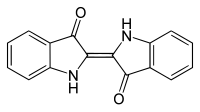
indigo
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | indigo | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 16 H 10 N 2 O 2 | ||||||||||||||||||
| Brief description |
dark blue, odorless crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| characteristics | |||||||||||||||||||
| Molar mass | 262.27 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
390-392 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Indigo (from ancient Greek ἰνδικόν indikón , German 'the Indian' ; after the region of origin East India ) is a deep blue, crystalline organic-chemical compound . It is an organic pigment with high color strength and sparingly soluble in water. Indigo is the namesake for the group of indigoid dyes , whose chemical structure is closely related to that of indigo.
The color shade of the same name, indigo, is also named after him. It can best be described as the last recognizable shade of blue before it turns into a bluish purple . Indigo is listed in the Color Index as a pigment under the name CI Pigment Blue 66 and as a vat dye under CI Vat Blue 1.
Indigo is one of the oldest and best-known pigments and was used to color textiles as early as prehistoric times. In the past, indigo was obtained from vegetable sources such as the leaves of woad or from the indigo plant . From 1865, Adolf von Baeyer carried out a series of studies in which he developed various synthetic routes for indigo and determined its chemical structure. For his work on dye chemistry , he received the Nobel Prize in Chemistry in 1905 . Indigo played an important role in the further development of organic chemistry , especially the relationship between the color and the structure of the molecule and its derivatives was intensively studied and thus contributed to the development of a general theory of dyes.
With the development of industrial synthesis processes towards the end of the 19th century, the large-scale and thus inexpensive production of indigo began, whereupon the market for natural indigo collapsed. Nowadays, tens of thousands of tons of indigo are produced synthetically each year, mainly used for dyeing denim cotton fabrics for the manufacture of blue jeans .
Occurrence
Indigo can be obtained from the Indian indigo plant ( Indigofera tinctoria ) or from woad ( Isatis tinctoria ), which was naturalized in Europe in ancient times . The indigo plant in India supplied thirty times the amount of dye compared to woad, so that cultivation in Europe in the 17th century became unprofitable. American settlers used the bastard indigo ( Amorpha fruticosa ) as a substitute for indigo, and in Japan the dyer's knotweed ( Polygonum tinctorium , syn. Persicaria tinctoria ) was used for dyeing blue. A number of other plants are suitable for the production of indigo, including the common devil's bite ( Succisa pratensis ), the indigolupine ( Baptisia australis ), the dyer's oleander ( Wrightia tinctoria ) or the ternwort ( Marsdenia tinctoria ). In China, the Chinese woad ( Isatis indigotica ) was used. Indigo is still cultivated in Brazil and El Salvador . The dye-rich species Indigofera arrecta and Indigofera sumatrana are used . The UNESCO promotes various projects to plant indigo bearing plants, including the Jordan Valley and the Aral Sea .
history
Indigo was and is one of the most widespread and most frequently used colorants since ancient times . Before the start of synthetic production, it was obtained from various plants containing indigo. These are often referred to with the species-specific epithet tinctoria , a Latin word for coloring.
In Europe, woad, Isatis tinctoria , from the cruciferous family was used to dye blue until the 17th century . It originally comes from the Middle East , but was already cultivated as a dye plant in pre-Christian times in Europe . In Asia and South America, species of the genus Indigofera were used, such as the tropical indigo plant Indigofera tinctoria , the European settlers in North America used hybrid indigo , Amorpha fruticosa , to dye the color blue.
Dyeing with indigo forms the basis for centuries-old textile traditions throughout West Africa. The Indigo-colored Tagelmust , worn by the Tuareg nomads of the Sahara, symbolizes wealth and health. Because of the water-poor dyeing process there, the indigo is not very abrasion-resistant and penetrates the wearer's skin, which is why the Tuareg are also known as the “blue men of the desert”. The Yoruba , Mandinka and Hausa also dye their clothes with indigo.
Antiquity to the late Middle Ages
The use of indigo can be traced back to 6000 year old cotton fabrics from the pre-ceramic factory of Huaca Prieta de Chicama on the north coast of Peru . Various Indigofera species native to South America probably served as indigo suppliers. In Egypt, mummies from the fifth dynasty of the Old Kingdom , about 4,400 years before the present, were found wrapped in indigo-colored mummy ribbons. Japan and Southeast Asian countries have used indigo as a dye for centuries. The colorant continued to be known in the early cultures of Mesopotamia , Iran, and Africa. Plants for the production of indigo were mainly grown in India , the first major center for its production and processing. The indigo was shipped to Egypt via Barbarikon on the Indus . From there it made its way to Greece and Italy, where it was viewed as a luxury product.
Caesar reported around 50 BC. BC in De bello Gallico that the Celts used woad before armed conflicts in order to cause their skin to turn blue. The Roman scholar Pliny the Elder described indigo around 77 AD in his work Naturalis historia . According to Pliny, indigo enjoyed the highest esteem after purple in the ancient world . He stated India as the country of origin, which is expressed in the Latin word indicum , from which the current name indigo is derived. Another term for the dye is anil , which is derived from the Arabic term for blue, an-nil , and is found as a component of names such as aniline . In the Middle Ages, no blue dye other than indigo was known in Europe. In the 8th century, Charlemagne regulated the cultivation of woad for indigo production in his country estate ordinance Capitulare de villis vel curtis imperii .
In Europe, the dye from the indigo plant was rare until the 12th century; it was only imported in small quantities from India via Syria and Alexandria . Up until the 17th century, woad was grown for indigo production in England, France and Germany. In Germany, the largest cultivation area was in Thuringia , with about 3750 hectares being cultivated with the plant.
Early modern age
With the discovery of the sea route to India by Vasco da Gama , the direct import of Indian indigo (also called “the just indigo that grows in Indian tubes”) to Europe by Portuguese seafarers began. The Dutch merchant companies founded the Dutch East India Company in 1602 and subsequently increased the imports of indigo from India and Indonesia. Spain started growing in Guatemala and Venezuela at this time , France had indigo grown in San Domingo, and England began growing in Carolina around 1700 . Since the indigo content of woad was only about 3–4% of the indigofera plant, the indigo eventually displaced the tropical Indigofera tinctoria , despite protectionist import regulations.
After the defeat in the American War of Independence in 1783 and the associated loss of the North American acreage, England increased indigo cultivation in Bengal . In particular, former employees of the East India Company , so-called planters, began to commercialize indigo cultivation in Bihar and Bengal. Through fraudulent contracts and excessive interest rates for the procurement of the seeds, the growers made large profits, while the farmers made little profit. The circumstances led to the Indigo Riots in 1859–1862 , the first peasant movement in Bengal and Bihar against the exploitative methods of the European planters. Although the riots ended with the abolition of compulsory cultivation of indigo, indigo cultivation in India continued to be an enormous branch of industry that was predominantly British-owned. Tunic skirts, sailors' uniforms and the blue work clothes of the workers were dyed with this dye. In Bihar alone, around 1.5 million people were involved in indigo cultivation. In 1897 the cultivation area in India was about 7,000 square kilometers and at that time around 8,000 tons of pure indigos worth 100 million marks were produced annually.
Chemical synthesis towards the end of the 19th century
With the first successful synthesis of the dye mauvein in 1856 by William Henry Perkin , a competition in science and industry for the development of new tar colors began . The synthesis of indigo, the "king of dyes", was achieved in 1878 by the German chemist Adolf von Baeyer, who in 1883 clarified its chemical structure . The chemical companies BASF and Farbwerke vorm. Meister, Lucius & Brüning , the later Farbwerke Hoechst, agreed in 1880 to develop Baeyer's synthesis routes into a technical process. However, Baeyer's synthetic routes were not suitable for industrial production, as the raw materials required were not inexpensive to produce at the time.
An important invention for vat dyeing with indigo was made by Paul Schützenberger in 1873 with the introduction of sodium dithionite as a reducing agent. With the reducing agent manufactured by BASF on an industrial scale from 1906, it was now possible to manufacture leuco indigo in a simple form.
In 1881, BASF started producing “Little Indigo”, in which a preliminary product of synthetic indigo was reduced to indigo directly on the textile fiber. However, due to poor market acceptance, BASF discontinued the process a short time later. A synthesis developed by Karl Heumann in 1890 based on N- phenylglycine , the so-called 1st Heumann synthesis , seemed more promising. However, since the yields in this process were very low, BASF stopped this process development again in 1893.
The poor yields of the first Heumann synthesis could be improved by using anthranilic acid as starting material. The accidental discovery by Eugen Sapper of the catalytic oxidation of naphthalene with oleum with the addition of mercury sulfate to phthalic acid gave BASF the raw material necessary for the synthesis of anthranilic acid. The sulfur dioxide produced could be reprocessed using the contact process developed by Rudolf Knietsch at BASF to industrial maturity . Based on this synthesis variant, BASF brought the first synthetic indigo onto the market in July 1897 with high investments.
In 1901 Johannes Pfleger found a variant of the Heumann synthesis at Degussa , which also allowed Degussa and Farbwerke Hoechst to commercialize indigo. As a result of competition between chemical companies, the price of indigo per kilogram fell from around 20 to 4 Reichsmarks . In 1904, BASF and Farbwerke Hoechst signed the “Indigo Convention”, a cartel to eliminate competition, which set a market price of 8 Reichsmarks per kilogram. With the start of industrial production, natural indigo lost large market shares within a few years. In 1914 natural indigo only had a 4% share of the world market.
A brief increase in vegetable indigo production came about as a result of the First World War and the associated sea blockade , which did not allow Germany to export. After the World War, the synthetic production of indigo completely replaced the laborious extraction from plant material. The synthetic indigo offered a higher and constant purity and was therefore easier to use. In addition, the dyers were no longer dependent on the outcome of the dye plant harvest.
After the First World War
Indigo increasingly lost market share due to newly developed synthetic blue dyes such as indanthrene . It was only with the spread of jeans fashion from the mid-1960s that there was renewed demand for indigo. In 2011, the dyeing of denim used more than 95% of the approximately 50,000 tons of synthetic indigos produced annually. This makes it one of the most widely used pigments for textile dyeing, with which over a billion blue jeans are colored every year.
Research in the field of indigo production and application now focuses on the development of low-water dyeing processes or the electrochemical reduction to leuco-indigo and the use of water as a solvent for the synthesis and recrystallization of indigo.
Manufacturing
Obtained from dye plants
The dye plants do not contain indigo, but a precursor, indican , a glycoside of indoxyl .
This is first converted into indoxyl, a derivative of indole , through fermentation with splitting off of the sugar residue .
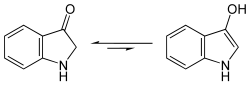
- Structural formula of indoxyl, equilibrium between keto and enol forms
Subsequent oxidation in air turns the yellow indoxyl into blue indigo.
The traditional extraction in India took place in so-called indigo factories . The extraction takes place in two height-offset basins. The freshly harvested indigo plant was layered in a first, higher basin and doused with water. The parts of the plant were weighted down with stones and beams to keep them completely under water. In this basin, the fermentation of the indicane to indoxyl started at correspondingly high ambient temperatures. After completion of the fermentation process, the indoxyl-containing water was drained into a lower basin and mechanically aerated there. The workers went into the pool and hit the aqueous solution with slats or the like to bring in air. The indigo formed by oxidation was then filtered off and boiled down. Portioning and final drying in air then took place. The purity of the indigo obtained in this way was between 15 and 70%. This was increased by fractional precipitation from sulfuric acid. In addition to organic impurities, natural indigo contains traces of inorganic components such as silica , phosphoric acid , clay and many others.
The biosynthesis of the indigo precursors in higher plants was researched with the help of isotope labeling and probably takes place via the shikimic acid route :
In this case, shikimate ( 1 in several stages to) chorismate ( 2 converted). This is transformed into anthranilate ( 3 ) by the anthranilate synthase . The enzyme anthranilate phosphoribosyl transferase (EC 2.4.2.18) catalyzes the reaction to N - (5-phospho- D -ribosyl) -anthranilate , which in turn by phosphoribosyl anthranilate isomerase (EC 5.3.1.24) to 1- (o-carboxyphenylamino) -1-deoxribulose-5-phosphate ( 4 ) reacts. Ring closure with decarboxylation gives the indole derivative 5 , which is finally converted into the amino acid tryptophan ( 6 ). The formation of indoxyl ( 7 ) from tryptophan, in which the enzymes tryptophanase and dioxygenase play a role, has not yet been fully clarified.
Syntheses according to Adolf von Baeyer
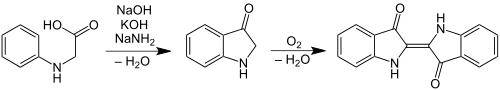
Adolf von Baeyer's first fully synthetic production of indigo was carried out by reduction of isatin , which he had previously been identified as a degradation product of indigo. Starting from phenylacetic acid , he arrived at oxindole in several steps , which he further oxidized to isatin. By chlorinating isatin with phosphorus pentachloride to form isatin chloride and then reducing it with zinc in acetic acid , he arrived at indigo.
A synthesis route developed by Baeyer and Drewsen ( Baeyer-Drewson reaction ) led to indigo via the aldol addition of ortho- nitrobenzaldehyde and acetone via the intermediate stage of o -nitrophenyl lactic acid ketone. This synthetic route is simple and useful for the preparation of indigo and many of its derivatives on a laboratory scale.
However, both synthesis routes could not be converted inexpensively into an industrial process.
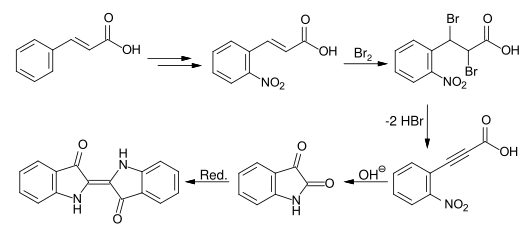
Baeyer developed another synthetic route that started from cinnamic acid . The nitration of the esterified cinnamic acid gives, after ester hydrolysis the o -Nitrozimtsäure. The o -nitrophenylpropiolic acid is obtained by bromination and double dehydrobromination , which can be converted into isatin by boiling with alkali and then reduced to indigo.
The head of research at BASF and close friend of Baeyers, Heinrich Caro , discovered that the isolable intermediate o -nitrophenylpropiolic acid could be converted to indigo directly on the fiber under mild alkaline conditions with sodium xanthate . BASF produced this so-called “little indigo” for a few years, but without any great market success.
Syntheses according to Karl Heumann
In 1890, the Zurich chemist Karl Heumann developed a new synthesis route based on N -phenylglycine (1st Heumann synthesis ). The chemical company BASF and Farbwerke Hoechst patented and further developed the process. The series of experiments with phenylglycine was discontinued by BASF in 1893 because the indigo yield was very low.

- 1. Heumann synthesis (aniline ( 1 ), chloroacetic acid ( 2 ), N -phenylglycine ( 3 ), indoxyl ( 4 ), indigo ( 5 ))
Heumann developed an alternative synthesis route in 1897. By reacting anthranilic acid with chloroacetic acid , Heumann arrived at phenylglycine- o- carboxylic acid. If this substance is heated to 200 ° C with potassium hydroxide in an inert atmosphere , 2-indoxylcarboxylic acid is formed. This material easily decarboxylates to indoxyl, which oxidizes to indigo in air (2nd Heumann synthesis).

- 2. Heumann synthesis (anthranilic acid ( 1 ), chloroacetic acid ( 2 ), phenylglycine o -carboxylic acid ( 3 ), 2-indoxylcarboxylic acid ( 4 ), indoxyl ( 5 ), indigo ( 6 ))
industrial production
A lucky coincidence with a broken thermometer led to the discovery that mercury sulfate was a suitable catalyst for the oxidation of naphthalene, which is produced in large quantities in the tar dye industry, to phthalic acid . The phthalic acid could be converted into the acid amide by reaction with ammonia . The subsequent Hofmann rearrangement gave BASF anthranilic acid, which was required on an industrial scale as a starting material for the Heumann synthesis.

- Production of anthranilic acid by means of Hofmann rearrangement of the phthalimide
By means of the 2nd Heumann synthesis, the anthranilic acid could be processed into indigo in yields of 70 to 90%. From 1897 onwards, BASF produced synthetic indigo on an industrial scale using this process.
In 1901 Degussa succeeded in using a process by Johannes Pfleger, using sodium amide , which was produced in the Castner-Kellner process to produce sodium cyanide , and an alkali melt N -phenylglycine at a temperature of around 200 ° C to obtain indigo in high yields (Heumann -Pfleger synthesis). The sodium amide serves as a dehydrating agent. This process was marketed together with Farbwerke Hoechst:
BASF has been producing indigo using this process since 1926.
In 1905, BASF developed a process variant of the Heumann-Pfleger synthesis in which the expensive sodium amide was replaced by cheaper calcium oxide . The process was carried over to a previously developed synthetic route. Aniline is reacted with ethylene chlorohydrin to form 2-anilinoethanol , which is converted into indoxyl in a sodium hydroxide - potassium hydroxide - calcium oxide melt at temperatures of about 280 ° C. with satisfactory yields . BASF produced according to this process from 1909 to 1924.
From 1924, indigo synthesis at BASF was based on phenylglycine nitrile, which was made from aniline. In all cases indoxyl is formed, which is oxidized to indigo by atmospheric oxygen. The obvious assumption that indigo is formed by base-catalyzed condensation between isatin and indoxyl could be ruled out by mechanistic studies. The oxidation of indoxyl in basic solution probably takes place via a radical intermediate . Whether the formation takes place via the coupling of two indoxyl radicals or the coupling of an indoxyl radical and an indoxyl anion could not be clearly established experimentally.
Microbiological synthesis
It was already observed in the 1920s that soil bacteria could synthesize indigo from indole . A number of microbial indigo producers, such as Pseudomonas putida , are now known that can form indigo from aromatic hydrocarbons such as naphthalene , cumene or styrene . The enzyme system responsible for indigo formation consists of one or more enzymes, typically monooxygenases , dioxygenases or hydroxylases .
The major problems of the microbiological route are the high dilution of the indigo and the effort involved in separating the considerable amount of organic material. So far, a microbiological synthesis route has not been commercialized.
Forms of trade
Major European producers of indigo are DyStar , originally a joint venture between Bayer , Hoechst and BASF and since 2010 in Sino-Indian ownership, and Archroma , a spin-off of the Swiss company Clariant . There are a large number of manufacturers in Asia, including TaiFeng Chemical Industrial , Zhejiang Runtu and Bodal Chemicals . Indigo is available in a non-reduced or pre-reduced form. The non-reduced grades are available as granules , powder or alkaline paste. Typical commercial forms of the paste contain 20 to 30% indigo. The pre-reduced solutions are available in concentrations from 20 to 60%.
characteristics
Physical Properties
Indigo has a fairly high melting point of around 390–392 ° C and sublimates at a temperature of 170 ° C. It is poorly soluble in water, ethanol and diethyl ether. This is due to the fact that indigo forms a hydrogen bridge polymer in the solid state . X-ray structure analyzes have shown that each indigo molecule is bound to four surrounding molecules. Indigo crystallizes monoclinically in the space group P 2 1 / c (No. 14) with the lattice parameters a = 924 pm ; b = 577 pm; c = 1222 pm and β = 117.0 °, Z = 2.
The infrared spectrum can be determined using ATR infrared spectroscopy . The stretching vibration of the carbonyl group at a wave number of 1623 cm −1 is characteristic . An intense band at 1065 cm −1 is assigned to the vibration of the five-membered ring.
Molecular Properties
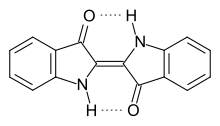
Indigo has a planar structure with a C 2h symmetry . The photochemical stability of indigo is due to intramolecular hydrogen bonds between the neighboring carbonyl and secondary amino groups . These stabilize the molecule in a planar trans configuration and thus inhibit a photochemical cis-trans isomerization .
The color of a molecule results from its ability to absorb electromagnetic radiation. If this takes place in the range of visible light, the fabric appears colored. The complementary color to the color of the absorbed light is always perceived as the color. The condition for color is therefore the occurrence of electrons that are excited by visible light. According to Witt's dye theory , a dye consists of a chromophore ( ancient Greek χρῶμα chrṓma , German 'color' , ancient Greek φορός phorós , German 'carrying' ), for example a delocalized π-electron system that makes the color possible, and an auxochrome (from ancient Greek αὐξεῖν auxein , German 'grow' and ancient Greek χρῶμα chrṓma , German 'color' ), for example an electron donor with a + M effect that shifts the absorption maximum of the chromophore into the longer-wave range of the spectrum. Indigo has two conjugated carbonyl groups as a chromophore. Because of this structural element, indigo belongs to the group of carbonyl dyes .
The absorption of electromagnetic radiation then causes an electron transfer from the highest occupied molecular orbital ( HOMO ) to the lowest unoccupied molecular orbital ( LUMO ). If there is no auxochromic group, the energy difference between HOMO and LUMO is relatively large and the absorption through the π → π * transition occurs in the invisible range. The lone pair of electrons of the secondary amino group of the indigo serves as an electron donor and interacts with the π system of the chromophore. This creates three new molecular orbitals π 1 , π 2 and π * 3 , whereby the energy difference between HOMO and LUMO becomes smaller. The π 2 → π * 3 transition occurs in indigo through light in the orange area. Indigo therefore appears blue.
Chemical properties
The elemental analysis of indigo provides an empirical molecular formula of C 8 H 5 NO. By cryoscopic measurements results in a molecular formula of C 16 H 10 N 2 O 2 . Indigo is a very light and temperature stable molecule. At higher temperatures of around 460 ° C, it rearranges to form dibenzonaphthyridinedione , breaking the bonds between the carbonyl groups and the central carbon-carbon double bond .
In the alkaline, the molecule breaks down at higher temperatures into compounds such as aniline and anthranilic acid. The sulphonation to indigo carmine takes place in concentrated sulfuric acid . Oxidation with potassium permanganate provides isatin.
Indigo can be easily reduced in the weakly acidic and alkaline range. The leuco form is in the keto form below a pH value of 5.5. The mono-enolate is present in the range between 5.5 and 11 and the di-enolate from a pH value of 11.

- Reduction of indigo to the leuco form (depending on the pH value as di-enolate, mono-enolate or diketone)
With chlorosulfonic acid , the leuco form forms sodium salts of sulfuric acid esters , so-called indigosols . The indigosols are water-soluble and are suitable for dyeing wool, which is then oxidized with nitrous acid , with saponification taking place at the same time . As a mono-chelating ligand , indigo forms soluble metal complexes with palladium and platinum salts that have neither intra- nor intermolecular hydrogen bonds.
use
Vat coloring
Indigo is used for dyeing, among other things, because of its excellent light stability. The compound is strongly absorbed by cotton fibers and is very washable. Indigo itself is almost insoluble in water and must be converted into the water-soluble leuco indigo (from Greek leukós : white, shiny) by reduction before dyeing , for example using sodium dithionite , the so-called vatting.
Before sodium dithionite was used, the so-called fermentation vat traditionally consisted of a fermentable material such as syrup or other carbohydrate-containing substances and an alkaline additive such as lime or urine . The carbohydrates served as a reducing agent . Other reducing agents such as arsenic sulfide , iron (II) sulfate or zinc dust were used later .
For dyeing, the cotton fabric is then placed in the aqueous vat solution with the reduced, colorless leuco form. The soluble component is absorbed by the fiber and when it is dried in the air, indigo is created again through oxidation. Accordingly, the blue coloration only occurs after contact with oxygen. The indigo does not form a chemical bond with the fiber, but adheres to it through adhesive forces .
This process is known as vat dyeing and is also used for other textile dyes. In the past, to oxidize the dye, the fabrics were placed in the sun on a meadow, where the indigo was oxidized by bleaching the lawn . Synthetically produced indigo is widely used as a vat dye in the textile industry. For the most part, indigo is used to dye denim fabrics.
Other textile dyeing methods
In England, dyers developed two other methods of indigo dyeing in the 18th and 19th centuries. The first method is known as "Pencil Blue". Arsenic trisulfide and a thickener were added to the leuco indigo for stabilization . The arsenic compound delayed the oxidation of the indigo so that it could be applied to textiles with pencils or brushes.
In the second method, the insoluble indigo was printed directly on the fabric and then reduced using iron (II) sulfate . Oxidation with air then took place again. This so-called "China blue" process produced clear designs. Towards the end of the 19th century, John Bracewell developed the glucose process, which allowed direct printing with indigo.
The blueprint is a traditional textile printing technology as well as the name for the dark blue fabric with white patterns produced with this printing method. It is a reserve print in which the fabric is printed with a protective compound to keep it white. After printing with the protective mass, the fabric is dyed with indigo.
Traditional Chinese medicine
In traditional Chinese medicine , Indigo Naturalis is used in combination with realgar containing arsenic in the treatment of promyelocytic leukemia . Indigo Naturalis is also used against psoriasis . The active ingredient in Indigo Naturalis preparations, however, appears to be indirubin , which acts as an inhibitor of cyclin-dependent kinases . Banlangen , a traditional Chinese remedy, is made from the roots of woad or indigo plants, for example, and is used to treat sore throat and larynx and a variety of other diseases.
Other uses
The Maya have been making the Maya blue pigment since around 800 AD. The pigment has been identified as a composite of palygorskite and indigo, probably derived from the leaves of a native species of indigofera. A current recipe for making Maya blue was published in 1993.
For technical purposes , indigo can be used in the form of thin organic films for the construction of solar cells . Research has shown that indigo can be used in field effect transistors . A thin film of semi-crystalline indigo is a semiconductor with a band gap of 1.7 eV and thus a potential material for organic electronics .
Indigo was rarely used in oil painting until around the end of the 17th century. One of the most famous examples is Vermeer's work “ Christ with Maria and Martha ”, in which both the blue cloak of Christ and the skirt of Maria are painted with indigo.
Indigo can be used to measure the concentration of ozone in the air. For this purpose, chromatography paper is soaked in indigo. Isatin is formed when it comes into contact with the ozone in the air. This is determined photometrically after elution . Another reaction product is isatoic anhydride. Indigoid dyes can also be used to detect ozone.
In Japan, Indigo Naturalis was used in traditional medicine as an anti-inflammatory substance. The samurai wore clothing dyed with indigo to heal wounds and injuries. Due to the suspected positive health effects, indigo-colored blankets and clothing are still traditional gifts for newborns to protect them from disease. Roman and Greek remedies contained indigo in part.
Indigoid dyes
Indigoid dyes are structurally related to indigo. These include, for example, the variants that are created by changing the basic indigo structure, for example by replacing the secondary amino group with other electron donors such as selenium , sulfur or oxygen . The table shows the comparison of the longest-wave absorption band of these compounds in comparison with indigo (λ max. At 606 nm).
| Surname | Basic structure | X | λ max (nm) (in ethanol ) |
|---|---|---|---|
| indigo |

|
NH | 606 |
| Selenium indigo | Se | 562 | |
| Thioindigo | S. | 543 | |
| Oxindigo | O | 432 |
The synthesis of structural isomers or the derivatization of the benzene ring open up further possibilities for color variation. The structural isomer indirubin is a red-violet dye and indigo carmine (5,5'-indigodisulfonic acid disodium salt) is a blue dye. Ring-substituted derivatives of indigo can be synthesized in various ways. The use of bromine-substituted nitrobenzaldehyde in the classical synthesis according to Baeyer leads to the formation of purple (6,6'-dibromoindigo), an ancient dye obtained from purple snails . Its structure was clarified in 1909 by Paul Friedlaender . Further examples of indigoid dyes are 5,5 ', 7,7'-tetrabromoindigo (brilliant indigo B) or 2- (5-bromoindole) -5-bromo-2'-thionaphthene indigo (Cibaviolet 3B). Many different indigoid dyes such as thioindigo or tetrachloroindigo are commercially available. Compared to indigo, however, the economic importance of the derivatives is rather minor.
Toxicology and environmental aspects
Indigo has low mammalian toxicity. The LD 50 value in the mouse is 32 g / kg. There is no evidence of sensitization in humans after repeated skin applications. Feeding experiments on rats and dogs with up to 3% by weight indigo in the feed did not show any serious adverse health consequences.
The chemicals used in the production of indigo, such as aniline, formaldehyde or hydrogen cyanide, are partly toxic and environmentally hazardous and are therefore only handled in closed systems. Indigo has no adverse effects on activated sludge systems in biological wastewater treatment plants due to its low solubility, but the rate of biodegradation is low. Ultrafiltration systems are suitable for removing the pigment before it is introduced into the sewage treatment plant. Unreacted raw materials such as aniline or anthranilic acid are easily biodegradable. To reduce the salt load in the actual dyeing process, pre-reduced applications are offered that make the use of sodium dithionite as a reducing agent unnecessary. In recent years, a new form of trade for indigo has therefore been established. An indigo suspension is catalytically reduced to the leuco form with hydrogen and marketed as a pre-reduced, concentrated liquid indigo brand.
literature
- Elmar Steingruber: Indigo and indigo colorants. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2004 doi: 10.1002 / 14356007.a14 149.pub2 .
- Renate Kaiser-Alexnat: Indigo. The king of dyes. In: Southeast Asia Magazine . Volume 3, 2008, pp. 110–121, (PDF; 3.3 MB) , (special print) .
- Helmut Schmidt: Indigo. 100 years of industrial synthesis. In: Chemistry in Our Time . Volume 3, 1997, pp. 121-128, doi: 10.1002 / ciuz.19970310304 .
- Paul Rys, Heinrich Zollinger: Dye Chemistry - A Guide. 3rd revised edition, Wiley-VCH, Weinheim 1982, ISBN 3-527-25964-3 .
Web links
- Cultivation of indigofera and the traditional extraction of indigo in India (9:47 min.) On YouTube .
Individual evidence
- ↑ a b c d Entry on indigo in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d e f g h Elmar Steingruber: Indigo and indigo colorants . In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2004, doi: 10.1002 / 14356007.a14 149.pub2 .
- ↑ a b Entry on Indigo. In: Römpp Online . Georg Thieme Verlag, accessed on May 4, 2014.
- ↑ Albert Gossauer: Structure and reactivity of biomolecules. Verlag Helvetica Chimica Acta, Zurich 2006, p. 477, ISBN 978-3-906390-29-1 .
- ↑ Christian-Herbert Fischer: Historical organic dyes. In: Spectrum of Science . October 1997, p. 104.
- ↑ a b c d e f g Renate Kaiser-Alexnat: Indigo - The King of Dyes . In: Southeast Asia Magazine . 3/2008; Pp. 110-121, ISBN 978-3-7375-9664-0 .
- ^ Reintroducing Indigo .
- ↑ Can a blue dye help save the Aral Sea?
- ↑ a b R. Hegnauer, M. Hegnauer: Chemotaxonomie der Pflanzen. Volume XI: Leguminosae. Birkhäuser, Basel / Boston / Berlin 1994, ISBN 978-3-0348-9392-3 , p. 1047.
- ↑ a b Colleen E. Kriger: Cloth in West African History. Altamira Press, New York 2006, ISBN 978-0-7591-0421-1 , pp. 120-124.
- ↑ Jeffrey C. Splitstoser, Tom D. Dillehay, Jan Wouters, Ana Claro: Early pre-Hispanic use of indigo blue in Peru. In: Science Advances . Volume 2, 2016, p. E1501623; doi: 10.1126 / sciadv.1501623 .
- ^ W. Conard Fernelius, Edgar E. Renfrew: Indigo. In: Journal of Chemical Education . Volume 60, 1983, p. 633, doi : 10.1021 / ed060p633 .
- ↑ Gaius Iulius Caesar, De bello Gallico 5,14,2 ( online ).
- ^ M. Van der Veen, AR Hall, J. May: Woad and the Britons Painted Blue. In: Oxford Journal of Archeology . Volume 12, No. 3, 1993, pp. 367-371; doi: 10.1111 / j.1468-0092.1993.tb00340.x .
- ↑ Margarete Bruns: From azurite, indigo and aniline. The history of the blue color. In: Emil Ernst Ploß: A book of old colors. Technology of textile colors in the Middle Ages with an outlook on solid colors. 6th edition, Impuls Verlag, Munich 1989, ISBN 978-3-89164-060-9 , pp. 14-20.
- ↑ Reinhard Schneider : Capitulare de villis. In: Concise dictionary on German legal history . Volume 1, 2nd edition, Schmidt, Berlin, ISBN 978-3-503-07912-4 , pp. 809-811.
- Jump up ↑ Robin JH Clark, Christopher J. Cooksey, Marcus AM Daniels, Robert Withnall: Indigo, woad, and Tyrian Purple: important vat dyes from antiquity to the present. In: Endeavor . Neue Serie, Volume 17/4, 1993, 192, pp. 191-199; doi: 10.1016 / 0160-9327 (93) 90062-8 .
- ^ Wilhelm Hassenstein, Hermann Virl : The fireworks book from 1420. 600 years of German powder weapons and gunsmithing. Reprint of the first print from 1529 with translation into High German and explanations by Wilhelm Hassenstein. Verlag der Deutsche Technik, Munich 1941, p. 108 ( Indig, indicum or Waidtsblau and Waidblum ).
- ↑ a b c d e f g h i j k l Helmut Schmidt: Indigo - 100 years of industrial synthesis. In: Chemistry in Our Time . Volume 31, 1997, pp. 121-128; doi: 10.1002 / ciuz.19970310304 .
- ^ David Paterson: Concerning Indigo, Natural and Artificial. In: Oil and Colourman's Journal . August 1905, Nos. 857 and 358, Volume XXVIII.
- ↑ Sabine Struckmeier: Textile dyeing from the late Middle Ages to the early modern period . Waxmann, Münster 2011, ISBN 978-3-8309-2527-9 , pp. 177-178.
- ↑ Werner Abelshauser: The BASF: A company history. 2nd edition, Beck, Munich 2003, ISBN 3-406-49526-5 , p. 132.
- ↑ Lauren Wolf: What's That Stuff? Blue jeans . In: Chemical & Engineering News . October 24, 2011, Volume 89, Number 43, p. 44.
- ^ AD Kinghorn, H. Falk, J. Kobayashi: Progress in the Chemistry of Organic Natural Products 99.Springer -Verlag, Heidelberg / New York 2014, ISBN 978-3-319-04899-4 , p. 88.
- ↑ Wrangler denim Adopts the Industry's First "Dry-Dyeing" Process.
- ^ Electrochemical indigo machine debuts in Pakistan .
- ↑ M. Josef Taublaender, Florian Glöcklhofer, Martina Marchetti-Deschmann, Miriam M. Unterlass: Inside Cover: Green and Rapid Hydrothermal Crystallization and Synthesis of Fully Conjugated Aromatic Compounds. In: Angewandte Chemie International Edition . Volume 57, 2018, p. 12180; doi: 10.1002 / anie.201808280 .
- ↑ Entry in the Enzyme nomenclature database for anthranilate phosphoribosyltransferase .
- ^ A b A. D. Kinghorn, H. Falk, J. Kobayashi: Progress in the Chemistry of Organic Natural Products 99.Springer -Verlag, Heidelberg / New York 2016, ISBN 978-3-319-34645-8 , p. 82.
- ↑ Entry in the Enzyme nomenclature database for phosphoribosyl anthranilate isomerase .
- ↑ a b Arne Andersen: Historical technology assessment using the example of metalworking and the chemical industry 1850-1933 (== Zeitschrift Fur Unternehmensgeschichte - Supplements. Volume 90). Steiner, Stuttgart 1996, p. 238.
- ↑ Adolf Baeyer, Viggo Drewsen: Representation of indig blue from orthonitrobenzaldehyde. In: Reports of the German Chemical Society . Volume 15, 1882, pp. 2856-2864; doi: 10.1002 / cber.188201502274 .
- ^ Fritz Mayer: Chemistry of organic dyes . Springer-Verlag, Berlin, Heidelberg 1921, ISBN 978-3-662-05490-1 , pp. 199 ( limited preview in Google Book search).
- ↑ BASF Patent DE 171,172, filed on January 23, 1904th
- ^ Glen Allan Russell, Gerd Kaupp: Reactions of resonance stabilized carbanions. XXXI. Oxidation of carbanions. 4. Oxidation of indoxyl to indigo in basic solution. In: Journal of the American Chemical Society . Volume 91, 1969, pp. 3851-3859; doi: 10.1021 / ja01042a028 .
- ↑ PHH Gray: The Formation of Indigotin from Indol by Soil Bacteria. In: Proceedings of the Royal Society B: Biological Sciences . Vol. 102, 1928, pp. 263-281; doi: 10.1098 / rspb.1928.0003 .
- ↑ B. Bhushan, SK Samanta, RK Jain: Indigo production by naphthalene-degrading bacteria. In: Letters in Applied Microbiology . Volume 31, 2000, p. 5; doi: 10.1046 / j.1472-765x.2000.00754.x .
- ^ Klaus Hunger: Industrial Dyes: Chemistry, Properties, Applications. Wiley-VCH, Weinheim 2003, ISBN 3-527-30426-6 , p. 231.
- ^ Paul Rys, Heinrich Zollinger: Dye chemistry. A guide. 3rd revised edition, Wiley-VCH, Weinheim 1982, ISBN 3-527-25964-3 , p. 137.
- ↑ H. v. Eller In: Bulletin de la Société Chimique de France . Volume 106, 1955, p. 1426.
- ↑ Peter Süsse, Manfred Steins, Vladimir Kupcik: Indigo: Crystal structure refinement based on synchrotron data. In: Journal of Crystallography - Crystalline Materials. Volume 184, 1988, pp. 269-273; doi: 10.1524 / zkri.1988.184.14.269 .
- ↑ Anna Baran, Andrea Fiedler, Hartwig Schulz, Malgorzata Baranska: In situ Raman and IR spectroscopic analysis of indigo dye. In: Analytical Methods . Volume 2, 2010, p. 1372; doi: 10.1039 / c0ay00311e .
- ^ A b Stefan Bienz, Laurent Bigler, Thomas Fox, Herbert Meier: Spectroscopic methods in organic chemistry. 9th edition, Thieme, Stuttgart / New York 2016, ISBN 978-3-13-576109-1 .
- ↑ Vu Thi Ngan, G. Gopakumar, Tran Thanh Hue, Minh Tho Nguyen: The triplet state of indigo: Electronic structure calculations . In: Chemical Physics Letters . Volume 449, 2007, pp. 11-17; doi: 10.1016 / j.cplett.2007.10.015 .
- ^ J. Seixas de Melo, AP Moura, MJ Melo: Photophysical and spectroscopic studies of indigo derivatives in their keto and leuco forms . In: Journal of Physical Chemistry A . Volume 108, No. 34, 2004, pp. 6975-6981; doi: 10.1021 / jp040753y .
- ↑ Eberhard Breitmaier, Günther Jung: Organic chemistry. Edition 5, Thieme, Stuttgart / New York 2005, ISBN 978-3-13-541505-5 , pp. 728-734.
- ↑ Günter Hauke, Gerhard Graneß: Thermal Isomerization of indigo. In: Angewandte Chemie International Edition in English . Volume 34, 1995, p. 67; doi: 10.1002 / anie.199500671 .
- ↑ GA Baig: Dyeing nylon with indigo in various pH regions . In: AUTEX Research Journal . Volume 10, 2010, pp. 21-25.
- ↑ F. Weiss: The indigo sols and their use . In: The vat dyes and their use in dyeing and fabric printing. Springer-Verlag, Vienna 1953, ISBN 978-3-7091-7827-0 , pp. 313-364.
- ↑ Wolfgang Beck, Christoph Schmidt, Rolf Wienold, Manfred Steimann, Barbara Wagner: Indigo-Metal Complexes: Synthesis and Structure of Pd II and Pt II Compounds Containing the Anions of Indigo and Octahydroindigo as Mono- and Bis-Chelate Ligands. In: Angewandte Chemie International Edition in English. Volume 28, 1989, pp. 1529-1531; doi: 10.1002 / anie.198915291 .
- ↑ Izumi Iwakura, Atushi Yabushita, Takayoshi Kobayashi: Why is Indigo Photostable over Extremely Long Periods ?. In: Chemistry Letters . Volume 38, 2009, p. 1020; doi: 10.1246 / cl.2009.1020 .
- ↑ Laksanawadee Saikhao, Jantip Setthayanond, Thitinun Karpkird, Thomas Bechtold, Potjanart Suwanruji: Green reducing agents for indigo dyeing on cotton fabrics. In: Journal of Cleaner Production. Volume 197, 2018, pp. 106-113; doi: 10.1016 / j.jclepro.2018.06.199 .
- ^ KG Gilbert nee Stoker, DT Cooke: Dyes from plants: Past usage, present understanding and potential . In: Plant Growth Regulation . Volume 34, Number 1, 2001, pp. 57-69; doi: 10.1023 / A: 1013374618870 .
- ^ DS Balan, RT Monteiro: Decolorization of textile indigo dye by ligninolytic fungi . In: Journal of Biotechnology . Volume 89, Numbers 2-3, 2001, pp. 141-145; PMID 11500207 .
- ↑ a b P. C. Floud: The English contribution to the Early History of Indigo printing. In: Journal of the Society of Dyers and Colourists . Volume 76, 1960, pp. 344-349; doi: 10.1111 / j.1478-4408.1960.tb02382.x .
- ↑ Patent US409906 : Process of printing dark blue colors.
- ↑ Nationwide directory of intangible cultural heritage: blueprint .
- ↑ L. Wang, G.-B. Zhou, P. Liu, J.-H. Song, Y. Liang, X.-J. Yan, F. Xu, B.-S. Wang, J.-H. Mao, Z.-X. Shen, S.-J. Chen, Z. Chen: Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. In: Proceedings of the National Academy of Sciences . Volume 105, 2008, pp. 4826-4831; doi: 10.1073 / pnas.0712365105 .
- ↑ John Koo, Sumaira Arain: Traditional Chinese Medicine for the Treatment of Dermatologic Disorders. In: Archives of Dermatology . Volume 134, 1998, pp. 1388-1393. doi: 10.1001 / archderm.134.11.1388 .
- ↑ Ralph Hoessel, Sophie Leclerc, Jane A. Endicott, Martin EM Nobel, Alison Lawrie, Paul Tunnah, Maryse Leost, Eve Damiens, Dominique Marie, Doris Marko, Ellen Niederberger, Weici Tang, Gerhard Eisenbrand, Laurent Meijer: Indirubin, the active constituent of a Chinese antileukemia medicine, inhibits cyclin-dependent kinases. In: Nature Cell Biology . Volume 1, 1999, pp. 60-67; doi: 10.1038 / 9035 .
- Jump up ↑ Antonio Doménech-Carbó, Sigrid Holmwood, Francesca Di Turo, Noemí Montoya, Francisco Manuel Valle-Algarra, Howell GM Edwards, María Teresa Doménech-Carbó: Composition and Color of Maya Blue: Reexamination of Literature Data Based On the Dehydroindigo Model. In: Journal of Physical Chemistry C . Volume 123, 2018, pp. 770-782; doi: 10.1021 / acs.jpcc.8b08448 .
- ^ Constantino Reyes-Valerio: De Bonampak al Templo Mayor. In: El azul maya en Mesoamerica. Siglo XXI Editores, Mexico DF 1993, p. 157. Azul Maya - Maya Blue Pigment. (English).
- ↑ K. Uehara, K. Takagishi, M. Tanaka: The Al / Indigo / Au photovoltaic cell. In: Solar Cells . Volume 22, 1987, pp. 295-301; doi: 10.1016 / 0379-6787 (87) 90059-7 .
- ↑ Roswitha Harrer: Indigo on memory chips. In: Chemistry in Our Time . Volume 46, 2012, p. 136; doi: 10.1002 / ciuz.201290032 .
- ↑ Mihai Irimia-Vladu, Eric D. Głowacki, Pavel A. Troshin, Günther Schwabegger, Lucia Leonat, Diana K. Susarova, Olga Krystal, Mujeeb Ullah, Yasin Kanbur, Marius A. Bodea, Vladimir F. Razumov, Helmut Sitter, Siegfried Bauer, Niyazi Serdar Sariciftci: Indigo - A Natural Pigment for High Performance Ambipolar Organic Field Effect Transistors and Circuits. In: Advanced Materials . Volume 24, 2012, pp. 375-380; doi: 10.1002 / adma.201102619 .
- ^ H. Schweppe: Indigo and Woad . In: EW FitzHugh (Ed.): Artists' Pigments. A Handbook of Their History and Characteristics. Volume 3, Oxford University Press, Oxford 1997, pp. 81-107.
- ^ Vermeer, Christ in the House of Martha and Mary , ColourLex.
- ↑ P. Remler, W. Kosmus: Integral long-term method for determining ozone in the atmosphere. In: Fresenius' magazine for analytical chemistry . Volume 329, 1988, pp. 871-874; doi: 10.1007 / BF00471974 .
- ^ Daniel Grosjean, Paul M. Whitmore, Glen R. Cass, James R. Druzik: Ozone fading of natural organic colorants: mechanisms and products of the reaction of ozone with indigos. In: Environmental Science & Technology . Volume 22, 1988, pp. 292-298; doi: 10.1021 / es00168a009 .
- ↑ Samurai Blue: A History of Indigo
- ↑ Rebecca Futo Kennedy, Molly Jones-Lewis: The Routledge Handbook of Identity and the Environment in the Classical and Medieval Worlds . Routledge, New York 2016, ISBN 978-0-415-73805-7 , pp. 155-161.
- ^ Paul Rys, Heinrich Zollinger: Dye chemistry. A guide. 3rd revised edition, Wiley-VCH, Weinheim 1982, ISBN 3-527-25964-3 , p. 136.
- ↑ Gundula Voss, Hans Gerlach: Regioselective bromine / lithium exchange with 2,5-dibromo-1-nitrobenzene. - A simple synthesis of 4-bromo-2-nitrobenzaldehyde and 6,6′-dibromoindigo. In: Chemical Reports . Vol. 122, 1989, pp. 1199-1201; doi: 10.1002 / cber.19891220628 .
- ↑ Paul Friedländer: About the dye of ancient purple from murex brandaris. In: Reports of the German Chemical Society . Volume 42, 1909, pp. 765-770; doi: 10.1002 / cber.190904201122 .
- ↑ E. Grandmougin, P. Seyder: About Indigo. V: About halogenated indigo and derivatives. In: Reports of the German Chemical Society . Volume 47, 1914, pp. 2365-2373; doi: 10.1002 / cber.191404702154 .
- ↑ Data sheet Indigo (CI 73000) synth. (PDF) from Carl Roth , accessed on November 3, 2019.
- ↑ Anne Vuorema: Reduction and Analysis Methods of indigo. University of Turku, 2008, ISBN 978-9-51293781-3 , p. 14.
- ^ Abraham Reife, Harold S. Freeman: Environmental Chemistry of Dyes and Pigments. John Wiley and Sons, New York 1996, ISBN 0-471-58927-6 , pp. 208-209.
- ↑ Patent application DE19831291 : Concentrated leuco-indigo solution, especially for dyeing cotton warp yarn for blue denim, contains leuco-indigo in the form of a mixture of salts of at least two alkali metal hydroxidesl. Registered on July 13, 1998 , published on February 20, 2000 , applicant: BASF AG, inventor: Manfred Gäng, Rudolf Krüger, Peter Miederer.