual layers consist of different materials and are therefore adapted to a different wavelength range of light. The cells at the top absorb only part of the light spectrum , the rest can pass through and be used by the layer below. By making greater use of the sun's light spectrum , these cells are more efficient than simple solar cells. Some of them are used in photovoltaic systems, but are still relatively expensive.
Manufactured from silicon blocks or bars
Solar cells can be manufactured using various methods.

The basic material silicon is the second most common chemical element found in the earth's crust. It is in the form of silicates or quartz . Crude silicon, so-called metallurgical silicon , with impurities of approximately 1 to 2% can be produced from quartz sand in a smelting reduction furnace . In 2005, 4.7 million tons of silicon were produced in this way. A large part of it goes into the steel industry and the chemical industry . Only a small proportion of the metallurgical silicon is used for microelectronics and photovoltaics.
Polycrystalline hyperpure silicon is then produced from the raw silicon using a multi-stage process based on trichlorosilane . The previously used Siemens process , a CVD process (CVD = chemical vapor deposition, German: chemical gas phase deposition), was however developed and optimized for microelectronics. There the demands on the quality of the silicon are sometimes completely different from those in photovoltaics. For solar cells, for example, the purity of the wafer in its entire thickness is important in order to ensure the longest possible charge carrier life. In microelectronics, on the other hand, only the upper 20 to 30 µm should in principle be highly pure. Since the consumption of high-purity silicon for photovoltaics has now exceeded the consumption in microelectronics, intensive work is currently underway on special, more cost-effective manufacturing processes for solar silicon that are optimized for photovoltaics.
The entire manufacturing process for high-purity silicon is very energy-intensive, but the solar cells used today can compensate for the amount of energy required for their production - depending on the design - within 1.5 to 5 years. So you have a positive energy balance.
The hyperpure silicon can be further processed in different ways. For polycrystalline cells, the casting process , the Bridgman process and the edge-limited tape drawing process (EFG process, from English edge-defined film-fed growth ) are mostly used. Monocrystalline cells are almost always made using the Czochralski process . In all processes, the doping with boron (see below) is already carried out when the blocks ( ingots ) or rods are manufactured.
Ingot casting process
This process is used to produce polycrystalline silicon. The ultra-pure silicon is melted in a crucible with the aid of induction heating and then poured into a square tub, in which it is cooled as slowly as possible. The largest possible crystallites should arise in the blocks. The edge length of the tub is about 50 cm, the height of the solidified melt about 30 cm. The large block is divided into several small blocks about 30 cm long.
Another casting process is continuous casting, where the mass is applied to the carrier material in the thickness required at the end. The advantage is that there is no need for a sawing process with its losses.
Bridgman method
The Bridgman process is used to produce polycrystalline silicon and is named after Percy Williams Bridgman . It is not to be confused with the Bridgman-Stockbarger method , which is used to produce monocrystals. The ultra-pure silicon is also melted in a crucible with the help of induction heating at over 1400 ° C. The slow cooling of the melt , during which large zones of uniform crystals form, takes place here in the same crucible. The heated zone is slowly raised from bottom to top in the crucible, so that liquid silicon is at the top until the end, while solidification takes place from the bottom of the crucible. Here the edge lengths are slightly larger than in the casting process (mostly standard size 690 mm), the height of the block is around 20 to 25 cm. The large block is also divided into several small blocks, mostly with an edge length of 156 mm. This step is called briquetting .
Czochralski method
The Czochralski process is used to manufacture long monocrystalline rods. The so-called seed crystal determines the orientation in the crystal. Before the cells are manufactured, the resulting cylinder is cut to size.
Zone melting process
The zone melting process , also known as the float zone process, is also used to manufacture monocrystalline silicon rods. The purity achieved with this process is normally higher than required for solar technology and is also associated with very high costs. This is why this technology is rarely used for solar technology. The only company that uses significant quantities of float zone wafers for solar cells is the US company SunPower .
Wafer manufacturing
The crystal rods now have to be cut into wafers using a wire sawing process. This creates sawdust from a large part of the silicon, the use of which has been researched since 2013. The thickness of the resulting wafers was around 0.4 mm in the early days of solar cell production and has been steadily reduced since then in order to increase the yield (number of wafers per kg of Si). Since 2008, the typical wafer thickness has been around 0.18 mm, which, given the currently common wafer size with an edge length of 156 mm, represents an optimum between the competing requirements of a high yield on the one hand and avoiding losses due to an excessively high breakage rate on the other. A further increase in the yield is possible by reducing the sawing losses (parts of the Si block that become sawdust; English "kerf loss"); In addition to the increase in cell efficiency, this also contributes to the decline in Si demand per watt of peak power (measured in g / Wp) that has been observed since 2008.
Another source for wafers was originally the scrap of blanks for the manufacture of integrated circuits in semiconductor manufacture, whose blanks that are unsuitable for further processing can be used as solar cells.
The monocrystalline cells are characterized by a homogeneous surface, while with the polycrystalline cells the individual zones with different crystal orientations can be easily distinguished - they form an ice flower-like pattern on the surface.
At the wafer stage, the front and back of the cell are not yet determined.
Wafer processing
The sawn wafers now pass through several chemical baths to repair saw damage and to create a surface that is suitable for capturing light. Normally, the wafers are already provided with basic boron doping. This causes excess defect electrons (positive charges) to exist, which means that electrons can be captured. This is also called p-doping. On the way to the finished solar cell with pn junction, the surface must now be given an n-doping, which is done by heating the cell in a furnace in a phosphorus atmosphere. The phosphorus atoms create a zone with an excess of electrons on the cell surface that is about 1 µm deep. After diffusion with phosphorus, phosphor glass is formed on the surface of the wafer. To remove this, another very short etching step with hydrofluoric acid is necessary. Then the anti-reflective layer is applied in another oven using PECVD , which gives the cell its typical color.
Then the cell is printed, e.g. B. by screen printing , with the necessary soldering zones and the structure, which ensures better tapping of the generated electrical current. The front side usually has two wider strips on which the ribbons for connecting several cells are attached later. In addition, a very thin, electrically conductive grid is applied, which on the one hand hampers the incidence of light as little as possible and on the other hand should reduce the ohmic resistance of the cover electrode. The back is usually coated over the entire surface with a highly conductive material.
After this preprocessing, the cells are classified according to optical and electrical characteristics, sorted and put together for the production of solar modules .
Direct production of panels or layers
In order to avoid the detour of sawing wafers from crystal blocks, there are extensive activities to produce solar cells directly.
EFG procedure
In the EFG process (from English edge-defined film-fed growth , approximate translation: " edge-defined film growth "), octagonal tubes about 6 to 7 m in length are drawn upwards from an electrically heated graphite tank made of liquid hyperpure silicon . The pulling speed is in the range of approx. 1 mm / s. The edge length of the individual sides is 10 or 12.5 cm, the wall thickness approx. 280 µm. After completion of the tube, it is cut along the edges with Nd: YAG lasers and then in a certain grid across the width of the respective side. This results in the possibility of producing cells with different edge lengths (for example 12.5 cm × 15 cm or 12.5 cm × 12.5 cm). A yield of about 80% of the starting material is achieved. The cells produced in this way are also made of polycrystalline material, which clearly differs in appearance from the sawn cells. Among other things, the surface of the cells is more wavy. One advantage compared to sawing from blocks is the extensive avoidance of waste, which is also not contaminated with cutting fluid ( slurry , see colloids ). This process is also known as the tape drawing or octagon process .
The EFG process was used by Schott Solar (Germany) until 2009 . No further use had to be made, as Schott Solar, as the only user of this process, could not advance the further development quickly enough compared to other processes with more developers in the background. The process was developed by ASE Solar (USA).
String-Ribbon Process
There is also the string-ribbon process by the insolvent US company Evergreen Solar , in which the wafers are pulled directly from the silicon melt between two threads. This creates less waste (such as chips, etc., which are normally disposed of directly) than with conventional methods. As a German company, Sovello AG used the string-ribbon process to produce wafers.
Layer transfer process
In the layer transfer process, a layer of monocrystalline silicon only approx. 20 µm thick is grown directly flat on a substrate . Ceramic substrates or specially surface-treated silicon are suitable as carrier material, as a result of which the resulting wafer can be detached and the carrier can be reused. The advantages of this process are the significantly lower silicon requirement due to the small thickness and the elimination of sawing losses. There is no sawing as an additional process step. The achievable efficiency is high and is in the range of monocrystalline cells.
Solar cells made from “dirty” silicon
The process of zone melting and doping can also be relocated to an already manufactured, flat plate or layer. The principle is that the impurities are concentrated in a few places by heat treatment (multiple laterally progressing remelting, e.g. with laser radiation) of the silicon.
Solar cells made from special silicon structures
Since the 2000s, various research groups have been working on solar cells based on long “silicon rods” (sometimes also called “silicon microwires”) on a micrometer scale. The individual silicon rods are usually a few micrometers thick and approximately 200 micrometers long. Structures made of rods arranged perpendicular to a carrier surface show, compared to conventional solar cells made of silicon, an increased absorption of sunlight in a broad spectral range, cf. Black silicon .
An example of such a solar cell was presented in 2010 by a working group led by Harry Atwater from the California Institute of Technology . They produced rods over 100 micrometers long using what is known as VLS ( vapor - liquid - solid ) technology, then poured transparent, flexible plastic ( polydimethylsiloxane , PDMS) over them to stabilize them , and then detached the finished cell from the plate. As mentioned before, these cells show an increased absorption of up to 85% of the incident light over a large spectral range. The solar cells produced in this way, in laboratory status, have a high degree of efficiency. Their production only consumes 1 percent of the amount of silicon that is normally used in solar cell production, and these solar cells can also be bent.
Perovskite solar cells
The development of solar modules based on perovskite is judged to be very promising due to their inexpensive manufacture . The cells can be made much thinner than silicon cells. Because perovskite cells can also utilize green and blue light well, while silicon cells mainly convert the red and infrared range of light, they are also considered promising candidates for tandem solar cells. So far, however, the problem is the short shelf life, the protection against moisture and the proportion of lead required in some perovskite cells, since the RoHS directive calls into question their economic usability.
Although it is basically possible to replace lead with other elements such as tin , attempts of this kind were largely unsuccessful as of 2016, as tin gradually oxidizes and the crystal structure of the perovskite is lost. In 2017, however, a promising material was identified with bismuth iodide oxide , with which efficient and stable perovskite solar cells could be manufactured without lead. In addition to tin and bismuth iodide oxide, other elements such as germanium , copper , manganese , iron , cobalt and nickel can also be used to manufacture lead-free perovskite cells; however, their efficiencies are currently significantly lower. For example, the efficiency of lead-free Perovskite cells based on a CH 3 NH 3 SnI 3 structure was a good 6% in 2014. A decisive step in the development of lead-free perovskite cells is the prevention of the oxidation of the tin content in the cell in order to ensure long-term stability. If this is successful, lead-free perovskite cells could be developed within a few years, which not only consist of non-toxic materials, but also have a higher degree of efficiency than lead-containing perovskite cells.
A review article published in the journal Energy and Environmental Science in 2015 came to the conclusion that, after the constant increases in efficiency in the past few years, perovskite modules must be viewed as a serious potential challenger for other solar technologies. The efficiency has risen from 3.8% to 20.1% in just 5 years and will probably increase to 25% in the next few years, and the technology is cheap. Although they are still at the beginning of their development, they have shown an outstanding potential for sustainability. They already have the lowest energy payback time of all solar modules (0.22 years were determined for a perovskite module, i.e. just under 3 months) and could be the most environmentally friendly photovoltaic technology if further development can increase the degree of use and durability.
Other solar cells
Thin-film cells
Thin-film cells are available in different designs, depending on the substrate and the vapor-deposited materials. The range of physical properties and degrees of efficiency is correspondingly large. Thin-film cells differ from traditional solar cells (crystalline solar cells based on silicon wafers) primarily in their production processes and the layer thicknesses of the materials used. The physical properties of amorphous silicon, which are different from crystalline silicon, affect the solar cell properties. Some properties are also not yet fully understood.
Even with crystalline solar cells, the light is already absorbed in a thin surface layer (approx. 10 µm). It therefore makes sense to make solar cells very thin. Compared to crystalline solar cells made from silicon wafers, thin-film cells are around 100 times thinner. These thin-film cells are usually applied directly to a carrier material by deposition from the gas phase. This can be glass, sheet metal, plastic or some other material. The complex process of cutting silicon blocks described in the previous chapter can therefore be avoided.
The most common material for thin-film cells so far is amorphous silicon (a-Si: H) behind glass. Such thin-film modules are long-lasting products. Outdoor tests show stable efficiencies for more than ten years. In sunlight they are 9 ... 10%, which is significantly below crystalline Si cells. However, the efficiency does not decrease as quickly in diffuse, low light as that of polycrystalline Si cells, which is why they are also used to a large extent to power watches and pocket calculators.
Other possible materials are microcrystalline silicon (µc-Si: H), gallium arsenide (GaAs), cadmium telluride (CdTe) or copper-indium (gallium) -sulfur-selenium compounds, the so-called CIGS solar cells or CIS Cells, whereby depending on the cell type S can stand for sulfur or selenium. A new material that is newly used in thin-film technology is CZTS .
Efficiency levels in the range of 20% (21.7% with CIGS solar cells, see) for small CIGS laboratory cells (≈ 0.5 cm²) are possible. CIGS thin-film modules meanwhile achieve similar efficiencies as modules made of polycrystalline silicon (11–12%).
For cadmium telluride cells, the efficiency of laboratory cells was 21% in August 2014.
Often more important are the costs at which electricity can be generated from the solar cells, and there are also important criteria such as the emission of pollutants. Some studies show that cadmium telluride thin-film solar cells have a better balance here.
Another strength of thin-film modules is that they can be produced more easily and with a larger area, especially thin-film cells made of amorphous silicon. Thin-film modules do not depend on a rigid substrate such as glass or aluminum. With roll-up solar cells for the hiking backpack or sewn into clothes, a lower level of efficiency is accepted; the weight factor is more important than the optimal light conversion.
Machines that are also used for the production of flat screens are suitable for production. Coating areas of over 5 m² are achieved. The process for the production of amorphous silicon can also be used to produce crystalline silicon in thin layers, so-called microcrystalline silicon. It combines the properties of crystalline silicon as a cell material with the methods of thin-film technology . In the combination of amorphous and microcrystalline silicon, considerable increases in efficiency were achieved for some time, but the efficiency is currently stagnating; the technology has been losing market share noticeably since 2012.
A special process for the production of crystalline thin-film cells from silicon was used for CSG modules (CSG: Crystalline Silicon on Glass ). With these modules, a silicon layer less than two micrometers thick is applied directly to a glass substrate ; the crystalline structure is only achieved after heat treatment. The power supply is applied using laser and inkjet printing technology . For this purpose, a production facility was built in Germany in 2005 by the CSG Solar company . Because the process could not be operated economically, the company had to stop production after a short time. The Chinese solar group Suntech acquired the company and its technology, but gave up activities in this area in 2011 and closed the company.
Thin-film solar cells made of black silicon are currently being developed, which should achieve roughly double the efficiency.
Concentrator cells
With concentrator cells (also concentrator photovoltaics , English : Concentrated PV, CPV), semiconductor space is saved by initially concentrating the incident sunlight on a smaller area. This can be achieved through geometrical optics as described in this section or through fluorescent cells with light guide bodies that use total reflection .
The bundling of light is z. B. achieved with lenses , mostly Fresnel lenses , or mirrors. Light guides are sometimes used to guide the concentrated light.
Concentrator cells are supposed to save semiconductor material, which allows the use of more efficient, more expensive materials. The solar radiation of a larger area can therefore often be used even at lower costs. Frequently used materials for concentrator solar cells are III-V semiconductors . Mostly, multi-junction solar cells are used (see next section), which would be uneconomical for full-area solar cells. They still work reliably at more than 500 times the sun's intensity. Concentrator solar cells have to track the position of the sun so that their optics can concentrate the solar radiation on the cells. The US energy authority has achieved efficiencies of over 40% with this technology.
Multi-junction solar cells
Multi-junction solar cells consist of two or more solar cells with different materials that are monolithically layered on top of each other. The purpose of this arrangement is to increase the efficiency of the entire arrangement. The efficiency of laboratory samples of tandem concentrator solar cells reached over 40% in 2008 and 2009. In 2014, the Fraunhofer Institute for Solar Energy Systems ISE achieved an efficiency of 46% with a quadruple solar cell and 508 times the concentration.
Electrochemical dye solar cell
In the case of dye solar cells, also known as Grätzel cells, the current is obtained, in contrast to the cells listed above, via the light absorption of a dye ; Titanium dioxide is used as a semiconductor . Complexes of the rare metal ruthenium are mainly used as dyes, but for demonstration purposes even organic dyes, for example the leaf dye chlorophyll or anthocyanins (from blackberries ), can be used as light acceptors (however, these have a short lifespan). The functioning of the cell has not yet been clarified in detail; the commercial application is considered to be quite safe, but is not yet in sight in terms of production technology.
Conventional n-type dye solar cells work with a photoanode, a positive electrode that is connected to an n- type semiconductor , e.g. B. titanium dioxide , and a dye is coated. When light hits it, the dye molecules are excited and release electrons. A redox mediator, which is part of the electrolyte and can move freely between the electrodes, regenerates the dye. With the p-type (p-DSC, p-dye-sensitized solar cell), the process is exactly the opposite. A special dye and a p-semiconductor are on a photocathode. The light-excited dye sucks electrons from the valence band of the p-semiconductor, e.g. B. nickel oxide out. Scientists from Monash University , the Commonwealth Scientific and Industrial Research Organization (Australia) and Ulm University replaced the commonly used system of iodide and tri-iodide with the cobalt complex tris (1,2-diaminoethane) cobalt (II / III), in which the cobalt can switch between the +2 and +3 oxidation states . Cells on this basis achieve a higher energy conversion efficiency. Another approach to increasing the performance of photovoltaic cells is to combine an n- and a p-type dye solar cell into a tandem solar cell .
Organic solar cells
An organic solar cell is a solar cell made of materials used in organic chemistry ; H. from hydrocarbon - compounds ( plastics ). These compounds have electrically semiconducting properties. The efficiency with which solar energy is converted into electrical energy was 12.0% in 2013, below that of solar cells made of inorganic semiconductor material . Organic solar cells or plastic solar cells, as they are also called, are a current research topic due to the possibilities with regard to inexpensive and versatile manufacturing processes. The advantages over silicon solar cells mentioned by the manufacturers of these plastic-based cells are:
- Low manufacturing costs due to cheap production technologies
- High current yields thanks to thin-film large-area technologies for plastics
- Greater flexibility, transparency and easy handling (mechanical properties of plastics)
- High environmental compatibility (carbon-based plastics)
- Adaptation to the solar spectrum through targeted polymer synthesis
- "Colorful" solar cells for architectural style elements
The material for this type of solar cell is based on organic hydrocarbon compounds with a specific structure, the conjugated π-electron system , which gives the materials in question the essential properties of semiconductors. Typical representatives of organic semiconductors are conjugated polymers and molecules, whereby specially synthesized hybrid structures are also used. The first plastic solar cells made from conjugated polymers (electron donors) and fullerenes (electron acceptors) were two-layer solar cells. These cells consist of a thin layer of the conjugated polymer on which another thin layer of fullerenes is applied. From a technological point of view, conjugated polymers and functionalized molecules are attractive base materials for the cost-effective mass production of flexible PV elements with a comparatively simple structure due to their processability from the liquid phase. Molecular semiconductors are usually processed into well-defined multilayer systems in vacuum-assisted evaporation processes and allow the production of sequentially deposited semiconductor layers and thus more complex cell types (e.g. tandem cells).
Organic photovoltaics (OPV) has the technological potential to find its way into mobile power supply as a so-called “ low-cost energy source ”; This is also due to the cost-effective mass production based on established printing processes. This could open up a new area of application with low investment costs at the same time. The company Konarka Technologies GmbH in Nuremberg in 2009 had brought the first organic panels for mobile devices on the market.
Hybrid solar cell
A hybrid solar cell is a solar cell that contains organic and inorganic components.
Fluorescent cell
Fluorescence cells are solar cells that first generate light of greater wavelengths using fluorescence in a plate ( Stokes shift ), in order to convert it through cells sitting on the edges of the plate. A large part of the longer-wave light generated in the plate only reaches the edges of the plate due to total reflection.
Solar cells based on this principle are also part of the concentrator solar cells. The advantage is that they do not have to be tracked like those with geometrical optics and that shorter wavelengths can be better used. Concentration factors of over 30 can be achieved. Such solar cells are also used for the power supply in poor lighting conditions in rooms and have two effects in particular:
- Conversion of short wavelengths into longer ones, which are converted more effectively by silicon solar cells
- Concentration so that the solar cells work effectively even in low light
Cells for thermal photovoltaics (TPV)
Cells for thermal photovoltaics (TPV) based on InP (formerly GaSb ) do not use visible sunlight, but heat radiation, i.e. light of a much higher wavelength. The efficiency has been increased to 12% through more recent work (previously a maximum of 9%). A potential application of such cells would be the utilization of heat, as it arises in large quantities in large-scale technical applications and which previously had to be disposed of with additional effort.
history
The beginning of the use of the sun for the production of electrical energy can roughly be dated to the year 1839. The Frenchman Alexandre Edmond Becquerel found that when a battery is exposed to sunlight, it has a higher performance than without it. He used the potential difference between a darkened and an exposed side of a chemical solution in which he dipped two platinum electrodes. When he placed this construction in the sun, he observed that a current developed between the two electrodes. This is how he discovered the photovoltaic effect, but could not yet explain it. It was later shown that other materials such as copper are also photoconductive.
The photoconductivity was demonstrated for selenium in 1873. Ten years later, the first “classic” photocell was made from selenium. Another ten years later, in 1893, the first solar cell for generating electricity was built.
In 1904, the Austro-Hungarian physicist Philipp Lenard discovered that rays of light release electrons from the surface when they hit certain metals and thus provided the first explanations for the effect of photovoltaics. A year later he received the Nobel Prize in Physics for studying the passage of cathode rays through matter and for his electron theory.
Albert Einstein achieved the final breakthrough in 1905 when he was able to explain the simultaneous existence of light both as a wave and as a particle with the help of quantum theory. Until then it was believed that light only occurs as energy with different wavelengths. But Einstein found in his attempts to explain photovoltaics that light behaves exactly like a particle in some situations, and that the energy of each light particle or photon depends only on the wavelength of the light. He described the light as a collection of projectiles hitting the metal. When these projectiles have enough energy, a free electron that is in the metal and is hit by a photon is released from the metal. He also discovered that the maximum kinetic energy of the detached electrons is independent of the intensity of the light and is only determined by the energy of the photon that hits it. This energy in turn only depends on the wavelength (or the frequency) of the light. In 1921 he received the Nobel Prize in Physics for his work on the photoelectric effect.
The discovery of the pn junction (crystal rectifier) in 1947 by William B. Shockley , Walther H. Brattain and John Bardeen was another big step towards the solar cell in its present form. After these discoveries, nothing stood in the way of building a solar cell in its current form. However, it is thanks to a lucky coincidence that this first solar cell was built in 1954 in the laboratories of the American company Bell. The company's employees (under team leader Morton Price) observed, when they examined a rectifier that worked with the help of silicon, that it delivered more electricity when it was in the sun than when it was covered. Bell quickly recognized the benefit of this discovery for supplying the telephone network in rural regions with electricity, which until then had been done with batteries. The Bell company , more precisely Daryl Chapin , Calvin Souther Fuller and Gerald Pearson , developed the first arsenic- doped silicon-based solar cell in 1953 , which had an efficiency of around 4%. By changing the dopant, the efficiency could be increased to about 6%.
Space travel recognized the benefits of solar technology very quickly and in 1958 it equipped a satellite with solar cells for the first time . Vanguard 1 launched on March 17, 1958 and was only the fourth satellite ever. He owned a solar panel, which was equipped with 108 silicon solar cells. These only served as a charging station for the batteries and not for direct power supply. It was calculated that the cells had an efficiency of 10.5%. The designers had assumed a lower energy yield and a shorter service life, so that this satellite was not provided with an "off switch". Only after eight years did the satellite cease to operate due to radiation damage.
Shortly afterwards, the CdS-Cu2S solar cell was created , which was still used in satellites until the early 1990s. In comparison with Vanguard I, today's satellites are equipped with around 40,000 solar cells.
In space, nothing stands in the way of natural solar radiation compared to the earth's surface, no blankets of clouds and no radiation-absorbing and more or less polluted atmosphere that hinders sunlight. On the other hand, the extreme radiation conditions in space lead to a stronger degradation of the solar cells than is the case on earth. Since then, industry and research have been trying to achieve ever greater levels of efficiency and at the same time to improve degradation and radiation resistance.
Usually space probes in the inner solar system are supplied with electricity by solar cells. Since solar cells used today for space travel are not only 50% more efficient, but also more radiation-resistant than the silicon cells used 20 years ago, the Juno space probe will be able to launch in 2011 as the first space probe equipped with solar cells to the planet Jupiter , which is submerged in radiation .
By using purer silicon and better doping options, the efficiency has been increased and the service life increased. In 1972, Mandelkorn and Lamneck improved the service life of the cells by reflecting the minority charge carriers by introducing a so-called back surface field (BSF) into the p-conducting layer. In 1973 Lindmayer and Ellison presented the so-called violet cell, which already had an efficiency of 14%. By reducing the reflectivity, the efficiency was increased to 16% in 1975. These cells are called CNR solar cells ( Comsat Non Reflection ; Comsat = telephone satellite) and were developed for satellites. In the meantime, Green, Stanford University and Telefunken have developed solar cells with efficiencies of around 20%. The theoretical efficiency for silicon solar cells is 29% for the radiation conditions in middle latitudes. For the efficiencies see also technical characteristics .
The main impetus for this development was the quadrupling of the oil price in the early 1970s . After this price increase, Richard Nixon started a research program in 1974 that dealt with renewable energies. Until then, each watt cost $ 200 and was therefore not competitive. In order to gain acceptance and trust among the population, races with solar cars were held in the early 1980s, and in July 1981 a solar powered airplane crossed the English Channel.
Thin-film modules made of amorphous silicon enabled the autonomous supply of pocket calculators, watches and other small consumers.
Modules with crystalline cells were initially used for island systems with a 12 V system voltage based on a lead battery. From 1990 onwards, large-scale use in grid-connected systems began in Germany with the 1000 roofs program .
Until the late 1990s, cells with an edge length of around 100 mm ( also called four-inch cells in technical jargon ) and 36 cells per module were the most common size for 12 V systems. Thereafter, 125 mm cells (5 ") were increasingly used for modules with 72 cells for 24 V system voltage, and since around 2002 156 mm cells (edge length 156 mm or 6 inches) have been the common choice for standard modules with typically 60 cells Attempts to introduce 8 "cells were discontinued because the mechanical stability would have required an increase in the wafer thickness and thus the use of material.
From 2007 onwards, thin-film modules with cells made of CdTe from First Solar triggered a price slide for solar modules. Plants for modules with CIS and CIGS cells were set up. Chinese modules made of crystalline silicon have dominated the market since 2012 because of their low price.
Cost reduction and growth of worldwide installations
Adjusted for inflation, the module costs were US $ 96 per watt in the mid-1970s. Improvements in production and an enormous increase in the amount produced led to a reduction to around 70 US cents per watt at the beginning of 2016. The costs for the system peripherals (balance of system, BOS) were even higher than those of the modules for some time . In 2010, large open-space systems could be built for 3.40 US dollars per watt, with roughly the same module and BOS costs.
Because of the industrial use of ever larger Si single crystals, the older machines became cheaper. The cell size increased according to the availability of the appropriate equipment. While cells with an edge length of 125 mm were still installed in the modules in the 1990s and early 2000s, cells with an edge length of 156 mm then prevailed. The mass production of flat screens made large glass plates available at low cost.
Swanson's law is not a physical law in the strictest sense, but merely a compilation of data similar to Moore's law : The cell prices decrease by 20% when the production volume is doubled. The term was first used in an article in The Economist weekly.
During the 1990s, more and more cells were made from multicrystalline material. Although these cells are less efficient than the monocrystalline cells, they are much cheaper to manufacture, which is partly due to the lower energy consumption. In the mid-2000s, multi-cells dominated the low-cost module market. The high silicon prices in the mid-2000s also led to a decline in silicon consumption: in 2004 it was 16 grams per watt, with wafer thicknesses around 300 micrometers. In contrast, in 2010 it was only 7 grams per watt, with wafer thicknesses of around 180 micrometers.
Modules made of crystalline silicon dominate the world market (2015: approx. 93% market share according to ISE Photovoltaics Report , page 4), the largest quantities are produced in China and Taiwan. At the end of 2011, demand in Europe collapsed, as a result of which module prices also fell, to 1.10 US dollars per watt; by the end of 2012, prices were already reaching $ 0.62 / watt.
The worldwide installed PV capacity reached around 177 gigawatts peak in 2014 , which is enough to meet 1 percent of the global demand for electrical energy. The expansion is currently the fastest in Asia; In 2014, annual production went to China and a quarter to Japan.
Shapes and sizes
At the beginning of the commercialization of solar technology , round cells were often used, the origin of which stems from the mostly round silicon rods used in the computer industry. In the meantime, this cell shape is no longer found, instead square cells or almost square cells with more or less beveled corners are used. The standard format currently processed is wafers with an edge length of 156 mm. Cells with a larger edge length (210 mm) were advised for a while, but they have a higher breakage rate for the same wafer thickness and, because of the higher current strength, potentially lead to greater ohmic losses; therefore it is not worth making them.
By sawing the finished processed cells, cells with smaller edge lengths are also created for special applications in the small appliance sector. They deliver almost the same voltage as the large cells, but a smaller current according to the smaller area.
In the EFG process, which is no longer used, cells were also produced in which the sides of the resulting rectangle did not have the same lengths.
Efficiency
The efficiency of a solar cell is the ratio of the electrical power it generates and the power of the incident radiation .
The maximum efficiency of a solar cell depends on the band gap and the number of pn junctions optimized for different spectral ranges. In the case of a pn junction, with an optimal band gap and light tuned to the wavelength, an efficiency of up to 41% can theoretically be achieved. In practical applications, the efficiencies that can actually be achieved are around and below 25%. In the case of tandem solar cells , which can cover larger spectral ranges through several different pn junctions, the total efficiency of all pn junctions can also be above the theoretical limit of 41%.
In space, on the one hand, the solar constant is greater than the global radiation on earth, on the other hand, the solar cells age faster. Solar panels for satellites currently (2005) achieve an efficiency of almost 25% with an operating time of 15 years.
| Cell material | Maximum cell efficiency in the laboratory | Maximum efficiency (series production) | Typical module efficiency | Space requirement per kWp |
|---|---|---|---|---|
| Monocrystalline silicon | 26.1% | 24% | 19% | 5.3 m² |
| polycrystalline silicon | 22.3% | 20% | 17% | 5.9 m² |
| Amorphous silicon | 14.0% | 8th % | 6% | 16.7 m² |
| CIS / CIGS | 22.6% | 16% | 15% | 6.7 m² |
| CdTe | 22.1% | 17% | 16% | 6.3 m² |
| Concentrator cell | 46.0% | 40% | 30% | 3.3 m² |
A high degree of efficiency is desirable because it leads to a greater yield of electrical current with the same light conditions and the same area. There is a thermodynamic limit for every machine that generates mechanical or electrical work on earth from sunlight or in some other way (e.g. solar thermal power plants, Stirling engines, etc.) .
Thermodynamic Limit I.
The roughest estimate of the efficiency is obtained from the Carnot efficiency . It describes the maximum efficiency that any physical machine can achieve if it draws its energy from the temperature difference between a heat source and a heat sink. The Carnot efficiency results from the temperature of the warmer source and the temperature of the colder sink according to:
In the case of the solar cell, the heat source is the sun's surface with a temperature of around 5,800 K and the heat sink is the solar cell with a temperature of 300 K. This results in a Carnot efficiency of 95%. Solar cells used in space have a correspondingly higher degree of efficiency due to the higher temperature difference.
Thermodynamic Limit II
The estimate in the section above neglects that the energy from the sun to the solar cell is transmitted by radiation. In a more detailed model, an absorber is placed in front of the solar cell. This absorbs the radiation of the sun and radiates a small part of the heat radiation back to the sun. According to the Stefan-Boltzmann law , the total heat output flows
from the sun to the absorber, where is the Stefan-Boltzmann constant . According to the Carnot efficiency , the absorber can only use the part of this heat
convert into electrical work. The efficiency is now calculated from this share and the total radiated power from the sun to
At a temperature of 5800 K for the sun's surface and 300 K ambient temperature, the efficiency is at its maximum at an absorber temperature of around 2,500 K and is 85%.
Shockley-Queisser limit

The Shockley-Queisser limit considers the excitation process of electrons in a semiconductor that is typical for solar cells. In a solar cell, light is converted into electrical energy by exciting electrons from the valence band into the conduction band. Only a narrow section of the energy spectrum offered is used. The theoretical limit value of energy-selective cells is therefore smaller than the thermodynamic limit of an overall system.
The size of the band gap of the semiconductor is decisive for the energy that can be gained per excited electron . Regardless of how far the electron is excited over the lower edge of the conduction band, the maximum energy of the band gap per electron is obtained as electrical energy. With the electrical power that is obtained from all excited electrons, one has to take into account that more electrons are generated with a small band gap. With a large band gap, each individual electron has more energy. A compromise must therefore be found between the following borderline cases:
- Large band gap: only high-energy light (blue and ultraviolet light) can generate electrons, since longer wavelengths are not absorbed. Because of the large band gap, each electron has a high energy.
- Small band gap: Even long-wave light can excite electrons, so that a large number of electrons are excited into the conduction band. However, due to collision processes with the crystal lattice, these lose part of their energy in a few hundred femtoseconds until they only have the energy of the band gap.
The Shockley-Queisser limit applies to the case of a cell with only one pn junction. With so-called tandem solar cells ( English multi-junction solar cell ) in which several pn junctions are combined with different band gaps, principle, higher efficiencies can be achieved, see section multijunction solar cells .
technical features
The parameters of a solar cell are specified for standardized conditions, the standard test conditions , often abbreviated to STC ( English Standard Test Conditions ):
- Irradiance of 1000 W / m² at module level,
- Solar cell temperature constant 25 ° C,
- Radiation spectrum AM 1.5 global; DIN EN 61215, IEC 1215, DIN EN 60904, IEC 904.
AM 1.5 stands for the term Air Mass , 1.5 for the fact that the sun's rays pass through 1.5 times the height of the atmosphere because they hit at an angle. This corresponds very well to the summer conditions in Central Europe from Northern Italy to Central Sweden or the solar spectrum at a location at sea level at 48 ° latitude during an equinox . In winter, the sun is much lower in temperate latitudes, and a value of AM 4 to AM 6 is more realistic here.
The absorption in the atmosphere also shifts the spectrum of the light hitting the module. "Global" stands for global radiation , which is made up of the diffuse and direct radiation components of the sun.
It should be noted that in reality the cell temperature in particular with such irradiation, which is reached in Germany in summer at midday, is significantly higher during normal operation (depending on installation, wind flow, etc., it can be between about 30 and 60 ° C lie). However, an increased cell temperature also means a reduced efficiency of the solar cell. For this reason, another reference value was created, P NOCT , the performance at normal operating temperature . NOCT (normal operating cell temperature).
Common abbreviations for the names are
- SC: Short Circuit
- OC: Open Circuit - idle
- MPP: Maximum Power Point - operating point of maximum power
- PR: performance ratio; Quality factor that indicates what part of the electricity yield generated by the solar generator (under nominal conditions) is actually available.
The characteristics of a solar cell are
- Open circuit voltage (also )
- Short circuit current
- Voltage at the best possible operating point (also )
- Current at the operating point with maximum power
- Maximum achievable power Solar cell manufacturers state this maximum achievable power under standardized conditions in the unit kWp, in other words "kilowatt peak"
- Fill factor
- Coefficient for the change in power with cell temperature, typically the maximum power drops by 0.4% per degree Celsius above NOCT (25 ° C).
- Cell efficiency with the irradiated area and the irradiance
Solar cells can therefore deliver an output of very roughly 160 W / m² . When installed in a module, the output per area is lower, as there are clearances between the cells and the edge of the module. An output of 220 watts per square meter is achieved in space. Since no atmosphere absorbs part of the solar radiation, more radiation arrives at the solar cell in earth orbit , namely the solar constant on average . That is why solar cells suitable for space are qualified directly in the AM0 spectrum .
Circuit diagrams
The circuit symbol of a solar cell, like the circuit symbol of a diode or photodiode , uses an arrow to indicate the technical current direction for the connection. However, the characteristic curve of a real solar cell differs from that of an ideal photodiode. There are several equivalent circuit diagrams to model these deviations .
Simplified equivalent circuit diagram
The circuit diagram initially only consists of a current source that is connected in parallel to an ideal diode. This produces a current that depends on the irradiance and models the photocurrent . The total current strength results from the diode current (see diode )
- .
Extended equivalent circuit diagram (one- and two-diode model)
The extended equivalent circuit takes into account real factors of the component that arise during manufacture . These models are intended to create a model of the actual solar cell that is as realistic as possible. In the single-diode model , the simplified equivalent circuit diagram is initially only supplemented by a resistor connected in parallel and one in series .
- The parallel resistance Rp symbolizes crystal defects , non-ideal doping distributions and other material defects that cause leakage currents that bridge the pn junction . This resistance is relatively high in solar cells that are well manufactured.
- The series resistance Rs summarizes all the effects that result in a higher overall resistance of the component. These are mainly the resistance of the semiconductor material, the resistance at the contacts and the leads . This size should be as small as possible for manufactured solar cells.
The formula for the total current is already a recursive function for this model and is:
In the transition to the two-diode model , another diode with a different ideality factor n is added. Usually these are set using the values 1 and 2. Furthermore, all these models can be supplemented by a voltage-controlled current source when operated in reverse direction in order to model the avalanche breakdown. The formulas for the currents in the two-diode model are then, with adaptation conductance g b , breakdown voltage U b and avalanche breakdown exponent n b :
Energetic amortization and harvest factors
The energetic amortization period is the point in time at which the primary energy used to manufacture a photovoltaic system was generated again by the same. Since the electrical energy used in production is classified as secondary energy, it is converted into primary energy using the efficiency of a fossil power plant. This conversion is also carried out accordingly for the electrical energy generated by the solar cell. Similarly, one can imagine that the photovoltaic system replaces the electricity from a conventional power plant.
According to a study by Peng et al., The energetic amortization period of photovoltaic systems is currently (as of 2013). globally between 0.75 and 3.5 years, depending on the location and photovoltaic technology used. The mean value fluctuates in the range from approx. 1.5 to 2.5 years. This means that during this period the photovoltaic system has again brought in the energy that was consumed during its entire life cycle. The manufacture of the systems, their transport, construction, operation and dismantling or recycling are therefore taken into account . The calculated CO 2 emissions from photovoltaic systems are between 10.5 and 50 g CO 2 / kWh, depending on the technology and location , with averages in the range from 35 to 45 g CO 2 / kWh. The study assumed 30 years for modules based on crystalline silicon cells and 20-25 years for thin-film modules, and 15 years for the inverter. During their operating time, photovoltaic systems therefore provide a multiple of the energy that was originally used to produce them.
environmental Protection
The manufacture of photovoltaic solar cells is a chemical process in which gaseous, liquid and solid chemicals are used that are harmful to health and the environment. For this reason, a high standard of process security is essential. Safe exhaust air collection and cleaning must be guaranteed under occupational safety aspects.
Some types of solar cells use substances such as silicon instead of silicon . B. toxic or carcinogenic cadmium , arsenic or their compounds and copper-indium-gallium-diselenide . A typical cadmium telluride solar module (CdTe), for example, contains approx. 22 g of the dangerous heavy metal cadmium and 25 g of tellurium per m² of cell surface. A CdTe solar cell usually consists of five individual layers: Specifically, these are an approx. 8 μm thick CdTe absorber layer, an approx. 100 nm thick cadmium sulfide intermediate layer and two 20 or 100 nm thick antimony telluride (Sb 2 Te 3 ) thin layers . During production, both substances are applied to the carrier material by co-evaporation. Co-evaporation is a non-directional process in which the entire inner surface of the coating chamber is coated with thin layers of tellurium, cadmium telluride, cadmium sulphide and antimony telluride. This increases material consumption by at least another 40%.
During regular cleaning of the production chamber with aqueous acid - cleaning is done manually - the residues are converted into an aqueous solution. In addition to the actual cell production, cleaning the production facilities also represents a challenge in terms of environmental protection and occupational safety . Production residues and emissions can lead to long-term damage and contaminated sites . When disposing of old cells, particular attention should be paid to the fact that they can contain dangerous heavy metals.
Investigations from 2008 came to the result that during production and operation, CdTe cells release 90 to 300 times less cadmium per kilowatt hour into the atmosphere than coal-fired power plants with a service life estimated at 30 years. Taking into account the energy required for production and assuming that this comes from conventional power plants, the emission of cadmium is still a factor of 10 below that of coal-fired power plants. The problem with perovskites as solar cell material is the necessary proportion of lead .
Safety assessment
For protective measures in the event of fire and lightning strikes, see photovoltaic system .
See also
literature
- Christoph Brabec: Organic photovoltaics - materials, device physics, and manufacturing technologies . Wiley-VCH, Weinheim 2008, ISBN 978-3-527-31675-5 .
- Guillermo Diaz-Santanilla: Technology of the solar cell - physical principles, properties and applications . Franzis, Munich 1984, ISBN 3-7723-7371-2 .
- Heinrich Häberlin: Photovoltaics: Electricity from sunlight for network and island systems . 2nd, significantly expanded and updated edition. VDE / Electrosuisse, Berlin / Fehraltorf 2010, ISBN 978-3-8007-3205-0 .
- Tom Markvart, Luis Castañer: Solar cells - materials, manufacture and operation . Elsevier, Oxford 2006, ISBN 1-85617-457-3 (English).
- Volker Quaschning , Regenerative Energy Systems. 9th edition. Hanser, Munich 2015, ISBN 978-3-446-44267-2 .
- Viktor Wesselak , Sebastian Voswinckel: Photovoltaics: How the sun becomes electricity. Data, facts, background . Springer Vieweg, Berlin 2012, ISBN 978-3-642-24296-0 (= technology in focus ).
- Viktor Wesselak , Thomas Schabbach , Thomas Link, Joachim Fischer: Handbuch Regenerative Energietechnik , 3rd updated and expanded edition, Berlin / Heidelberg 2017, ISBN 978-3-662-53072-6 .
- Peter Würfel: Physics of Solar Cells . Spectrum Academic Publishing House, Heidelberg 2000, ISBN 3-8274-0598-X ( spectrum university paperback ).
Web links
- The solar mouse - creation of the solar cell with Armin Maiwald ( YouTube video)
- PV Education - detailed online lecture with basics and applications for PV (English)
- Photovoltaics - Innovations (BINE Information Service)
- Solar cells explained on YouTube , accessed October 7, 2018.
Individual evidence
- ^ Photovoltaics Report . Fraunhofer ISE website . Retrieved June 5, 2015.
- ^ Orbital Sciences Corporation (Ed.): Dawn. Investigating the "Dawn" of Our Solar System (fact sheet) (PDF; 1.4 MB). 2009 (English).
- ↑ Zhiliang Ku et al., Full Printable Processed Mesoscopic CH3NH3PbI3 / TiO2 Heterojunction Solar Cells with Carbon Counter Electrode . In: Scientific Reports 3, (2013), doi: 10.1038 / srep03132
- ↑ Volker Quaschning: Renewable energies and climate protection. 3rd edition, Munich 2013, p. 126.
- ↑ Polycrystalline silicon thin film solar cells on glass. Archived from the original on December 2, 2013 ; Retrieved October 25, 2016 .
- ^ MA Green , K. Emery, DL King, Y. Hishikawa, W. Warta: Solar Cell Efficiency Tables (Version 28) . In: Progress in Photovoltaics . tape 14 , 2006, p. 455-461 , doi : 10.1002 / pip.720 .
- ↑ Michael D. Kelzenberg, Shannon W. Boettcher, Jan A. Petykiewicz, Daniel B. Turner-Evans, Morgan C. Putnam, Emily L. Warren, Joshua M. Spurgeon, Ryan M. Briggs, Nathan S. Lewis, Harry A. Atwater: Enhanced absorption and carrier collection in Si wire arrays for photovoltaic applications . In: Nat Mater . tape 9 , no. 3 , February 2010, p. 239-244 , doi : 10.1038 / nmat2635 .
- ↑ Solar power light. In: Image of Science. February 15, 2010, accessed September 9, 2019 (news item).
- ↑ SolarServer: Photovoltaic research: Caltech develops flexible solar cells with rows of silicon wire and high absorption ( Memento of the original from January 6, 2014 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed May 31, 2012.
- ↑ World record: 41.1% efficiency for multiple solar cells. In: pro-physik.de. Fraunhofer Institute for Solar Energy Systems ISE, accessed on August 9, 2009 .
- ↑ heise: 28.2% efficiency achieved (accessed June 24, 2011)
- ^ Martin A. Green , Keith Emery, Yoshihiro Hishikawa, Wilhelm Warta, Ewan D. Dunlop: Solar cell efficiency tables (version 43) . In: Progress in Photovoltaics : Research and Applications . tape 22 , no. 1 , 2014, p. 1-9 , doi : 10.1002 / pip.2452 .
- ↑ CIGS DÜNSCHICHT-TECHNOLOGIE ACHIEVES A WORLD RECORDER EFFICIENCY OF 17.4% ( Memento of the original from February 22, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , Q-Cells press release dated November 29, 2011, accessed on February 14, 2012
- ↑ New world record for organic solar cells: Heliatek asserts itself as the technology leader with 12% cell efficiency ( memento of the original from March 29, 2014 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , Press release from Heliatek from January 16, 2013
- ↑ Antonio García-Olivares, Substituting silver in solar photovoltaics is feasible and allows for decentralization in smart regional grids . In: Environmental Innovation and Societal Transitions (2015), doi: 10.1016 / j.eist.2015.05.004 .
- ↑ Dmitrii Bogdanov et al .: Radical transformation pathway towards sustainable electricity via evolutionary steps . In: Nature Communications . tape 10 , 2019, doi : 10.1038 / s41467-019-08855-1 .
- ↑ USGS Minerals Information
- ↑ Lars Fischer: Raw materials: The raw material crises of the future , spectrum-direct, March 29, 2011, accessed on September 12, 2011
- ↑ Indium stocks according to USGS Mineral Commodity Summaries (2006) (PDF; 74 kB)
- ↑ About the availability of indium and gallium. (PDF) Retrieved October 25, 2016 .
- ↑ Indium and Gallium Supply Sustainability September 2007 Update , 22nd EU PV Conference, Milan, Italy, February 16, 2009.
- ↑ Prof. H. Föll, Fundamental Functioning of a Solar Cell , script "Materialwissenschaft II", accessed on October 16, 2014.
- ↑ Kazuo Nakajima, Noritaka Usami: Crystal Growth of Si for Solar Cells . Springer, 2009, ISBN 978-3-642-02043-8 , pp. 4-5 .
- ↑ Silicon Manufacturing - Bridgman Process Info Page on renewable-energy-concepts.com, accessed April 17, 2010
- ↑ Crystal growing ( Memento of the original from February 15, 2010 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Info page from Swiss Wafers, accessed on April 17, 2010
- ↑ Squaring and briquetting ( memento of the original from February 15, 2010 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Info page from Swiss Wafers, accessed on April 17, 2010.
- ↑ Recycling of silicon is supposed to reduce manufacturing costs for solar cells. Retrieved October 25, 2016 .
- ↑ BINE Information Service: Subject information: Photovoltaics - Innovations - Solar cells made from silicon wafers. In: www.bine.info. Retrieved April 7, 2016 .
- ↑ a b Dr. S. Philipps (Fraunhofer ISE), W. Warmuth (PSE AG): Fraunhofer ISE publishes "Photovoltaics Report". In: www.ise.fraunhofer.de. P. 30 , accessed on April 7, 2016 (English).
- ↑ a b Pulling or sawing - a system comparison ( Memento from October 17, 2007 in the Internet Archive )
- ↑ Eicke Weber new head of the Fraunhofer Institute for Solar Energy Systems ISE ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. . Fraunhofer Society. July 6, 2006, accessed April 22, 2010 (press release).
- ↑ a b Michael D. Kelzenberg, Shannon W. Boettcher, Jan A. Petykiewicz, Daniel B. Turner-Evans, Morgan C. Putnam, Emily L. Warren, Joshua M. Spurgeon, Ryan M. Briggs, Nathan S. Lewis, Harry A. Atwater: Enhanced absorption and carrier collection in Si wire arrays for photovoltaic applications . In: Nature Materials . advance online publication, 2010, doi : 10.1038 / nmat2635 .
- ↑ Christel Budzinski: Black and Efficient - Black Silicon for Solar Cells. In: World of Photonics. Retrieved February 19, 2010 .
- ^ RS Wagner, WC Ellis: Vapor-liquid-solid mechanism of single crystal growth . In: Applied Physics Letters . tape 4 , no. 5 , 1964, pp. 89-90 , doi : 10.1063 / 1.1753975 .
- ↑ Christoph Seidler: Photovoltaics, tiny silicon wires catch sunlight . Spiegel Online, February 15, 2010, accessed: February 19, 2010.
- ↑ Inexpensive solar alternative . In: Süddeutsche Zeitung , August 14, 2014. Accessed August 14, 2014.
- ^ A b Nicola Armaroli , Vincenzo Balzani : Solar Electricity and Solar Fuels: Status and Perspectives in the Context of the Energy Transition . In: Chemistry - A European Journal . tape 22 , no. 1 , 2016, p. 32-57 , doi : 10.1002 / chem . 201503580 .
- ↑ Boris Hänßler: Solar cells made of perovskite At: Spektrum.de from December 19, 2013.
- ^ Robert LZ Hoye et al .: Strongly Enhanced Photovoltaic Performance and Defect Physics of Air-Stable Bismuth Oxyiodide (BiOI) . In: Advanced Materials . 2017, doi : 10.1002 / adma.201702176 .
- ↑ Pablo P. Boix et al .: Perovskite Solar Cells: Beyond Methylammonium Lead Iodide . In: The Journal of Physical Chemistry Letters . tape 6 , 2015, p. 898-907 , doi : 10.1021 / jz502547f .
- ↑ Nakita K. Noel et al .: Lead-free organic-inorganic tin halide perovskites for photovoltaic applications . In: Energy and Environmental Science . tape 7 , 2014, p. 3061-3068 , doi : 10.1039 / c4ee01076k .
- ↑ Jian Gong et al., Perovskite photovoltaics: life-cycle assessment of energy and environmental impacts . In: Energy and Environmental Science 8, (2015), 1953–1968, doi: 10.1039 / c5ee00615e .
- ↑ Thin-film solar cells. Retrieved October 25, 2016 .
- ^ ZSW Brings World Record Back to Stuttgart. (PDF) Archived from the original on November 29, 2014 ; Retrieved October 25, 2016 .
- ↑ M. Powalla, B. Dimmler, R. Schäffler, G. Voorwinden, U. Stone, H.-D. Mohring, F. Kessler, D. Hariskos: CIGS solar modules - progress in pilot production, new developments and applications. In: 19th European Photovoltaic Solar Energy Conference, (2004) Paris ( PDF ( Memento of the original from October 9, 2006 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and remove then this note. ).
- ↑ First Solar hits 21.0% thin-film PV record
- ^ A b Vasilis M. Fthenakis, Hyung Chul Kim, Erik Alsema: Emissions from Photovoltaic Life Cycles. In: Environmental Science & Technology. 42, No. 6, 2008, pp. 2168–2174 ( PDF ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. , Doi: 10.1021 / es071763q ).
- ↑ CSG Solar AG Discontinues Operations ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. , Press release of July 1, 2011
- ↑ Alok Jha: 'Black silicon' boosts solar cell efficiency . guardian.co.uk, October 15, 2008.
- ↑ Andreas Mühlbauer: New solar concentrator promises cheaper electricity , Elektronikpraxis, February 23, 2009
- ^ Tyler Hamilton: A Cheaper Solar Concentrator , Technology Review, February 20, 2009
- ^ NREL: Concentrating Solar Power Research - Concentrating Photovoltaic Technology. (No longer available online.) In: nrel.gov. Archived from the original on August 22, 2011 ; accessed on December 30, 2014 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Sarah Kurtz: Opportunities and Challenges for Development of a Mature Concentrating Photovoltaic Power Industry (PDF; 130 kB) National Renewable Energy Laboratory. November 2012. Retrieved February 8, 2012.
- ↑ 41.1% solar cell with 46% efficiency - new world record French-German cooperation confirms the competitiveness of the European photovoltaic industry . Fraunhofer ISE press release dated December 1, 2014. Accessed August 14, 2015.
- ↑ Satvasheel Powar, Torben Daeneke, Michelle T. Ma, Dongchuan Fu, Noel W. Duffy, Günther Götz, Martin Weidelener, Amaresh Mishra, Peter Bäuerle, Leone Spiccia1, Udo Bach: Highly Efficient p-Type Dye-Sensitized Solar Cells based on Tris (1,2-diaminoethane) cobalt (II) / (III) electrolytes . In: Angewandte Chemie . tape 124 , no. 50 , 2012, ISSN 1521-3757 , doi : 10.1002 / anie.201206219 .
- ↑ New world record in organic photovoltaics: Heliatek achieves 12% solar cell efficiency. SolarServer, January 17, 2013, accessed September 11, 2013 .
- ↑ Konarka announces availability of solar cells for portable chargers at the European Photovoltaic Solar Energy Conference. Konarka, September 20, 2009; Archived from the original on October 21, 2009 ; Retrieved December 9, 2009 (press release).
- ↑ Hybrid solar cells based on inorganic semiconductor nanoparticles and conductive polymers ( Memento of the original of December 10, 2009 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. . Carl von Ossietzky University of Oldenburg. Institute for Physics.
- ^ Made from Solar Concentrate. Retrieved October 25, 2016 .
- ↑ FLUKO * solar clock. (PDF) Retrieved October 25, 2016 .
- ↑ PDF file on cells for thermal photovoltaics (English)
- ^ Spacecraft and Instruments
- ↑ Monocrystalline solar cells made of silicon Article on solarintegration.de, accessed on June 6, 2010
- ↑ Musk vs. Buffett: The Billionaire Battle to Own the Sun. Retrieved October 25, 2016 .
- ↑ $ 1 / W Photovoltaic Systems DOE whitepaper August 2010
- ↑ Sunny Uplands: Alternative energy will no longer be alternative , The Economist. November 21, 2012. Retrieved August 22, 2016.
- ↑ a b ISE Photovoltaics Report, June 2016, page 30
- ^ Giles Parkinson: Plunging Cost Of Solar PV (Graphs). Clean Technica, March 7, 2013, accessed August 22, 2016 .
- ↑ Snapshot of Global PV 1992-2014 . International Energy Agency - Photovoltaic Power Systems Program. March 30, 2015. Archived from the original on March 30, 2015.
- ↑ In: esa.int Technical and Quality Management - Home - European solar cell efficiency reaches new high ( Memento from October 1, 2008 in the Internet Archive )
- ↑ Volker Quaschning : Renewable energies and climate protection. 4th edition, Munich 2018, p. 134.
- ↑ Jenny Nelson: The physics of solar cells . Imperial College Press, 2003, ISBN 1-86094-349-7 , pp. 291 (English, limited preview in Google Book search).
- ^ A b Günther La Roche: Solar generators for space travel: Basics of photovoltaic solar generator technology for space travel applications . Ed .: Otto Mildenberger. 1st edition. Vieweg, Braunschweig 1997, ISBN 3-528-06945-7 , pp. 253 .
- ↑ Data sheet Solarwatt Vision 60M style. (PDF) April 11, 2019, accessed on May 13, 2019 .
- ↑ This is how solar energy is converted into electricity by satellites. (No longer available online.) Archived from the original on January 6, 2014 ; Retrieved October 25, 2016 .
- ^ V. Quaschning: Energy expenditure for the production of regenerative systems . 2002, Retrieved August 10, 2009.
- ↑ Jinqing Peng, Lin Lu, Hongxing Yang, Review on lifecycle assessment of energy payback and greenhouse gas emission of solar photovoltaic systems in: Renewable and Sustainable Energy Reviews 19, (2013) 255–274, in particular p. 256 u. 269. doi: 10.1016 / j.rser.2012.11.035 .
- ↑ VM Fthenakis, SC Morris, PD Moskowitz, DL Morgan: Toxicity of cadmium telluride, copper indium diselenide, and copper gallium diselenide. In: Progress in Photovoltaics 7, No. 6, 1999, pp. 489-497.
- ↑ Boris Hänßler: Solar cells made of perovskite At: Spektrum.de from December 19, 2013.

















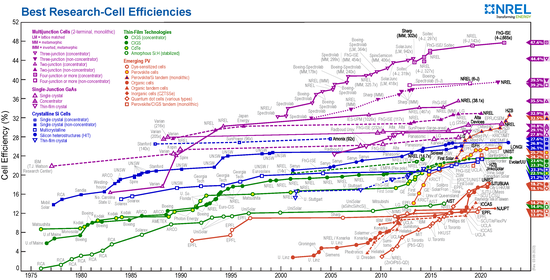













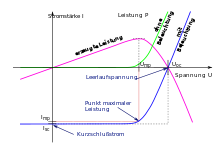
















![I = I_ \ mathrm {ph} - I_ \ mathrm {D} = I_ \ mathrm {ph} - I_ \ mathrm {S} \ left [\ mathrm {e} ^ {\ frac {U_ \ mathrm {D}} { n \ cdot U_ \ mathrm {T}}} - 1 \ right]](https://wikimedia.org/api/rest_v1/media/math/render/svg/26194b470da8ec2b516edd33772a85c1857ab037)

![I = I_ \ mathrm {ph} -I_ \ mathrm {d} - \ frac {U_ \ mathrm {p}} {R_ \ mathrm {p}} = I_ \ mathrm {ph} -I_ \ mathrm {S} \ left [\ mathrm {e} ^ {\ frac {U + R_ \ mathrm {s} \ cdot I} {n \ cdot U_ \ mathrm {T}}} - 1 \ right] - \ frac {U + R_ \ mathrm { s} \ cdot I} {R_ \ mathrm {p}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4cd7201efe317e687a20f0b43c2cac0eb0b739e3)

![I = I_ \ mathrm {ph} -I_ \ mathrm {b} -I_ \ mathrm {S1} \ left [\ mathrm {e} ^ {\ frac {U + R_ \ mathrm {s} \ cdot I} {n_1 \ cdot U_ \ mathrm {T}}} - 1 \ right] -I_ \ mathrm {S2} \ left [\ mathrm {e} ^ {\ frac {U + R_ \ mathrm {s} \ cdot I} {n_2 \ cdot U_ \ mathrm {T}}} - 1 \ right] - \ frac {U + R_ \ mathrm {s} \ cdot I} {R_ \ mathrm {p}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1391b0c97902611b77958f5708d1b806d9c7e203)
