Prostate cancer
| Classification according to ICD-10 | |
|---|---|
| C61 | Malignant neoplasm of the prostate |
| ICD-10 online (WHO version 2019) | |
The Prostate Cancer (medical prostate cancer , prostate cancer) is a malignant tumor disease and passes from the glandular tissue of the prostate ( prostate ) from. In Germany , almost three out of a hundred men die of prostate cancer. Prostate cancer is one of the most common cancers in men: within the group of men who have died of cancer, it is responsible for around ten percent of deaths, making it the third most fatal cancer after lung and colon cancer.
The disease is symptom-free in the early stages. At an advanced stage, symptoms such as urination disorders, bone pain and later weight loss and anemia can occur. If the diagnosis is only made when symptoms have already occurred, metastasis has often already taken place, primarily in the local lymph nodes or in the skeleton ( bone metastases ).
Treatment with the prospect of healing is only possible if the degenerated tissue has not yet crossed the organ borders and there are no metastases. Since symptoms usually only arise when the disease is advanced, regular screening tests are offered in Germany for men over 45 years of age (from the age of 41 for men with a positive family history ) in order to make the cancer diagnosis as early as possible in a still curable stage .
Prostate cancer occurs predominantly in older men, many of whom would no longer experience the symptoms . Since around the beginning of this millennium, "active observation" (see below) has developed as a useful concept for men who (at least initially) do not want to undergo invasive therapy. The decision on treatment is difficult and depends on the individual case. Therapeutic options include surgery to completely remove the prostate ( prostatectomy ) , radiation therapy , hormone therapy, and in some cases chemotherapy . Therapeutic hyperthermia (“nanotherapy”) is still in the test phase.
Prostate cancer has also been described in animals; among domestic animals it is most common in dogs .
The prostate
The prostate or prostate gland is an accessory sex gland of all male mammals including humans. In humans, it lies below the urinary bladder and surrounds the urethra down to the pelvic floor . In men, it resembles a chestnut in size and shape. At the back of the prostate which borders the rectum ( rectum ). This is why it can be felt and assessed with the fingers from the rectum. The task of the prostate is to release a secretion that, together with that of the vesicle gland , the bulbourethral gland and the sperm cells from the testes , forms the sperm . The growth and function of the prostate gland are mainly controlled by the sex hormone testosterone .
Epidemiology
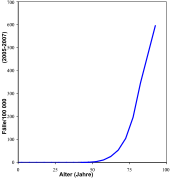
Prostate carcinoma is the most common cancer diagnosed in men in Germany and is the third most common cancer- related cause of death in men after bronchial carcinoma and colorectal carcinoma . Around 26 percent of all cancers that occur annually in men affect the prostate. This corresponds to an age-standardized incidence rate of around 110 per 100,000 males. According to current statistics for 2019, 60,700 men develop prostate cancer in Germany every year. The supposedly strong increase that has been observed over the past few decades is more due to improved diagnostic methods and a generally higher life expectancy than to an actual increase in the number of cases.
| year | 1980 | 1985 | 1990 | 1995 | 2000 | 2012 |
|---|---|---|---|---|---|---|
| Annual new cases in Germany (estimated) |
16,800 | 21,600 | 27,500 | 35,400 | 44,800 | 63,700 |
The annual mortality (total number of deaths) is around 12,000. According to a 2015 review study, the rate of "dormant" ( latent ) prostate cancer at autopsy of people who died from other causes rose from 5% in the 30-year-old age group to 59% in the 80-year old age group. A similar overview study, also from 2015, showed the same trend on a slightly different data basis.
There are large geographic and ethnic differences in the incidence: Black Americans have the highest incidence rate , while it is lowest among Asians.
The data for the worldwide incidence rates are not reliable because they are partly based on estimates and the diagnostic possibilities in the individual regions differ greatly. The GLOBOCAN survey, based on data from the International Agency for Research on Cancer (IARC), indicates a total of almost 680,000 new cases and around 220,000 deaths for 2002. According to this, the annual incidence rate is lowest in Central Asia with less than 3 / 100,000 inhabitants, the highest is recorded on the North American continent with over 160 / 100,000 inhabitants.
Risk factors and protective factors
genetics
The cause of the disease is largely unknown. The genetic disposition plays a role in the development of the disease (familial accumulation). Therefore, men whose fathers or brothers had prostate cancer are considered high-risk patients with about twice the risk of the disease. These men should start the early detection of cancer , which is usually only required from the age of 50, by checking the prostate-specific antigen from the age of 45. A study has now shown that adenocarcinoma of the prostate that occurs in young men is based on a different pathophysiology. It has been shown that the testosterone-binding androgen receptor has increased activity, which changes a number of genes and facilitates the development of carcinoma.
Way of life
The great differences in the frequency of illness among different ethnic groups are also attributed to their lifestyle habits, especially since the descendants of emigrants do not bear the risk of illness from their ancestors, but assume that of their new home country. A certain role is ascribed to nutrition.
The following correlation shows how important the lifestyle is: Although the prostate cancers detected in autopsies occur around the world with roughly the same frequency, the actual occurrence ( incidence ) of prostate cancer is characterized by large geographical differences: while in the USA about 120 (whites) or 200 (Colored) and in Germany 100 out of 100,000 inhabitants get prostate cancer, in Japan it is only about twelve out of 100,000 inhabitants. However, if the Japanese move from Japan to California and become "Americanized", their prostate cancer risk increases significantly and approaches US levels. The Asian diet and lifestyle are primarily blamed for the significantly lower incidence. Current studies indicate the importance of a plant-rich diet and certain plant substances in relation to the prevention, progression and survival of prostate cancer.
No evidence could be found that sterilization ( vasectomy ) increases the risk of disease. However, the testosterone level is a sure influencing factor , since the tumor cells depend on the stimulation by androgens : eunuchs do not develop prostate cancer. In contrast, the benign enlargement of the prostate ( benign prostatic hyperplasia ), which is common in advanced age, and prostate inflammation , whether chronic or acute , are not independent risk factors . The current data on possible cancer promotion through increased levels of the tissue hormone IGF-1 (insulin-like Growth factor).
According to a study published in 2003, frequent ejaculating in younger years is said to reduce the risk of the disease. Australian scientists compared data on sexual practices from 1,079 prostate cancer patients with those from 1,259 healthy men between the ages of 20 and 50. Their result: Twenty-year-olds who ejaculate more than four times a week reduce their risk of prostate cancer by a third. Methodologically, the cause-effect relationship has not been clarified; it could be that men with healthy, efficient genital tracts ejaculate more often and that genital health is the root cause, which is why they do not get sick as often later. In contrast, previous studies had linked frequent sexual contact with a significantly increased risk of prostate cancer. However, according to the Australian researchers, this could be due to the higher risk of infection . If you look at the total number of ejaculations, they would have a protective effect because the frequent formation of seminal fluid causes carcinogenic substances to be washed out of the prostate. In this way, the prostate cells would also be stimulated to mature, which could make them less susceptible to carcinogens . A study published in 2016 (database of over 30,000 men) also concludes that the risk of developing the disease decreases with increasing ejaculation frequency. However, the authors also admit that sexually active men may go to preventive examinations less often, so that potential diseases in this study group go undetected more often. Various physiological reasons are suggested for a reduced risk of disease. Frequent ejaculation can influence the function and division speed of the marginal epithelial cells, so that early tumorigenesis is hindered.
Another possible risk factor is lack of sun. In North America and Europe there is a noticeable north-south divide in the incidence of prostate cancer, which can currently only be explained by the different levels of sun exposure of the male population. Cholecalciferol (vitamin D 3 ), which has a chemopreventive effect , is probably responsible for this. Most of the cholecalciferol available in the body is formed in the skin through UV-B radiation . A lack of vitamin D significantly increases the risk of prostate cancer. However, it is still unclear which blood levels of calcidiol and which UV dose are optimal. The “lack of sun theory ” is to be viewed with reservations, because on the other hand it has been scientifically proven that too much sun exposure is a risk factor for the occurrence of skin cancer . However, the benefits of tanning may outweigh this.
nutrition
There is controversial debate as to whether diet has an influence on the incidence of prostate cancer.
It is the subject of much controversy whether the risk of prostate cancer increases with the consumption of meat, especially red meat. There are data from various case-control and cohort studies from the late 1990s that could lead to the conclusion that the consumption of red meat increases the risk of prostate cancer by at least 30%. In a similar period of time, a prospective study of 51,529 men in the health professions showed that the risk of metastatic prostate cancer increases by 60% with the consumption of red meat (relative risk = 1.6 for the highest quintile, compared with lowest, 95% confidence interval = 1.0–2.5). Animal fats resulted in a 1.63 fold risk. Apparently, the classic carcinogens such as heterocyclic amines (HMA) and polycyclic aromatic hydrocarbons (PAH), which are produced when meat is roasted, braised or grilled, also play a role. High meat consumption is said to not only increase the risk of prostate cancer, but has also been shown to increase the risk of breast and colon cancer. Epidemiological connections have also been proven with kidney , lung and pancreatic cancer . In a cohort study of around 1,000 patients, it was shown that the likelihood of recurrence in patients who have already had an operation decreases when they switch their diet from red meat and eggs to poultry and fish.
In later analyzes a not so uniform picture emerged. The World Cancer Research Fund (WCRF) published a report on “Diet, Physical Activity and Cancer Prevention: A Global Perspective” in 2007; the data on prostate cancer was last updated in 2014 (in the Continuous Update Project , CUP). On the basis of the data available at the time, the authors came to the conclusion that statements about the risk of illness and the consumption of red and processed meat are only true to a limited extent at best. A meta-analysis carried out in 2015 confirmed this: The consumption of (red) meat does not lead to an increased risk of developing prostate cancer. The hypothesis that HMAs and the way the meat is heated at high temperatures are risk factors for prostate cancer could not be shown in the meta-analysis. A meta-analysis published in 2016 also showed that increased consumption of poultry meat slightly reduced the risk of fatal prostate cancer, while increased consumption of eggs increased this slightly. In addition, no significant effects were observed after consuming fish - regardless of the degree or stage of the disease.
- Food supplements
In connection with the chemopreventive effect of vitamin D 3 , possible negative effects of high calcium intake were also considered. A high calcium intake reduces the body's own cholecalciferol production . Two prospective cohort studies showed that the consumption of calcium doses> 2000 mg per day is associated with an increased risk of prostate cancer. Two other prospective cohort studies found no association for calcium doses of 1330 and 1840 mg per day. A deficient production of vitamin D 3 is suspected as the background for the increased risk . Milk and many dairy products are good sources of calcium . Sun u. a. found a review of nine prospective studies in 2005, five of which established a connection between the consumption of dairy products and the risk of prostate cancer. The extent to which calcium consumption contributes to the risk in relation to fat consumption from milk and dairy products is unclear. A meta-analysis by Gao et al. a. came to the conclusion in 2005 that high intake of dairy products and calcium could be linked to a slightly increased risk of prostate cancer. According to Severi et al. a. however, to “relatively weak statistical evidence” and a “very small effect size”. In addition, the critics of the meta-analysis were able to provide study data that did not support the conclusion of Gao et al. a. fit. According to Michael de Vrese from the Max Rubner Institute , the prostate cancer risk from milk consumption has not yet been conclusively assessed.
A benefit of so-called phytohormones from soy has not been proven, and phytochemicals given in isolation do not show any effectiveness. The DKFZ also warns against examples in advertising whose effectiveness has not been proven (e.g. lycopene from tomatoes). The subject of numerous studies is pomegranate juice . The phytochemical plant substances contained in it are effective in reducing oxidative stress and modulating inflammatory pathways. In 2016, numerous studies were evaluated in a meta-analysis that examined the administration of various extracts and plant substances (e.g. lycopene, isoflavones or pomegranate juice) to prostate cancer patients. The PSA value (prostate-specific antigen) was evaluated as a central marker for possible therapeutic success. After application of the evaluation criteria, five of several studies were approved for the meta-analysis, including two placebo-controlled studies. The authors came to the conclusion that - although these five evaluated studies were of good quality - the case numbers were predominantly small and the investigation periods were short. The general problem is that no high-quality studies on the subject were published until 2016. Overall, the plant substances given are well tolerated and safe. There is also evidence that this could affect PSA dynamics. However, the data situation is too limited to make statements about a possible form of therapy (as a supplement or as a replacement for the classic form of therapy). This also applies to 2019.
General
The report published by the WCRF / CUP in 2014 showed that, in contrast to diet, body dimensions (height and amount of body fat) have a “convincing influence” on the risk of developing prostate cancer. The studies used by the WCRF-CUP were examined in a meta-analysis in 2016. Here, however, the authors came to the conclusion that there was no convincing evidence for a connection between diet, body size and physical activity on the risk of prostate cancer. While these factors may influence the risk, major effects cannot be inferred.
Molecular biological aspects of prostate cancer
Like all neoplasms , prostate cancer is ultimately based on the irreversible change in the genetic make-up of a single cell. All cancer cells are descendants ( clones ) of this cell. A further complicating factor is that their genetic makeup continues to change, since the physiological processes that would repair DNA damage or cause mutated normal body cells to die ( apoptosis ) do not take effect in cancer cells. Over time, therefore, a mosaic of cells with differently modified genomes develops. Clinically, this corresponds to an inhomogeneous appearance of the tumor and an increasing "malignancy" over the years.
In contrast to many other epithelial malignancies , prostate carcinoma does not have a typical adenoma-carcinoma sequence or a specific mutation pattern. Instead, there are very heterogeneous genomic changes in the form of point mutations in different places, loss of alleles or entire chromosomes and sometimes polyploidy . Often, however, at a later (metastatic) stage, at least classic tumor suppressor genes such as TP53 are also affected by deletions or mutations. Changes in the gene coding for the androgen receptor seem to play an important role . At least 17 genetically different prostate cancer cell lines are currently known.
Symptoms
In the early stages, prostate cancer is almost always asymptomatic. In advanced carcinoma, the main complaints result from the blockage of the urine flow and thus consist of disorders of micturition (urination). Delayed onset, prolonged micturition with a weak stream, dripping or interruption of the urine stream while urinating are possible. Often, residual urine remains in the bladder. Irritant complaints are an increased or predominantly nocturnal urge to urinate ( nocturia ), frequent passing of small amounts of urine ( pollakiuria ), difficult urination ( dysuria ) or painful urination (alguria). Erectile dysfunction can result from pressure damage to nerves in the sacrum area . Visible blood in the urine ( hematuria ) or ejaculate ( hematospermia ) is rare.
In an advanced stage with metastasis, symptoms can also arise primarily from the metastases, while prostate cancer remains clinically silent ( occult cancer ). The most common here are pain in the spine and pelvis. With strong metastatic penetration, spontaneous bone fractures without trauma, so-called pathological fractures , can occur. Since the spine is often the first line of sowing, complex neurological deficits due to spinal cord injuries such as paraplegic syndromes or the cauda equina syndrome are not uncommon. Lymph node metastases can lead to lymphedema of the legs or scrotum . Overall, however, bone metastases are the predominant manifestation of the disease for most patients and are also the main cause of the morbidity and mortality of prostate cancer.
Advanced metastatic tumors usually lead to general symptoms such as anemia and unwanted weight loss .
Diagnosis
Physical examination
With the digital rectal examination, an experienced examiner can already make the suspected diagnosis, since the tactile findings are typical. However, in this way the rarer tumors in the anterior organ regions may be overlooked and the more advanced stages are generally recognized.
Imaging
Ultrasonic
Ultrasound examination ( transrectal sonography ) allows for more precise localization and size determination . Tumors with a diameter of ten millimeters or more can be reliably found, but only around 20% of the smaller ones.
HistoScanning is an ultrasound-based method that has not yet been fully evaluated, but it also promises significantly higher sensitivity and specificity than conventional ultrasound or digital rectal examinations.
Magnetic resonance imaging
The magnetic resonance imaging has proved to the transrectal ultrasound as roughly equivalent, however, is much more complex and costly to implement. The primary tumor is shown in the T 2 weighting in the MRI as a circumscribed dark region with a relatively light surrounding zone.
Modern multi-parametric MRT examinations with endorectal coil, diffusion (ADC) or DTI , perfusion (DCE) and 1H spectroscopy have an average sensitivity of 86% and a specificity of 94%. Negative predictive value is 95%. Due to the rapid development of MRT technology, the numbers are potentially significantly higher, depending on the technology used and the level of knowledge of the radiologist. The multi-parametric MRT is therefore very powerful and harmless to health, but quite complex.
Positron emission tomography
In addition, positron emission tomography (PET) with 18 F- choline (radioactively labeled tracer ) is becoming more and more established . With 18 F-choline PET / CT in particular , prostate cancer tissue can be reliably differentiated from benign hyperplasia , chronic prostatitis and healthy prostate tissue.
Laboratory diagnostics
Established procedures
A laboratory parameter often used in prostate cancer diagnostics is the prostate-specific antigen (PSA). It is specific for the prostate, but not for a tumor disease, but can also be used in the case of inflammation, for example in the case of a urinary tract infection , benign prostate enlargement , urinary retention or, sometimes for several days, after any mechanical stress in the pelvic area, e.g. from sport, v . a. Bicycling, sex, or medical procedures such as digital rectal exam (DRE), transrectal prostate ultrasound , or urinary catheters , may be increased. A value above 4 ng / ml is in need of clarification. A differentiation, if an infectious cause (prostatitis, urinary tract infection) has been excluded, allows the complexed and free proportion of PSA to be determined. If the proportion of free PSA is below 10%, prostate cancer is probable, a proportion of 10–20% is considered a gray area, and more than 20% can be assumed to be benign. The PSA is the decisive parameter in tumor follow-up care after surgery or radiation therapy. The same importance is attached to it in the follow-up of an antiandrogenic treatment (hormone therapy).
The prostatic acid phosphatase (PAP) today only of secondary importance.
Not established practices
Furthermore, a protein pattern diagnosis and the PCA3 test are available, urine is used as material. Both procedures are not established, the costs are usually not covered by the statutory health insurance in Germany.
histology
Proving of prostate cancer is only the detection of cancer cells in a biopsy tissue sample taken. The biopsy is performed transrectally under ultrasound guidance. At least three tissue samples are taken from each side of the organ with a hollow needle. With a large prostate, the number of biopsies should naturally be higher. A pathologist examines the prostate tissue and makes his diagnosis.
However, the value of this so-called systematic biopsy is very low. Even with a sample count of 12, only about 25-50% of actual cancer cases are detected. Even increasing the number of samples to 24 increases the rate only insignificantly. In addition, the risk of an incorrect classification of the dangerousness of an identified cancer infestation is considerable with approx. 20-50%.
Diagnostics to determine the stage of a proven disease
If the diagnosis of “prostate cancer” is confirmed, a staging , known as staging , is required. Here it is determined whether the tumor has already spread or whether it is a carcinoma limited to the prostate.
The necessary examinations include an ultrasound examination of the organs in the abdomen, especially the liver , kidneys and lymph nodes , as well as an X-ray examination of the lungs. A skeletal scintigraphy is performed to rule out bone metastases depending on the PSA level. In addition, a computed tomography of the abdomen and lungs as well as an excretory urography of the kidneys with contrast agent to assess the course of the ureter and a cystoscopy can be performed.
No imaging method (CT, MRI with endorectal coil, transrectal ultrasound examination) has been able to establish itself to date for the exact assessment of growth beyond the organ (stage T3) in prostate cancer confirmed by punch biopsy .
Pathology and histopathology
Punch biopsies and surgical specimens are examined by a pathologist .
Macroscopic pathology

Macroscopically (with the naked eye) the carcinoma usually appears yellow or whitish, relatively homogeneous and indistinct. The majority of it starts from the epithelia of the peripheral gland parts , about 85% in the rear (rectal) parts of the prostate gland and spreads in the outer zones of the organ. To lay the urethra with complaints of urination it comes late, usually after the organ capsule was already broken.
Extensive stage T3 / T4 cancers can infiltrate the seminal vesicles , urinary bladder , pelvic floor, or rectum . The metastasis is initially lymphogenic (via the lymphatic channels ) into the local lymph nodes. Hematogenous sowing often takes place later (via the bloodstream). Typical are bone metastases in the pelvis , sacrum and lumbar spine , thighbones , thoracic spine and ribs . In 80% to 90% of patients with metastatic prostate cancer, the axial skeleton (spine, pelvis and structures on or near the trunk) are affected. These are almost always osteoblastic (bone-forming). Distant metastases in the lungs and liver due to hematogenous seeding (via the bloodstream ) are less common.
Microscopic pathology
97% of all prostate tumors are adenocarcinomas , which means that they arise from degenerate gland cells. A transition stage to manifest cancer is known as prostatic intraepithelial neoplasia (PIN) and corresponds to a carcinoma in situ . In the actual carcinoma, different histopathological growth patterns occur, also at the same time: glandular or acinar (gland-like), cribriform (sieve-like) and solid. The degree of dedifferentiation is the basis of the grading . 40–50% of tumors are multifocal at diagnosis.
The non-adenocarcinomas (less than three percent) are mostly of urothelial origin, i.e. they are derived from the transitional tissue of the urethra or bladder (see bladder cancer ). Sarcomas ( leiomyosarcoma , fibrosarcoma , rhabdomyosarcoma ) of the stroma in adults are extremely rare . Rhabdomyosarcomas are the most common form of prostate cancer in children, but are not regarded as prostate cancer, as in adult patients, but rather as soft tissue sarcoma .
Tumor grade and Gleason score
During the microscopic examination of the removed tissue, the biological properties of the tumor are determined more precisely and its malignancy is determined. A special classification scheme (G: histopathological grading ) describes how strongly the tumor cells differ microscopically from normal, "mature" cells.
To grade prostate cancer, the Gleason score is used in accordance with the S3 guidelines for the early detection, diagnosis and treatment of prostate cancer . According to the histological picture in the punch biopsy, the most poorly differentiated and the most frequently occurring tumor tissue is assessed and added up with scores between 1 and 5 (together between 2 and 10). If an operation has already been performed, the most common and the second most common Gleason grade in the entire prostate is added. The two added Gleason grades must be stated in the correct order and in the punch biopsy as a percentage (of the total tumor and the total tissue obtained). A Gleason score 3 + 4 represents a better differentiated grade than Gleason score 4 + 3, although the sum is the same. Their sum is therefore interpreted differently in the punch biopsy than in the surgical specimen. A special feature is that Gleason grades 1 and 2 cannot be diagnosed in the punch biopsy.
The Gleason score is an important prognostic factor in addition to the tumor size and the presence of lymph node and distant metastases ( TNM classification ) . Sometimes additional information about grading (e.g. DNA cytometry ) can be helpful. A Gleason score can only be established in the presence of a previously untreated adenocarcinoma of the prostate; a different grading system is used for urothelial carcinoma and neuroendocrine tumors.
DNA cytometry
If the Gleason grade is low, the sample taken as a biopsy can also be examined for the so-called ploidy grade . This procedure is called DNA cytometry and is used by individual chief urologists at clinics and pathologists. In the case of a low degree of malignancy of the carcinoma, it can provide further information on the aggressiveness of the tumor cells and can thus be used as an aid to therapy decision-making.
Tumor stages
Stages of manifestation
According to Mostofi, a distinction is made between the following stages of manifestation:
| Stage of manifestation | description |
|---|---|
| Manifest carcinoma | The primary tumor causes symptoms or can be clinically diagnosed ( palpable ). |
| Occult carcinoma | The metastases can be diagnosed symptomatically or clinically, but not the primary tumor. |
| Incidental carcinoma | Incidental finding during the examination or operation with a different question. |
| Latent carcinoma | Accidental autopsy finding in someone who died from another cause. |
Staging (TNM system)
In assessing the tumor stage according to the TNM system is the size and local extent of the prostate tumor (T), lymph node involvement (N, of Engl. Node , Node ') and metastases (M) into account. The numbers behind the letters stand for the size and extent of the primary tumor (T1-T4), the presence of affected lymph nodes (N0-N1) and the presence and distribution of distant metastases (M0-M1c). A fairly good predictor of dedifferentiation, local invasion of neighboring organs and the likelihood of distant metastasis is also the tumor size. The “threshold of curability”, i.e. the size of the tumor up to which one considers treatment with the aim of healing (curative treatment) to be possible, is set at 4 cm³. If this threshold is exceeded, healing is usually no longer possible. However, smaller tumors can already have metastasized and thus evade curative treatment.
| stage | description |
|---|---|
| Tx | No statement can be made on the extent of the primary tumor. |
| T1 | The tumor is small and cannot be felt. It is found by chance during prostate surgery because of BPH or elevated PSA levels (incidental tumor). |
| T1a | The tumor affects less than 5% of the tissue. |
| T1b | The tumor affects more than 5% of the tissue. |
| T1c | The tumor was diagnosed through a needle biopsy. |
| T2 | The tumor is still within the prostate capsule. |
| T2a | The tumor affects less than 50% of a side lobe. |
| T2b | The tumor affects more than 50% of a side lobe. |
| T2c | The tumor affects both side lobes. |
| T3 | The tumor has spread beyond the prostate capsule. |
| T3a | The tumor has spread through the prostate capsule without affecting the seminal vesicles . |
| T3b | The tumor has spread over the prostate capsule and affects the seminal vesicles. |
| T4 | The tumor has affected neighboring structures (infiltrated) or is fixed (immovable). |
| Nx | No statement can be made on regional lymph node metastases. |
| N0 | No metastases in the regional lymph nodes. |
| N1 | Metastases in the regional lymph nodes. |
| M0 | No distant metastases detectable. |
| M1 | The tumor has formed distant metastases. |
| M1a | Metastases in other lymph nodes (non-regional lymph nodes). |
| M1b | Metastases in the bones. |
| M1c | Metastases in other organs and / or structures. |
The treatment is based on the classification in the TNM scheme. The prognosis can also be estimated using additional parameters.
Another scheme of staging is that of Whitmore-Jewett (modified from Hopkins ). Here grades A (microscopic carcinoma, almost always incidental - corresponds to T1), B (macroscopic, limited to the prostate - corresponds to T2); A distinction is made between C (transcending organs, limited to the small pelvis - corresponds to T3 / 4M0) and D (with distant metastases - corresponds to T1-4M1). This scheme is preferred in Anglo-American countries, but is not common in Germany.
Prognostic factors
The most important factors for assessing the prognosis are the tumor stage according to TNM , the PSA blood level and the differentiation of the tumor, the Gleason score .
therapy
Active surveillance, radiation therapy or surgery: results of the UK ProtecT study
Until recently it was not clear whether surgery or radiation therapy should be regarded as equivalent; there was no direct comparison of the two therapy methods. It was also unclear whether treatment (regardless of which one) had an advantage over active monitoring. The PREFERE study carried out in Germany on this question was closed in autumn 2016. Almost at the same time, however, a British study was published (the so-called ProtecT study), in which three methods were compared, namely active monitoring, surgery and radiation therapy (external radiation with a linear accelerator, combined with six-month anti-hormonal therapy).
Important results of the ProtecT study are:
- Active monitoring is possible. There is no increased death rate in the first ten years after diagnosis.
- However, metastases increased in patients who were initially only monitored and not treated immediately. Since this is disadvantageous in the long term, therapy should be considered in younger patients and / or in tumors with a risk constellation.
- In the patient group that was initially only actively monitored, half of the patients had to be treated with radiation or surgery within ten years because the cancer had progressed measurably. With active monitoring, the following applies to further treatment: postponed, but not finally canceled.
- An important finding concerns the comparison of surgery and radiation therapy. Both procedures were equally good with regard to the control of the tumor disease (the trend was that radiation was even slightly better). Both therapy methods were well tolerated; However, the side effects of radiation therapy were significantly lower than those of surgery, although the radiation had not yet been carried out with the more precise radiation techniques that are customary today.
The two original publications of the study, a summary by the Austrian Cochrane , a comment from the New England Journal of Medicine and a press release from the German Society for Radiation Oncology are freely available. The statements only apply to patients with the corresponding criteria, especially a tumor with a less aggressive classification.
Further results on the question of which treatment method offers the best chances of success come from the so-called “Grimm Study” published in 2012. In this meta-analysis, 11 methods (surgical procedures, radiation methods, HiFu, etc.) were examined and compared on the basis of data from approx. 52,000 patients with localized prostate cancer. The best results in all three risk groups were always provided by an irradiation method. The most pronounced advantage in terms of long-term tumor control was shown by radiation compared to surgery in patients with high-risk prostate cancer.
surgery
For localized prostate cancer (T1 / 2) and a good constitution, radical (complete) surgery of the prostate, in which the prostate, seminal vesicles and regional lymph nodes are removed, is the classic method. This so-called "radical prostatectomy" (RPE) can be performed in four different ways:
- as a retropubic radical prostatectomy (RRP)
- as a radical perineal prostatectomy (RPP) through an incision on the perineum (between anus and scrotum)
- minimally invasive - laparoscopic
- robot-assisted (RARP)
RRP and RPP are performed extraperitoneally , i.e. without opening the abdominal cavity . RPP is less time consuming and less bleeding than RRP, but the access is relatively narrow. One drawback is the lack of removal of the pelvic lymph nodes at the RPP, so that a second engagement means of laparoscopic pelvic (on the tank -related) lymphadenectomy is sometimes necessary. Individual centers have published techniques that enable lymph node removal.
In the minimally invasive method, the prostate is operated on using a laparoscopy technique through a few small incisions. The main advantage here compared to “open” surgery is the mostly lower blood loss and the small wounds.
A further development of the "minimally invasive" technique is the robot-assisted prostatectomy. Here, the laparoscopic technique is carried out with the help of a surgical robot. The robot is remote-controlled by the surgeon using a special console and does not make any independent movements. The advantage for the surgeon compared to standard laparoscopy is the almost unlimited mobility of the instruments, the excellent view (three-dimensional via a double optic system) and the delicacy of the movement, as the robot compensates for the natural trembling of the hand. The disadvantage of robot technology compared to "standard laparoscopy" is the comparatively high acquisition and maintenance costs for the hospital, so that so far only a few centers in Germany have been able to use this technology. In some departments, a private additional payment is required to cover the material costs the health insurance companies currently do not cover this. An improvement of the surgical results through the robot technology could not be scientifically proven so far.
If the operation succeeds in completely removing the tumor, a cure is possible and the prognosis for long-term survival (five years or more) is between 80 and 90 percent. According to the world's largest urological study (52,000 patients) for the treatment of localized prostate cancer, surgery in patients with a low or medium risk of radiation is slightly inferior after five years. Radiation showed the greatest advantage compared to surgery, especially in patients who are at high risk of developing metastases.
The risk of dying from the operation or its consequences is around 1.5 percent. A relevant risk of the operation is primarily the risk of long-term urinary incontinence and is between 2 and 40 percent depending on the study. In a British study from 2006, for example, 27.8% of patients required up to one insole per day. Loss of sexuality through erectile dysfunction as a result of an injury to the cavernous nerves (branches of the parasympathetic pelvic innervation) occurs in around 80% of cases. Injuries to the obturator nerve , which can occur as part of the lymph node removal, or the rectum are rare. A lymphocele occurs in five to 20 percent of cases due to the removal of the lymph nodes . As a long-term consequence, in about 32% of cases, a narrowing of the connection point between the urethra and bladder ( anastomosis ) occurs, the so-called anastomotic stricture.
A number of centers offer a "nerve-conserving" surgical method (according to Patrick Craig Walsh ), in which the cavernous nerves that run in the immediate vicinity of the prostate are spared. The risk of postoperative erectile dysfunction can thus be reduced to ten percent (in young patients) to 50% (in older patients), but the success depends significantly on the surgeon's experience. In addition, the technology harbors the risk of insufficiently radical clearance of the tumor. This increases the long-term risk of local recurrence.
The type of surgical method used does not seem to have any significant influence on the oncological results (survival rate, recurrence-free survival, etc.) of the procedure, rather the experience of the individual surgeon seems to be decisive.
Radiation Therapy (Radiation)
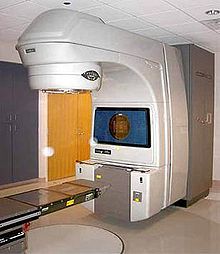
An equivalent alternative to surgery with fewer side effects is radiation therapy alone for locally limited prostate cancer with low and medium risk factors (see prognostic factors). In a comparative study, over 1,600 patients were treated with either radical surgery, percutaneous radiation therapy, or a wait and see strategy. After a follow-up period of ten years, there was no advantage of one method in terms of cancer-specific survival, i.e. the probability of dying from prostate cancer. In the treatment group with a wait-and-see strategy, there was a doubling of the likelihood of remote settlements (metastases). With regard to the most important side effects for patients, incontinence and sexual function (potency), prostate surgery often resulted in a significant deterioration in both functions. Radiation therapy showed significantly more intestinal side effects. There were no differences between the groups with regard to quality of life.
Radiation therapy can be used for different groups of patients, for example after the recurrence of an operated tumor, for metastatic tumors or as a method that competes with surgery. It takes place either from the outside (percutaneous radiation therapy ) or by “spiking” ( brachytherapy ) (from the Greek brachýs = near) the prostate with radioactive material. Percutaneous irradiation is carried out using a linear accelerator . In brachytherapy, a distinction is made between the implantation of “seeds” (radioactive particles with a short half-life, also known as “low-dose-rate brachytherapy”, LDR brachytherapy) and “afterloading” (“high-dose-rate brachytherapy”, HDR- Brachytherapy), whereby a radioactive source is inserted into hollow needles that are stuck in the diseased tissue to be irradiated and then removed again for a time calculated in advance (with a special planning program via PC). HDR brachytherapy can be combined with percutaneous radiation or used as a single therapy. LDR brachytherapy (“seeds”) cannot be combined with other forms of radiation; this therapy is a direct alternative to surgery. The advantages of radiation are the elimination of the risk of surgery and the possibility of outpatient treatment. Disadvantages are side effects such as temporary diarrhea and indigestion. In a meta-analysis, the influence of brachytherapy alone or in combination with percutaneous radiation in terms of tumor-free survival was rated as superior to all other compared methods. The aim of this so-called “Grimm Study”, which was published in 2012, was to compare all of the then common treatment methods for non-metastatic prostate cancer. The best results in all three risk groups were achieved by brachytherapy alone or in combination. Combined brachytherapy compared to surgery in patients with high-risk prostate cancer showed the most pronounced advantage in terms of long-term tumor control. The study was criticized for the fact that new processes such as B. dose-escalated percutaneous irradiation or hypofractionated proton therapy, which meanwhile yielded at least equivalent results, was not included in the meta-analysis. In order to determine the significance of the operation again in direct comparison with radiation procedures, the so-called “PREFERE” study was launched in 2013, but had to be discontinued in 2016.
In a small number of patients, after radiation therapy for prostate cancer, permanent damage to the intestines (radio proctitis, radiocolitis) and urinary bladder (radiocystitis) occurs. Loss of limb stiffness (erectile dysfunction) and a disruption of the sphincter muscle function of the anus or the urinary bladder have also been observed in a few patients after irradiation for prostate cancer. The radiation exposure of adjacent organs can be reduced by using so-called gold markers, so that the risk of side effects is also reduced. These three "seeds", which are deposited in the prostate before the start of radiation therapy, allow the prostate to be indirectly localized during each radiation session (so-called Image Guided Radio Therapy , IGRT), so that more targeted radiation is possible with a smaller "safety margin". So far, however, this has been an individual health service .
A new therapeutic method is high-precision irradiation of the prostate. The entire radiation dose is administered in just five treatment sessions over a period of around one and a half weeks. The US Society for Radiation Therapy (ASTRO) already sees this method as an alternative to the standard procedure. In Germany, this therapy is currently being offered in a clinical study approved by the Federal Office for Radiation Protection at the University Hospitals in Kiel, Lübeck, Rostock and Frankfurt and carried out with the radiation robot Cyberknife (in cooperation with Saphir Radiosurgery ).
If, at the time of diagnosis, it has already spread to other organs, the disease is usually no longer curable. However, radiation therapy can at least delay the spread of the cancer. The main application here is the irradiation of bone metastases, which stabilizes endangered bone areas and thus contributes significantly to mobility and freedom from pain in patients with metastatic tumors.
Proton therapy
A new form of radiation therapy is treatment with protons. The physical phenomenon of the bragg peak is used here , in which the radiation energy is only released at a predetermined point in the body. The advantage of proton therapy lies in the accuracy of treating the cancer focus effectively and yet protecting the closely adjacent healthy tissue. In March 2014, 5-year long-term results of proton irradiation in prostate cancer were published for the first time. In the three studies summarized here, a total of 211 patients with non-metastatic prostate cancer were treated with protons and followed up for an average of 5 years (5.2 years). The five-year tumor control rate (cPFS) was 99% for low-risk and medium-risk prostate cancer and 76% for high-risk prostate cancer. The rate of severe side effects according to the international classification guideline CTCAE version 3.0 (or current version 4.0) was 1% (e.g. bleeding of the rectum) in the intestines (e.g. incontinence) and around 1% in the urinary tract (e.g. incontinence) 5.4% (1%).
Prostate Rectum Hydrogel Spacers

For prostate cancer, radiation therapy , either in the form of brachytherapy or external radiation therapy, is one of the most commonly chosen treatments.
Although radiation therapy for prostate cancer is more effective than other treatment methods, injury to the rectum from radiation (radiation-induced proctitis) is dangerous because the rectum is located directly behind the prostate . The damage to the rectum from radiation can lead to diarrhea , rectal pressure, mucosal production, and bleeding. It can take six to twelve months for these symptoms to appear. However, symptoms can appear at any time for up to 30 years after radiation therapy.
An emerging strategy to mitigate this rectal damage is to place a spacer between these two structures, effectively pushing the rectum away from the high-dose radiation field. Researchers were able to make these spacers by placing various materials in the potential space between the prostate and the rectum.
Injection of hyaluronic acid into the space between the prostate and rectum resulted in an additional space of more than an inch with no discomfort from tenesmas or the feeling of rectal filling. In patients treated with hyaluronic acid, rectal mucosal damage (5% vs. 36%, p = 0.002) and no macroscopic rectal bleeding (0%) occurred significantly less frequently in proctoscopic examinations compared to patients who had not received a hyaluronic acid spacer. vs. 12%, p = 0.047). A similar study was conducted using collagen injections in the same room. This method created a space averaging 1.1 cm between the prostate and anterior rectum, resulting in a more than 50 percent reduction in rectal radiation dose during radiation therapy to the prostate.
Researchers evaluated an absorbable balloon placed in the space between the prostate and rectum and found a space nearly two centimeters with a calculated reduction in rectal radiation. Studies of absorbable hydrogel injected into the same space (currently in clinical trials in the US) resulted in an additional one centimeter space with a 60% reduction in rectal radiation (V70).
While the prostate - rectum spacers are still in the clinical trial phase, there is great promise that they will not only help reduce unintended rectal exposure and its complications, but also enable higher doses in radiation therapy for cancer patients. Thus, the patient's survival rate can be improved. In addition, with a higher dosage per treatment, a complete treatment with fewer visits to the doctor can be realized, which is on the one hand more pleasant for the patient and on the other hand leads to considerable savings in medical costs.
In contrast to the USA, where the hydrogel is still in clinical testing, the hydrogel is already CE-certified in Europe and is available on the market in some countries.
High intensity focused ultrasound
One method that has been used in Germany since 1996 is high-intensity focused ultrasound (HIFU). The method is based on the fact that the entire prostate is heated from the rectum with directed ultrasound waves and the carcinoma is thus destroyed. To do this, the transducer is inserted into the rectum . The treatment takes place in one session, the hospital stay is only three to five days. In several studies with follow-up periods of up to ten years, the effectiveness and safety of the procedure have been proven. The HIFU therapy is used by more than 30 centers in Germany, the treatment costs are covered by the statutory health insurance companies as part of the DRG system . The procedure can be used both curatively and palliatively. In contrast to radiotherapy methods, it can be repeated in the event of a relapse, so it does not represent a “therapeutic dead end”. Due to the low stress on the patient, HIFU is particularly suitable for older patients as well as for patients who have other serious diseases in addition to cancer Suffer. According to the current S3 guideline on prostate cancer (version 2.0, 1st update 09.2011), however, “... HIFU therapy for locally limited prostate cancer is an experimental procedure. The HIFU therapy should only be used in the context of prospective studies. "
Therapy with HIFU has been offered for the first time in Austria at the Wels Clinic since August 2017.
Hormone therapy
Hormone therapy, also known as androgen deprivation therapy, is based on the dependency of prostate cancer on testosterone, which is usually present. It is used as a palliative therapy for metastatic tumors or as a supplement to other therapeutic measures such as B. radiation therapy. Hormone therapy in the form of hormone withdrawal can be carried out as (mostly reversible) chemical castration by administering GnRH agonists or antagonists . The aim is to lower the testosterone level to below 50 ng / dl. Since prostate cancer is mostly highly testosterone-dependent, both procedures usually lead to a significant decrease or standstill of the disease, so that the patient often has no cancer-related symptoms for years. Chemical castration using GnRH agonists can lead to a so-called flare-up phenomenon, a short-term, strongly accelerated course of the disease caused by a short-term increase in testosterone. This can be prevented by the short-term administration of antiandrogens such as cyproterone acetate , which is particularly recommended in cases of pronounced metastasis. Current study data indicate that treatment options should be carefully considered in patients with cardiovascular diseases. In addition, there are studies that have shown that GnRH antagonists are associated with a significantly lower risk of cardiovascular disease than the GnRH agonists.
The side effects of both castration procedures are mostly testosterone deficiency symptoms, including hot flashes, depressive states, anemia , muscle breakdown and, as a long-term effect, osteoporosis , with orchiectomy both the psychological stress caused by irreversible surgical castration and osteoporosis the hormone LH, which is not suppressed at the same time, becomes more apparent. In addition, erectile dysfunction occurs , which is usually not perceived as too bad, as the libido also decreases. In order to minimize these therapy-related side effects, intermittent hormone blockade (intermittent androgen deprivation) can be used in some patients , i.e. treatment-free intervals are deliberately planned. Another alternative for selected patients is the administration of an antiandrogen alone. In the course of therapy, the prostate carcinoma may become castration-resistant, i.e. despite testosterone that is suppressed to below 50 ng / dl, the disease will progress.
The uncritical use of hormone therapy as the sole therapy for localized, i.e. non-metastatic prostate cancer, does not lead to an extension of life.
chemotherapy
The chemotherapy was in prostate cancer long as ineffective. However, some patients (responders) with metastatic prostate cancer may benefit from chemotherapy. The response rate is around 20%. Chemotherapy usually has its place in the treatment of tumor recurrence and failed hormone therapy (so - called castration - resistant prostate cancer ). So far it has also been purely palliative. Therapeutic agents used are cyclophosphamide , doxorubicin ( adriamycin ), 5-fluorouracil , suramin and others; however, no survival advantage has yet been shown for these. In a work published in the renowned journal The New England Journal of Medicine in 2004 , a statistically significant survival benefit of a median of 2.5 months was demonstrated for the first time for those patients who received the drug docetaxel every three weeks . One first at the 2014 American Society of Clinical Oncology annual meeting . (ASCO) in Chicago, 2015 also in the New England Journal of Medicine. The published study also showed a survival benefit in patients with metastatic, hormone-sensitive prostate cancer through early docetaxel chemotherapy.
In 2011, new drugs were approved in Germany that showed good results in phase III studies for patients with advanced castration-refractory prostate cancer, even after administration of docetaxel-based chemotherapy: Cabazitaxel (trade name Jevtana), approved on March 17, 2011 and abiraterone ( Zytiga), approved on September 5, 2011. Further drugs are currently in development. Enzalutamide was approved in Europe in June 2013 under the trade name Xtandi. Both enzalutamide and abiraterone have now also been approved for use before chemotherapy in the castration-resistant stage.
Strictly speaking, these two drugs are not chemotherapeutic agents, but preparations with an antiandrogenic effect, i.e. a form of hormone therapy.
Palliative therapy
In the advanced stage, which no longer allows curative (healing) treatment, medical measures can still alleviate the symptoms and keep the quality of life at a reasonable level. Bisphosphonates , such as zoledronate , have been shown to be effective in reducing osteoporotic changes in the course of antiandrogen therapy as well as fractures caused by skeletal metastases . Opioids such as morphine or oxycodone are used to relieve bone pain . External radiation to bone metastases can also reduce pain for some time. The injection of certain radioisotopes , such as strontium -89, phosphorus -32, samarium -153 or radium -223 (Alpharadin, Xofigo), which accumulate in metabolically active bone metastases, has a similar effect (see radionuclide therapy of bone metastases ).
Active observation
Under certain circumstances, a strategy of "active observation" can (Engl. Active surveillance ) can be considered. In addition, watchful waiting , “ observation and waiting ”, is used. By definition, active surveillance in otherwise healthy patients who are suitable for curative therapy aims to postpone the timely curative treatment until a point in time at which the tumor biology or the patient's wishes may change, under close monitoring including regular control biopsies. In most cases, radical prostatectomy is chosen as a secondary treatment (48%). On the other hand, patients under watchful waiting are only treated palliatively if they have symptomatic progression.
Factors that make active observation seem sensible are, for example, the age of the patient, the other state of health and the so-called degree of ploidy measured with methods of DNA cytometry , a parameter for the chromosomal changes in cancer cells, which is also suitable as a follow-up control. DNA cytometry is a very inexpensive method that is paid for by health insurance companies and can be made from all types of biopsies ( punch biopsy and fine needle aspiration biopsy, FNAB for short). The simplest method of monitoring the progress is to regularly determine the PSA doubling time.
Patients who pursue an active observation perspective try to actively slow the progression of a cancer, for example by taking nutritional supplements. On the medical side, these endeavors are sometimes very critically observed and referred to as a " gamble ", but on the other hand they are also supported in order to avoid so-called overtreatment.
Patients who opt for the active surveillance method weigh up the side effects and benefits of early, invasive therapy (surgery, radiation). Through active observation under the supervision of a doctor, curative steps can still be taken in good time if these should become necessary due to the progression of the disease. A current review by Weißbach and Altwein comes to the following conclusion: 88 viewed studies on active surveillance confirm consistently high tumor-specific survival rates (99–100%) with treatment through active observation. All seven research guidelines for the treatment of prostate cancer since 2006 mention active observation as a treatment option for PCa with a low risk of progression in their recommendations. In this case, the National Institute for Health and Clinical Excellence (in Great Britain) recommends only "active surveillance" as a treatment strategy.
Under appropriate circumstances, the prognosis for active observation can correspond to that for conventional therapies without having to accept the side effects of other prostate cancer treatments. If this strategy is successful, the patient dies with, and not from, their cancer.
Experimental procedure
Irreversible electroporation
With the technology of irreversible electroporation (IRE), a non-thermal, tissue-selective ablation is possible in prostate cancer. IRE can be used both focally and over a large area. Potentially, due to the intrinsic tissue selectivity of the technique, maintaining potency and maintaining continence are very likely. IRE can also potentially be used for complicated relapses - even after radical prostatectomies and radiation therapy . For physical reasons, necrosis does not occur, which means that no pain or intraprostatic scarring or inflammation are to be expected. The first study on the use of IRE for the prostate dates back to 2007, so the procedure is still very new. There are no long-term data for this yet. The number of available studies in this regard is also thin. Prostate cancer treatments with IRE are carried out and further developed in Germany by university clinics and a private institute. Statutory health insurance companies have not yet covered the treatment costs (as of March 2013).
Photodynamic Therapy
Photodynamic therapy (PDT) is a therapy that is currently being tested. The basic idea is, the photoelectric effect between the generated light of lasers and a light-sensitive, which accumulate only on cancer chemotherapeutic agent ( photosensitizing agent ) to use, in order to cause a local cytotoxic effect. A 1 O 2 is released that can directly kill tumor cells through necrosis and / or apoptosis. WST-11 and 5-ALA are two photosensitizers located in the experimental stadium.
Advantages of this method are: repeatability, presumably low cost, real cell selectivity, minimal local damage. Disadvantages are: previously unknown side effects of chemotherapeutic agents, unknown influence of possible tumor hypoxicity .
Immunotherapy
A new therapeutic approach is “vaccination” with antigen-presenting cells that are loaded with a recombinant fusion protein ( PA2024 ) and stimulate the body's own immune response . Trials are currently underway with some success in patients with androgen-independent prostate cancer. These patients have so far been difficult to treat because they do not respond to hormone therapy. The mostly well-tolerated immunotherapeutic treatment ( cancer immunotherapy ) led in the context of the studies to significant remissions and to an increase in survival time. This treatment is not yet approved in Germany .
Aftercare
According to the “patient guidelines”, follow-up examinations are to be carried out as follows: Every three months in the first and second year; every six months in the third and fourth year and annually from the fifth year. Above all, this includes regular checks of the PSA value. Imaging methods are only used if there is a corresponding clinical suspicion of tumor recurrence or disease progression.
Forecast and impact on life expectancy
The prognosis for prostate cancer is relatively favorable for a malignant tumor or cancer. At least in the localized stage (the term “pet cancer” is also used here as an illustration) life expectancy is hardly shortened. It is assumed that ultimately less than a fifth of those suffering from prostate cancer also die from it, i.e. the mortality rate is less than 20%. The reason for this is the late manifestation and the comorbidity that usually exists at this time . In a study it could be shown that in the localized stage the prognosis when waiting (see the section “Active observation”) is no worse than with immediate therapy. The high mortality is therefore primarily due to the strikingly high prevalence in older people.
The diagnosis-specific five - year survival after diagnosis is 80 to 99% for tumors that are confined to the gland. In the case of scattered tumors, however, this value is significantly lower at around 31%. The prospect of a cure (i.e. destroying all cancer cells) is only given in the case of non-metastatic carcinomas, and there it is quite good under aggressive therapy: carcinomas limited to the prostate can be cured to almost 90%, those crossing the organ capsule to about 50%. In the case of locally limited prostate cancer, the chance of a cure is around 70%. The so-called Partin tables, in which a combination of PSA value, Gleason score and T stage are used to estimate the prognosis, are used to estimate the prognosis more precisely.
The statements made here apply to adenocarcinoma of the prostate. The rare neuroendocrine and small cell prostate carcinomas have a significantly poorer prognosis with an average survival time of one year.
early detection
Examinations for the early detection of prostate cancer are not a service of the statutory health insurance companies. However, many medical practices offer them as an individual health service (IGeL). Because of the uncertain results, the unnecessary uncertainty of the patient and the risk of overdiagnosis and overtreatment, the relevant medical societies expressly do not recommend examinations for early diagnosis, but only inform the patient - with advantages and disadvantages - that such examinations are possible. For general practitioners , it is even recommended that doctors do not address this option of their own accord, but only when a patient expresses a corresponding request.
In December 2019, the Institute for Quality and Efficiency in Health Care (IQWiG) published a preliminary report under the heading: Prostate cancer screening using a PSA test . The following conclusions were drawn:
“Prostate cancer screening using a PSA test harms significantly more men through overdiagnosis than it does men. In summary, it is therefore stated that the benefits of prostate cancer screening using the PSA test do not outweigh the damage. "
In an interview with Deutschlandfunk (broadcast on January 4, 2006) Robert Allan Weinberg , cancer researcher at the Whitehead Institute for Biomedical Research in Cambridge near Boston , pointed out that prostate cancer is six times more likely to be diagnosed in the United States than in Denmark . However, mortality is the same in both countries.
The National Cancer Institute of the USA has presented the following statistics for prostate screening (as of December 2015): If 1,000 men between the ages of 55 and 69 have a PSA test for ten years at intervals of one to four years, then
- 100–120 men will receive a false positive result, which may lead to fears and further diagnostic measures;
- 110 men will get a positive result (a correct diagnosis) for prostate cancer;
- 4–5 of men with correctly diagnosed prostate cancer will die of complications from prostate cancer within the 10-year period despite screening;
- in the best case scenario, the screening prevents death;
- At least 50 of the men screened will develop complications from the treatments, including erectile dysfunction, incontinence and, less commonly, cardiovascular disease
history
The oldest documented case of metastatic prostate cancer was diagnosed on the basis of typical bone changes in a 2700 year old skeleton of a Scythian prince in Siberia . The discoverers were researchers at the University of Göttingen around Michael Schultz.
The prostate was first described by the Venetian anatomist Niccolò Massa in 1536. Andreas Vesalius published the first illustration two years later. Nevertheless, prostate cancer was unknown until 1853. It was considered a rare disease in the 19th century due to poor diagnostic options and lower general life expectancy. The first orchiectomies had been attempted as early as 1890, but with modest success. The first surgical interventions on the gland itself were aimed at improving urination in the case of urethral obstruction. The first radical prostatectomy was performed in 1904 by Hugh Young at Johns Hopkins Hospital . In the middle of the 20th century, palliative transurethral resection (via the urethra) was introduced to eliminate urethral obstruction. Radical retropubic prostatectomy was developed by Patrick Craig Walsh in 1983 .
In 1941 Charles Brenton Huggins published his study results in which he used (chemically coupled) estrogens to inhibit testosterone production in patients with unresectable cancer. The discovery of this "chemical castration" earned him the 1966 Nobel Prize in Physiology or Medicine .
Radiation therapy was developed in the early 20th century and initially consisted of the implantation of radium implants. Percutaneous radiation has been performed since the middle of the century. The first description of brachytherapy dates back to 1983.
Veterinary medicine
For pets prostate tumors are much less common than found in humans. In cats so far only five cases of the disease have been described. Dogs are the most common . Here around 0.2–0.6% of all neoplasms are tumors of the prostate. Among the neoplasms of the male urinary and reproductive organs in dogs, the proportion is six percent. Dogs of medium to large breeds with an average age of eight to ten years tend to get sick. Castration does not reduce the incidence of the disease; there is rather evidence that the disease occurs more frequently in neutered males. In terms of differential diagnosis, the disease must be differentiated from the benign prostate enlargement that occurs much more frequently in dogs. A tumor of the prostate is almost always a malignant neoplasm in dogs , in most cases it is adenocarcinoma , as in humans . On the other hand, castrated males show undifferentiated carcinomas in around 50% of cases. Squamous cell carcinomas, transitional cell carcinomas and leiomyosarcomas have sporadically been described as further malignant neoplasms. Benign fibromas , adenomas or leiomyomas are only isolated cases . In accordance with their aggressive nature, metastases are already present in 70 to 80% of cases at the time of diagnosis. The tumor cells are spread via the lymphatic system and affect the lungs in 66% of cases. This course seems to be more common in neutered males than in intact males. In addition, metastases affect the lymph nodes in the pelvic area, as well as the liver, spleen, heart, kidneys, more distant lymph nodes, bones and the adrenal glands.
Symptoms
The clinical presentation of the disease is variable. Defecation disorders such as tenesmus or constipation occur much more frequently than problems with micturition . The new formation can cause pain symptoms in the area of the hindquarters, which can even appear as paralysis if metastasized into the spine. In some cases, painfulness occurs on rectal palpation . However, enlargement of the organ is not detectable in all cases. In two thirds of the cases, signs of inflammation or bleeding can be found in the urine . However, tumor cells themselves are rarely found here. Use of the human medical markers acid phosphatase and prostate-specific antigen is controversial, especially since the human medical tests cannot be used in dogs.
Diagnosis
In the case of metastasis, changes in the affected organs can often be detected in the X-ray image. The prostate itself is often enlarged and has foci of calcification. In addition to an enlargement of the organ, an increased echogenicity and, in some cases, the presence of cysts can often be detected on ultrasound . The definitive diagnosis is made by means of a transabdominal or transrectal prostate biopsy and a subsequent pathohistological examination. Another possibility is to perform a catheter suction biopsy, in which cells are sucked in from the prostate area by means of a urethral catheter.
therapy
Since most dogs with a tumor of the prostate are only presented when metastases are present, the prognosis is in most cases unfavorable from the outset. Early detection measures such as B. PSA tests are not currently available. The mean survival time after diagnosis is three months. Surgical removal of the prostate is often not possible due to the large extent of the tumor. In intact males, the survival time is not improved even by castration or the administration of antiandrogens . Even chemotherapy protocols or radiation therapies that have been tested in various ways have not proven to improve the prognosis.
See also
literature
Guidelines
- Prostate Cancer Patient Guide (PDF; 1 MB), Patient Guideline Locally Limited Prostate Cancer (PDF; 440 kB), Patient Guideline Locally Advanced and Metastatic Prostate Cancer (PDF; 430 kB) ( German Cancer Society )
- S3 guideline for prostate cancer: early detection, diagnosis and therapy of the various stages of the German Society for Urology (DGU). In: AWMF online (as of 2018)
Reference books
- Emil A. Tanagho, Jack W. McAninch: Smith's General Urology. 17th edition. Mcgraw-Hill Professional, 2008, ISBN 978-0-07-145737-8 .
- Hans Ulrich Schmelz, Christoph Sparwasser, Wolfgang Weidner: Urology specialist knowledge. Springer, Heidelberg 2006, ISBN 3-540-20009-6 .
- Herbert Rübben: uro-oncology. Springer, Berlin 2001, ISBN 3-540-67310-5 .
- M. Kessler: Small Animal Oncology. Parey-Verlag, Munich 2005, ISBN 3-8304-4103-7 .
Magazine articles
- Lothar Weißbach, Jens Altwein: Active monitoring or active therapy for local prostate cancer? In: Dtsch. Doctor bl. Int . No. 106 (22) , 2009, pp. 371-376 ( Article ).
- DG Bostwick et al .: Human prostate cancer risk factors. In: Cancer . Volume 101, Number 10 Suppl, November 2004, pp. 2371-2490, doi: 10.1002 / cncr.20408 . PMID 15495199 . (Review).
Web links
- PathoPic - Image database of the University of Basel: Adenocarcinoma of the prostate (image of a specimen)
- PathoPic - image database of the University of Basel: tumor infiltration of the urinary bladder (image of a specimen)
- Prostate cancer . German Society for Urology
- Center for cancer registry data at the Robert Koch Institute
- Prostate cancer . National Cancer Institute
- Prostate cancer . Online urology textbook for doctors and healthcare professionals
- Prostate Cancer Institute for Quality and Efficiency in Health Care
- Patient information for early detection of prostate cancer Medical Center for Quality in Medicine
Individual evidence
- ↑ Federal Statistical Office of Germany: Marriages, those born and died 1946–2011 . ( Memento from November 13, 2012 in the Internet Archive ) ( MS Excel ; 242 kB) - In 2005 a total of 388,554 men died.
- ↑ a b Issue 36 Prostate Diseases - Federal Health Reporting . ( Memento from December 18, 2013 in the Internet Archive ) (PDF; 0.6 MB) Robert Koch Institute . - Around 11,000 men die of prostate cancer in Germany each year (2005), that is 22.4% of those affected.
- ↑ a b estimate by the Robert Koch Institute
- ↑ krebshilfe.de accessed on April 15, 2019
- ^ Deutsche Krebshilfe Bonn, April 2019.
- ↑ T. Schelhase et al .: The cause of death statistics - methodology and results 2004. ( Memento of November 15, 2010 in the Internet Archive ) (PDF; 753 kB) Federal Statistical Office (editor), p. 625.
- ^ KJ Bell, C. Del Mar, G. Wright, J. Dickinson, P. Glasziou: Prevalence of incidental prostate cancer: A systematic review of autopsy studies. In: International Journal of Cancer . Volume 137, number 7, October 2015, pp. 1749–1757, doi: 10.1002 / ijc.29538 , PMID 25821151 , PMC 4682465 (free full text) (review).
- ↑ JL Jahn, EL Giovannucci, MJ Stampfer: The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era. In: International Journal of Cancer . Volume 137, number 12, December 2015, pp. 2795-2802, doi: 10.1002 / ijc.29408 , PMID 25557753 , PMC 4485977 (free full text) (review).
- ^ RM Hoffman et al .: Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. In: J Natl Cancer Inst . 93, 2001, pp. 388-395. PMID 11238701 .
- ↑ a b Overview: Prostate Cancer - What Causes Prostate Cancer? American Cancer Society, Aug. 8, 2009; Retrieved October 19, 2012.
- ↑ International Agency for Research on Cancer: GLOBOCAN - Estimated Cancer Incidence, Mortality, Prevalence and Disability-Adjusted Life Years (DALYs). Database queries.
- ^ GD Steinberg et al .: Family history and the risk of prostate cancer. In: The Prostate . Volume 17, Number 4, 1990, pp. 337-347, PMID 2251225 .
- ↑ AF Olumi: Commentary on "integrative genomic analyzes reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer."; Genome Biology Unit, European Molecular Biology Laboratory (EMBL), Heidelberg, Germany .: Cancer Cell 2013; 23 (2): 159-70. In: Urologic oncology. Volume 32, number 2, February 2014, p. 212, doi: 10.1016 / j.urolonc.2013.08.018 . PMID 24445294 .
- ^ N. Breslow et al .: Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. In: International Journal of Cancer . Volume 20, Number 5, November 1977, pp. 680-688, PMID 924691 .
- ^ T. Matsuda, K. Saika: Comparison of time trends in prostate cancer incidence (1973–1997) in East Asia, Europe and USA, from Cancer Incidence in Five Continents Vols IV VIII. In: Japanese journal of clinical oncology. Volume 37, Number 7, July 2007, pp. 556-557, doi: 10.1093 / jjco / hym100 . PMID 17720742 .
- ↑ California Cancer Registry 2002
- ↑ SE Berkow et al .: Diet and survival after prostate cancer diagnosis. In: Nutrition reviews. Volume 65, Number 9, September 2007, pp. 391-403, PMID 17958206 . (Review).
- ^ MA Khan, AW Partin: Vasectomy and prostate cancer. In: Reviews in urology. Volume 6, Number 1, 2004, pp. 46-47, PMID 16985574 . PMC 1472683 (free full text).
- ^ GG Giles et al .: Sexual factors and prostate cancer. In: BJU International . 2003, 92, pp. 211-216. PMID 12887469 . doi : 10.1046 / j.1464-410X.2003.04319.x
- ↑ Jennifer R. Rider et al .: Ejaculation Frequency and Risk of Prostate Cancer: Updated Results with an Additional Decade of Follow-up . In: European Urology . tape 70 , no. 6 , December 1, 2016, p. 974-982 , doi : 10.1016 / j.eururo.2016.03.027 , PMID 27033442 .
- ^ GG Schwartz, CL Hanchette: UV, latitude, and spatial trends in prostate cancer mortality: all sunlight is not the same (United States). In: Cancer causes & control. Volume 17, Number 8, October 2006, pp. 1091-1101, doi: 10.1007 / s10552-006-0050-6 . PMID 16933060 .
- ^ MM Ali, V. Vaidya: Vitamin D and cancer. In: Journal of cancer research and therapeutics. Volume 3, Number 4, Oct-Dec 2007, pp. 225-230, PMID 18270398 . (Review).
- ^ YR Lou et al .: The role of Vitamin D3 metabolism in prostate cancer. In: The Journal of steroid biochemistry and molecular biology. Volume 92, Number 4, November 2004, pp. 317-325, doi: 10.1016 / j.jsbmb.2004.10.007 . PMID 15663995 . (Review).
- ↑ a b R. Krause et al .: UV radiation and cancer prevention: what is the evidence? In: Anticancer Research . Volume 26, Number 4 A, July-August 2006, pp. 2723-2727, PMID 16886683 . (Review).
- ^ H. Glossmann: Vitamin D, UV, and skin cancer in the elderly: to expose or not to expose? In: Gerontology. Volume 57, number 4, 2011, pp. 350-353, doi: 10.1159 / 000322521 . PMID 21196703 . (Review).
- ↑ Prostate cancer: risk factors and prevention - few triggers known , information portal of the DKFZ, accessed on January 23, 2016
- ↑ LN Kolonel: Fat, meat, and prostate cancer. In: Epidemiologic reviews. Volume 23, Number 1, 2001, pp. 72-81, PMID 11588857 . (Review).
- ^ DS Michaud et al .: A prospective study on intake of animal products and risk of prostate cancer. In: Cancer Causes & Control . Volume 12, Number 6, August 2001, pp. 557-567, PMID 11519764 .
- ↑ E. Giovannucci et al .: A prospective study of dietary fat and risk of prostate cancer. In: Journal of the National Cancer Institute . Volume 85, Number 19, October 1993, pp. 1571-1579, PMID 8105097 .
- ^ Health Education Authority: Nutritional Aspects of the Development of Cancer ( Memento of December 9, 2008 in the Internet Archive ) (PDF; 4 MB), London 1999.
- ↑ Kathryn M. Wilson et al .: Meat, Fish, Poultry, and Egg Intake at Diagnosis and Risk of Prostate Cancer Progression . (PDF) In: Eur J Cancer . 9, No. 12, 2016, pp. 933-941. doi : 10.1158 / 1940-6207.CAPR-16-0070 . PMID 27651069 .
- ↑ Verena Katzke, Rudolf Kaaks: Cancer and nutrition . (PDF) In: Bundesverband Prostatakrebs Selbsthilfe e. V., 1/2016, pages 17-19
- ↑ LC Bylsma, DD Alexander: A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer . In: Nutr J. , 2015, 21, 14 pp. 933-941, doi: 10.1186 / s12937-015-0111-3 . PMID 26689289 , PMC 4687294 (free full text)
- ↑ K. Wu et al .: Associations between unprocessed red and processed meat, poultry, seafood and egg intake and the risk of prostate cancer: A pooled analysis of 15 prospective cohort studies . In: Int J Cancer. . 138, No. 10, 2016, pp. 2368-2382. doi : 10.1002 / ijc.29973 . PMID 26685908 . PMC 4837898 (free full text).
- ↑ GA Sonn et al .: Impact of diet on prostate cancer: a review. In: Prostate Cancer and Prostatic Diseases. 8, 2005, pp. 304-310. doi: 10.1038 / sj.pcan.4500825 PMID 16130015 .
- ^ Xiang Gao et al .: Prospective Studies of Dairy Product and Calcium Intakes and Prostate Cancer Risk: A Meta-Analysis. In: J Natl Cancer Inst. 97 (23), December 7, 2005, pp. 1768-1777. doi: 10.1093 / jnci / dji402 PMID 16333032 .
- ↑ Gianluca Severi et al .: Re: Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. In: J Natl Cancer Inst. 98 (11), June 7, 2006, pp. 794-795. doi: 10.1093 / jnci / djj215 PMID 16757704 .
- ↑ NDR, Plus Minus: Myth Milk. ( Memento of April 27, 2006 in the Internet Archive ) April 25, 2006.
- ↑ AJ Pantuck, CA Pettaway et al. a .: A randomized, double-blind, placebo-controlled study of the effects of pomegranate extract on rising PSA levels in men following primary therapy for prostate cancer. In: Prostate Cancer and Prostatic Disease. 18, 2015, p. 242, doi: 10.1038 / pcan.2015.32 .
- ↑ Margaret Diana van Die, Kerry M. Bone et al. a .: Phytotherapeutic interventions in the management of biochemically recurrent prostate cancer: a systematic review of randomized trials. In: BJU International. 117, 2016, p. 17, doi: 10.1111 / bju.13361 .
- Jump up ↑ R. Campi, SD Brookman-May, JD Subiela Henríquez, B. Akdoğan, M. Brausi, T. Klatte, JF Langenhuijsen, E. Linares-Espinos, M. Marszalek, M. Roupret, CG Stief, A. Volpe, A. Minervini, O. Rodriguez-Faba: Impact of Metabolic Diseases, Drugs, and Dietary Factors on Prostate Cancer Risk, Recurrence, and Survival: A Systematic Review by the European Association of Urology Section of Oncological Urology. In: European urology focus. Volume 5, number 6, November 2019, pp. 1029-1057, doi : 10.1016 / j.euf.2018.04.001 , PMID 29661588 (review), PDF .
- ↑ G. Markozannes et al .: Diet, body size, physical activity and risk of prostate cancer: An umbrella review of the evidence . In: Eur J Cancer. . 69, 2016, pp. 61-69. doi : 10.1016 / j.ejca.2016.09.026 . PMID 27816833 .
- ↑ A. van Bokhoven et al .: Molecular characterization of human prostate carcinoma cell lines. In: The Prostate. Volume 57, Number 3, November 2003, pp. 205-225, doi: 10.1002 / pros.10290 . PMID 14518029 .
- ↑ a b A. B. Apolo, N. Pandit-Taskar, MJ Morris: Novel tracers and their development for the imaging of metastatic prostate cancer. In: Journal of nuclear medicine. Volume 49, Number 12, December 2008, pp. 2031-2041, doi: 10.2967 / jnumed.108.050658 . PMID 18997047 . (Review).
- ^ MD Krahn et al .: Screening for prostate cancer. A decision analytic view. In: JAMA. 272 (10), 1994, pp. 773-780. PMID 7521400 .
- ^ A b c d Ronald J. Zagoria, Glenn A. Tung: Genitourinary Radiology. Mosby, St. Louis 1997.
- ↑ LAM Simmons, P. Autier, F. Zát'ura, J. Braeckman, A. Peltier, I. Romic, A. Stenzl, K. Treurnicht, T. Walker, D. Nir, CM Moore, M. Emberton: Detection , Localization and characterization of prostate cancer by Prostate HistoScanning. In: BJU Int. Epub 2011 Nov. 17
- ^ MRI in addition to or as a substitute for prostate biopsy: The clinician's point of view. In: Diagnostic and Interventional Imaging. 93, 2012, pp. 262-267.
- ↑ NB Delongchamps et al .: Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: Combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. In: BJU Int . 107 (9), 2011, pp. 1411-1418.
- ↑ How Good is MRI at Detecting and Characterizing Cancer within the Prostate? In: European Urology . 50, 2006, pp. 1163-1175.
- ↑ Combined prostate diffusion tensor imaging and dynamic contrast enhanced MRI at 3T - quantitative correlation with biopsy. In: Magnetic Resonance Imaging. 28, 2010, pp. 621-628.
- ↑ B. Scher et al .: Value of 11C-choline-PET and PET-CT in patients with suspected prostate cancer. In: European Journal of Nuclear Medicine. 34, 2007, pp. 45-53.
- ↑ SN Reske et al .: Imaging Prostate Cancer with 11C-Choline. In: Journal of Nuclear Medicine. 47, 2006, pp. 1249-1254.
- ↑ Analysis directory | Laboratory crown. Retrieved on October 12, 2017 (German).
- ↑ LADR : Prostate Carcinoma - Prevention and early detection using prostate cancer-specific PCA3 test (PDF)
- ↑ MA Bjurlin, SS Taneja: Standards for prostate biopsy. In: Current opinion in urology. Volume 24, number 2, March 2014, pp. 155–161, doi: 10.1097 / MOU.0000000000000031 , PMID 24451092 , PMC 4142196 (free full text) (review).
- ↑ M. Brock, C. von Bodman, J. Palisaar, W. Becker, P. Martin-Seidel, J. Noldus: Detecting Prostate Cancer. In: Deutsches Arzteblatt international. Volume 112, number 37, September 2015, pp. 605–611, doi: 10.3238 / arztebl.2015.0605 , PMID 26396046 , PMC 4581108 (free full text), German version: Detection des Prostatakarzinom , PDF .
- ↑ AM Hoogland, CF Kweldam, GJ van Leenders: Prognostic histopathological and molecular markers on prostate cancer needle-biopsies: a review. In: BioMed research international. Volume 2014, 2014, p. 341324, doi: 10.1155 / 2014/341324 , PMID 25243131 , PMC 4163394 (free full text) (review).
- ↑ Ursus-Nikolaus Riede, Hans-Eckart Schaefer: General and special pathology. Thieme, Stuttgart 1999, ISBN 3-13-683304-X .
- ^ HY Wu, HM Snyder: Pediatric urologic oncology: bladder, prostate, testis. In: Urol Clin North Am. 31 (3), 2004, pp. 619-627, xi. PMID 15313070 .
- ^ DB Groff: Pelvic neoplasms in children. In: J Surg Oncol. 77 (1), 2001, pp. 65-71. PMID 11344486 .
- ↑ a b Guideline "Prostate Carcinoma" of the German Cancer Society and the German Cancer Aid, link list, accessed on November 12, 2018
- ↑ See also the website prostapath.org ( Memento of March 28, 2010 in the Internet Archive ) Gleason School - Intraductal Prostate Cancer; PCa variants; Gleason Grading in Punch Biopsies; Reproducibility and correlation. Retrieved February 9, 2012.
- ^ So Strohmaier, Clinic Coburg, in the GPK special issue from June 2007 "Cancer early detection without myth".
- ^ Alfred Böcking: With cells instead of scalpels. Lehmanns Fachbuchhandlung, 2006, ISBN 3-86541-177-0 .
- ^ Correspondence on DNA cytometry. Accessed May 13, 2009.
- ↑ FK Mostofi, CJ Davis, IA Sesterhenn: Pathology of carcinoma of the prostate. In: Cancer. Volume 70, Number 1 Suppl, July 1992, pp. 235-253, PMID 1376194 . (Review).
- ↑ Prostate Cancer Coding Aid. Tumor Center Freiburg 2007 ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. (PDF)
- ↑ UICC: What are the changes between the 6th and 7th editions? (PDF; 2.1 MB) ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. (PDF)
- ↑ Christian Wittekind, Hans-Joachim Meyer (Ed.): TNM: Classification of malignant tumors. 7th edition. Wiley-VCH Verlag, 2010, ISBN 978-3-527-32759-1 .
- ↑ FC Hamdy, JL Donovan u. a .: 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. In: The New England Journal of Medicine . Volume 375, number 15, 10 2016, pp. 1415-1424, doi: 10.1056 / NEJMoa1606220 , PMID 27626136 .
- ↑ Prostate cancer: wait instead of intervening . Medicine Transparent , January 15, 2018; accessed on March 16, 2018
- ^ Jenny L. Donovan, Freddie C. Hamdy, J. Athene Lane, Malcolm Mason, Chris Metcalfe: Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer . In: The New England Journal of Medicine . tape 375 , no. 15 , October 13, 2016, p. 1425-1437 , doi : 10.1056 / NEJMoa1606221 , PMID 27626365 , PMC 5134995 (free full text).
- ↑ Long-term study on prostate cancer shows: Patients benefit from early treatment - radiation as effective as surgery, but gentler - Degro. Retrieved April 12, 2017 .
- ↑ a b c P. Grimm et al .: Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. In: BJU international. Volume 109 Suppl 1, February 2012, pp. 22-29, doi: 10.1111 / j.1464-410X.2011.10827.x . PMID 22239226 . (Review). praxis-margareten.at (PDF, full text)
- ↑ H. Keller, J. Lehmann, J. Beier: Radical perineal prostatectomy and simultaneous extended pelvic lymph node dissection via the same incision. In: European urology. Volume 52, number 2, August 2007, pp. 384-388, doi: 10.1016 / j.eururo.2006.09.045 . PMID 17084507 .
- ↑ a b P. J. Davidson, D. van den Ouden, FH Schroeder: Radical prostatectomy: prospective assessment of mortality and morbidity. In: European urology. Volume 29, Number 2, 1996, pp. 168-173. PMID 8647142 .
- ^ Brian McGlynn et al .: Management of Urinary Incontinence Following Radical Prostatectomy. In: Urol Nurs. 24 (6), 2004.
- ^ E. Sacco, T. Prayer-Galetti, F. Pinto, S. Fracalanza, G. Betto, F. Pagano, W. Artibani: Urinary incontinence after radical prostatectomy: incidence by definition, risk factors and temporal trend in a large series with a long-term follow-up. In: BJU international. Volume 97, Number 6, June 2006, pp. 1234-1241, doi: 10.1111 / j.1464-410X.2006.06185.x . PMID 16686718 .
- ↑ Mechanism of the erection on www.urologielehrbuch.de
- ↑ Freddie C. Hamdy, Jenny L. Donovan, J. Athene Lane, Malcolm Mason, Chris Metcalfe: 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer . In: The New England Journal of Medicine . tape 375 , no. 15 , October 13, 2016, ISSN 1533-4406 , p. 1415-1424 , doi : 10.1056 / NEJMoa1606220 , PMID 27626136 .
- ↑ JL Donovan, FC Hamdy, JA Lane, M. Mason, C. Metcalfe: Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer . In: The New England journal of medicine . tape 375 , no. 15 , October 13, 2016, ISSN 0028-4793 , p. 1425-1437 , doi : 10.1056 / NEJMoa1606221 , PMID 27626365 , PMC 5134995 (free full text).
- ↑ CM Yablon et al .: Complications of prostate cancer treatment: spectrum of imaging findings. In: Radiographics. Volume 24 Suppl 1, October 2004, pp. S181-S194, doi: 10.1148 / rg.24si045502 . PMID 15486240 . (Review).
- ↑ RS Kushwaha et al .: Physiologic changes of the anorectum after pelvic radiotherapy for the treatment of prostate and bladder cancer. In: Diseases of the Colon and Rectum . Volume 46, Number 9, September 2003, pp. 1182-1188, doi: 10.1007 / s10350-004-6712-0 . PMID 12972961 .
- ↑ DE Spratt et al .: Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. In: Int J Radiat Oncol Biol Phys. 85 (3), March 1, 2013, pp. 686-692. doi: 10.1016 / j.ijrobp.2012.05.023 . Epub 2012 Jul 12.
- ↑ a b N. P. Mendenhall et al .: Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. In: International journal of radiation oncology, biology, physics. Volume 88, Number 3, March 2014, pp. 596-602, doi: 10.1016 / j.ijrobp.2013.11.007 . PMID 24521677 .
- ↑ PREFERE large-scale study evaluates therapies. In: Aktuel Urol. 44 (02), 2013, p. 105 doi: 10.1055 / s-0033-1343926
- ↑ GS Merrick, WM Butler: Rectal function following permanent prostate brachytherapy. In: The West Virginia medical journal. Volume 100, Number 1, January-February 2004, pp. 18-20, PMID 15119492 .
- ↑ GM Duchesne et al .: Outcome, morbidity, and prognostic factors in post-prostatectomy radiotherapy: an Australian multicenter study. In: Urology. Volume 61, Number 1, January 2003, pp. 179-183, PMID 12559292 .
- ↑ ASTRO press release from October 2012.
- ↑ Information on the study ( Memento of the original from March 6, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Press release from the University of Lübeck
- ↑ TE Schultheiss, WR Lee, MA Hunt, AL Hanlon, RS Peter, GE Hanks: Late GI and GU complications in the treatment of prostate cancer. In: Int. J. Radiat. Oncol. Biol. Phys. 37 (1), Jan 1997, pp. 3-11. PMID 9054871 .
- ↑ NH Gilinsky, DG Burns, GO Barbezat, W. Levin, HS Myers, IN Marks: The natural history of radiation-induced proctosigmoiditis: patients on analysis of the 88th In: QJ Med. 52 (205), 1983, pp. 40-53. PMID 6603628 .
- ↑ PJ Prada et al .: Transperineal injection of hyaluronic acid in the anterior perirectal fat to decrease rectal toxicity from radiation delivered with low-dose-rate brachytherapy for prostate cancer patients. In: Brachytherapy. 8 (2), 2009, pp. 210-217. doi: 10.1016 / j.brachy.2008.11.010 . PMID 19213607 .
- ^ WR Noyes, CC Hosford, SE Schultz: Human collagen injections to reduce rectal dose during radiotherapy. In: Int. J. Radiat. Oncol. Biol. Phys. 82 (5), April 2012, pp. 1918-1922. doi: 10.1016 / j.ijrobp.2011.02.034 . PMID 21514738
- ↑ G. Kovacs et al .: Significant Rectal Dose Reduction During Prostate Cancer Radiotherapy Using Novel Biodegradable Inflatable Balloon System: Interim Results of a Prospective Multi-Center Study. Abstract, ASTRO 2011.
- ↑ DY Song et al .: A multi-institutional clinical trial of rectal dose reduction via injected polyethylene-glycol hydrogel during intensity modulated radiation therapy for prostate cancer: analysis of dosimetric outcomes. In: International journal of radiation oncology, biology, physics. Volume 87, number 1, September 2013, pp. 81-87, doi: 10.1016 / j.ijrobp.2012.12.019 . PMID 23414766 . PMC 3737267 (free full text).
- ↑ M. Pinkawa et al .: Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. In: Radiother Oncol . 100 (3), September 2011, pp. 436-441. doi: 10.1016 / j.radonc.2011.09.005 . PMID 21963289 .
- ↑ MJ Zelefsky et al .: Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. In: Int. J. Radiat. Oncol. Biol. Phys. 71 (4), July 2008, pp. 1028-1033. doi: 10.1016 / j.ijrobp.2007.11.066 . PMID 18280056 .
- ↑ M. Ritter, J. Forman, P. Kupelian, C. Lawton, D. Petereit: Hypofractionation for prostate cancer. In: Cancer J. 15 (1), 2009, pp. 1-6. doi: 10.1097 / PPO.0b013e3181976614 . PMC 3039916 (free full text). PMID 19197165 .
- ↑ Gentle form of prostate surgery orf.at, August 27, 2017, accessed August 27, 2017.
- ↑ D. Margel et al .: Cardiovascular morbidity in a randomized trial comparing GnRH-agonist and GnRH-antagonist among patients with advanced prostate-cancer and pre-existing cardiovascular disease . In: American Urological Association (Ed.): The Journal of Urology . Elsevier-Verlag, 2019, doi : 10.1097 / JU.0000000000000384 .
- ↑ PC Albertsen et al .: Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist . In: European Association of Urology (Ed.): European Urology . tape 65 , no. 3 . Elsevier-Verlag, 2014, p. 565-573 , doi : 10.1016 / j.eururo.2013.10.032 .
- ↑ UW tunn, N. Bruchovsky, H. Renneberg, JM Wolff, R. Kurek: Intermittent androgen deprivation. In: Der Urologe A. Volume 39, No. 1, January 2000.
- ↑ S. Madersbacher, UW Tunn: Update on intermittent hormone therapy for prostate cancer. (PDF; 974 kB) In: Journal for Urology and Urogynaecology. 14, 2007, (special issue 3)
- ^ GL Lu-Yao et al .: Survival following primary androgen deprivation therapy among men with localized prostate cancer. In: JAMA. 300, 2008, pp. 173-181. PMID 18612114
- ^ IF Tannock et al .: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. In: NEJM. 351, 2004, pp. 1502-1512. PMID 15470213
- ^ CJ Sweeney et al .: Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. In: N Engl J Med. 373, 2015, pp. 737-746. doi: 10.1056 / NEJMoa1503747
- ↑ New drugs: Xtandi (enzalutamide). (PDF) In: akdae.de Medicines Commission of the German Medical Association. November 29, 2013. Retrieved November 29, 2013 .
- ↑ D. Mukherji, CJ Pezaro, JS De-Bono: MDV3100 for the treatment of prostate cancer. In: Expert Opinion on Investigational Drugs . Volume 21, Number 2, February 2012, pp. 227-233, doi: 10.1517 / 13543784.2012.651125 . PMID 22229405 .
- ↑ F. Saad et al .: A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. In: Journal of the National Cancer Institute. Volume 94, Number 19, October 2002, pp. 1458-1468, PMID 12359855 .
- ↑ European Medicines Agency : Xofigo. Radium-223 dichloride (PDF; 75 kB). (PDF) EMA / 579264/2013, EMEA / H / C / 002653, November 13, 2013.
- ↑ Interdisciplinary guideline of quality S3 for early detection, diagnosis and therapy of the different stages of prostate cancer , leading professional society: Deutsche Gesellschaft für Urologie e. V. (Ed.), Version 5.0, 2018,
- ↑ L. Weissbach, J. Altwein: Active monitoring or active therapy for local prostate cancer? In: Deutsches Ärzteblatt international. Volume 106, number 22, May 2009, pp. 371–376, doi: 10.3238 / arztebl.2009.0371 . PMID 19623304 . PMC 2704359 (free full text). (Review).
- ↑ Health and Clinical Excellence Guidance: Localized Prostate Cancer ( Memento of the original from March 15, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , United Kingdom. Retrieved October 18, 2012.
- ↑ Edward W. Lee, Susan Thai, Stephen T. Kee: Irreversible electroporation: a novel image-guided cancer therapy. In: Gut and Liver. 4. Suppl 1, 2010, pp. 99-104.
- ↑ Boris Rubinsky: Irreversible electroporation: implications for prostate ablation. In: Technology in cancer research & treatment. 6.4, 2007.
- ↑ CLINICS in Germany that offer irreversible electroporation - IRE (“NanoKnife”) (as of June 2015). (PDF) concerns: Prostate cancer - hope through gentle therapy. SWR, July 22, 2015, accessed on July 24, 2015 .
- ↑ G. Bozzini et al .: Focal therapy of prostate cancer: energies and procedures. In: Urologic oncology. Volume 31, Number 2, February 2013, pp. 155-167, doi: 10.1016 / j.urolonc.2012.05.011 . PMID 22795500 . (Review).
- ↑ PA Burch et al .: Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a Phase 2 trial. In: Prostate. 60, 2004, pp. 197-204. PMID 15176049 .
- ^ JE Johansson et al .: Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. In: JAMA: the journal of the American Medical Association. Volume 277, Number 6, February 1997, pp. 467-471, PMID 9020270 . Abstract (German) ( Memento of the original from October 18, 2004 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ CR Porter et al .: 25-year prostate cancer control and survival outcomes: a 40-year radical prostatectomy single institution series. In: The Journal of urology. Volume 176, number 2, August 2006, pp. 569-574, doi: 10.1016 / j.juro . 2006.03.094 . PMID 16813891 .
- ^ Survival rates for prostate cancer. American Cancer Society, accessed February 9, 2012.
- ↑ P. Enders et al .: Prostate Cancer I - Locally Limited Prostate Cancer (PDF; 1125 kB) An evidence-based patient guide to the S3 guideline on early detection, diagnosis and treatment of the various stages of prostate cancer, German Cancer Society, 2015.
- ^ AW Partin et al .: Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. In: Urology. Volume 58, Number 6, December 2001, pp. 843-848, PMID 11744442 .
- ↑ S3 guideline for prostate cancer: early detection, diagnosis and therapy of the various stages of the German Society for Urology (DGU). In: AWMF online (as of 2018)
- ↑ Prostate cancer screening using PSA test , IQWiG, December 20, 2019.
- ↑ DLF interview with cancer researcher Robert Weinberg. In: Research News. January 4, 2006.
- ↑ Emily Sohn: Screening: Diagnostic dilemma. In: Nature. Volume 528, No. 7582, 2015, pp. S120-S122 (here: pp. S121), doi: 10.1038 / 528S120a
- ^ Metastases in the Scythian prince. In: image of science . 3/2008. Konradin Medien GmbH, Leinfelden-Echterdingen.
- ^ J. Adams: The case of scirrhous of the prostate gland with corresponding affliction of the lymphatic glands in the lumbar region and in the pelvis. In: Lancet. (1853) 1, p. 393.
- ^ HH Young: Four cases of radical prostatectomy. In: Johns Hopkins Bull. 16, 1905, p. 315.
- ↑ C. Huggins, CV Hodges: Studies on Prostatic Cancer - 1. The effect of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. In: Cancer Res. 1, 1941, pp. 203-297.
- ↑ RW Nelson, CG Couto: Internal medicine of small animals. Urban & Fischer, Munich / Jena 2006, p. 1004.