Real stranglers
| Real stranglers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Northern gray shrike ( Lanius excubitor ) |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Lanius | ||||||||||||
| Linnaeus , 1758 |
The real shrike ( Lanius ) are a genus of passerine birds within the family of the shrike (Laniidae). The genus was first described by Carl von Linné in 1758 . The Latin Lanius means “butcher, butcher” and refers to the predominantly carnivorous diet of the species and the property of some representatives of spearing prey or jamming them in branch forks. The German name Würger and the occasional English name Butcherbird are in the same context, as is Fiscal , the English part of the name of some African representatives. The main English name Shrike refers to the harsh, harsh calls of many types of stranglers. (English shriek = scream, screech)
The number of species within the genus fluctuates between 25 and over 30, depending on the scientific opinion. Most of them are currently not endangered, although for some species, especially in Central Europe, significant population declines have been recorded, some of which are still ongoing. The Philippines shrike is potentially endangered, the São Tomé shrike , an island endangered on São Tomé , is threatened with extinction.
Most of the sparrow -to- blackbird-sized birds show the typical long-tailed and relatively large-headed shrike shape with a dark hooked bill and a black face mask. Black, gray, white and reddish brown plumage colors dominate. Shrikes feed primarily on large insects and small vertebrates , which they also use to raise their young. The species of the northern temperate zone are often migratory birds , some extreme long-distance migrants , most African and the species of southern and southeastern Asia are resident birds .
The genus is represented in Europe, Asia, North America, Africa and with a subspecies in New Guinea . The Afrotropic and the Palearctic Ocean have the greatest biodiversity.
features
The genus includes small to medium-sized passerine birds, the most important common features of which are the dark face mask and the powerful, not very long hooked bill. Some representatives of the genus have a black upper head color; the face mask is hardly noticeable for them. The beak has a so-called falcon tooth , a sharp point in the upper beak near the end curvature, which in some species corresponds to a slight, particularly sharp-edged indentation in the lower beak . This adaptation to the predominantly carnivorous diet enables an effective killing bite. The species are relatively long-tailed, with some the tail is particularly elongated, which is occasionally expressed in a trivial name. ( Long-tailed shrike , long-tailed shrike English. Long-tailed Shrike) Strangler act großköpfig, this is partly due to the highly developed jaw muscles. This large-headedness can also, as with the buffalo head shrike or the Louisiana shrike , engl. Loggerhead Shrike becomes eponymous.
The smallest species, the red-eared shrike, is 15 centimeters long and weighs 20-25 grams, about the same size as a house sparrow , but much lighter than this, the largest species, the wedge-tailed shrike (especially the highland subspecies L. sphenocercus giganteus ) is 32 Centimeters tall and weighing up to 100 grams. It almost reaches the size of a cuckoo with a slightly lower weight .
Four colors predominate in the plumage: white, black, gray (in many shades) and reddish brown in various shades. Yellowish ( masked shrike and São Tomé shrike), pink and salmon red shades ( southern shrike , Louisiana, chess shrike , especially the subspecies Lanius schach erythronotus ) occur sporadically in the abdomen and flank area of some species, some females of African black and white shrike species, such as fiscal shrike , Long-tailed Shrike or Mackinnon Shrike have dark reddish brown, elongated flank feathers. The coloring is intense and rich in contrast, at least in the males. The face mask is very distinctive in all species in which it is not invisible due to the dark color of the top of the head, such as in the Taita strangler . It usually extends from the base of the beak over the ear covers to the neck. In some species (Louisiana strangler , tiger shrike ) it also covers a narrow forehead strip above the beak, in others (chess shrike, black- fronted shrike ) almost the entire forehead. The mask is often additionally accentuated by a light, fine stripe over the eyes. Very many species have white wing fields, which are particularly noticeable in flight, only a few ( red- backed shrike, tiger shrike, brown shrike ) are completely without white markings on the upper side of the wings. Melanistic , less often leucistic morphs occur, often in some regions. In Eastern China, the melanistic form of the chess strangler led to the creation of a subspecies (incorrectly L. schach fuscatus - actually melanistic morph of L. schach schach ). The tail is graduated in all species (especially clear and eponymous with the wedge-tailed shrike) in a few ( red-headed shrike , red-backed shrike) also rounded spoon-shaped. In some of the reddish brown species it is reddish brown or black, in the gray species it is mostly black. The shorter outer feathers are often lighter or white. The slightly protruding, relatively far forward eyes are black in all species, and the beak of adult birds is also colored. The quite long legs are gray-brown, gray or dark gray, as are the four sharply clawed toes.
A size or weight dimorphism, if present at all, is only weak and not uniform between the species. The color dimorphism is usually slight, only in a few species, such as the Isabellus shrike , the buffalo head shrike or the red-rumped shrike it is clear, in the red-backed shrike it is very clear. Females of many species are less contrasting in color, the face mask is shorter and narrower, the breast and upper plumage show a slight, dark undulation, as it is characteristic of the juvenile plumage of almost all species. The beak is often a bit lighter, especially the lower beak can have light brown or flesh-colored tones at the base of the beak. In some black-and-white, African species, the sexes differ by an elongated, reddish-brown flank plumage that only the females wear. This feature also occurs in the yellow-billed shrike ( Corvinella corvina ). Older females of many species increasingly resemble the males in their plumage.
Birds in their first youthful plumage always differ significantly from adults . Although they usually already show the basic elements of the plumage coloring of adult birds, the colors are paler, usually weakened to gray or gray-brown. There are no strong contrasts. The face mask is often indicated (especially in the area behind the eyes), but never drawn through. In almost all species the juvenile plumage is darkly wavy, flocked or pearled, sometimes with fine lines; Features that also characterize the plumage of many female Lanius representatives. In adult females, these features increasingly fade with successive moults . The youth clothes of the southern shrike and the wedge-tailed shrike show no or only a hint of sparrowing.
Morphological features
The hooked beak is the clearest adaptation of the Lanius species to a way of life as a predator . The beak is high, not particularly long, and looks compressed at the side; it has a falcon tooth, a sharp horny point in the upper beak that fits into a notch in the lower beak; it facilitates the rapid killing of prey, especially the severing of the spine of small vertebrates . The falcon tooth is not already embryonic , as is the case with the falcon ( Falco sp.) , But only develops in young birds that are around four weeks old. The chewing and jaw muscles are very strong, which makes some species appear large-headed in relation to the trunk. The legs are strong, relatively long, paneled on the front (covered with horn scales) on the back rather smaller. The toe arrangement is anisodactyl like in almost all passerine birds . The basal limbs of the middle toe and the 4th toe (outer toe) are fused. The claws are strongly curved, long and pointed. The 1st and 3rd toes are the same length, which enables a pincer-like, firm grip when the foot is closed, in conjunction with the corrugated toe balls. The slightly protruding eyes are positioned relatively far forward in the head, which supports spatial vision. The face mask present in all species, but hardly recognizable in those with a black upper head color, may help to reduce the glare of the sunlight. Most species have 10 hand wings , some, like many species of crows , have a stunted 11.
Mauser
The type and extent of moulting differ significantly between migratory and resident birds. There are also differences between long-distance, short-distance and medium-distance migrants. This also applies within a species if, for example in the case of the northern gray shrike or the Louisiana shrike, northern populations are migratory birds, whereas southern populations remain in the breeding area all year round. The moulting sequences essentially correspond to those of most passerine birds.
Young birds largely leave the nest in their first juvenile plumage when molted. In migrating species, the moulting of the small plumage begins before the wings and tail feathers of the 1st juvenile plumage have reached their full length. The moulting is then interrupted for the duration of the train. In the winter quarters, the large plumage is then renewed until late winter. In resident birds, the moulting of the small plumage begins about a month after the flight and continues with the change of the large plumage into early spring. The moulting sequence is similar in adults: resident birds begin moulting after breeding and usually continue with pauses when changing their plumage until late winter; Migratory birds interrupt moulting after changing their small plumage and change their large plumage in the wintering areas.
The tiger shrike and the brown shrike, both East Asian medium to long-distance migrants, seem to change their entire plumage twice a year, once shortly before they leave the breeding area and then in their winter quarters.
Vocalizations
Stranglers are known for their loud, harsh, and croaking vocalizations. However, this only applies to their monosyllabic or multiple-ranked calls, the utterance of which is almost always in a territorial and / or antagonistic context. The actual singing of most shrike species, on the other hand, is relatively quiet and can only be heard in the pairing period and the pre-breeding season. It consists of short, modulated trills and clear, sometimes whistled, melodious tone sequences that are varied and repeated in different ways, sometimes chatting quietly. Both sexes sing, but the male more persistently and more frequently. Many species incorporate singing elements from other bird species into their song. With the start of breeding, only the harsh signal sounds of the different species can be heard. In addition, there are a series of short, sharp shouts that warn of enemies; these are often differentiated according to the type of threat. Voice imitations are apparently also used by some species to attract potential prey.
Distribution, habitat and migrations
distribution
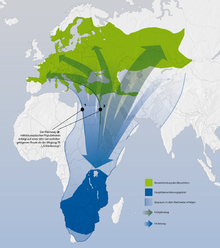
The genus is distributed worldwide except for the polar and subpolar regions as well as the Neotropics. The Australis is reached with a subspecies of the chess strangler ( L. schach stresemanni ) in New Guinea, L. ludovicianus mexicanus , a subspecies of the Louisiana strangler, breeds in southern Mexico in the border area to the Neotropic. The greatest biodiversity is in the Afrotropic and the Palearctic. 10 species breed exclusively in the Afrotropic, 8 species only in the Palearctic. There is also a high concentration of species in the Southeast Asian border area between the Palearctic and Oriental . 2 types (São Tomé shrike and Philippines shrike) are island endings ( São Tomé or Philippines ). 6 species breed in 2 fauna kingdoms , 2 species (southern gray shrike and chess shrike) in three.
In Europe the genus is represented with 6 species, in Central Europe with 4, of which only the red-backed shrike has a largely closed distribution in this region. The other species of strangler are very rare in Central Europe and their occurrence is highly fragmented.
According to climatic zones, the genus is mainly represented in the temperate and subtropical zones as well as in the tropics. Some species are very climate tolerant and inhabit different climatic areas. This is especially true for the migratory birds among the Lanius species. The northernmost breeding occurrences of the northern gray shrike are in Alaska near the Arctic Circle, the southernmost in the hot summer steppe areas of southern Central Asia.
Representatives of the genus occur vertically from sea level to altitudes of over 4000 meters. Species that are native to the Central Asian Mountains ( Tian Shan , Alai Mountains , Altai and Pamir Mountains ) as well as the Himalayan region have been found to be breeding birds at altitudes between 3000 and 4000 meters, the Tibetan shrike is in Nepal only widespread from 2700 meters and ascends at least up to 4500 meters. The subspecies of the wedge-tailed shrike ( Lanius sphenocercus giganteus ) also lives exclusively at high altitudes . But also African species, such as the fiscal strangler, can be found at altitudes of over 3000 meters. Most species, however, inhabit colline and submontane altitudes.
habitat
According to the large and climatically differentiated distribution area of the genus, shrike occur in very different habitats. Most species, however, prefer dry, heat-exposed, open or semi-open landscape structures with the most diverse, mosaic-like vegetation composition possible. For some species, the habitat preferences differ significantly between the breeding season and the non-breeding season, especially of course for migratory birds. There must always be areas with short grass or no vegetation at all, bushes, isolated trees, hedges, telegraph masts and power lines in treeless steppe areas as a hide and dense, preferably thorny bushes as a breeding ground and place to spear prey. Since only an area of 10–15 meters in the vicinity can be searched intensively for prey from a single control room, depending on the vegetation and the relief of the ground, the number, position and type of perches are essential indicators of the habitat quality. This is also improved by pastures or regularly mowed areas as well as by well-sunlit, warm sections, such as vineyards. In the prairie belt of the USA , the Louisiana strangler prefers sections with the largest possible proportion of stony, uncultivated soil. The diversity and composition of the bush vegetation also play a role in the frequency of the species. Many species have got used to the proximity of humans and colonize built-up, but if possible extensively used, agricultural land, pasture land, orchards, olive groves and large parks. Busy roadsides, ruderal areas along railway lines or on military training areas , clearing or burn areas in forests can be just as suitable habitats as heather or marshland . In the Central Asian steppe areas, regions that are loosely overgrown with Saxaul or woody trees that accompany rivers are preferred; in the Near and Middle East , pistachio or tamarisk bushes often serve as breeding grounds. The high mountain species of Central Asia and the Himalayan region often occur in the strongly sun-exposed rhododendron wilderness or in juniper stands near or above the tree line . Shrike that penetrate to high latitudes mainly colonize large clearings within the taiga or the transition zone between taiga and tundra . The species native to Africa live in different, mostly dry to almost semi-desert habitats, in dry and wet savannas as well as on the edge of forest areas, or in large cleared areas or extensive clearings. They often colonize agriculturally used land and also occur on the edges of or within plantations. The São Tomé shrike, which apparently only colonizes the remains of the primary forest on São Tomé, seems to be the only obligatory forest dweller. Relatively dense tree population also prefer mask shrike, tiger shrike, rust-mantle shrike and the Philippines shrike . In many cases, shrike species, especially in West African and Southeast Asian areas of distribution, have been able to significantly expand their breeding areas because deforestation, plantation management and urbanization created new habitats.
The space requirements of the individual species are very different; it depends on many factors, the general quality of the habitat (number of waiting areas, density of prey, land cover), as well as the competitive pressure from the same species, or from the related species, for example with the buffalo head shrike and brown shrike that occurs in some regions of Japan, are particularly important Differences both in size and structure also exist between breeding grounds and the territories used outside of the breeding season. Small and medium-sized species such as the red-backed shrike, with good habitat quality, require areas of half a hectare or 100–150 meters of usable area with a linear extension of the area (Isabellus shrike in a strip of bush along a dry valley.) Large and very large species, especially those that predominantly feed on mammals and birds and accordingly require more living space; the territories of the northern gray shrike can extend over 30–40 hectares and, if the habitat quality is not optimal, can grow to 100 hectares.
hikes
In simple terms, it can be said that the species of the subtropics and tropics remain in their respective breeding areas throughout the year; this applies to all African representatives of the genus as well as to the South and Southeast Asian species. Some of them, especially young birds, strip except brut time around small-scale, with some few have been at least observed in subpopulations migration ( Gray-backed fiscal , rust coat Strangler ). The Central and East Asian species of the high mountains undertake vertical migrations, unless they are migratory birds at all. The stranglers of the northern temperate zone are partly migratory birds. Since many of them are at home in several climatic areas, long-distance migrants and resident birds can occur within a species in the extreme, with all the gradations in between. Not only the geographical latitude of the breeding area, but also the prevailing eating habits influence the degree of willingness to migrate. Large species, such as the northern gray shrike, feed primarily on small vertebrates such as small rodents and small birds. These are available all year round, so it is not absolutely necessary to leave the breeding areas and only becomes necessary when the prey animals leave the habitat or are no longer accessible due to weather conditions.
Of the 29 species considered here, 18 at least in subpopulations are migratory birds; this number corresponds to the species that breed in the Holarctic . With the exception of the southern gray shrike, which is largely resident on the Iberian Peninsula , the shrike occurring in Europe are obligatory, or in the case of the northern shrike optional migratory birds. The red-backed shrike and the black-fronted shrike are extreme long-distance migrants who travel 10,000 and more kilometers from their breeding areas to the wintering areas in southern Africa, while red-headed shrike and masked shrike usually migrate over medium distances of up to 6000 kilometers. In East Asia, some populations of the brown shrike migrate from the northernmost breeding areas on the Arctic Circle in Siberia to the wintering areas in South and Southeast Asia, also covering distances of around 8,000 kilometers. The other Central and East Asian shrike species are medium- or short-distance migrants or optional migratory birds that only leave their breeding area when the food situation requires it.
As far as is known, most species migrate individually or in small groups and during the night; nocturnal migration for the Louisiana strangler is questionable. Stranglers seem to have no or only insignificant fat reserves before the move. Apparently they can feed themselves adequately on the migration, possibly with exhausted, migrating small birds. They themselves are also often prey to birds of prey, especially those that have synchronized their breeding seasons with the migration times of the Palearctic passerine birds, as is the case with the shale falcon and Eleanor falcon .
Food and nutrition acquisition
food

The food of the shrike consists of arthropods and other invertebrates as well as small vertebrates . The vast majority of the arthropods captured are insects , including beetles , grasshoppers , crickets and bees , especially larger species such as bumblebees . Medium-sized and large insect prey is clearly preferred, but even very large shrike species will take prey under one centimeter in length if they are in abundance. Quantitatively, less significant, but seasonal and species-specific are not unimportant spiders , hundreds - and millipedes and snails and worms . Among the vertebrates, mice , voles and shrews as well as small passerines and reptiles such as snakes , lizards and geckos , and occasionally bats, predominate . Some species fish for tadpoles and fry from pools . Even frogs and toads are among the rare indeed, but regularly captured prey. Occasionally, stranglers go to carrion or eat suitable food at feeding places. Fruits and berries are seasonal complementary foods that are not very important in terms of quantity. In almost all species there are significant differences in the food composition between the breeding season and the rest of the year.
In terms of the number of prey, arthropods predominate for most species. Some, especially small and medium-sized shrike species, feed almost exclusively on them. Vertebrates play a more important role in larger ones. They always outnumber arthropods in ingested biomass and become the staple food in the winter months when insects are not available. Reptiles, and in some cases frogs, play a special role for the stranglers native to the South Palearctic, the Afrotropic and the Oriental.
Shrike are able to beat vertebrates that are not insignificantly larger than their own body size, but mostly the prey animals are small rodents, small lizards or geckos, young snakes or small passerine birds. Sick or injured animals are particularly often captured.
The northern gray shrike, a very large species, needs a daily amount of food of 44.1–62.6 grams, smaller species accordingly less. Indigestible food residues are choked out in spittle balls.
Food acquisition
Shrike are not food specialists, but feed on prey animals that are available in sufficient quantities and can be captured without using too much energy. Only during the breeding season is the selection of prey adapted to the requirements of rearing young.

Shrike are primarily high seat hunters, but all species are able to adapt the hunting method to the respective situation. They usually spot the prey in a very upright posture and clearly visible from a low viewing point and hit it on the ground. This hunting method is by far the most energy efficient. Those species that specialize in capturing nestlings, such as the fiscal strangler, hunt from hidden hides. Stranglers prey on their prey in flight, to varying degrees from species to species, especially when too high a ground cover prevents sufficient ground visibility. Birds are usually hunted in the air and pursued over long distances if the first attack was unsuccessful. Occasionally prey animals are read from the substrate (bark, leaves, branches) - the tiger shrike in particular prefers this method of obtaining food; Only in the case of a few stranglers, such as the southern gray shrike , does groundhunting on foot play a major role. Some species search the ground for potential prey while vibrating . Although most species seem to have mastered the shaking flight, this energy-intensive hunting method is only used more often when hides are missing or far apart.
Some stranglers prey on and consume poisonous species on a regular basis. The Louisiana strangler often eats the not inconsiderably poisonous species of horror Romalea microptera , which is avoided by other birds; the captured animals are impaled and only eaten after a certain time after the apparently temporally unstable poison has lost its effectiveness. Individual individuals of the Makinnon shrike skin poisonous toads before they eat them; this behavior appears to be acquired as it is only exhibited by individual birds within a population. Stinging bees and wasps are either squeezed out of the poisonous bladder before they eat, like the bee-eater, by hitting a hard surface or by manipulation with the beak, or the stinger and poisonous bladder are completely removed with the beak.
The impaling of prey
The impaling of prey is the best-known behavior of this bird species. It is probably used both to fix the prey in order to be able to tear small pieces out of them when consumed, and to keep them in stock. Apart from Lanius, it was found in the genus Cracticus , which is widespread in Australia , and in some representatives of the bush shrike . Two methods are used: the impaling of prey, especially vertebrates, on thorns or sharp twigs, and wedging in branch forks. Not all species use these methods. In stranglers that mainly live on insects, impaling and pinching is observed less often or not at all, for example in the red-headed shrike. When it does occur, it is primarily used to keep supplies. Larger species such as the gray shrike, the wedge-tailed shrike, the fiscal shrike or the Louisiana shrike often spear their prey. Both sexes show this behavior; Well-filled skewers for a male seem to play a not insignificant role for females when choosing a partner. After the courtship season, during which the spear places are often clearly visible at the territorial boundaries and the prey animals are not always consumed in their entirety, the main spear place is later mostly very hidden near the breeding place, but usually not in the same bush, some more distributed in the Territory.
behavior
Shrike are diurnal, year-round, to varying degrees, territorial birds. Your activity phase begins well before sunrise and ends at dusk. Species living close to the Arctic Circle (brown shrike, northern gray shrike) also keep a virtual day-night rhythm during the summer months. They spend the night inside a thick, if possible thorny bush, which offers protection from nocturnal predators as well as from the weather. Most species live in seasonal breeding partnerships and establish breeding territories, which are defended against conspecifics and food competitors with overflights, shouts and threatening gestures (crouched posture, elongated beak, fanned tail and flapping wings). Contact fights are rare. The extent of the territoriality varies, with some species only the immediate vicinity of the spit-hole and the nest is intensively claimed and, if necessary, defended. Some types of stranglers tend to loosen up colony formation, to form so-called clusters of territory, whereby neighboring territories often overlap without causing hostilities. Birds of such a cluster regularly gather at the territorial boundaries. This behavior is particularly pronounced in the black-necked shrike, but was also found in the red-backed shrike, Louisiana shrike, brown shrike and buffalo-head shrike. One species, the gray-coat shrike , breeds in a social association with only one dominant breeding pair and a few group members as helpers . The social organization is similar in L. cabanisi and in the related genera Eurocephalus and Corvinella . Cooperative breeding is also suspected in the red-rump shrike. Some species often live in close proximity to other passerine birds (shrike, red- backed shrike - sparrowhawk warbler ; red headed shrike - orpheus warbler ; northern gray shrike - fieldfare ; long-tailed shrike - buffalo weaver ); however, these mutualistic relationships have not yet been sufficiently explored.
Stranglers warn of enemies with shouts and threatening gestures. They usually flee to safe cover from skilled air fighters, while other predators are also approached and bullied . In territorial clusters, the couples jointly participate in this defense against the enemy. During the breeding season, stranglers are very secretive and silent. If an intruder approaches the nest, the female remains seated for a long time, while the male follows the approach from a certain distance, also without warning calls. Only when there is immediate danger do both partners emit loud, glaring panic calls and fly straight at the enemy. In such situations, skipping reactions such as sham cleaning or begging calls can also be observed. Once the danger is over, the female often approaches the nest again from the opposite direction.
Stranglers take both dust baths and water baths. Entire body immersion was observed. They take great care of their plumage when they rest, after meals the beak is cleaned by rubbing the side on a surface.
Breeding biology
Pair formation
Except for the gray-coat shrike, which is a common breeder with a dominant breeding pair, all other representatives of the genus Lanius lead a largely monogamous seasonal partnership. Reparations of last year's breeding partners occur, even over several years. These are naturally more common in resident populations than in migrating species. Overall, polygyny appears to be rare; it has so far been found occasionally in the southern gray shrike, northern gray shrike, red backed shrike and Louisiana shrike. Except for pair copulations, however, were regularly observed in some species, especially common in black-necked shrike and Louisiana shrike. A study of 44 broods of the Louisiana strangler in Oklahoma showed that 4% of the nestlings did not have their mate as a father. In order to prevent such incidents, the aggressive behavior of the males is significantly increased during the fertile days of the females. The rituals of pair formation differ from species to species in their individual elements and also differ somewhat between migrating and resident populations, but are very similar in their course. The male always first establishes a territory and draws attention to his presence by sitting upright in exposed places, sightseeing, conspicuously placed spit spots and calling series. If a female appears, she approaches immediately and performs the species-specific courtship dances in her immediate vicinity, the elements of which consist primarily of nodding, spreading or stilting the tail, flapping wings and turning the body and which are usually accompanied by soft, murmuring singing. The female is mostly passive in this phase. Inspection of spit places and handing over of food, as well as flying over the territory together, confirm the pair formation and lead to the search for nesting places and nest building.
Nesting place and nest
Shrike nest either at a low height above the ground in a dense, ideally thorny bush, or usually a little higher in the outer branches of trees on the edge. For the different species, preferences for one or the other breeding site option can be determined. Many of the shrikes of the superspecies around L. collurio nest primarily in bushes, while the great gray shrike , the red-headed shrike or the black-headed shrike prefer trees. Ground broods or nesting sites on telegraph poles or other elevated waiting areas of anthropogenic origin are rare, especially when there are no natural nesting opportunities in bushes or trees. Some East Asian species prefer the fringes of bamboo stands. In Central Asia pistachio bushes and Saxaulsträucher frequent nest carrier in the arid zones of North America the Sagebrush .
The nest is usually built in 4–12 days, in rare individual cases in just one or two days. The participation of the partners in nest building varies from species to species, but mostly relatively equivalent. Panov distinguishes between two basic schemes: the three-layer, externally unclad nest type and the two-layer, which is clad and camouflaged on the outside with different materials. The outer layer of the first type consists of coarse twigs that form a loosely interlocked framework and support the second layer and the actual nest bowl. Nests of this type appear sloppily assembled, but are usually very stable constructions. Nests of the second type differ from the first only in the absence of the first framework layer. The actual nest bowl is very densely woven from blades of grass, fine roots, animal and plant wool, moss and various other materials and is clad and camouflaged on the outside with cobwebs, moss, leaf veins, dry leaves and lichen. Feathers as well as animal and plant wool predominate in the loosely lying interior cladding. The two types of construction are overall uniform, the materials used are naturally very different. Nests of the first type are mainly built by the gray shrike , the wedge-tailed shrike , the representatives of the L. collurio species, the Louisiana and the buffalo-head shrike, while masked shrike, red-headed shrike, black-necked shrike, tiger shrike and most African species build nests of the second construction type. Perhaps a special case is the nest of the rust-mantled shrike, which is a small, finely crafted, compact bowl with a thick cobweb clad on the outside, very similar to the nests of Eurocephalus sp. or Prionops sp.
Stranglers usually build a new nest for each brood, even if an initial brood fails. The second nest is often built at the same nest location, but a little higher, using materials from the first nest. Re-uses of old nests also occur, and they have often been found in fiscal stranglers.
Clutch and brood
Strangler clutches consist of 1–9 eggs. Species or populations at higher latitudes lay significantly more eggs than those in the tropics. These differences also exist within a species: The clutches of the chess shrike (subspecies L. s. Stresemanni ) in New Guinea always contain 2 eggs, while the same species (subspecies L. s. Tricolor ) lays an average of 5 eggs in the temperate zones of Central Asia. Northern gray shrike clutches of 8 or 9 eggs are common in Finland and Alaska , while those of the southern gray shrike in the Maghreb rarely contain more than 5 eggs. Most African species have clutches of two or three eggs. The number of broods also varies with latitude. Species of the temperate zones breed only once a year, while those of the subtropics and tropics regularly raise two broods, and occasionally three. Mostly smaller replacement clutches were found in all species when the clutch was lost early.
The eggs are laid in the daily rhythm in the early morning hours. The eggs are mostly pointed-oval, in some species (red-headed shrike, northern gray shrike, Louisiana shrike) also approximately round-oval, often creamy white in their basic color, but altogether quite variable in color and usually spotted and spotted irregularly towards the blunt end. It breeds almost exclusively by the female, the male only seldom and for a short time replaces the female. The breeding season is 15–20 days; it depends on the species, the clutch size and climatic factors. The female is provided with food by the male during the breeding season. If the intensive brood does not begin until the penultimate or last egg, the young hatch within a relatively short period of time and show only slight differences in development. Occasionally, if the brood begins earlier, the weaker last hatched often die. Their carcasses are removed, but more often fed. The chicks hatch naked and blind. Around the fourth day they start to open their eyes, a little later the first feathers open, mostly in the chest area. At around 16 days, the first youth dress is largely formed and the control feathers have reached about half their final length. At this time the first half-fledged young birds leave the nest. For the first time they stay in the immediate vicinity of the nest and are looked after by their parents there. The lead time, in which they gradually expand their radius of action, lasts at least 40 days, with some species longer. In species with two annual broods, the female begins to build a new nest about one and a half to two weeks after the last youngster has fled out, while the male continues to take care of the young, and later also of the breeding female. After becoming self-employed, the young birds leave their parents' territory and form loose youth groups.
Breeding success
Information on breeding success is not available for all species. The available data indicate rather high breeding losses. Cold and wet weather during the breeding season and a high density of potential nest predators reduce the breeding success, as does the location of the nest, in particular its height above the ground, influences the escape rate. For species that prefer to breed in clusters of territory, the breeding success increases to a certain extent with the number of breeding pairs in such a cluster. Overall, however, all information should be interpreted carefully, as they are influenced by a number of variables and are therefore subject to considerable fluctuations.
An escape rate of 56% was calculated for Louisiana stranglers from 11 US states, i.e. 56% of all eggs laid reached the age of escape. It is around 50% in the brown shrike and buffalo head shrike, a little less in the red backed shrike and the predatory shrike. The average breeding success for some African species appears to be very low, but the information is based on only a small number of examined nests. With 36% young birds flown out, the red-headed shrike of a Spanish population also have a very low escape rate.
Systematics
External system
The family is believed to have originated in Africa. Corvidae and Laniidae are probably sister families and form a clade with the Monarchidae. The two Eurocephalus species are considered to be the most primitive shrike, their assignment to these is the subject of scientific discussion. It is also unclear whether the monotypic genus Platylophus can be assigned to the Laniidae or the Corvidae. At present, four genera are united in this family with Lanius , Eurocephalus , Urolestes and Corvinella , of which Eurocephalus has two species and the latter two are monotypical.
Internal system
The genus Lanius was first described by Carl von Linné in 1758 on the basis of a bellows of the northern gray shrike. In the 10th edition of the Systema naturae he describes some Lanius species (including 5 species that are valid today and 5 from other families), among them the waxwing ( Bombycilla garrulus ). He put the genus together with vultures , falcons and owls to the birds of prey (Accipitres). In 1815 the genus was placed by Constantine Samuel Rafinesque-Schmaltz in the Laniidae family, which he defined. Lanius is a very homogeneous genus from an anatomical, morphological and behavioral point of view. Nevertheless, at the genus level, many questions remain unanswered - above all with regard to the general relationships within the genus and with regard to radiation , number of species and number and assignment of subspecies. Within the genus there are some species groups that have very closely related species. Often the distribution areas overlap, so that hybrid populations emerge, some of which were viewed as independent species or as races that are very advanced on the way to speciation. For example, L. tephronotus lahulensis , a subspecies of the Tibetan shrike, appears to be a stabilized hybrid between the chess shrike and the Tibetan shrike. Similar delineations exist in the other species groups, primarily in the L. collurio - L. phoenicuroides - L. isabellinus complex in the group of large, gray palaearctic and holarctic shrike and in the species group around L. collaris . The difficult definition is reinforced by the fact that some species have a large number of subspecies in their extensive distribution areas, often with very clear differentiation.
Also molecular genetic testing methods have only partially helped to answer open questions. For example, the investigations by Olsson and others showed that the currently valid separation of L. excubitor into a northern (northern gray shrike) and a southern species (southern gray shrike) is not supported by molecular genetics, and the L. excubitor complex according to the Authors should consist of at least 6 species. However, they add that other interpretations of their results are also possible. Deviating from this, Peer et al. Species rank for the nearctic subspecies of L. excubitor and the nominate form of L. meridionalis . But they also advise waiting for further results. Overall, it is to be expected that the number of species will increase, especially in the group of gray shrike some taxa - now still treated as subspecies - will receive species rank.
According to the IOC species list and the revaluations that have been made since the publication of the work of Olsson, Alström, Peer and others, 30 species have been included in the following species list. Essentially, this corresponds to those of Harris and Franklin, Panov and that of Josep del Hoyo et al. In its last taxonomic revision (2017), the HBW lists L. giganteus with the German name Sichuan Shrike as an independent species. IOU and other authorities are of this opinion September 2018 not yet followed. Even at the end of 2018, the taxonomic situation within the L. excubitor / L.meridionalis complex is confusing . As a result of the work by Alström et al. the shrike native to the Holarctic taiga zone were classified as L. borealis ; the 8 or more subspecies of L. meridionalis were added to L. excubitor , so that L. meridonalis is currently monotypic and restricted to the Iberian Peninsula and southern France, while L. excubitor became a giant species that occurs on three continents and comprises at least 12 subspecies . However, it must be assumed that the systematics of this species group will be subjected to further revisions; As of the end of 2018, the position of the subspecies in the group around L. e. elegans and around L. e. lahorta especially discussed.
| German name | Scientific name | distribution | Hazard level Red List of IUCN |
Remarks | image |
|---|---|---|---|---|---|
| Tiger shrike |
Lanius tigrinus Linnaeus , 1758 |
Far Southeast Russia , Northeast and East China , Korea , Japan |
|
monotypic - Probably closely related to the superspecies from L. collurio , L. cristatus and L. isabellinus . |

|
| Buffalo head shrike |
Lanius bucephalus Temminck & Schlegel , 1845 |
Southeast Russia , Northeast, Southeast and Central China , Korea , Japan , Sakhalin , Kuril Islands , Ryūkyū Islands , Daitō Islands , Ogasawara Islands |
|
2 subspecies |

|
| Red backs |
Lanius collurio Linnaeus , 1758 |
Europe up to the SW and higher north, Asia Minor , eastwards to Central Asia |
|
at least three subspecies - Forms a superspecies with L. cristatus and L. isabellinus |

|
| Isabel Shrike |
Lanius isabellinus Hemprich & Ehrenberg , 1833 |
East of the Caspian Sea from Kazakhstan to NW Pakistan , north to NW China and NW Mongolia . |
|
at least 4 subspecies. Subspecies L. i. phoenicuroides mostly viewed as an independent species - forms a superspecies with L. cristatus and L. collurio |

|
| Red-tailed shrike |
Lanius phoenicuroides ( Schalow , 1875) |
Eastern Iran, Central and Northern Afghanistan, Northwest Pakistan, Turkmenistan , Uzbekistan , Kyrgyzstan, and Central and Southern Kazakhstan |
|
monotypical - former subspecies L. isabellinus phoenicuroides placed in species rank in 2005 - not generally recognized. |

|
| Brown shrike |
Lanius cristatus Linnaeus , 1758 |
Central Asia east to the Pacific , north to the Arctic Circle and southeast to Korea . Kamchatka , Sakhalin and Japan. |
|
4 subspecies - Forms a superspecies with L. tigrinus , L. isabellinus and L. collurio |

|
| Burmese strangler |
Lanius collurioides Lesson , 1831 |
Not well known. Northeast India and Mainland - Southeast Asia (excluding Malay Peninsula ) |
|
2 subspecies - presumably superspecies with L. vittatus . |

|
| Red-necked Shrike |
Lanius gubernator Hartlaub , 1882 |
Very local to the south of the Sahara and north of the equator from South Mali eastwards to southern South Sudan . |
|
monotypic - Presumably forms a superspecies with L. souzae . | |
| Rust-coated shrike |
Lanius souzae Bocage , 1878 |
Western and central Africa south of the rainforest belt . southwest to southern Angola , southeast to northern Mozambique . |
|
3 subspecies - Presumably forms a superspecies with L. gubernator |

|
| Red-shouldered shrike |
Lanius vittatus Valenciennes , 1826 |
Fragmented distribution from southern and central Turkmenistan, Iran, Afghanistan, to Nepal and India. |
|
2 subspecies - Presumably superspecies with L. collurioides , but overall unexplained relationships. |

|
| Chess strangler |
Lanius schach Linnaeus , 1758 |
Very extensive distribution area from SW Iran over India to Southeast Asia, Taiwan , Hainan , Sumatra , Java and some small Sunda islands , New Guinea. |
|
9 subspecies - superspecies with L. tephronotus - considered conspecific by some authors . |

|
| Tibetan strangler |
Lanius tephronotus ( Vigors , 1831) |
Himalayan region from Kashmir eastwards to southern China. |
|
2 subspecies - superspecies with L. schach , sometimes viewed as conspecific with this. |

|
| Philippines shrike |
Lanius validirostris Olgivie-Grant , 1894 |
Endemic to the Philippines . Luzon , Mindanao and Mindoro . |
|
3 or 4 subspecies | |
| Black-fronted Shrike |
Lanius minor Gmelin , 1788 |
Gaps in Europe, more closed to the east and south-east. East to NW China, in the southeast Asia Minor, Syria , Iraq , Afghanistan. |
|
2 subspecies |

|
| Louisiana strangler |
Lanius ludovicianus Linnaeus , 1766 |
Canada south to Mexico ( Oaxaca ) |
|
7 subspecies - Forms a superspecies with L. excubitor , L. meridionalis and L. sphenocercus , L. borealis and L. giganteus . |

|
|
Gray shrike previously Northern gray shrike |
Lanius excubitor Linnaeus , 1758 |
fragments Central Europe and Western Europe . Asia Minor, Caucasus ; Africa up to 15 ° N, Arabian Peninsula , Central Asia, N and Central India, Mongolia , NW China |
|
Geographical variation and position of the subspecies not clear. Reassessed in 2016; associated with the subspecies of meridionalis ; forms a superspecies with L. ludovicianus , L. meridionalis , L. sphenocercus , L. borealis and L. giganteus |

|
|
Taiga shrike previously Northern shrike |
Lanius borealis Vieillot , 1808 |
Holarctic belt of coniferous forests from Alaska to Western Mongolia |
|
Geographical variation and position of the subspecies not clear. Reassessed in 2016; 6 subspecies; forms a superspecies with L. ludovicianus , L. meridionalis , L. sphenocercus , L. excubitor and L. giganteus |

|
|
Iberian shrike previously southern shrike |
Lanius meridionalis Temminck , 1820 |
Iberian Peninsula; Mediterranean coast and hinterland in southern France |
|
monotypical; Reassessed in 2016; previous subspecies put to excubitor . Forms a superspecies with L. ludovicianus , L. excubitor and L. sphenocercus |

|
| Wedge-tailed shrike |
Lanius sphenocercus Cabanis , 1873 |
Distribution limits not very clear: Southeast Russia, south and south-central China; northern North Korea (?) |
|
1 subspecies or monotypic. L. s. giganteus is often regarded as a species ( L. giganteus - Sichuan shrike) - forms a superspecies with L. ludovicianus , L. excubitor and L. meridionalis |

|
| Sichuan strangler |
Lanius giganteus Przevalski , 1887 |
Distribution limits are not very clear: high mountain type Central China (eastern Qinghai , southwestern Gansu , eastern Xizang , northern and western Sichuan ) |
|
monotypical. Separated from L. sphenocercus in 2016 and placed in species rank superspecies with L. sphenocercus , L. ludovicianus , L. excubitor and L. meridionalis | |
| Gray-coat shrike |
Lanius excubitoroides Prévost & Des Murs , 1847 Syn .: Lanius excubitorius |
Sahel zone south of the Sahara, eastwards to Tanzania , Rwanda and Burundi . |
|
3 subspecies - The first descriptors named the species L. excubitoroides , but changed the species name to exbubitorius because they wrongly assumed that excubitoroides was already taken. |

|
| Long-tailed shrike |
Lanius cabanisi Hartert , 1906 |
South Somalia , Central and Southeast Kenya, Northeast Tanzania to Dar es Salaam |
|
monotypical |

|
| Taita Strangler |
Lanius dorsalis Cabanis , 1878 |
Eastern Africa from southeastern South Sudan south to northeast Tanzania. |
|
monotypical |

|
| Somali strangler |
Lanius somalicus Hartlaub & Heuglin , 1859 |
Southern South Sudan, Ethiopia , Somalia and Kenya |
|
monotypical |

|
| Mackinnon Shrike |
Lanius mackinnoni Sharpe , 1891 |
Probably fragmented - western part from South Cameroon to northern Angola , eastern part, eastern Democratic Republic of the Congo , Uganda , north-western Kenya. |
|
monotypical |

|
| Northern fiscal strangler |
Lanius humeralis Stanley , 1814 |
Western Africa from South Mauritania eastwards Central Eritrea south-eastwards to north Mozambique , southeast to NE Zambia , southwest to Sierra Leone and Liberia . |
|
3 subspecies. Previously a subspecies of Lanius collaris , raised in species rank in 2012. The subspecies L. humeralis marwitzi Reichenow , 1901 is considered a species by some authors. |

|
| Southern fiscal strangler |
Lanius collaris Linnaeus , 1766 |
Much of Africa south of the equator |
|
4 subspecies. Lanius collaris humeralis (with two other subspecies) ranked species in 2012 |

|
| Sao Tome Shrike |
Lanius Newtoni Bocage , 1891 |
Endemic to São Tomé |
|
monotypic - Occasionally considered conspecific with L. collaris . |

|
| Red-headed shrike |
Lanius senator Linnaeus , 1758 |
Mediterranean area, eastward to Iraq and western Iran. |
|
4 subspecies. |

|
| Masked Shrike |
Lanius nubicus Lichtenstein , 1823 |
Balkans , Levant , eastward to Iraq and western Iran. Eastern and north-eastern distribution limits unclear. |
|
monotypical |

|
Existence and endangerment
The IUCN lists only two species as endangered worldwide: the Philippines shrike and São Tomé shrike, both island endangered species, which naturally react sensitively to interventions in their mostly spatially restricted habitat. The Philippines shrike is potentially endangered (NT = near threatened), mainly because its habitat on the three Philippine islands of Luzon , Mindanao and Mindoro is exposed to strong human interference, especially in the lowland areas. However, recent studies show that the species is quite common in middle and higher altitudes. The stock situation of the São Tomé shrike is much more critical. The species was thought to be extinct until it was rediscovered in 1990. Since then, further observations have been made, but the population is estimated to be less than 50 individuals. Decisive for this development is the destruction of the habitat, especially the conversion of the primary forest into cocoa plantations, as well as the spread of invasive animal species such as the black rat ( Rattus rattus ) and the monkey cat ( Cercopithecus mona ).
All other species are globally regarded as harmless (LC = least concern). Nevertheless, some species such as black-necked shrike, red-headed shrike and northern gray shrike, which were quite common in Central Europe in the past, have become rarer in this area or have disappeared at all. The serious decline in the population of these shrike species is related to the strong decline or regional extinction of other thermophilic large insect hunters such as hoopoe , blue roller , red hawk and little owl . Climate conditions that are more strongly influenced by the Atlantic as well as strong interventions in the habitats of the various species, such as land consolidation, destruction of niche and fringed habitats, conversion of small-scale building structures into large-scale monocultures and intensified use of fertilizers and pesticides are discussed as causes. The stock slump started in the 1960s and intensified until the end of the century. At the moment, stabilization at a low level can be observed for the species mentioned, provided they have not completely disappeared. The red-backed shrike has also suffered population and area losses, above all the species has disappeared from many lowland breeding areas.
The buffalo head shrike and some other Asian species were also found to suffer severe populations during this period.
In the Nearctic, the Louisiana strangler has seen a serious decline in the population. The species is also safe in its total population, but has become rare in individual regions. In Canada and 14 states of the USA the subspecies L. ludovicianus migrans is listed as endangered, the subspecies L. ludovicianus mearnsi , which only occurs on San Clemente , was threatened with extinction ; extensive protective measures, however, let the population grow again to over 200 individuals.
The status of most African and some East Asian species is not very well understood. Their habitats are often threatened. Red-eared shrike ( L. gubernator ) and rust-mantled shrike ( L. souzae ) in particular do not seem to be common.
The main causes of danger are of an anthropogenic nature. Habitat destruction and pesticide input come first here. In some regions, especially in East Asia, stranglers are hunted or caught in nets; although this is increasingly prohibited and criminalized, the losses it causes are still substantial.
Like other small songbirds, shrike have many natural enemies. Above all, birds of prey such as sparrowhawks and hawks, as well as different types of falcons, strike adult shrikers; a number of species of crows destroy clutches and prey on nestlings and young birds. Different species of snakes, as well as martens and foxes also play a role as nest robbers. A total of 9 cuckoo species as well as the brown-headed cowbird reduce the breeding success as breeding parasites , but the ability to recognize and remove foreign eggs is very highly developed in many species of strangler.
Shrike species are attacked by a number of ecto- and endoparasites . Often ticks are found on them, which can transmit diseases that are dangerous for humans, such as TBE , Omsk fever and Kyasanur forest fever and can cause babesiosis in horses .
literature
- Josep del Hoyo , Andrew Elliot, Jordi Sargatal (Eds.): Handbook of the Birds of the World. Volume 13: Penduline-Tits to Shrikes. Lynx Edicions, Barcelona 2008, ISBN 978-84-96553-45-3 .
- Urs N. Glutz von Blotzheim (Hrsg.): Handbook of the birds of Central Europe. Volume 13: Passeriformes. 4th part: Sittidae - Laniidae. Edited by Kurt M. Bauer and Urs N. Glutz von Blotzheim, among others. Volume 13/2, Akademische Verlagsgesellschaft, Frankfurt am Main 1993; Aula-Verlag, Wiesbaden 1993, ISBN 3-89104-535-2 .
- Tony Harris, Kim Franklin: Shrikes & Bush-Shrikes. Including wood-shrikes, helmet-shrikes, flycather-shrikes, philentomas, batises and wattle-eyes. Christopher Helm, London 2000, ISBN 0-7136-3861-3 , pp. 21, 56-57, 139-142.
- Norbert Lefranc, Tim Worfolk: Shrikes A Guide to the Shrikes of the World. Pica Press, 1997, ISBN 1-4081-3505-1 .
- Evgenij N. Panov: The True Shrikes (Laniidae) of the World - Ecology, Behavior and Evolution. Pensoft Publishers, Sofia 2011, ISBN 978-954-642-576-8 .
Individual evidence
- ↑ James A. Jobling: Helm Dictionary of Scientific Bird Names . Helm, London 2011, ISBN 978-1-4081-2501-4 , p. 153.
- ↑ Note: Stranglers are sometimes referred to as Butcherbird in English. Mainly, however, this name is used for birds of the genus Cracticus , which is particularly common in Australia , which are probably related to Lanius , and share some behaviors, such as the impaling of prey, with them.
- ↑ Note: The Fiscal ( Afrikaans ) is a South African official who also played the role of executioner and wore a black and white uniform
- ↑ Data sheet ITIS - Lanius
- ↑ Data sheet IUCN Lanius validirostris
- ↑ Data sheet IUCN Lanius newtoni
- ↑ N. Lefranc, T. Worfolk: Shrikes. A Guide to ... 1997, p. 17.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 739.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 71.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 43.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 45.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 737.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 71.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, pp. 71-73.
- ↑ N. Lefranc, T. Worfolk: Shrikes. A Guide to ... 1997, p. 18.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 746.
- ↑ Voice files at xeno-canto
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 782.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 792.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, pp. 739-744.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, pp. 75-78.
- ↑ v. Blotzheim (Ed.): Sittidae - Laniidae. 1993 Volume 13/2 (1993) p. 1180 ff.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 559.
- ↑ v. Blotzheim (Ed.): Sittidae - Laniidae. 1993 Volume 13/2 (1993) p. 1291.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 82.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, pp. 763-766.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 113.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 748.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 114.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 113.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 751.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 117.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 119 (note)
- ↑ v. Blotzheim (Ed.): Sittidae - Laniidae. 1993 Volume 13/2 (1992) p. 1305 ff.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 756.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 95
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, pp. 85 and 86
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 754.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 754.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 88.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 725.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, pp. 88-93.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, pp. 88-93.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 758.
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 92
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, pp. 93 and 94
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 95
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, pp. 95 and 96
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 99
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, p. 293
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, pp. 761-763.
- ↑ TiF Checklist (2014)
- ^ Knud A. Jønsson, Jon Fjeldså: A Phylogenetic Supertree of Oscine Passerine Birds (Aves: Passeri). In: Zoologica Scripta. 35, 2006, pp. 149-186.
- ^ TiF Checklist
- ^ T. Harris, K. Franklin: Shrikes & Bush-Shrikes. 2000, p. 21.
- ↑ EN Panov: The True Shrikes (Laniidae). 2011, pp. 155-166.
- ↑ Marie Agger Beck, Jon Fjeldså, Les Christidis, Pierre-Henri Fabre, Knud Andreas Jønsson: Resolving deep lineage divergences in core corvoid passerine birds supports a proto-Papuan origin Iceland . In: Molecular Phylogenetics and Evolution 70 (Jan. 2014) pp. 272-285
- ^ William Turton (transl.): A General System of Nature (...) by Sir Charles Linné. London 1806, pp. 177-181. Turton's work is a translation and a strong extension of the Systema naturae
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 734.
- ↑ EN Panov: The True Shrikes (Laniidae). 2011, pp. 37-43; 153 ff.
- ↑ TiF Checklist - Corvidae
- ↑ Note: The species circles, i.e. groups of very closely related species, some of which also hybridize, are mentioned in the table as super species.
- ↑ Urban Olsson, Per Alström, Lars Svensson, Mansour Aliabadian, Per Sundberg: The Lanius excubitor (Aves, Passeriformes) conundrum — Taxonomic dilemma when molecular and non-molecular data tell different stories In: Molecular Phylogenetics and Evolution. (2010) Vol. 55/2, pp. 347-357.
- ↑ Jelmer Poelstra: Speciation in shades of gray: the great gray shrike complex. In: Dutch Birding 32: 229-250, 2010
- ↑ Urban Olsson, Per Alström, Lars Svensson, Mansour Aliabadian, Per Sundberg: The Lanius excubitor (Aves, Passeriformes) conundrum — Taxonomic dilemma when molecular and non-molecular data tell different stories In: Molecular Phylogenetics and Evolution. (2010) Vol. 55/2, pp. 347-357.
- ^ Brian D. Peer, Carl E. Mc Intosh, Michael J. Kuehn, Stephen I. Rothstein and Robert C. Fleischer: Complex biogeographic History of Lanius Shrikes and its Implication for the Evolution of Defenses of Avian Brood Parasitism. In: The Condor 113 (2): 385-394 (2011)
- ↑ TiF Checklist - Corvidae
- ↑ IOC Checklist 8.2 (June 2018)
- ↑ Urban Olsson, Per Alström, Lars Svensson, Mansour Aliabadian, Per Sundberg: The Lanius excubitor (Aves, Passeriformes) conundrum — Taxonomic dilemma when molecular and non-molecular data tell different stories In: Molecular Phylogenetics and Evolution. (2010) Vol. 55/2, pp. 347-357.
- ^ Brian D. Peer, Carl E. Mc Intosh, Michael J. Kuehn, Stephen I. Rothstein and Robert C. Fleischer: Complex biogeographic History of Lanius Shrikes and its Implication for the Evolution of Defenses of Avian Brood Parasitism. In: The Condor 113 (2): 385-394 (2011)
- ^ Del Hoyo, J., Collar, N. & Kirwan, GM (2018). Giant Gray Shrike (Lanius giganteus). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, DA & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona. (retrieved from https://www.hbw.com/node/1343839 on August 30, 2018).
- ^ IOC list June 2018
- ↑ Clements checklist 6.8 (August 2013) p. 570.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 788.
- ↑ Clements checklist - Revision 6.7 Sept. 2012
- ↑ Jérôme Fuchs, Timothy M. Crowe and Rauri CK Bowie: Phylogeography of the fiscal shrike (Lanius collaris): a novel pattern of genetic structure across the arid zones and savannas of Africa. In: Journal of Biogeography (J. Biogeogr.) (2011) pp. 1-13
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 793.
- ↑ Clements checklist - Revision 6.7 Sept. 2012
- ^ T. Harris, K. Franklin: Shrikes & Bush-Shrikes ... 2000, p. 212.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, p. 768.
- ^ Josep del Hoyo and others: Penduline-Tits to Shrikes. 2008, pp. 767-768.
- ↑ See population development of the species mentioned in Baden-Württemberg. In: Jochen Hölzinger (arr.): The birds of Baden-Württemberg. Volume 3: Songbirds. Teilband 2, Ulmer, Stuttgart 1997, ISBN 3-8001-3483-7 , pp. 242-330.
- ^ T. Harris, K. Franklin: Shrikes & Bush-Shrikes ... 2000, p. 185.
- ↑ San Clemente Loggerhead Shrike ( Lanius ludovicianus mearnsi ). 5-Year Review: Summary and Evaluation. US Fish and Wildlife Service, Carlsbad Fish and Wildlife Office. Carlsbad, CA, June 17, 2009.
- ^ T. Harris, K. Franklin: Shrikes & Bush-Shrikes ... 2000, pp. 146-212.
- ↑ Peter E. Lowther: Host List of Avian Brood Parasites - 2 - CUCULIFORMES - Old World Cuckoos Field Museum 2013
- ^ Brian D. Peer, Carl E. Mc Intosh, Michael J. Kuehn, Stephen I. Rothstein and Robert C. Fleischer: Complex biogeographic History of Lanius Shrikes and its Implication for the Evolution of Defenses of Avian Brood Parasitism. In: The Condor 113 (2): 385-394 (2011)
- ↑ EN Panov: The True Shrikes (Laniidae) ... 2011, pp. 134-139.