amphetamine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Simplified structural formula of amphetamine without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Amphetamine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 9 H 13 N | |||||||||||||||||||||
| Brief description |
Amine-like smelling, pungent tasting liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
indirect sympathomimetic |
|||||||||||||||||||||
| Mechanism of action |
Norepinephrine / dopamine release |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 135.21 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.93 g cm −3 |
|||||||||||||||||||||
| boiling point |
200-203 ° C |
|||||||||||||||||||||
| Vapor pressure |
2.9-3.5 h Pa (20 ° C) |
|||||||||||||||||||||
| pK s value |
10.13 |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.518 (26 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Amphetamine (also: phenylisopropylamine or amfetamine ) is a synthetic chemical compound from the phenylethylamine group and is one of the wake-up amines ( amines with a "waking" effect). It is used in medicine as a drug to treat attention deficit / hyperactivity disorder (ADHD) and narcolepsy .
Amphetamine has a strong stimulating and stimulating effect . Like all amphetamine derivatives and many stimulants, it suppresses the appetite and, in high doses, is euphoric . It is therefore particularly popular in the drug scene and widely used under names such as speed or pep .
Amphetamine is the parent compound of the amphetamine class of substances . It includes a number of other psychoactive substances , including methamphetamine and the naturally occurring ephedrine . Amphetamine is a stimulant and indirect sympathomimetic , i.e. that is, it stimulates the sympathetic parts of the autonomic nervous system .
There are two different enantiomers of amphetamine: D- amphetamine and L- amphetamine. Pure D- amphetamine is often used medicinally because it is more effective than L- amphetamine. If only “amphetamine” is spoken of without a more precise name, the 1: 1 mixture of the two enantiomers is meant ( DL -amphetamine).
In the Federal Republic of Germany, amphetamine is listed in Appendix III of the Narcotics Act and is therefore a marketable and prescription narcotic; Trade and possession without a permit will be prosecuted.
overview
The first synthesis of amphetamine was achieved in 1887 by the Romanian chemist Lazar Edeleanu at Berlin University . In 1927, the American chemist Gordon Alles coined the name amphetamine , which is derived from the now outdated chemical name a lpha- M ethyl ph en et hyl amine .
Originally used as a bronchospasmolytic and appetite suppressant , today it is only used medically to treat narcolepsy and attention deficit / hyperactivity disorder ( ADHD) due to its addictive potential and other side effects . Amphetamine is being used more and more again in ADHD treatment; in Germany, two finished medicinal products came onto the market in 2011 and 2013, and in the USA the number of amphetamine prescriptions rose significantly again in the 1990s. In Germany and most other countries, drugs with similar effects are still preferred: methylphenidate for ADHD and modafinil for narcolepsy .
As an intoxicant, amphetamine is particularly widespread in the party scene due to its effects such as suppressing tiredness and increasing self-confidence. It is also used as a doping agent . The amount of amphetamines seized in the European Union has been increasing more or less steadily since 1985; while a certain stagnation was reached from 1999, the number continued to rise in the Scandinavian countries .
Timetable
Before 1900 to 1950
- In 1887, Lazar Edeleanu was the first to synthesize amphetamine as part of his doctoral thesis.
- In 1893 the Japanese chemist Nagayoshi Nagai synthesized the amphetamine derivative methamphetamine .
- In 1910, the English physiologists Barger and Dale discovered that amphetamine and the body's own hormone adrenaline are similar in their chemical structure.
- 1927 coined Gordon Alles, who after a cheaper and easier to synthesize substitute for the course in Ephedra occurring ephedrine was looking for the term amphetamine .
- 1932 brought Smith, Kline & French amphetamine sulphate under the name Benzedrine on the US market. It was used in the form of an inhaler to widen the bronchi in respiratory diseases such as asthma . In Germany, the drug was sold as amphetamines.
- In 1935 the stimulating effect was recognized and used to treat narcolepsy .
- In 1937, the psychiatrist Charles Bradley administered amphetamines to children with behavioral problems, and their symptoms improved as a result. He repeated the study in 1941. Bradley's studies are considered milestones in psychotropic therapy in children.
- In 1938 the Berlin Temmler -Werke brought methamphetamine onto the market under the brand name Pervitin , which was manufactured until 1988.
- Amphetamines became widespread by the end of the 1930s and were prescribed for a variety of purposes: for example as an appetite suppressant , for colds , for narcolepsy , depression , vomiting, and after excessive alcohol consumption (“hangovers”). At that time there were combination preparations (e.g. dexamyl) which, in addition to amphetamine, contained a strong sedative (mostly barbiturates ) to counteract its side effects. Such combinations are now viewed as not very sensible and risky.
- During World War II , amphetamine derivatives were used in the armies of Germany (methamphetamine, pervitin ), the United States (D, L-amphetamine, amphetamine), Great Britain, and Japan to increase alertness, perseverance, and mood among soldiers.
- In 1941, due to the increasing number of cases of abuse and addiction, it was made subject to the Reich Opium Act in Germany , which regulated traffic with the substance.
- In 1948 Glaxo-Wellcome brought Dexedrine (pure dexamphetamine ) onto the market in the USA as an agent against ADHD .
1950 until today
- By the 1950s, stimulant use and abuse in Japan reached enormous levels, with 0.7% of the population being chronic users and 2.5% ex-users in 1954. In Europe (particularly in Sweden ) and the USA, stimulant abuse also increased.
- In 1959 there were first reports of users in the USA who injected themselves with the contents of the amphetamines inhalers , as a result of which inhalers that could be used for injection were withdrawn from the market. The first cases of illegally produced amphetamine became known.
- In 1970, amphetamine was included in Schedule II of the Controlled Substances Act in the United States, making trade, possession, and manufacture without authorization a criminal offense; a doctor can still prescribe it.
- in the 1981 revised Narcotics Act (BtMG) amphetamine was listed in Appendix III, which makes trading, possession and production without a permit a criminal offense; it could still be prescribed by doctors. Amphetamine had previously been prescribable in Germany without any special requirements. Today (as of December 2016) the amphetamine racemate and dexamphetamine are still marketable and prescribable (see legal status ).
- In 1996, Adderall (a mixture of various amphetamine salts) was approved as a drug for the treatment of ADHD in the United States.
application areas
Amphetamine and dexamphetamine be in Germany for the treatment of attention-deficit /
Amphetamine is once again becoming increasingly popular in the United States (USA) for the drug treatment of ADHD, with the number of prescriptions rising from less than 1 million prescriptions to almost 6 million in the 1990s. According to a 2001 study commissioned by the US Congress, there is no evidence of an accumulation of abuse among schoolchildren in the US.
Amphetamine was used earlier as an asthma medication, as it causes the mucous membranes to swell and, above all, allows the bronchi to breathe more freely. It was also used as an appetite suppressant and an antidepressant .
Effects and side effects
Amphetamine is a central sympathomimetic and has a stimulating effect on the sympathetic nervous system, part of the autonomic nervous system, in the brain and spinal cord . A sufficiently high dose of amphetamine puts the organism into an ergotropic state, a stressful state that enables all emergency functions of the organism to be activated for increased readiness to act, which is useful in life-threatening situations.
Depending on the dose and dosage form, the following effects can occur after taking amphetamine:
- increased wakefulness , less tiredness , decreased need for sleep , sleep disorders and nervousness
- increased attention span and ability to concentrate , involuntary focus up to tunnel vision
- increased physical and mental stamina
- Reduction or suppression of hunger and thirst
- increased heart rate up to palpitations or ventricular fibrillation
- cerebral seizure
- (Central) anticholinergic syndrome
- Rise in blood pressure due to narrowing of the blood vessels up to high blood pressure
- Dilatation of the bronchi and decongestion of the mucous membranes , dry mouth
- Increase in self-confidence up to euphoria
- increased willingness to take risks , decreased aggression threshold
- decreased pain sensation
- Agitation (erratic movements), increased urge to move, restlessness and symptoms of restless legs syndrome
- Tremor (tremors), increased muscle tone (tension), nystagmus (eye tremors) and bruxism (teeth grinding )
- Symptoms of hyperhidrosis (increased sweat secretion)
- increased sexual desire
- Dilatation of the pupils
- Urinary retention (inability to empty the bladder despite the urge to urinate )
- Logorrhea (increased need to communicate)
Chronic consumption can also have the following effects:
pharmacology
General
The official IUPAC name is 1-phenylpropan-2-amine. It is a homologue of phenylethylamine . The amphetamine base, a colorless to very slightly yellowish, oily liquid, is sparingly soluble in water, soluble in alcohols , ethers and weak acids such as acetic acid. It reacts with diluted alcoholic sulfuric acid and forms the precipitating sulfate salt. The base has a characteristic amine odor. At higher concentrations in the air, there is more burning of the mucous membranes (eyes, nose).
Enantiomers
Amphetamine has a stereocenter on carbon atom C 2 and is therefore chiral . Therefore there are two enantiomers, the dextroisomer (dexamphetamine, D- amphetamine) and the levoisomer (levamphetamine, L- amphetamine). The effects of both enantiomers are similar, but dexamphetamine has about twice the psychoactive effectiveness of levamphetamine and is therefore considered a eutomer . As "amphetamine" or D, L -amphetamine, the racemate , a 1: 1 mixture of L -amphetamine and D -amphetamine designated.
Mode of action
Amphetamine is very similar in its chemical structure to catecholamines , but cannot activate either adrenergic or dopaminergic receptors directly. It therefore works indirectly by causing the natural neurotransmitters norepinephrine and dopamine to be released . It also binds equally well to the transporters of dopamine (DA) and noradrenaline (NA), where it acts as a reuptake inhibitor.
Amphetamine also inhibits monoamine oxidase (MAO) and activates TAAR1 (Trace amine-associated receptor 1) , a trace amine receptor.
The main effect of amphetamine, however, is to release the neurotransmitters norepinephrine (NA) and dopamine (DA). The substance therefore causes NA to be released in the peripheral nervous system . In the brain, however, amphetamine appears to be more likely to cause dopamine than norepinephrine. It seems that dopaminergic transmission in particular is responsible for most of the psychostimulatory effects. In contrast, no significant release of serotonin (5-HT) is observed.
The release mechanism consists of three steps:
- the influx of amphetamine into the presynaptic cell via the transporter
- the release of neurotransmitters from the vesicles (storage vesicles within the cell) into the cell interior ( cytosol )
- the active transport of the transmitter from the inside of the cell into the extracellular space ( synaptic gap ). This is accomplished by reversing the direction of the cell-membrane transporter ( inversion ).
In this way, the extracellular neurotransmitter level is increased. In contrast to the principle of reuptake inhibition, this happens independently of the signal impulse from the nerve cell.
The repeated intake (in rapid succession) of amphetamine leads to a short-term development of tolerance due to tachyphylaxis . The storage vesicles in the neurons are exhausted after repeated stimulation, so that after the onset of tachyphylaxis, noradrenaline and dopamine are no longer available for release. The tachyphylaxis does not end until a few hours later, when the storage vesicles have refilled with the neurotransmitters.
Metabolism
The plasma half-life of dextroamphetamine is around ten hours, so it takes just under three days for the amount in the organism to drop to one percent of the intake. The lipid solubility is LogP = 1.799, so it is distributed preferentially in adipose tissue. Its protein binding is between 25 and 40%, and it is metabolized in the liver by the cytochrome P450 isoenzyme 2D6 .
The water-soluble acid formed is excreted in the urine . The amount of excretion depends on the pH value of the urine; the more acidic the urine (e.g. by taking ascorbic acid or acidic fruit juices), the faster the excretion.
Interactions
With the following drugs (incomplete listing) are partially lethal drug interactions known: chlorpromazine , fluoxetine , fluphenazine , fluvoxamine , guanethidine , isocarboxazid , mesoridazine, methotrimeprazine, paroxetine , perphenazine , phenelzine , prochlorperazine, promethazine , Propericiazin, rasagiline , thioridazine and trifluoperazine. Interactions include psychotic symptoms , risk of hypertensive crisis, and possible occurrence of serotonin syndrome . When monoamine oxidase inhibitors are used at the same time , the breakdown of amphetamine can be inhibited, which also causes life-threatening interactions.
toxicology
The lethal dose in humans is 1.3 mg / kg (LD Lo , lowest published lethal dose); at a body weight of 75 kg this would correspond to about 100 mg. If there is tolerance , the dose is significantly higher, so cases of single doses of 1000 mg and daily doses of up to 5000 mg are known. Experiments with monkeys showed a significantly higher relative toxicity in young animals; the LD 50 in mg / kg there was about 65 to 75% below that of adult animals.
In Germany, 20/16/21 deaths were counted in 2010/2013/2014 in the respective year that were directly related to the consumption of amphetamine alone. Methamphetamine / amphetamine and other drugs were involved in 54/32/35 further deaths in 2010/2013/2014.
Neurotoxicity
There is no evidence that therapeutically used amphetamine doses have a damaging effect on brain cells. In animal experiments, high doses of parenterally administered amphetamine showed changes in dopaminergic neurons in the caudate nucleus and putamen (parts of the striatum ). It is unknown whether long-term administration of high doses of amphetamine can produce similar changes in humans. Long-term administration of lower doses showed no such changes in the animal model.
Manufacturing and analytics
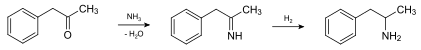
There are a number of different synthesis routes. In the pharmaceutical industry amphetamine is usually in the condensation of 1-phenyl-2-propanone (phenylacetone / P2P) with ammonia and subsequent reduction prepared. This produces racemic ( RS ) -amphetamine [( RS ) -1-phenylpropan-2-amine], i.e. a 1: 1 mixture of ( R ) -amphetamine [( R ) -1-phenylpropan-2-amine] and ( S ) -Amphetamine [( S ) -1-Phenylpropan-2-amine]:
In the USA, the production volume approved by the DEA in 2000 was 15,000 kg, corresponding to 500,000,000 individual doses of 30 mg.
The reliable qualitative and quantitative analysis of amphetamines is possible in the various test materials such as blood , blood serum , blood plasma , hair , urine or saliva or waste water after suitable sample preparation through the coupling of chromatographic methods such as gas chromatography or HPLC with mass spectrometry . Also enzyme immunoassays are as screening tests available, but should for forensic be supplemented purposes by the aforementioned specific methods.
Non-medical use
For recreational consumption , amphetamines are consumed as powder, bombs (speed wrapped in paper) or, less often, in pill form . The powder is mostly absorbed through the nose, but oral , parenteral and rectal consumption are also possible . While oral intake is the common dosage form for medical use, it is otherwise not very common. This is probably due to the fact that the effect occurs more slowly when consumed orally and the slower surge leads to a less sudden onset of the effect (less "kick"). However, the effect lasts longer overall. Amphetamine has an oral bioavailability of approximately 75%.
Unlike methamphetamine , it is not possible to smoke amphetamine because the amphetamine sulfate it contains has such a high boiling point that it first decomposes through pyrolysis .
The European Monitoring Center for Drugs and Drug Addiction reports that in 2009 the usual retail price of amphetamines in half of the reporting countries in Europe was between 5 and 30 euros per gram. According to the Federal Criminal Police Office , around 1,200 kilograms of amphetamines were seized in Germany in 2010, 33,482 crimes (1% of all crimes in that year) were related to amphetamine derivatives.
In Germany and Europe, amphetamine is mainly consumed in the techno scene in order to be able to dance longer, among other things. It has a slightly euphoric effect, keeps you awake and enables activities to be extended for several hours. If the effect wanes, there is nervousness and exhaustion ("turn off"); the body demands the much needed rest, but the not yet degraded amphetamine prevents this. For this reason, it is common to calm down (“smoke down”) by consuming cannabis . Sometimes strong sedatives from the group of benzodiazepines such as Rohypnol or Valium are taken to calm down.
doping
Amphetamine doping has been widespread since the 1930s. Since amphetamine also heats up the body in sports, it is particularly suitable for competitions in damp and cold weather. Since the Olympic Games in 1972, the use of controls at competitions has been largely put to a stop; for example, the doping case Jan Ullrichs , who consumed amphetamines during rehabilitation, led to a six-month ban in the spring of 2002. As an international anti-doping agency, WADA now allows amphetamines to be taken during training and only forbids it in competitions.
Health hazards
The health risks associated with amphetamine consumption include increased aggressiveness , cramps , tremors , circulatory collapse , palpitations and heart attacks . In a dependency syndrome can collapse of the muscles , kidney failure , memory disorders , stroke , paranoid delusions and depression to, altered mental status ranging coma and chronic psychoses occur. It can lead to a neglect of social obligations (family, school, job, relationship). If amphetamines are sniffed frequently, damage to the nasal septum may result.
The risk of developing addiction depends on genetic factors and on the person's psychosocial situation. In the animal model, some individuals were able to regulate their amphetamine consumption flexibly for life, in 50%, on the other hand, after a certain period of time a dependency with a massive increase in dose and acquisition of a tolerance developed , which persisted even after forced withdrawal.
Often, the users get into a vicious circle of alternating use of activating and calming drugs, with each agent intended to alleviate the after-effects of the other.
Amphetamine addicts experience withdrawal symptoms after stopping the amphetamine . Symptoms of amphetamine withdrawal are lethargy , depression and even suicide , tactile hallucinations (English crank bugs ), apathy , anxiety and sleep disorders . The substance may cause muscle pain , abdominal pain and excessive appetite. Withdrawal symptoms peak after about three days and then slowly subside. Compared to benzodiazepine withdrawal, amphetamine withdrawal is physically harmless.
In amphetamine addicts to find high rates of comorbidity with schizophrenic psychosis, bipolar disorder , antisocial personality disorder and ADHD . In American studies, comorbidity with schizophrenia was found in up to 25% of cases. In contrast to amphetamine-induced psychosis, in the combination of amphetamine dependence and schizophrenia, psychotic symptoms persist for more than six months after controlled abstinence. In addition, there are strong fluctuations in affect between unadapted euphoria and severe depression due to consumption.
There is evidence that amphetamine abuse significantly increases the risk of later developing Parkinson's disease .
Extended amphetamine
The white-yellowish / pink powder that is illegally offered to drug users as speed consists only partly of amphetamine. Usually caffeine or neutral diluents such as glucose or lactose are included, but other psychoactive substances such as paracetamol , ephedrine or methamphetamine can also be added. Amphetamine is also illegally traded as “paste”, which is often slightly damp and lumpy and smells like amine (smell of fish that is beginning to rot). The mass is usually a mixture of amphetamine salt and solvents.
In contrast to the European countries , it happened more often in the USA that speed was added to methamphetamine, which was possibly due to the better availability of the starting materials required for the synthesis ( ephedrine preparations were available without a prescription in the USA until March 2005).
Since the respective proportion of amphetamines is unclear, there is always the risk of overdosing and intolerance to excipients (a fatal dose for a person weighing 75 kg can be around 100 mg of amphetamine). Drugchecking is therefore very important for risk reduction.
Illegal synthesis
In the illegal manufacture of amphetamine is, for example, by reduction of norephedrine (phenylpropanolamine) with iodine and red phosphorus , or from phenylacetone recovered. While amphetamine could previously be synthesized relatively unhindered by private individuals from precursors such as phenylacetone and hydroxylamine , these chemicals were increasingly monitored by the authorities and the unauthorized manufacture and trade of phenylacetone and norephedrine was made a criminal offense ( Basic Substance Monitoring Act ). This created a need for illegally working producers for substitutes that were not monitored. For example, phenylacetic acid was gradually included in illegal production. For decades there have been new directions for manufacturing amphetamines using substances that are not yet suspect. The authorities finally become aware of these production methods and the cycle continues. So-called “OTC methods” (over the counter, English for “over-the-counter”, which means “freely available”) are therefore spreading increasingly. The designation stands for the extraction of required precursor substances from non-prescription drugs or other freely available goods (cleaning agents, car accessories), the release of which, unlike pure substances, cannot be effectively regulated. For example, norephedrine (PPA) was obtained from over-the-counter appetite suppressants in the United States until 2002.
Amphetamine is produced illegally mainly by reducing phenyl-2-nitropropene with Al (Hg) or LiAlH 4 or by reductive amination of phenylacetone and ammonia + Al (Hg). Benzaldehyde and nitroethane or the esters of phenylacetic acid are readily available starting materials . The chemicals produced during this production are mostly disposed of illegally: Solvents ( acetone , ether , methanol and others) and acids ( sulfuric acid , hydrochloric acid ) are usually dumped in containers in the open at night or emptied into rivers, sometimes (including hydrogen cartridges) on fire plugged. In the USA and the Netherlands, among others - both countries with high levels of illegal (meth) amphetamine production - the environmental damage caused by toxic by-products is growing in some cases into serious problems. The production of 1 kilogram of amphetamine generates 5 to 20 liters of waste, depending on the synthesis route. In addition to the quantity, the type and toxicity of the waste depend on the particular synthesis route.
Legal status
In the Federal Republic of Germany, amphetamine is listed in the Narcotics Act (BtMG): The racemate D, L-amphetamine and dextroamphetamine are classified as marketable and prescribable in Appendix III. The pure levoisomer levamphetamine is listed in Appendix II as marketable , but not as a prescription. Trade and possession without a prescription or permit is a criminal offense. In the US, amphetamine is on Schedule II of the Controlled Substances Act , which makes it a criminal offense to possess and trade without a prescription or authorization. It is approved there for the indications narcolepsy and ADHD.
For a patient, doctors in the Federal Republic of Germany may prescribe 600 mg amphetamine or 600 mg dexamphetamine within 30 days. In justified individual cases and while maintaining the required safety of narcotics traffic, the doctor may deviate from this provision with regard to the maximum amount for a patient who is undergoing continuous treatment. Such a prescription must be marked with the letter " A " ( Section 2 of the Narcotics Prescription Ordinance, BtMVV). Until the new version of the BtMVV of January 20, 1998 (which came into force on February 1, 1998), doctors were allowed to prescribe 10 times as much as today for one patient per unit of time.
In Austria, amphetamine, dexamphetamine and Levamphetamin regarded as narcotic drugs within the meaning of the Narcotic Substances Act , as they in the UN - Convention on Psychotropic Substances are included. According to the Narcotics Ordinance, all three can be prescribed on drug prescriptions, but no maximum quantities are set.
Since January 1998, the official spelling in the Federal Republic of Germany has been amfetamine , so it has been adapted to the WHO nomenclature.
Amphetamine and Traffic Law (Germany)
Driving a motor vehicle in traffic is prosecuted as an administrative offense (Section 24a Paragraph 2.3, Section 25 Paragraph 2a StVG; Section 4 Paragraph 3 BKatV) if the limit value of 25 ng / ml in the blood is exceeded. A first-time offender is regularly fined EUR 500 and a driving ban for a period of one month. In addition, there is a threat of the driver's license being withdrawn .
Trade names
Finished preparations ( monopreparations )
In Germany, "Attentin" (dexamphetamine hemisulfate) has been available for children and adolescents since December 2011. A prodrug of dexamphetamine, lisdexamfetamine , is available in the United States, Germany, Switzerland, and other countries for the treatment of ADHD.
In the United States, finished medicinal products are approved under brand names such as "Dexedrine", "ProCentra" and "Zenzedi" (active ingredient dexamphetamine). "Adderall" contains a mixture of enantiomers of dexamphetamine (72.7%) and levamphetamine (27.3%) and is available in both immediate-release and delayed-release forms.
- Recipes
In Germany, amphetamine sulfate can also be prepared as an individual recipe in the pharmacy . The New Formulation Form (NRF) therefore contains entries on amphetamine sulphate (juice according to NRF 22.4 or capsules according to NRF 22.5) and dexamphetamine sulphate (2.5% drops according to NRF 22.9). In Switzerland, corresponding to extemporaneous prescribed or ordered abroad. In Austria, all public pharmacies are allowed to dispense the corresponding preparations.
literature
- Walter Reginald Bett u. a .: Amphetamine in clinical medicine. Springer, Berlin 1956.
- Sean Connolly: Amphetamines. Heinemann Library, Chicago 2000, ISBN 1-57572-254-2 .
- Hans Cousto: Mixed drug use. The most important things in brief about the most common (party) drugs. Nightshade, Solothurn 2003, ISBN 3-03788-119-4 .
- Hans-Christian Dany: Speed. A society on drugs. Edition Nautilus, Hamburg 2008, ISBN 978-3-89401-569-5 .
- AK Cho, David S. Segal: Amphetamine and Its Analogs. Psychopharmacology, Toxicology, and Abuse. Academic Press, San Diego 1994, ISBN 0-12-173375-0 .
- Nicolas Rasmussen: On Speed. The Many Lives of Amphetamines. New York University Press, New York 2008, ISBN 978-0-8147-7601-8 .
- Stephen Smith: Addiction. The story of Stephen Smith. Ullstein, Berlin 1998, ISBN 3-548-31215-2 .
- Leslie Iversen: Speed, Ecstasy, Ritalin. Amphetamines - Theory and Practice. Verlag Hans Huber, 2006, ISBN 3-456-84519-7 .
Web links
- Drug enjoyment culture: information on amphetamine and methamphetamine
- Drugscouts: Information on the risks and effects of speed
- Shire Pharmaceuticals (as of 2015): Package Insert for Adderall Extended Release (XR)
Individual evidence
- ↑ a b c d e entry on amphetamine. In: Römpp Online . Georg Thieme Verlag, accessed on June 5, 2014.
- ↑ a b Entry on amphetamine in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b Entry on amphetamine in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-432.
- ↑ Data sheet DL-Amphetamine sulfate salt from Sigma-Aldrich , accessed on March 20, 2011 ( PDF ).
- ↑ a b Lazăr Edeleano: About some derivatives of phenyl methacrylic acid and phenyl isobutyric acid . In: Reports of the German Chemical Society in Berlin; 20th vol. (1887), Volume 3, pp. 616-622. doi: 10.1002 / cber.188702001142
- ↑ EMCDDA 2001 Indicators for the Drug Market - Seizures, Price, Purity
- ↑ UNODC (Ed.): Ecstasy and Amphetamines Global Survey . 2003, ISBN 92-1148164-3 (English, unodc.org [PDF; accessed July 17, 2016]).
- ↑ Nagayoshi Nagai: Kanyaku maou seibun kenkyuu seiseki (zoku). In: Yakugaku Zashi. Volume 13, 1893, p. 901.
- ↑ a b D. J. Heal, SL Smith, J. Gosden, DJ Nutt: Amphetamine, past and present - a pharmacological and clinical perspective. In: Journal of Psychopharmacology . tape 27 , no. 6 , 2013, p. 479–496 , doi : 10.1177 / 0269881113482532 , PMID 23539642 , PMC 3666194 (free full text) - (English).
- ^ Edward M. Brecher and the Editors of Consumer Reports Magazine, 1972: The Consumers Union Report on Licit and Illicit Drugs . Chapter 36. The amphetamines. , Consumers Union (English)
- ^ C. Bradley: The Behavior of Children Recieving Benzedrine. In: American Journal of Psychiatry. 1937, No. 94, pp. 577-581.
- ^ Madeleine P. Strohl: Bradley's Benzedrine Studies on Children with Behavioral Disorders. In: Yale Journal of Biology and Medicine. 2011, No. 84, pp. 27-33.
- ^ Leslie Iversen (2008): Speed, Ecstasy, Ritalin. Amphetamines - theory and practice (see literature).
- ^ A b c Center for Substance Abuse Research, University of Maryland: Amphetamines
- ↑ 550,000 / 80 million or 2 million / 80 million M. Tamura: Japan: stimulant epidemics past and present. In: Bulletin on Narcotics. United Nations Office on Drugs and Crime, January 1, 1989, pp. 83-93 , accessed July 14, 2006 .
- ^ Regulatory News: Richwood's Adderall. In: Health News Daily. February 22, 1996, accessed May 29, 2013 .
- ↑ a b PBS Statistics on stimulant use (English).
- ↑ Attention Disorder Drugs. Few Incidents of Diversion or Abuse Identified by Schools , United States General Accounting Office 2001 (English, PDF; 1.9 MB).
- ↑ a b Attentin prescribing information , Medice Arzneimittel Putter GmbH.
- ↑ technical information ( Memento of 18 January 2017 Internet Archive ) of Elvanse, Shire Pharmaceuticals
- ↑ RC Smith, JM Davis: Comparative effects of d-amphetamine, l-amphetamine and methylphenidate on mood in man . In: Psychopharmacology . tape 53 , no. 1 , 1977, pp. 1-12 , doi : 10.1007 / BF00426687 , PMID 407607 .
- ↑ a b Leslie L. Iversen : Speed, ecstasy, ritalin: the science of amphetamines . Oxford University Press, 2008, ISBN 978-0-19-853090-9 , 2.3. How do amphetamines work ?, p. 9.11–12 ( limited preview in Google Book Search).
- ↑ David Sulzer et al. a .: Mechanisms of neurotransmitter release by amphetamines: a review . In: Progress in Neurobiology . tape 75 , no. 6 , 2005, p. 406-433 , doi : 10.1016 / j.pneurobio.2005.04.003 ( pharmacology.ucsd.edu ( Memento from June 30, 2010 in the Internet Archive ) [PDF]).
- ^ RB Rothman, MH Baumann: Therapeutic and adverse actions of serotonin transporter substrates. In: Pharmacology & Therapeutics . Volume 95, Number 1, July 2002, pp. 73-88, PMID 12163129 . (Review).
- ^ D. Sulzer, MS Sonders, NW Poulsen, A. Galli: Mechanisms of neurotransmitter release by amphetamines: a review . In: Prog. Neurobiol. tape 75 , no. 6 , April 2005, p. 406-433 , doi : 10.1016 / j.pneurobio.2005.04.003 , PMID 15955613 . Full text: on Google Docs ( Memento from June 30, 2010 in the Internet Archive )
- ↑ Entry on Amphetamine in the DrugBank of the University of Alberta .
- ↑ Rafael de la Torre et al. a .: Clinical Pharmacokinetics of amfetamine and related substances: monitoring in conventional and non-conventional matrices . In: Clinical Pharmacokinetics . tape 43 , no. 3 , March 2004, p. 177 , doi : 10.2165 / 00003088-200443030-00002 , PMID 14871155 .
- ↑ entry for amphetamines in the DrugBank the University of Alberta , accessed on 14 November 2016th
- ↑ Poisons Information Monograph (PIM) for Amphetamines , accessed December 8, 2014.
- ↑ Drug Commissioner of the Federal Government: Narcotics deaths by cause of death 2010 - country survey ( memento of December 23, 2015 in the Internet Archive ) , published on March 24, 2011, accessed on October 14, 2015.
- ↑ Drug Commissioner of the Federal Government: Narcotics deaths by cause of death in 2013 - country query ( memento of February 9, 2016 in the Internet Archive ) , published on April 17, 2014, accessed on October 14, 2015.
- ↑ Drug Commissioner of the Federal Government: Narcotics deaths by cause of death in 2014 - country survey ( memento of April 7, 2016 in the Internet Archive ) , published in 2015, accessed on April 28, 2016.
- ↑ S. Berman et al. a .: Abuse of amphetamines and structural abnormalities in the brain . In: Ann NY Acad Sci . tape 1141 , 2008, p. 195–220 , doi : 10.1196 / annals.1441.031 , PMID 18991959 , PMC 2769923 (free full text).
- ↑ C. Kde Mariotti, RS Schuh, P. Ferranti, RS Ortiz, DZ Souza, F. Pechansky, PE Froehlich, RP Limberger: Simultaneous analysis of amphetamine-type stimulants in plasma by solid-phase microextraction and gas chromatography-mass spectrometry. In: J Anal Toxicol . 38 (7), 2014 Sep, pp. 432-437. PMID 25038769
- ↑ S. Pichini, O. García-Algar, AT Alvarez, M. Mercadal, C. Mortali, M. Gottardi, F. Svaizer, R. Pacifici: Pediatric exposure to drugs of abuse by hair testing: monitoring 15 years of evolution in Spain. In: Int J Environ Res Public Health. 11 (8), 2014 Aug 14, pp. 8267-8275. PMID 25153461
- ↑ ML Smith, DC Nichols, P. Underwood, Z. Fuller, MA Moser, R. Flegel, DA Gorelick, MN Newmeyer, M. Concheiro, MA Huestis: Methamphetamine and amphetamine isomer concentrations in human urine following controlled vicks vapoinhaler administration. In: J Anal Toxicol. 38 (8), 2014 Oct, pp. 524-527. PMID 25217541
- ↑ HR Lin, CC Liao, TC Lin: Improved identification of multiple drugs of abuse and relative metabolites in urine samples using liquid chromatography / triple quadrupole mass spectrometry coupled with a library search. In: Rapid Commun Mass Spectrom. 28 (19), 2014 Oct 15, pp. 2043-2053. PMID 25156593
- ↑ S. Macdonald, CJ Cherpitel, T. Stockwell, G. Martin, S. Ishiguro, K. Vallance, J. Brubacher: Concordance of self-reported drug use and saliva drug tests in a sample of emergency department patients. In: J Subst Use. 19 (1-2), 2014 Mar 1, pp. 147-151. PMID 25104914
- ↑ T. Mackuľak, J. Skubák, R. Grabic, J. Ryba, L. Birošová, G. Fedorova, V. Spalková, I. Bodík: National study of illicit drug use in Slovakia based on wastewater analysis. In: Sci Total Environ. 2014 Oct 1; 494-495, pp. 158-165. PMID 25046607
- ↑ M. Sundström, A. Pelander, I. Ojanperä: Comparison between drug screening by immunoassay and ultra-high performance liquid chromatography / high-resolution time-of-flight mass spectrometry in post-mortem urine. In: Drug Test Anal. 2014 Jun 20. PMID 24953563
- ↑ Nicolas Rasmussen: On Speed: The Many Lives of Amphetamine. NYU Press, 2008, ISBN 978-0-8147-7627-8 .
- ↑ Drug raid: Largest amount of amphetamines confiscated in 15 years. In: Berliner Morgenpost. June 8, 2012. Retrieved November 22, 2013.
- ↑ European Monitoring Center for Drugs and Drug Addiction: Drugs Problems in Europe 2009 (PDF; 4 MB).
- ^ Annual summary drug 2010 ( memento from January 11, 2012 in the Internet Archive ), data on drug crime from the BKA.
- ↑ Data on crime in Germany 2010 .
- ↑ Arnd Krüger : The classification of Roger Bannister's performance in the history of training for medium and long distances. In: J. Buschmann, S. Wassong (Hrsg.): Cross-country skiing through the Olympic history. Festschrift for Karl Lennartz. Carl and Liselott Diem - Archive, Cologne 2005, ISBN 3-88338-015-6 , pp. 349–372.
- ↑ Ullrich admits taking amphetamine. on: berlinonline.de , July 8, 2002, accessed on August 8, 2008.
- ↑ NADA (Ed.): The 2014 Prohibited List International Standard. Prohibited List 2014 (PDF).
- ↑ Drugcom: Frequently Asked Questions: What are the acute risks associated with speed consumption? .
- ↑ Gabriel Galli, Jochen Wolffgramm: Long-term voluntary D -amphetamine consumption and behavioral predictors for subsequent D -amphetamine addiction in rats . In: Drug and Alcohol Dependence . tape 73 , no. 1 , 2004, p. 51-60 , doi : 10.1016 / j.drugalcdep.2003.09.003 .
- ^ Jan Dirk Blom: A Dictionary of Hallucinations . Springer 2009, ISBN 978-1-4419-1223-7 , p. 122.
- ^ A. Testa, R. Giannuzzi, F. Sollazzo, L. Petrongolo, L. Bernardini, S. Dain: Psychiatric emergencies (part II): psychiatric disorders coexisting with organic diseases. In: European Review for Medical and Pharmacological Sciences . Volume 17, Suppl 1, February 2013, pp. 65-85, PMID 23436669 . PDF .
- ↑ Gouzoulis-Mayfrank E .: "Comorbidity psychosis and addiction. From the basics to practice." Steinkopff, Darmstadt, 2003
- ↑ R. Thomasius, E. Gouzoulis-Mayfrank a. a .: AWMF treatment guideline: Mental and behavioral disorders caused by cocaine, amphetamines, ecstasy and hallucinogens. In: Advances in Neurology - Psychiatry. 72, 2004, p. 679, doi: 10.1055 / s-2004-818531 .
- ↑ S2 guideline : Mental and behavioral disorders caused by cocaine, amphetamines, ecstasy and hallucinogens , AWMF register number 076/007 (online: full text ( memento of January 2, 2005 in the Internet Archive )), status 10/2004.
- ↑ JG Bramness u. a .: Amphetamine-induced psychosis - a separate diagnostic entity or primary psychosis triggered in the vulnerable? In: BMC psychiatry. Volume 12, 2012, p. 221, doi: 10.1186 / 1471-244X-12-221 . PMID 23216941 . PMC 3554477 (free full text).
- ↑ Using Amphetamines May Increase Risk of Parkinson's Disease ( October 22, 2012 memento on the Internet Archive ), American Academy of Neurology, February 20, 2011, accessed February 22, 2011.
- ^ A b The "Dirty" and Dangerous Side Effects of Synthetic Drugs Production. ( Memento of February 3, 2006 in the Internet Archive ) Europol (English).
- ↑ Appendix III BtMG - individual standard .
- ↑ Appendix II to Section 1 Paragraph 1 BtMG .
- ↑ Schedule II Section d of the CSA ( Memento from August 9, 2017 in the Internet Archive ) (English).
- ↑ Federal Drug Administration (FDA): Adderall (as of October 2015)
-
↑ 200 mg / day = 6000 mg / 30 days = 10 x (600 mg / 30 days)
4. BtMÄndV Art. 4 of 23 December 1992 (BGBl. 1992 I p. 2483; 2487). - ↑ a b Legal information system of the Austrian Federal Chancellery, Federal law consolidated: Entire legal provision for the Narcotics Ordinance , version of March 30, 2014 .
- ↑ 10. BtMÄndV Art. 1 No. 1 letter b; Art. 1 No. 3; Art. 3 ( BGBl. I, p. 74).
- ↑ Dexamfetamine, Attentin® (Medice Arzneimittel) . In: Pharmaceutical newspaper online . Retrieved August 5, 2013.
- ↑ Lisdexamfetamine, Elvanse® (Shire) . ( Memento from April 26, 2014 in the Internet Archive ) In: Pharmazeutische Zeitung online. Retrieved August 5, 2013.
- ↑ Elaine Moore: The Amphetamine Debate: The Use of Adderall, Ritalin and Related Drugs for Behavior Modification, Neuroenhancement and Anti-Aging Purposes . McFarland, 2010, ISBN 978-0-7864-8012-8 ( limited preview in Google book search), p. 90, Corrigendum : Dexamphetamine instead of Lisdexamphetamine .
- ^ Govi-Verlag: Standardized recipes. Govi-Verlag, 2017, ISBN 978-3-7741-1350-3 , p. 92 (capsules and juice), p. 93 (drops) ( limited preview in Google book search).
- ↑ Entry on dexamphetamine in Pharmawiki , accessed on January 28, 2017.