Mosses

Mosses (regionally also moor , Mies and Miesch ; from Middle High German mos / mies ) are green land plants that usually do not develop any supporting or conducting tissue . According to today's view, they developed from green algae in the intertidal zone around 400 to 450 million years ago . The mosses are characterized by a generation change in which the sexual generation ( gametophyte ) dominates over the asexual ( sporophyte ). The haploid gametophyte is the actual moss plant, it can be lobed (thalless) or leafy (folios). Mosses are characterized by the photosynthetic pigments chlorophyll a and b, starch as a storage substance and cell walls made of cellulose , but without lignin . There are around 16,000 known species. The science of the moss is called bryology . The three classic clans hornworts , liverworts and deciduous mosses individually each form lines of descent , but the mosses as a whole are not a natural family group.
Development cycle

Mosses are diplohaplons and have a heteromorphic , heterophasic generation change : The two generations have a different structure (heteromorphic) and they have different nuclear phases (heterophasic). This kind of generation change is shared by the mosses with the ferns and seed plants. The gametophyte is the actual moss plant and is photoautotrophic and haploid (has a simple set of chromosomes ). The sporophyte is dependent on the gametophyte for development and nutrition and is diploid (has a double set of chromosomes).
Gender generation

A filamentous, rarely lobed protonema (pre-germ or juvenile gametophyte) develops from the haploid meiospore . This is where the actual moss plants form from buds. As a rule, the protonema then dies. The protonema is used for vegetative reproduction , as a whole clone can arise from a spore. The gametophyte is usually leafy, less often thallos. The sexual organs ( gametangia ) arise on it : the male antheridia and the female archegonia . Depending on the species, mosses are dioecious (dioecious), that is, there are female and male plants, or monoecious (monoecious). In the latter, the antheridia and archegonia can occur in a gametangian state (synocial) or separately (parozian).
The haploid germ cells ( gametes ) develop in the sexual organs . Water is necessary for fertilization: the male mobile spermatozoids have to swim to the archegonia. They can actively cover up to 1.5 centimeters and are chemotactically attracted by sucrose . At greater distances, the spermatozoids are dependent on passive spreading, for example through rainwater splashes. The egg cell is fertilized in the archegonium.
Asex generation
The fertilized egg cell ( zygote ) is diploid and develops into an embryo and then into a sporophyte without a resting stage . The sporophyte is built very differently depending on the moss group, but remains connected to the gametophyte in all of them, from which it receives water and nutrients. A haustorium (foot) serves him for this purpose . The embryo grows upwards through the archegonium and forms a sporogon , at the tip of which the spore capsule ( sporangium ) sits. The tissue inside the sporangium is the archespor , whose cells divide into haploid spores through reduction division ( meiosis ) . Upon maturity, the spores are released from the spore capsule. To germinate, the spores swell, burst the exospore and develop into a protonema .
Construction and development
Gametophyte


The gametophyte is lobed (thallos) in the hornworts and part of the liverworts, and in the deciduous mosses and most liverworts it is leafy (folios). In leafy mosses, the gametophyte is divided into leaflets ( phylloids ), stems ( cauloids ) and root-like structures ( rhizoids ). These structures are indeed similar to those of ferns and seed plants; However, since they occur in gametophytes and not in sporophytes as they do in them, they are not homologous, which is why they have their own terms. However, only the rhizoid has prevailed, while phylloids and cauloids are usually referred to as leaf and stem. The gametophytes of the mosses are the most highly differentiated among the plants.
The thalli grow with two-, three- or four-edged apex cells, leafy plants with three-edged cells. As an exception, Takakia grows with a peak meristem . Both forms, thallous and foliose, can contain conductive tissue, which, however, is often without function. Horn mosses only have conductive tissue in the sporophytes. Liverworts rarely have hydroids in the gametophyte: these cells have sloping transverse walls and pits similar to the tracheids of ferns, but are not lignified . Mosses often have central strands with water-conducting hydroids in both gametophytes and sporophytes. Cells that conduct assimilates ( leptoids ) occur only in the Polytrichidae . The possession of water- and assimilate-conducting tissues is interpreted as an indication that the mosses developed from early tracheophytic land plants.
The leaflets grow by means of double-edged parietal cells. In liverworts, the leaflets are in three rows and have no ribs. The leaves of the moss are usually screw-shaped, rarely three or two lines. Original clans have ribbed leaves.
Gametangia and gametes

The formation of the sex cells ( gametes ) in sterile envelopes ( gametangia ) is an adaptation to rural life. The origin and structure of gametangia is quite similar in mosses and ferns, which is why both groups are combined as archegonies .
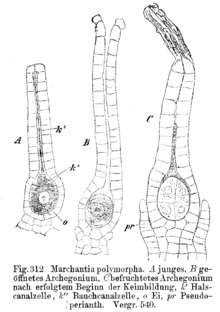
The female gametangia, the archegonia , are bottle-shaped. They have a thickened belly part made up of two to three layers of cells and a single-celled neck part. In the lower part lies the egg cell, above it the abdominal canal cell, and above it some cervical canal cells. When the archegonium matures, the two cover cells at the tip of the archegonium separate, and abdominal and cervical canal cells mucus. This clears the way to the egg cell.
The male antheridia arise from an epidermal cell, i.e. exogenous. They only arise endogenously in horn mosses. A single-layer, sterile envelope surrounds the spermatogenic cells. The latter divide into two cells, which detach from the cell structure and transform into flagellated spermatozoids . At the tip of the antheridium, some wall cells congeal. The spermatozoids are released through this opening. These have the shape of a corkscrew. The front end is specially designed and is called blepharoplast. This is where two long, smooth flagella attach , which are directed backwards. The flagella of the deciduous and liverworts are left-screwed, those of the hornworts are right-screwed. The flagella attach to a basal body. Below this lies a multi-layered structure (MLS) made up of parallel microtubules , a lamellar strip and a mitochondrion . Behind the nucleus, at the rear end of the spermatozoid, are the ribosomes , a plastid , endoplasmic reticulum and another mitochondrion. These ultrastructures are of great importance for the system as they are not subject to any functional pressure to adapt and are therefore considered to be very conservative features.
Embryo development
In the deciduous and liverwort, the fertilized egg cell ( zygote ) initially divides transversely. The actual embryo emerges from the upper cell, which further differentiates into foot, seta and sporangium. The lower cell usually dies. In horn mosses, the first cell division runs lengthways, after which the two cells then divide transversely. The two upper cells become the sporogon, the two lower cells develop into the foot.
The foot penetrates the gametophyte tissue and is connected to the gametophyte via a placenta . Water and nutrients are transported from the gametophyte to the sporophyte via the placenta. Transfer cells with wall protuberances are typical of the placenta, which are invaginations of the cell wall to increase the surface area. In deciduous and liverworts, the foot and gametophyte are separated by a placental gap. Transfer cells occur on both sides, only in the sporophyte, or are absent entirely. The placental gap is absent in horn mosses; here the foot penetrates the gametophytic transfer cells as a haustorium .
Sporophyte

The sporophyte represents the diploid generation of mosses. In horn mosses and a number of deciduous mosses, the sporophyte has stomata of the mnium type, which is also typical for ferns. In addition, the epidermis is cutinized. At least in the case of the Polytrichales, the structure and chemical composition of the cuticle correspond to those of the gymnosperms. The sporophytes of horn moss and moss also have a conductive tissue in the center, i.e. they have a protostele.
The sporophyte is pod-shaped in horn mosses. In deciduous and liverworts, it is divided into a stalk ( seta ) and a spore capsule ( sporangium ). The structure of the sporangium is very different depending on the large group. However, all have a spore-forming (sporogenic) tissue (the archespor) inside. In this, the spores ( meiospores ) develop from diploid spore mother cells through meiosis , typically in tetrads (in groups of four). The spores have a thin-walled endospor and a thick-walled exospor . A spore wall impregnated with sporopollenin is characteristic of the embryophytes . Through different, again taxon-specific mechanisms, the spores get into the open, where they in turn germinate into a protonema.
Propagation Biology
In mosses, there are two types of organs of expansion ( diaspores ): spores in sexual reproduction and brood bodies in vegetative reproduction.
Sexual reproduction
The role of sexual reproduction in increasing genetic diversity is considerably limited in mosses. Around half of the mosses are monoecious and predominantly self-pollinating (no self-incompatibility). In addition, many diocesan species occur only in all-female or all-male populations and cannot reproduce sexually.
The relatively low probability that the spermatozoids in the water reach the archegonia for fertilization is compensated for by the fact that in such a case usually very large numbers of spores are produced. Dawsonia holds the record with five million spores in a sporangium. Several hundred thousand are also not uncommon in other species. The spores are very widespread, usually much further than the actual species area. Therefore, many mosses can react very quickly to climatic changes and colonize new, suitable locations.
The size of the spores is mostly seven to 35 micrometers in deciduous mosses, 10 to 40 micrometers in jungermannial liverworts and 40 to 90 micrometers in marchanteal liverworts. The germination time of the spores lasts from a few hours (with some epiphyllic mosses) to many years, which is more the rule. In experiments spores germinated from 16 year old herbarium material. Some diocesan liverworts form connected spore tetrads during meiosis, so that male and female gametophytes are always together.
The vast majority of the spores spread through the wind ( anemochory ). However, some factors prevent the wind from spreading: very large spores; if spores are formed in the thallus (for example Riccia ) or the capsules do not open (cloistocarpic moss). This indicates a possible spread by animals ( zoochory ), which has also been proven experimentally in some species: Riella americana is spread in the intestines of ducks. The Splachnaceae , which are spread by insects , are a special case : The species grow on manure and animal corpses. They form clumped masses of spores and attract small dung flies ( Sphaeroceridae ) with their scent , which bring the spores back to new locations. Mosses in bodies of water are spread by the water ( hydrochory ). The spores of celistocarpic moss are only released after the capsule has rotted.
The position of the capsule plays a role in the spread: in xerophytic mosses, the capsule is often upright and opens in dry weather. This enables a very wide distribution. Forest mosses often have downward-facing capsules and release the spores in wet weather. As a result, they are not as widespread, but can germinate quickly in the damp conditions. The peristome of the capsule of many mosses also plays an important role in the spread. The peristome teeth open and close the capsule through hygroscopic movements. Depending on the type, the peristome is structured differently, so that it opens either in dry or in wet weather.
Vegetative propagation
Vegetative reproduction plays a much stronger role in mosses than in all other plant groups.
It compensates for the disadvantage that there is often only one gender at one location and therefore no sexual reproduction is possible. In addition, brood bodies are often formed under sub-optimal site conditions such as dry periods. Brood bodies also occur more frequently in the edge areas of the species area or the height distribution of a species. The induction mechanism for the formation of brood bodies is unknown, but it is believed that the plant hormone auxin plays a role. For a number of species no sporophytes are known at all. Such species usually colonize very small areas. But also some common forest mosses very rarely form sporophytes, such as Pleurozium schreberi , Hylocomium splendens and Dicranum scoparium .
In principle, vegetative reproduction can occur through all parts of the moss plant. If a gametophyte is passed through a sieve, complete gametophytes are created from all parts again. Usually, however, special means of dissemination are formed: flagella branches, derogatory, i.e. slightly sloping, leaves, stem tips, leaf tips, special brood bodies on leaves, ribs, rhizoids, axillary brood buds. In liver mosses, special brood bodies are rare, usually easily breaking leaves or branches are formed. The Marchantiales, however, form brood bodies in special breeding cups ( Marchantia polymorpha ). The brood bodies are hurled out of the cup by water droplets (splash-cup mechanism).
ecology
Mosses are usually small and grow relatively slowly. Therefore, they are poorly competitive compared to the higher plants . They therefore often switch to locations that cannot be populated by them: rocks, bark and leaves as almost nutrient-free locations, forest soils as very dark locations and open and disturbed locations.
Water balance
Moose can adjust its water content to a very limited extent, they are alternating wet (poikilohydre) plants.
According to the mechanism of water absorption and drainage, two groups of mosses are distinguished:
- Ectohydric species take up water all over their surface. Water conduction occurs only externally, for example capillary between rhizoids or leaflets and trunk. These species also make use of the humidity in the air. After drying out, they wet themselves with water within seconds.
- Endohydric species have water-conducting elements and a cuticle . They absorb water through the rhizoids and guide it up the stem. However, this mechanism is not sufficient for the water supply, so that external water uptake always occurs in these species.
There are different structures for water absorption and storage: possession of a central cord, outer water pipe, papillary leaf surfaces (easier wetting), water sacs (in some liverworts), leaf wing cells (water-storing cells on the lower leaf corners of some leaf mosses), cilia (long leaf lobes in some liverworts) ), Hyalocytes (large, dead cells in peat moss and other families), lamellae and filaments on leaf veins (store water in the gaps).
Evaporation protection
To reduce the loss of water through evaporation, some mosses form special structures that inhibit evaporation: Cuticula (especially in marchanteal liverworts). When rolled up, rolling blades reduce the evaporating surface. Glass hair reduces solar radiation. Papillae scatter incident light. The young sporogons are particularly sensitive to dehydration, their protection is the kalyptra .
Resistance to desiccation
The resistance to dehydration is very different depending on the species. The molecular causes of resistance have not been researched. A distinction is made between three groups (the names of which are similar to those of higher plants, but have a different meaning):
- Hygrophytes are already damaged by brief and slight dehydration. These include aquatic and swamp moss.
- Mesophytes endure dehydration for a shorter period of time.
- Xerophytes also survive prolonged drying out. Even after several years in the herbarium , re-moistened mosses can be reactivated without any problems. These include the rock and tree dwellers among the mosses.
temperature
The temperature optimum for growth is 15 to 20 ° C for temperate species and up to 25 ° C for tropical species. The upper compensation point for photosynthesis is between 25 and 30 ° C for all species, so at long-term higher temperatures they die due to excessive breathing losses.
The frost resistance is species-specific and independent of location and season. It correlates with the desiccation resistance of a species. In addition, mosses are much harder to frost when dried out. They can survive freezing in liquid nitrogen (−196 ° C).
For many mosses, the upper temperature limit for short-term exposure is 42 to 51 ° C when moist, 85 to 110 ° C when dry, and even higher for xerophytes.
nutrient
Nutrients are absorbed by the moss through precipitation. Only species with a well-developed guidance system take up nutrients from the soil. The source is dust and substances dissolved in the water, in the forest, for example, especially the trunk drain and the canopy . Mosses therefore bring nutrients from the atmosphere into the ecosystem. The absorption from the water is made possible by the following adjustments:
- The mosses have a high surface-to-volume ratio.
- The ion uptake is usually not hindered by a cuticle.
- The cell wall has a very high cation exchange capacity.
As with the higher plants, the elements potassium , calcium , magnesium , nitrogen , phosphorus and sulfur are essential for the mosses, but they need them in much lower concentrations. In addition, however, mosses also accumulate elements that are not found in higher plants. The reason is that mosses cannot control the uptake of ions. They also absorb metals such as niobium or scandium .
The recording takes place in three steps:
- Cation exchange on the cell wall
- The ions reach the cytoplasm via the semipermeable cell membrane. There are no studies of the uptake mechanisms.
- Particles can also be ingested via pinocytosis , for example lead.
A balance between potassium, calcium and magnesium is necessary for many types of moss, which is why many types only occur in acidic locations. Some species like Bryum argenteum and Marchantia polymorpha prefer nitrogen-rich locations, they are nitrophilous . They are strongly promoted by the high anthropogenic nitrogen emissions in Europe.
Special locations

Mosses have a dominant position in raised bogs , where peat moss ( Sphagnum ) play a key role in the development, structure and function of these ecosystems. However, a few other locations should be discussed here.
Tundra and polar regions
In the tundra , mosses play a major role in terms of species number, cover, phytomass and biomass production. Except in wet locations, however, their phytomass content never exceeds 30%. The reason is that the photosynthesis rate of the mosses is already saturated at low light intensities and therefore they achieve a significantly lower photosynthesis output than higher plants in full light. In compensation, mosses can still carry out effective photosynthesis at low temperatures , even at temperatures below 0 ° C. However, the mosses are sensitive to excessively low temperatures, so that they no longer occur in excessively cold locations. In the tundras, the mosses mainly form cushions, mats and dense lawns.
Deserts
In deserts , mosses only occur in places and are only recognizable in wet periods. In sandy deserts they grow partially covered by sand, where they have cooler and wetter conditions. In rubble deserts they can grow under transparent quartz rubble (for example, Aschisma carniolicum ). Species of the liverwort genus Riella , the only salt-tolerant mosses, grow in salt water pools . Special adaptations to dry locations are for marchanteal liverworts: a cuticle , respiratory pores for gas metabolism regulation, a water-storing sponge parenchyma, respiratory cavities, rolling mechanisms in case of dehydration, and abdominal scales for water absorption. The adaptations to radiation protection mentioned above are also often represented in desert mosses. Most desert mosses are acrocarpic mosses, especially species of the Pottiaceae family with the following adaptations: rolled leaves, thick leaves, ribs with water-storing cells, rolled-up leaf margins, glass hair. Often there are also annual or short-lived species that can go through their life cycle in short periods of wetness. These species survive dry periods as spores. Long-lived species are resistant to drought.
Rainforest

In the tropical rainforests there are around 3000 to 4000 species, 90% of which come from only 15 families. The greatest diversity is in Asia. There are great differences between the moss flora of Asia and Africa, so that the mosses cannot be referred to as a paleotropic . The amount of moss increases with the sea level. There are hardly any mosses in the lowland rainforest. The high temperatures combined with the low light intensities under the dense canopy prevent positive photosynthesis. Above 1000 meters above sea level, the number of species and phytomass rise sharply and reach their highest density between 1800 and 2800 meters, especially with epiphytic mosses. In the cloud forests, hanging mosses comb out the fog.
Epiphytes
The first fossil finds of epiphytic mosses come from the Tertiary and belong to today's genera or species. Since the epiphytic species are strongly derived forms, this way of life is relatively young. Epiphytic mosses are characterized by the following adaptations:
- The spores germinate in the sporangium to a chlorophyll-containing, multicellular stage. This leads to a development lead on the substrate.
- Diocese species form dwarf males that sit in the cushions of the female plants. In this way, fertilization can take place over the necessary short distance.
- They have structures that store water.
- Cushions (water reservoirs), fronds and tails as well as hanging mosses (for combing out fog) are life forms.
The supply of nutrients comes from the rain. As a result, epiphytes are particularly numerous in areas with high rainfall (tropical cloud forest).
Epiphylls
The conquest of living leaves as a habitat is likely to be even more recent than epiphytism. Epiphylle mosses show the most strongly derived characteristics, for example neoteny : In the genus Ephemeropsis the gametophyte is greatly reduced, the gametangia are formed in buds on the permanent protonema. Liverworts are predominant here, especially the Lejeuneaceae family . These have hackers to adhere to the rhizoid ends. Epiphylle mosses occur only in tropical and subtropical evergreen rainforests. Smooth, leathery leaves are preferred, which are initially populated by obligatory epiphylls, followed by facultative epiphylls and finally moss. The reproduction takes place mainly through brood bodies. At least one species ( Radula flaccida ) not only uses the leaf as a habitat, but also penetrates the leaf with the rhizoids and removes water and minerals, making it a hemiparasite .
Role for the ecosystem
Where mosses are common, such as in mountain forests and moors, they have an important ecological role in the nutrient cycle, as they filter the nutrients from the precipitation and also for the water cycle, as they can filter out fog on the one hand and to a certain extent also the Can store precipitation.
Mosses play a role as a habitat for small animals and as a germination bed for flowering plants. Some mosses form symbioses with cyanobacteria ( Blasia , hornworts) and fungi ( mycorrhiza , in many liverworts). One genus ( Cryptothallus ) is obligatory saprophytic under moss covers. Some liverworts (such as Colura zoophaga ) catch ciliates and other small animals in their water sacs. However, since they lack digestive proteases , there is no real carnivory , only zoophagia .
ingredients
In their assimilation pigments ( chlorophyll a and b), the carbohydrate reserves starch and sometimes fructans , and the cell walls made of cellulose, the mosses match all other green plants. In contrast to the fern and flowering plants, however, the cell wall substances cutin , suberin and lignin do not actually occur.
Flavonoids
Flavonoids were found in almost half of the mosses examined. They are absent in the horn moss and in the deciduous moss in the Polytrichidae, Andreaeopsida, Sphagnopsida and Tetraphididae. In the liverworts, characteristic flavonoid types are formed according to family or order, in the deciduous moss no systematically usable distribution of the flavonoids is recognizable. Flavone C- and O-glycosides, dihydroflavones , flavonols , dihydro chalcones and aurones occur . There are also flavonoids that appear as glucuronides and galacturonides . The spectrum of deciduous mosses is much larger than that of liverworts. They also contain isoflavones , biflavones and 3- deoxyanthocyanines . The occurrence of isoflavones in the deciduous mosses connects them with the fork-leaf plants , Selaginellales and the gymnosperms . The sphagnorubins of the peat moss are also among the flavonoids.
Phenolic substances
However, phenolic compounds, including cinnamic acid derivatives, do occur , which are similar to the degradation products of lignin in higher plants. An example is the sphagnum acid of peat moss (p-hydroxy-β- (carboxymethyl) -cinnamic acid). Bibenzyle occurs in liverworts, but not in deciduous mosses. Older information on the occurrence of lignin is doubtful; it is always likely to be about lignans , polyphenolic, lignin-like substances that are significantly less methylated than lignin.
Terpenes and terpenoids
In mosses, 24 mono terpenes , 172 sesquiterpenes, 44 diterpenoids, 14 triterpenoids and 13 steroids were detected. Mono- and sesquiterpenes occur only in liverworts, diterpenoids in liverworts and deciduous mosses, and triterpenoids only in deciduous mosses. These substances form the oil bodies characteristic of liverworts and are also responsible for the often species-specific odor of some mosses. The odorous substances include, above all, monoterpenes (for example limonene , pinene , geraniol , borneol ) and sesquiterpenes (of the Eleman, Eudesman, Germacran, and bisabolan types). The blue oil bodies of Calypogeia trichomanis are caused by azulene . Most sesquiterpenes are enantiomers of the compounds found in the higher plants. The diterpenes are of the labdan, pimaran, clerodan, and kaurant type, as well as the dolabellan and sacculate types, which only occur in mosses.
Biological activity of the ingredients
The ingredients listed above, especially the terpenes, are often biologically active, with the following effects being known to date:
- Antimicrobial (fungicidal and bactericidal) effect: Although little research has been done to date, the defense against fungi and bacteria is of great importance for mosses. It is therefore assumed that all mosses contain antimicrobial substances. The effect has been proven , for example, with Polygodial from Porella , Norpin-guison from Conocephalum conicum , Lunularin from Lunularia cruciata .
- Germination-promoting and inhibiting effect: Many moss extracts inhibit or promote - depending on the species - the growth of seedlings of higher plants. The germination rate increased in experiments by up to 70%. Inhibition of germination helps the mosses to prevent future competitors from emerging. But the promotion of germination is also interpreted as follows: Phytohormone-like substances allow the seeds to germinate quickly in a moss cushion, whereby it uses up its reserves before it reaches the appropriate substrate and thus dies. The responsible ingredients have not yet been isolated. The fungicidal effect of the mosses also reduces competition from other plants by preventing the formation of mycorrhizae.
- Biocidal and feeding-inhibiting effect: Most mosses contain feeding-inhibiting substances, so that mosses are rarely eaten by herbivores such as insects or snails. This effect was investigated with feeding experiments with extracts from deciduous, liver and peat moss as well as isolated active ingredients like the sesquiterpene pinguison from Aneura pinguis and plagiochilin A from Plagiochila . This feeding-inhibiting effect has the practical advantage that moss herbaria are rarely attacked by pests and do not have to be preserved.
- Some types of moss are allergenic to humans, such as Frullania tamarisci in the Mediterranean region. Triggers are usually lactones .
Areas
Mosses generally have larger areas than flowering plants, which is mainly due to their spreading mechanisms via spores.
Closed areas
- Marchantia polymorpha and Bryum argenteum are cosmopolitan .
- Around 60% of the families own a Panamanian area. They are distributed worldwide, tropical or extra-tropical. These families have likely existed since the Permian . Examples are Polytrichaceae , Sphagnaceae , Dicranaceae , Hypnaceae , Bryaceae .
- A laurasian ( holarctic ) area also has a lot of mosses, such as the Rhytidiaceae , Timmiaceae , Schistostegiaceae . The separation of North America by an arm of the sea in the Cretaceous can still be recognized today by the different moss flora on the east and west coast.
- Around 30% of families own a Gondwana area. They probably originated in the Mesozoic Era , before Gondwana broke up.
- Less common are species with circumscribed thetischer distribution (arid Moose Mesozoic origin) and pantropical areas.
Disjoint areas
Large disjunctions across continents occur very frequently in mosses and also at the species level, while they are less common in flowering plants and only occur at the genus level. These disjunctions can be interpreted as remnants of formerly coherent areas or as the result of long-distance distribution, but this can usually no longer be clearly determined in individual cases.
- Bipolar disjunctions affect species that occur in the extra-tropical areas of the northern and southern hemisphere. There are around 100 known species with this distribution, for example Conostomum tetragonum .
- Southern Hemisphere disjunction is the Circumantarctic distribution and is known from around 50 species, for example the genus Monoclea .
- Species with laurasian disjunction occur only in parts of the Holarctic, for example Plagiothecium undulatum only on the west coasts of North America and Europe, Campylopus atrovirens on the east and west coasts of North America and Eurasia. Further possibilities are an amphipacific distribution (East Asia - West North America, for example Takakia lepidozioides ) and the amphiatlantic distribution (East North America - West Europe, for example Diphyscium foliosum ).
- A disjunction neotropic - tropical Africa is known from currently 334 species, a disjunction tropical Africa - Southeast Asia from 52 species. The moss flora of Africa is therefore more similar to that of South America than that of Southeast Asia. The paleotropic concept therefore does not apply to the mosses .
Unexplained disjunctions
Some species have such extremely disjoint areas that their occurrence seems to elude any explanation. One example is Distichophyllum carinatum from the otherwise tropical family Hookeriaceae . It is only known from three locations on moist limestone cliffs in the northern Alps, where it was first described in 1908, and from Japan.
The species Hyophila involuta , widespread in the tropics, grows on moist limestone rocks. However, it also occurs in Swiss lakes, in Lake Constance and in Aare and Upper Rhine, but here as water moss in the splash zone.
Fossil history
The oldest moss fossil, the liverwort Pallavicinites devonicus from the lowest Upper Devonian , is around 350 million years old. It is assigned to today's Metzgeriidae . Only thaless liverworts are known from the Devonian. The oldest deciduous moss, Muscites plumatus , comes from the Lower Carboniferous England. The first peat moss-like moss appeared in the Permian . In Palaeophytic all large groups of bryophytes were already represented.
Only a few fossils are known from the Mesophytic , as this age was rather dry and therefore the fossilization conditions for mosses were much worse. During this time, however, the more dry- adapted clans such as the Marchantiidae developed: Marchantites cyathoides from the Middle Triassic is the first distinct species of the Marchantiales. The Jungermanniidae also appear here for the first time. At the end of the Cretaceous period , the first representative of a genus that is still alive today appears ( Campylopodium allonense ). From the Middle Cretaceous to the Tertiary , the first epiphytic and epiphyllic liverworts as well as epilithic (growing on rock) and pleurocarp clans among the deciduous mosses appear.
The majority of the tertiary fossils can already be assigned to living species, which means that many species are at least 40 million years old. In Europe there are many species from the Tertiary that are only found in subtropical areas today, such as the Canary Islands or Azores. In Europe they became extinct during the Ice Ages.
to survive
After lying frozen under the Antarctic ice for 1500 years, a moss plant began to sprout again after a few weeks under ideal breeding conditions. Until then, the viability of multicellular organisms had been estimated at a maximum of 20 years. Certain genes, which are activated when temperatures drop and only occur in mosses, help here.
Systematics
Until recently, mosses were seen as a single group and divided into two to three classes: liverworts, deciduous mosses and, more recently, hornworts. In recent years the profound differences between these groups have become clear, mainly due to ultrastructural research and molecular biological findings, so that they have been placed in ever higher taxonomic units . Most authors today regard the three large groups as monophyletic. Their position to one another and to the other land plants, the vascular plants , has not yet been finally clarified.
The mosses are probably not a monophyletic unit. Studies of spermatogenesis and of chloroplast genes postulate a monophyly of mosses and a basal dichotomy between mosses and vascular plants at the base of the embryophytes. However, most studies since around 1980, when moss paraphyly was first postulated, indicate moss paraphyly. However, a 2014 study concluded that this was due to an artifact caused primarily by silent mutations . The question of which moss group is the most basal within the embryophytes, liver or hornwort, has not been finally clarified. Many studies suggest that deciduous and liverworts are sister groups: especially studies on spermatogenesis, sperm ultrastructure, general morphology and genetic studies. However, there are also arguments based on sequence data and genome structures that the hornworts are the sister group of vascular plants. A broad-based study further substantiated this in 2006 and suggested the following cladogram:
|
|
|
||||||||||||||||||
|
|
Today the mosses are generally no longer regarded as a family group. The three groups are only seen as a common type of organization. The three departments with the subdivision up to the class are:
- Department liverworts (Marchantiophyta)
- Superclass I
- Class Treubiopsida
- Class Haplomitriopsida
- Superclass II
- Class Blasiopsida
- Class Marchantiopsida
- Superclass III
- Class Fossombroniopsida
- Class Pallaviciniopsida
- Class Pelliopsida
- Superclass IV
- Class Jungermanniopsida
- Superclass I
- Department mosses (Bryophyta)
- Takakiophytina subdivision
- Class Takakiopsida
- Subdivision Sphagnophytina
- Class Sphagnopsida
- Bryophytina subdivision
- Class Andreaeopsida
- Class Oedipodiopsida
- Class Tetraphidopsida
- Class Polytrichopsida
- Class Bryopsida
- Takakiophytina subdivision
- Department of Horn Mosses (Anthocerotophyta)
- Class Leiosporocerotopsida
- Class Anthocerotopsida
For a classification down to the family, see the moss system .
Hazard and protection
The endangerment of moss species is mainly due to the destruction of their habitat, in Central Europe particularly through the intensification of agriculture and forestry, which particularly endangers species of stubble fields, epiphytic mosses on deciduous trees and species on dead wood . Other reasons are the building up of wetlands in particular and the lowering of the water table . Air and water pollution should also be mentioned, with the improvement in air quality in Central Europe leading to a return of epiphytic mosses to the metropolitan areas.
The worldwide Red List , drawn up by the International Association of Bryologists, contains 91 species. There is also a red list for Europe and some European countries, including Belgium, Germany, Austria, Poland, Sweden and Switzerland. In Germany there are also red lists for most of the federal states. The risk is particularly high in metropolitan areas. In 1991, 33% of the species in West Berlin were lost or extinct and only 23% were not endangered, while 57% of the species were endangered in more rural areas such as Saxony (1995) and 50% in the Netherlands (1992).
|
Conservation status of the moss species of Annex II of the Habitats Directive in Germany (reporting period 2007–2012) |
||||
| Species name (German) | Species name (scientific) | ALP | ATL | CON |
|---|---|---|---|---|
| Vosges broken moss | Bruchia vogesiaca | k. A. | k. A. | k. A. |
| Green goblin moss | Buxbaumia viridis | --- |
|
|
| Hair Claw Moss | Dichelyma capillaceum | --- | --- | |
| Green fork tooth moss | Dicranum viride | --- |
|
|
| Keeled two-leaf moss | Distichophyllum carinatum | --- | --- | |
| Lapland sickle moss | Hamatocaulis lapponicus | k. A. | k. A. | k. A. |
| Glossy sickle moss | Hamatocaulis vernicosus | k. A. | k. A. | k. A. |
| Common white moss | Leucobryum glaucum |
|
||
| Three-man dwarf moss | Mannia triandra | --- |
|
|
| Long-handled gooseneck moss | Meesia longiseta | k. A. | k. A. | k. A. |
| Ball horn moss | Notothylas orbicularis | --- | --- |
|
| Roger's hooded moss | Orthotrichum rogeri | --- |
|
|
| Carinthian Spatenmoos | Scapania carinthiaca | --- | --- | |
| Rudolph's trumpet moss | Tayloria rudolphiana | --- | --- | |
|
ALP = alpine biogeographical region, ATL = Atlantic biogeographical region, CON = continental biogeographical region green = favorable conservation status, orange = inadequate conservation status, red = poor conservation status, gray = unknown conservation status, --- = the species does not occur in the respective biogeographical region k. A. = the species was not taken into account in the report |
||||
In Austria, 29% of the 762 known deciduous moss species are classified as endangered or extinct (of which 32 are extinct or lost and 24 are critically endangered), while a further 100 species are classified as potentially endangered. Of the 256 known liverworts (including hornworts), 23% are classified as endangered or extinct (of which 8 are extinct or lost and 10 are threatened with extinction), while a further 54 species are classified as potentially endangered.
In Switzerland, 38% of the 1093 species and subspecies are on the Red List. Of these, 15 species are extinct and 5.6% are threatened with extinction. Around 47% of the mosses are not considered to be endangered. Species of dry grassland and open-earth areas, such as fields, are particularly common. Species of wet locations are no longer as endangered, as a result of increased protection of moors.
In Germany, 54 of the 1121 species of moss are considered extinct, 28 as critically endangered, 104 as critically endangered and 203 species as endangered.
In the Washington Convention on the Protection of Species (CITES), no mosses are listed. Nine liverwort and 13 deciduous moss species are listed in the Bern Convention . In many countries, no moss species are protected, protection here is exclusively through habitat protection. In Germany, all species of the genera Hylocomium , Leucobryum and Sphagnum are protected by the Federal Species Protection Ordinance (version February 16, 2005, list ) . Any extraction from nature is therefore prohibited. In Austria, as in Belgium and parts of the Netherlands, the commercial use of peat moss is prohibited. Some species are specifically protected in a few other countries, including Estonia, Finland, Great Britain, Hungary, Japan, Latvia, Lithuania, Luxembourg, Mexico, Portugal, Spain and Ukraine.
In the countries of the European Union , 88 species of moss are subject to the protection regime of the Habitats Directive , of which 31 species are listed in Annex II of the Directive. The EU member states have to designate protected areas for these species listed in Appendix II .
In Germany there are 13 of the Appendix II moss species (see table opposite).
Mosses and humans
Use by humans
Mosses have been and are of use to humans for a long time. Some of the uses were as filling material for mattresses and upholstery, which is why Linnaeus named a genus Hypnum , sleeping moss. Eskimos and Japanese used mosses to fill coffins. The cracks in log houses were often stuffed with moss, as was the case with medieval boats. Dry moss was used as packaging material when shipping fragile objects, and damp moss when shipping garden plants. In Japan, small moss gardens are created in bonsai plant pots as well as large ones around Buddhist temples. Several types of moss such as white moss ( Leucobryum glaucum ) are popular in model making (architectural model for shrubs) and in floristry as decoration (e.g. moss wreaths for graves) and are a tradition in Christmas cribs .
In the aquarium hobby , aquatic mosses serve as decorative elements, spawning substrates and hiding places for aquarium inhabitants. Various species with a morphological resemblance to Taxiphyllum barbieri have been kept in the aquarium for decades under the collective name of “ Java moss ” , with their use as a spawning substrate being the main focus in the past. In the course of an increasing demand for the design of natural aquariums and nano aquariums , a large number of other species have been characterized and brought onto the market in recent years.
Peat moss was used as wound compresses until the First World War . In addition to the high water absorption capacity, the antimicrobial effect of the mosses was also important here. Some indigenous peoples also used these two properties by making baby diapers from moss, for example some Indian and Eskimo groups. It was also used as a menstrual pad . Excavations have also shown that mosses were used as toilet paper in Central Europe in the Middle Ages.
Liverworts in particular were also used in folk medicine, which is due to the antimicrobial effect. Some North American Indians used mosses to prepare ointments for wound care. In traditional Chinese medicine some 40 species of moss are used as for burns, eczema , angina and bronchitis .
Mosses such as Homalothecium sericeum grown on human skulls were considered to have magical powers and were used as a wound-cleansing and hemostatic medicine until the 17th century. For example, according to Tabernaemontanus (1558, edited 1664 by C. Bauhin ): “Mooß von Todtenkopff, Muscus ex craneo humano [...]. Quite a few Medici and pharmacists put a death head for a while on a damp Orth, from which finally a Mooß grows [...] to stop the bleeding ”.
The raised bog peat from peat moss has the greatest economic importance . It is mainly used as a growing medium in horticulture and as a fuel. In Russia, Ireland and Finland there are peat power plants for generating electricity.
In the construction industry, dried moss is used as insulation. In most cases, however, a chemical treatment against insect infestation is carried out beforehand, to reduce hygroscopicity and to improve the fire protection properties. In historical log construction (e.g. in the Spreewald region) the joints between the round timbers or square timbers were stuffed with moss and thus sealed, followed by a layer of clay. If moss settles on exposed concrete (masonry, concrete roof tiles), this can in the long term contribute to the destruction of the concrete surfaces. The high moisture storage capacity of moss prevents the concrete from drying out, which leads to sanding of the concrete in frosty conditions and, in the long term, to its surface destruction.
One particularly early known use by Romans in northern England is to insulate the voids under wooden floors. Sealing of wooden buildings was probably made worldwide with moss, which is still common in alpine huts in this country. The moss that was most often used for chimney seals was named after it: Antipyretica - Latin for against fire. Some mosses can absorb up to 30 times their dry weight water, which is why they are used as baby diapers and menstrual pads. During the First World War, bandages consisted primarily of peat moss in linen bags. Peat (from peat moss) is a good substrate for horticulture, but it should be conserved as a carbon store in the sense of climate protection. Under the microscope or even under a magnifying glass, some mosses look like forests, a microcosm with tardigrade swimming in them and other little or single-celled animals. Mosses rarely live in water, but they do live in splash water areas along rivers.
Use as bio-indicators
Several properties make mosses very good bio-indicators : They absorb water and nutrients through the surface and are thus exposed to the direct effects of pollutants; Their short life cycle leads to quick reactions to environmental changes; they can be determined macroscopically and are present all year round. However, up to now they are only used as bio-indicators in Europe, Canada, Japan and New Zealand. Active and passive biomonitoring using moss is standardized in some VDI guidelines . The EN 16414 standard also describes passive biomonitoring with mosses.
- In the case of water pollution, mosses indicate organic and chemical pollution, water acidification and heavy metal pollution.
- When the air is polluted, mosses react particularly to sulfur dioxide . In the past decades, the increasing pollution was mapped on the basis of the disappearance of mosses from industrial areas, today the decreasing pollution on the basis of repopulation, especially by epiphytes. The spatial distribution of nitrogen deposition can also be followed with mosses.
- Mosses accumulate heavy metals due to their high ion exchange capacities. The moss surface acts as an effective cation exchanger due to negatively charged groups . The moss species Hylocomium splendens , Hypnum cupressiforme and Pleurozium schreberi are being monitored throughout Europe . The Mossclone research consortium is testing which peat mosses are suitable for standardized air monitoring. The same mechanisms allow mosses to accumulate radionuclides so that they serve as long-term sensors for radioactive pollution.
- Due to their short life cycle and the wide spread of spores, mosses react quickly to climate fluctuations by shifting their area. In the temperate zones as in Central Europe, the main vegetation periods for mosses are autumn, the frost-free periods in winter and spring. Therefore, the mosses in this area react particularly strongly to changes in winter temperatures. In the last few years, between 1985 and 1999, 32 species of moss penetrated from the mild winter Atlantic and Mediterranean regions to Central Europe.
Production of biopharmaceuticals
The deciduous moss Physcomitrella patens is a model organism of developmental biology and molecular evolution of plants with increasing use by biotechnology . It can be cultivated in moss bioreactors and genetically modified in a targeted manner by homologous recombination . When using transgenic moss for the production of biopharmaceuticals , the recombinant protein can ideally be purified from the culture medium. By using certain knockout mosses , the glycosylation pattern can be changed in the sense of a humanization of the biopharmaceutical. An example of the production of biopharmaceuticals in moss is factor H: this molecule is part of the human complement system , a defect in the corresponding gene leads to various kidney and eye diseases. Biologically active, recombinant factor H was produced in moss bioreactors at the beginning of 2011.
literature
The article is mainly based on the following two books:
- Jan-Peter Frahm : Biology of Mosses . Spectrum Academic Publishing House, Heidelberg and Berlin 2001, ISBN 3-8274-0164-X
- Jan-Peter Frahm , Wolfgang Frey , J. Döring: Moosflora . (Stuttgart 1983) 4th, revised and expanded edition (UTB for Science, Volume 1250). Ulmer, Stuttgart 2004, ISBN 3-8001-2772-5 (Ulmer) & ISBN 3-8252-1250-5 (UTB)
- further reading
- Franz Fukarek et al .: Urania plant kingdom: mosses, ferns, naked seeds . Urania, Berlin 2000, ISBN 3-332-01168-5
- Janice M. Glime: Bryophyte Ecology. Volume 1. Physiological Ecology . Ebook sponsored by Michigan Technological University and the International Association of Bryologists. 2007 online
- Martin Hellbach: The use of mosses in Japanese and European garden culture: Representation and comparison . In: Die Gartenkunst 25 (2/2013), pp. 377–400.
- J. Shaw, K. Renzaglia: Phylogeny and diversification of bryophytes . American Journal of Botany 91 (10), 2004, pp. 1557-1581.
- Volkmar Wirth , Ruprecht Düll : Color Atlas of Lichen and Moss. Ulmer, Stuttgart 2000, ISBN 3-8001-3517-5 .
- Ruprecht Düll: Excursion pocket book of the most important mosses in Germany. An introduction to moss science, with special consideration of the biology and ecology of the mosses (for the magnification of the easily recognizable species in the area). Rheydt 1985.
Web links
- http://www.ijon.de/moose/
- http://www.blam-hp.eu/
- http://www.bryolich.ch/
- Mosses in Germany
- Conquest of the country by foreign DNA
Individual evidence
- ↑ Alfred Helfenstein: The Namengut Pilate territory. Keller, Luzern 1982, ISBN 3-85766-004-X , p. 26 f. ( Musflue ) and 49 ( Moss : "inconspicuous plant that prefers boggy soil, but also corridors, on which it thrives in abundance").
- ↑ See also Jürgen Martin: Die 'Ulmer Wundarznei'. Introduction - Text - Glossary on a monument to German specialist prose from the 15th century. Königshausen & Neumann, Würzburg 1991 (= Würzburg medical-historical research. Volume 52), ISBN 3-88479-801-4 (also medical dissertation Würzburg 1990), p. 151 ( mies / mieß [neuter and masculine]).
- ↑ Peter H. Raven, Ray F. Evert, Susan E. Eichhorn: Biology of plants. Gruyter, 2006, p. 415 ISBN 3-11-018531-8
- ^ Frahm: Biologie der Moose 2001, p. 195.
- ^ Frahm: Biologie der Moose 2001, p. 170.
- ↑ The whole section follows D. Frohne, U. Jensen: Systematics of the plant kingdom with special consideration of chemical characteristics and plant drugs . 4th edition, G. Fischer, Stuttgart, Jena, New York 1992, ISBN 3-437-20486-6 , pp. 70-74.
- ↑ Jan-Peter Frahm: Biology of the mosses . Spectrum Akademischer Verlag, Heidelberg and Berlin 2001, ISBN 3-8274-0164-X , pp. 262-266.
- ↑ a b Frahm: Biologie der Moose , 2001, p. 221 f.
- ↑ Moss plant survives 1,500 years under Antarctic ice , ORF.at of March 17, 2014
- ↑ "Tough as Old Moss" , The American Scholar from October 15, 2014
- ^ "Mosses survive climate catastrophes" , Laborpraxis from September 15, 2014
- ↑ a b J. Shaw, K. Renzaglia: Phylogeny and diversification of bryophytes . American Journal of Botany 91 (10), 2004, pp. 1557-1581.
- ↑ Garbary, Renzaglia: Bryophyte phylogeny and the evolution of land plants: evidence from development and ultrastructure . In: Bates, Ashton, Duckett (Eds.): Bryology for the twenty-first century . WS Mancy and Sons, Leeds 1998. Nishiyama et al .: Chloroplast Phylogeny Indicates that Bryophytes Are Monophyletic. Molecular Biology and Evolution 21 (10), 2004, pp. 1813-1819.
- ^ RJ Duff, DL Nickrent: Phylogenetic relationships of land plants using mitochondrial small-subunit rDNA sequences. American Journal of Botany 86 (3) 1999, pp. 372-386.
- ↑ Cymon J. Cox, Blaise Li, Peter G. Foster, T. Martin Embley, Peter Civan: Conflicting phylogenies for Early Land Plants are Caused by Composition biases among Synonymous substitution . In: Systematic Biology . 63, No. 2, 2014, pp. 272-279. doi : 10.1093 / sysbio / syt109 . PMID 24399481 . PMC 3926305 (free full text).
- ↑ DL Nickrent, CL Parkinson, JD Palmer, RJ Duff: Multigene Phylogeny of Land Plants with Special Reference to Bryophytes and the Earliest Land Plants . Molecular Biology and Evolution 17 (12) 2000, pp. 1885-1895.
- ^ Yin-Long Qiu et al .: The deepest divergences in land plants inferred from phylogenomic evidence. In: Proceedings of the National Academy of Sciences 103, (42), (2006), pp. 15511-15516, doi: 10.1073 / pnas.0603335103 .
- ↑ Wolfgang Frey, Eberhard Fischer, Michael Stech: Bryophytes and seedless Vascular Plants . In: Wolfgang Frey (Ed.): Syllabus of Plant Families - A. Engler's Syllabus of Plant Families . 13th edition. tape 3 . Borntraeger, Berlin / Stuttgart 2009, ISBN 978-3-443-01063-8 .
- ^ Tan, Geissler, Hallingbäck: Towards a World Red List of Bryophytes . Bryological Times (77), pp. 3-6.
- ↑ Schumacker & Martiny: Red Data Bood of European Bryophytes. Part 2: Threatened bryophytes in Europe including Macaronesia . Trondheim 1995
- ^ Ludwig et al .: Red List of Mosses (Anthocerophyta et Bryophyta) in Germany. In: G. Ludwig, M. Schnittler (edit.): Red List of Endangered Plants in Germany. Publication series Vegetationskunde Volume 28, pp. 189–306. ISBN 3-89624-001-3
- ↑ H. Niklfeld: Red Lists of Endangered Plants Austria. Green series of the Federal Ministry for the Environment, Youth and Family Volume 10, Vienna 1999, pp. 153–186. ISBN 3-85333-028-2
- ↑ Norbert Schnyder et al .: Red list of endangered species in Switzerland: Mosses. Edition 2004 ( PDF ).
- ↑ European Topic Center on Biological Diversity (2014): Species assessments at EU biogeographical level, as of February 28, 2014
- ↑ Grims, Köckinger: Red List of Endangered Mosses (Musci) Austria. 2nd version . In: H. Niklfeld: Red Lists of Endangered Plants Austria. Pp. 157-171.
- ↑ Saukel, Köckinger: Red List of endangered liverworts (Hepaticae) and hornworts (Anthocerotae) in Austria. 2nd version . In: H. Niklfeld: Red Lists of Endangered Plants Austria. Pp. 172-179.
- ↑ Federal Office for the Environment (FOEN): Red Lists: Moose. Status 2007 ( Memento from December 20, 2008 in the Internet Archive ).
- ↑ BFN table (PDF; 51 kB).
- ↑ a b Weddeling, K. Ludwig, G. & Hachtel, M. (2005): The Moose (Bryophyta, Marchantiophyta, Anthocerophyta) of the Habitats Directive , pp 207-329, in: Petersen, B., Ellwanger, G., Biewald, G., Boye, P., Hauke, U., Ludwig, G., Pretscher, P., Schröder, E., Symank, A. (2005): The European protected area system Natura 2000 - ecology and distribution of species of the Habitats Directive in Germany, Volume 1: Plants and Invertebrates, Series for Landscape Management and Nature Conservation , Volume 69, 743 S. (PDF; 48 kB)
- ↑ Glime, JM (2007): Economic and ethnic uses of bryophytes. In: Flora of North America Editorial Committee. (Ed.). Flora of North America North of Mexico. Vol. 27. Bryophyta, part 1. Oxford University Press, New York. pp. 14–41 ( PDF )
- ^ Karl-Heinz Weimann : The German medical terminology of Paracelsus. Philosophical dissertation, Erlangen 1951, p. 504 f.
- ↑ Jakob Grimm : German Mythology. I-III, Berlin 1835; 4th edition, obtained from Elard H. Meyer, Berlin 1875–1878; Reprint, with an introduction by Leopold Kretzenbacher, Graz 1968; Reprint Wiesbaden 1992, Volume III, p. 349.
- ↑ Philip Begardi: Index Sanitatits. Eyn schoens und vast useful Buechlin, called Zeyger der Gesuntheyt [...]. , Worms 1539, sheet 29.
- ↑ Dieter Beckmann, Barbara Beckmann: Mandrake, mugwort and other witch herbs. Everyday knowledge of bygone times. Frankfurt am Main / New York 1990, p. 196.
- ↑ Lothar Bodingbauer: From the life of nature: Survival artist in green upholstery: The botanist Harald Zechmeister on mosses. Part 3: A whole world in the microscope broadcast March 15, 2018, 8:55 am - 9:00 am and description. Listenable for 7 days, downloadable.
- ↑ VDI 3957 sheet 1: 2014-09 Biological measurement methods for determining and assessing the effects of air pollution on plants (biomonitoring); Basics and objectives (Biological measuring techniques for the determination and evaluation of effects of air pollutants on plants (biomonitoring); Fundamentals and aims). Beuth Verlag, Berlin, pp. 10-12.
- ↑ DIN EN 16414: 2014-08 outside air; Biomonitoring with mosses; Accumulation of air pollutants in mosses (passive monitoring): sampling and sample preparation; German version EN 16414: 2014. Beuth Verlag, Berlin, p. 6.
- ^ Karsten Mohr: Biomonitoring of nitrogen deposition with mosses. In: Hazardous substances - cleanliness. Air . 74, No. 6, 2014, ISSN 0949-8036 , pp. 263-265.
- ↑ VDI 3957 sheet 17: 2009-07 Biological measurement methods for determining and assessing the effects of air pollution (bioindication); Active monitoring of heavy metal load with peat moss (Sphagnum-bag-technique) (Biological measurement procedures to determine and assess effects of air pollutants on plants (bioindication); Active monitoring of the heavy metal load with peat moss (Sphagnum-bag-technique)). Beuth Verlag, Berlin, pp. 2–3.
- ↑ Video “Mosses should control air pollution” , Euronews , June 3, 2013, accessed on June 24, 2013
- ^ Ralf Reski (1998): Physcomitrella and Arabidopsis : the David and Goliath of reverse genetics . Trends in Plant Science 3, 209-210, doi: 10.1016 / S1360-1385 (98) 01257-6
- ↑ Baur, A., R. Reski, G. Gorr (2005): Enhanced recovery of a secreted recombinant human growth factor using stabilizing additives and by co-expression of human serum albumin in the moss Physcomitrella patens. Plant biotech. J. 3, 331-340 doi: 10.1111 / j.1467-7652.2005.00127.x
- ^ Eva L. Decker, Ralf Reski (2008): Current achievements in the production of complex biopharmaceuticals with moss bioreactors. Bioprocess and Biosystems Engineering 31 (1), 3-9 PMID 17701058
- ↑ Büttner-Mainik, A., J. Parsons, H. Jérôme, A. Hartmann, S. Lamer, A. Schaaf, A. Schlosser, PF Zipfel, R. Reski, EL Decker (2011): Production of biologically active recombinant human factor H in Physcomitrella. Plant Biotechnology Journal 9, 373-383. doi: 10.1111 / j.1467-7652.2010.00552.x