atom


Atoms (from ancient Greek ἄτομος átomos "indivisible") are the building blocks from which all solid , liquid or gaseous substances consist. All material properties of these substances as well as their behavior in chemical reactions are determined by the properties and the spatial arrangement of their atoms. Every atom belongs to a certain chemical element and forms its smallest unit. 118 elements are currently known, of which about 90 occur naturally on earth. Atoms of different elements differ in their size and mass and above all in their ability to react chemically with other atoms and to combine to form molecules or solid bodies . The diameters of atoms are in the range from 6 · 10 −11 m ( helium ) to 5 · 10 −10 m ( cesium ), their masses in a range from 1.7 · 10 −27 kg ( hydrogen ) to just under 5 · 10 −25 kg (currently the heaviest synthetically manufactured cores).
Atoms are not indivisible, as assumed at the time they were named, but show a well-defined structure made up of even smaller particles. They consist of an atomic nucleus and an atomic shell . The atomic nucleus has a diameter of about one ten to one hundred thousandth of the total atomic diameter, but contains over 99.9 percent of the atomic mass. It consists of positively charged protons and a number of roughly equally heavy, electrically neutral neutrons . These nucleons are bound to each other by the strong interaction . The shell consists of negatively charged electrons . It contributes less than 0.06 percent to the mass, but determines the size of the atom. The positive core and the negative shell are bound to one another by electrostatic attraction. In the electrically neutral basic form of the atom, the number of electrons in the shell is equal to the number of protons in the nucleus. This number determines the exact structure of the shell and thus also the chemical behavior of the atom and is therefore known as the chemical atomic number . All atoms of the same element have the same chemical atomic number. If additional electrons are present or missing, the atom is negatively or positively charged and is called an ion .
The idea of the atomic structure of matter already existed in antiquity , but was controversial until modern times . The final proof could not be provided until the beginning of the 20th century and is considered one of the most important discoveries in physics and chemistry . Individual atoms can not be seen even with the most powerful light microscopes . Direct observation of individual atoms has only been possible with field ion microscopes since the middle of the 20th century , and for some years also with scanning tunneling microscopes and high-resolution electron microscopes . The atomic physics that studies in addition to the structure of atoms and the processes in their interior and their interactions with other atoms, has significantly to the development of modern physics and in particular the quantum mechanics contributed.
History of exploration
The idea of the atomic structure of matter already existed in antiquity . Due to their extremely small size, individual atoms can not be seen even with the most powerful light microscopes ; their existence was still a matter of dispute at the beginning of the 20th century. The final proof is considered to be one of the most significant discoveries in physics and chemistry . Albert Einstein made a decisive contribution in 1905, when he explained the long-known Brownian movement of small grains, which is directly visible in the microscope, through accidental collisions of atoms or molecules in their surroundings. Only for a few decades have field ion microscopes and scanning tunneling microscopes , and for a few years also electron microscopes , been able to observe individual atoms directly.
Philosophical considerations
The concept of atomism , namely that matter is made up of basic units - "smallest particles" that cannot be broken down into smaller pieces - has existed for thousands of years, just like the counter-concept that matter is a continuum that can be divided at will. But these ideas were initially based solely on philosophical considerations and not on empirical experimental investigation. At the same time, different properties were ascribed to the atoms, very different properties depending on the age, culture and philosophical school.
An early mention of the atomic concept in philosophy is known from India. The Nyaya and Vaisheshika schools developed elaborate theories on how atoms combined to form more complex structures (first in pairs, then three pairs each).
Experimental natural scientists adopted the atom hypothesis at the end of the 18th century because this hypothesis offered an elegant explanation for new discoveries in chemistry within the framework of a particle model of matter. At the same time, however, the opposite notion that matter is a continuum was maintained by philosophers and also among natural scientists into the 20th century.
In Greek philosophy, the atomic concept was first used in the 5th century BC. At Leukippus . His pupil Democritus systematized it and introduced the term átomos ( ἄτομος ), which means something that cannot be cut up, i.e. an object that cannot be further divided. This name was adopted at the end of the 18th century for the then hypothetical smallest units of the chemical elements of the beginning modern chemistry, because with chemical methods atoms can in fact not be "cut up".
Scientific research
The existence of atoms has been confirmed in the course of scientific research. Many different atomic models have been developed to describe their structure. In particular, the hydrogen atom as the simplest of all atoms was important. Some of the models are no longer used today and are only of interest in the history of science. Others still apply today as an approximation, depending on the area of application. As a rule, the simplest model is used, which is still sufficient in the given context to clarify the questions that arise.
Confirmation of the atomic hypothesis

Robert Boyle , in his work The Skeptical Chymist in 1661, was of the opinion that matter was built up from various combinations of different corpuscules and not from the four elements of alchemy : water, earth, fire, air. In doing so, he prepared the overcoming of alchemy through the element and atom concepts of modern chemistry.
Daniel Bernoulli showed in 1740 that the even pressure of gases on the container walls and in particular the law of Boyle and Mariotte can be explained by countless collisions of the smallest particles. This made his research the forerunner of kinetic gas theory and statistical mechanics .
From the end of the 18th century, the idea of atoms was used to attribute the well-defined angles at the edges and corners of the gemstones to the various possible layers of hard spheres .
After Antoine Lavoisier had coined the current term of the chemical element in 1789 and correctly identified the first elements, John Dalton used the atomic concept in 1803 to explain why elements always react with one another in proportions of small whole numbers ( law of multiple proportions ). He assumed that every element consists of atoms of the same type, which can combine with one another according to fixed rules and thus form substances with different material properties. He also assumed that all atoms of an element had the same mass, and founded the term atomic weight .
Amedeo Avogadro was able to summarize the observations on the chemical and physical behavior of gases in 1811 in such a way that two approximately ideal gases always consist of the same number of identical particles ("molecules") with the same values of volume, pressure and temperature of the gas. In elementary gases such as hydrogen, oxygen or nitrogen, the molecules always consist of two atoms of the element ( Avogadro's law ).
In 1866 Johann Loschmidt was able to determine the size of the air molecules by evaluating the values measured by George Stokes for the internal friction in air using the formula obtained by James C. Maxwell from the kinetic gas theory . This enabled him to determine the weight of an air molecule. He also received the Loschmidt number named after him as the number of air molecules per cubic centimeter.
As a result of the work of Avogadro and Stanislao Cannizzaro , it was assumed that atoms do not appear as individual particles, but only as components of molecules made up of at least two atoms. But in 1876 August Kundt and Emil Warburg succeeded in making the first evidence of a monatomic gas. They determined the adiabatic exponent of mercury vapor at high temperature and obtained a value that, according to the kinetic gas theory, can only occur for particles in the form of real mass points . From 1895, corresponding observations were made on the newly discovered noble gases .
After his dissertation on the determination of molecular dimensions was published, Albert Einstein proposed an experiment in the same year in 1905 to quantitatively test the hypothesis of the existence of atoms based on the trembling motion of small particles in water. According to his theory, due to the irregularity of the impacts by the water molecules, the particles should perform small movements that are at least visible under the microscope. Einstein was initially not aware that he had quantitatively explained the Brownian movement of pollen, which had been known since 1827 , the cause of which Christian Wiener had first assumed molecular impacts as early as 1863 . The French physicist Jean Perrin determined the mass and size of molecules experimentally on the basis of Einstein's theory and found results similar to those of Loschmidt. This work made a decisive contribution to the general recognition of the so-called "atomic hypothesis" until then.
Divisibility and structure of the atoms
Joseph John Thomson discovered in 1897 that the cathode rays consist of particles of a certain charge and mass, the mass of which is less than a thousandth of the atomic mass. These particles were called electrons and turned out to be part of all matter, which contradicted the concept of the atom as an indivisible unit. Thomson believed that the electrons gave the atom its mass and that they were distributed in the atom in a massless, positively charged medium like "raisins in a cake" ( Thomson's atomic model ).
The recently discovered radioactivity was associated in 1903 by Ernest Rutherford and Frederick Soddy with the interconversion of different types of atoms. In 1908 they were able to prove that α-particles that are emitted by alpha radiation form helium atoms.
Together with his research group, Ernest Rutherford shot a gold foil with α-particles in 1909. He found that most of the particles penetrated the film almost unhindered, but a few were deflected by much larger angles than possible according to Thomson's model. Rutherford concluded from this that almost the entire mass of the atom is concentrated in a much smaller, charged atomic nucleus in the center of the atom ( Rutherford's atomic model ). The heavily deflected α-particles were those that happened to be closer to a nucleus than about a hundredth of the atomic radius. The charge number of the atomic nucleus turned out to be the chemical atomic number of the element in question, and α-particles turned out to be the atomic nuclei of helium.
In 1911, the chemist Frederick Soddy found that some of the natural radioactive elements had to consist of atoms of different masses and different levels of radioactivity. The term isotope for physically different atoms of the same chemical element was proposed by Margaret Todd in 1913 . Since the isotopes of the same element could not be distinguished by their chemical behavior, the physicist JJ Thomson developed a first mass spectrometer for their physical separation. In 1913 he was able to use neon as an example to demonstrate that there are also stable elements with several isotopes.
In 1918 Francis William Aston found out with a mass spectrometer of considerably greater accuracy that almost all elements are mixtures of several isotopes, with the masses of the individual isotopes always being (almost) whole-number multiples of the mass of the hydrogen atom. In the first observed nuclear reaction in 1919, Rutherford demonstrated that the nuclei of hydrogen atoms can be ejected from the nuclei of nitrogen atoms by bombarding them with α-particles. He gave these the name proton and developed an atomic model in which the atoms consist only of protons and electrons, with the protons and some of the electrons forming the small, heavy atomic nucleus, the remaining electrons forming the large, light atomic shell. The idea of electrons in the atomic nucleus, however, turned out to be wrong and was dropped after James Chadwick demonstrated the neutron as a neutral nucleus with roughly the same mass as the proton in 1932 . This is how today's atomic model was created: The atomic nucleus is composed of as many protons as the atomic number indicates, and also as many neutrons that the relevant isotope mass is reached.
Quantum mechanical atomic models

In 1913 , based on Rutherford's atomic model of core and shell, Niels Bohr was able to explain for the first time how the spectral lines in the optical spectra of pure elements come about that are absolutely characteristic for the respective element ( spectral analysis according to Robert Wilhelm Bunsen and Gustav Robert Kirchhoff 1859) . Bohr assumed that the electrons can only stay on certain quantized orbits (shells) and “jump” from one to the other, but cannot stay in between. During the quantum leap from an outer to an inner orbit, the electron has to release a certain amount of energy, which appears as a light quantum of a certain wavelength. In the Franck-Hertz experiment , the quantized energy absorption and release of mercury atoms could be confirmed experimentally. The Bohr model of the atom gave quantitatively correct results only for systems with only one electron (hydrogen and ionized helium). However, in the course of the following decade it formed the basis for a series of refinements that led to a qualitative understanding of the structure of the electron shells of all elements. The Bohr model of the atom thus became the basis of the popular image of the atom as a small planetary system.
In 1916 Gilbert Newton Lewis tried to explain the chemical bond through the interaction of the electrons of one atom with another atom using the Bohr model of the atom. In 1916, Walther Kossel first assumed that the noble gases had closed “electron shells” to explain that the chemical properties of the elements vary roughly periodically with the atomic number , with neighboring elements differing by one or two additional or missing electrons. This was further developed by Niels Bohr by 1921 into the " construction principle ", according to which, as the atomic number increases, every additional electron is absorbed into the energetically lowest electron shell of the atomic shell that still has free spaces without the electrons already present being rearranged significantly.

Based on the wave-particle dualism postulated by Louis de Broglie in 1924 , Erwin Schrödinger developed wave mechanics in 1926 . It describes the electrons not as mass points on certain orbits, but as three-dimensional waves of matter . As a result of this description, it is impermissible, among other things, to ascribe precise values for position and momentum to an electron at the same time . This fact was formulated in 1927 by Werner Heisenberg in the uncertainty principle. Accordingly, instead of the movement on certain trajectories, only probability distributions for value ranges of position and momentum can be given, an idea that is difficult to illustrate. The quantized orbits of Bohr's model correspond to standing matter waves or “ atomic orbitals ”. Among other things, they indicate how the probability of the electrons being concentrated near the atomic nucleus, and thus determine the real size of the atom.
The description of the properties of the atoms was much better with this first completely quantum mechanical atomic model than with the previous models. In particular, even with atoms with several electrons, the spectral lines and the structure of the atomic shell can be represented in spatial and energetic terms, including the exact possibilities of forming states bound with the atomic shells of other atoms, i.e. stable molecules. Therefore, Bohr's model of the atom was rejected in favor of the quantum mechanical orbital model of the atom.
The orbital model is still the basis and starting point for precise quantum mechanical calculations of almost all properties of atoms. This applies in particular to their ability to combine with other atoms to form individual molecules or large solids. In the case of atoms with several electrons, in addition to the Pauli principle , the electrostatic interaction of each electron with all the others must be taken into account. This depends u. a. on the shape of the occupied orbitals. On the other hand, the interaction has an opposite effect on the shape and energy of the orbitals. The problem arises of determining the orbitals in a self-consistent manner in such a way that a stable system results. The Hartree-Fock method is based on orbitals of a certain shape and varies them systematically until the calculation results in a minimal total energy. If one wants to determine the orbitals according to the density functional theory , one assumes a position-dependent total density of the electrons and from this forms a Schrödinger equation to determine the orbitals of the individual electrons. Here the initially assumed total density is varied until it agrees well with the total density, which is to be calculated from the occupied orbitals.
The orbital model for an atom with more than one electron can be described physically as an approximation, namely as a one-particle approximation . It consists in the fact that a certain orbital is assigned to each individual electron. A state formed in this way belongs to the simplest kind of multi-particle state and is referred to here as the configuration of the atom. More precise models take into account that, according to the rules of quantum mechanics, the shell can also be in a state that arises from the superposition of different configurations, i.e. where different electron configurations are present at the same time with different probability amplitudes (a so-called configuration mixture). This enables the most precise calculations of energy levels and interactions between the atoms. Because of the mathematical effort required for this, simpler atomic models will continue to be used wherever possible. Mention should be made here of the Thomas-Fermi model , in which the electron shell is treated as an ideal electron gas bound in the potential well, the Fermi gas , the density of which in turn determines the shape of the potential well.
Explanation of basic atomic properties
The electrons in the atomic shell are bound to the positive atomic nucleus by electrostatic attraction due to their negative charge. They clearly form an electron cloud without a sharp edge. A neutral atom has as many electrons in its shell as there are protons in its core. The shell is about ten to one hundred thousand times larger in diameter than the core, but contributes less than 0.06 percent of the atomic mass. It is very permeable to high-energy free particles (e.g. photons of X-rays or electrons and alpha particles of ionizing radiation ) with energies from a few hundred electron volts (eV). Hence the atom is sometimes described as “largely empty”.
For charged particles of low energy in the range of up to a few tens of eV, the shell is practically impenetrable. The kinetic energy and the binding energy of the electrons in the outer part of the shell are also in this range. Therefore, two atoms always experience a strong repulsive force when they approach each other so closely that their shells would noticeably overlap. The range of the kinetic energies of entire atoms and molecules, as they occur under normal conditions on earth, is still significantly lower. For example, the thermal energy ( Boltzmann constant , absolute temperature ), which is typical for the order of magnitude of this energy range, is only about 0.025 eV at room temperature. Under these conditions, the atomic shell is stable because no electrons are torn from it, and secondly, impenetrable because it does not noticeably overlap with the shells of other atoms. The atom thus becomes the universal building block of everyday macroscopic matter . It owes its size , albeit not very sharply defined, to the mutual impenetrability of the shells.
If the shells of two atoms only slightly overlap with their outer edge areas, an attractive force can develop between them. It is the cause of the formation of stable molecules, i.e. the smallest particles in a chemical compound . The condition is that an overall gain in binding energy is associated with the fact that one or two electrons transfer from one shell to the other shell or with a certain probability or are involved in both shells. This is only possible if the two cases are constructed exactly. Therefore, chemical bonds only occur with suitable combinations of atoms.
At greater distances, for example with a few atomic diameters, however, atoms of all types weakly attract each other, regardless of the possibility of entering into a chemical bond. These van der Waals forces cause any gas to condense into a liquid or solid at a sufficiently low temperature . They are therefore responsible for the change in the aggregate states and act between the neutral atoms or molecules, but are also of electrical origin. They are explained by the fact that two atoms induce electrical dipole moments that attract each other electrostatically by slightly shifting their electron clouds .
More discoveries
The chemist Otto Hahn , a student of Rutherford, tried in 1938 to produce atoms with greater mass ( transuranic elements ) by capturing neutrons on uranium nuclei , as had been the case for years with lighter elements. However, surprisingly, Fritz Straßmann proved that the much lighter barium was formed in the process. The physicists Lise Meitner and Otto Frisch were able to identify the process as nuclear fission by using an ionization chamber to detect several radioactive fission products .
From the 1950s, the development of improved particle accelerators and particle detectors made it possible to study atoms when bombarded with particles of very high energy. At the end of the 1960s, the “ deep inelastic scattering ” of electrons on atomic nuclei showed that neutrons and protons are not indivisible units, but are composed of quarks .
In 1951 Erwin Müller developed the field ion microscope and was thus able to create an image of the tip of a needle for the first time that was so magnified in a direct manner that individual atoms were visible in it (even if only as blurred spots). Developed in 1953 Wolfgang Paul , the magnetic ion trap ( Paul trap ), stored in the individual ions and are examined with ever greater precision could.
In 1985, a working group led by Steven Chu developed laser cooling , a process that uses laser radiation to greatly reduce the temperature of an accumulation of atoms . In the same year a group headed by William D. Phillips succeeded in locking neutral sodium atoms in a magneto-optical trap . By combining these processes with a method that uses the Doppler effect , a working group led by Claude Cohen-Tannoudji was able to cool small amounts of atoms to temperatures of a few microkelvins . With this method atoms can be examined with the highest accuracy; it also made possible the experimental realization of the Bose-Einstein condensation .
At the beginning of the 1980s Gerd Binnig and Heinrich Rohrer developed the scanning tunneling microscope , in which a needle tip scans a surface using the tunnel effect so finely that individual atoms become visible. This also made it possible to put atoms individually in certain places. In the 1990s, Serge Haroche and David Wineland were able to successfully examine the interaction of a single atom with a single photon in experiments . In the 2000s, the manageability of individual atoms was used, among other things, to produce a transistor from just one metal atom with organic ligands .
Many of these discoveries were awarded the Nobel Prize ( physics or chemistry ).
Classification
Elements, isotopes, nuclides
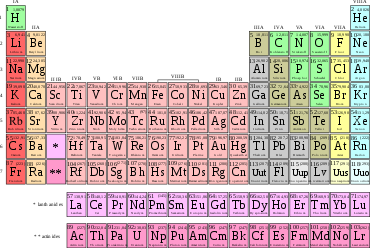
The differentiation and designation of different types of atoms is based initially on the structure of the atomic nucleus, while the state of the shell is indicated by additional symbols, if necessary. Indicators are the number of protons ( atomic number , atomic number) Z , the number of neutrons N of the core, and the formed therefrom mass number A = Z + N . Depending on their number of protons, the atoms belong to one of the 118 known chemical elements , from hydrogen with Z = 1 to Oganesson with Z = 118. Of these, 91 were discovered in natural occurrences, 27 only after artificial production through nuclear reactions . The order of the elements is graphically illustrated in the periodic table - important for chemistry. The elements are arranged in ascending order in the form of a table. Each line is called the period of the periodic table and ends when the respective orbital is fully occupied with electrons (noble gas). In the next lines, the chemical character of the elements is repeated due to the gradual electron occupation of the next orbitals. Elements with similar chemical properties are in one column below one another; they form a group of the periodic table .
Atoms of an element that differ in the number of neutrons belong to different isotopes of the element. In total, the 118 elements consist of around 2800 isotopes, 2500 of which were artificially created. With the exception of the hydrogen isotopes deuterium and tritium , isotopes are named after the chemical element and the mass number. The symbol for a particular isotope of the element X in the form , or X- A (examples: , , Pb-208). The specification of the number of protons Z is redundant, since Z results from the atomic number of the element.
Nuclide is the very general name for types of atoms, regardless of whether they belong to the same element or not. The nuclide map or isotope map - important for nuclear physics and its applications - is a table in which each type of atom has its own place. The number of protons is plotted on one axis and the number of neutrons on the other. Often the stability and, in the case of unstable nuclides, also the type of transformation or the magnitude of the half-life is represented by certain colors.
Stable and unstable (radioactive) atoms
The atomic nucleus of a nuclide can exist in the energetic ground state and in different excited states . If these include relatively long-lived, so-called metastable states, these are referred to as isomers and counted as separate nuclides (symbol , or similar). According to this definition, a total of around 3200 nuclides are known as of 2003.
In nuclear physics , nuclides with different numbers of protons but the same number of masses are called isobars . More rarely, isotonic nuclides with different numbers of protons but the same number of neutrons are grouped together.
Only about 250 isotopes of 80 elements have a stable nucleus. All other atoms are unstable and sooner or later transform into atoms of a stable isotope. Since they generally emit ionizing radiation , they are also called radioisotopes or radionuclides . In addition to all 250 stable isotopes, 30 radioisotopes were found in natural occurrences on earth, which are distributed over 10 radioactive elements and cause natural radioactivity . Many other short-lived isotopes exist inside stars, especially during the supernova phase.
Rare and theoretical forms
A Rydberg atom is an atom in which an electron is excited in such a high energy state that it orbits the atomic nucleus, sometimes also the entire core of the atom, consisting of the atomic nucleus and the remaining electrons, at a great distance and thus its behavior resembles a classical particle. Rydberg atoms can be over 100,000 times larger than unexcited atoms. Since they are extremely sensitive to external fields, you can use them e.g. B. examine the interaction with a single photon in detail. If two or more electrons are excited in such states, one speaks of planetary atoms .
In a partly figurative sense, exotic atoms are also used to denote systems that have certain physical similarities to ordinary atoms. In them z. B. one of the protons, neutrons or electrons has been replaced by another particle of the same charge. If, for example, an electron is replaced by a heavier muon , a muonic atom is formed. As positronium an exotic atom is referred to, in which an electron instead of a proton to a positron which is the positively charged anti-particle , is bound of the electron. Atoms that are made up entirely of antiparticles to normal matter are also possible. For example, anti-hydrogen atoms were artificially produced and detected for the first time in 1995 at CERN in Geneva . Among other things, fundamental physical theories can be tested on such exotic atoms.
Furthermore, the name atom is sometimes used for two-particle systems that are not held together by electromagnetic interaction, but by the strong interaction . Such a quarkonium is a short-lived elementary particle of the meson type , which is made up of a quark and its antiparticle. A quarkonium atom can be classified in its various metastable states by quantum numbers like the hydrogen atom.
Emergence
About a second after the Big Bang , the constant conversions between the elementary particles came to a standstill, leaving electrons, protons and neutrons. In the following three minutes, primordial nucleosynthesis combined the neutrons with protons to form the simplest nuclei: deuterium , helium , to a lesser extent also lithium and possibly even smaller amounts of beryllium and boron . The remaining protons (86 percent) were retained. The first neutral atoms with permanently bound electrons were only formed in the recombination phase 380,000 years after the Big Bang , when the universe had cooled down so much through expansion that the atoms were not immediately ionized again.
The nuclei of all heavier atoms were and are created by various processes of nuclear fusion . The most important is the stellar nucleosynthesis , through which helium is formed in stars, then the heavier elements down to iron . Elements with a higher atomic number than iron arise in explosive processes such as the r-process in supernovae and the s-process in AGB stars , which are about to reach the end of their lifespan.
Small amounts of different elements and isotopes are also formed by dividing heavy nuclei again. This happens through radioactive decays (see decay series ), which u. a. are responsible for part of the occurrence of helium and lead, and spallations , which are important for the formation of lithium, beryllium and boron.
Occurrence and distribution

The atoms in the observable universe have an average density of 0.25 atoms / m³. According to the big bang model ( Lambda CDM model ), they make up around 4.9 percent of the total energy density. The rest, the nature of which is still largely unclear, is made up of around 27 percent dark matter and 68 percent dark energy , as well as small contributions from neutrinos and electromagnetic radiation. In the interior of a galaxy such as the Milky Way , the density of atoms in the interstellar medium (ISM) is much higher and lies between 10 4 and 10 11 atoms / m 3 . The sun is located in the largely dust-free local bubble , so the density in the vicinity of the solar system is only about 10 3 atoms / m 3 . In solid celestial bodies such as the earth, the atomic density is about 10 29 atoms / m 3 .
In the distribution of elements in the universe, hydrogen dominates with around three quarters of the mass, followed by helium with around a quarter. All heavier elements are much rarer and only make up a small fraction of the atoms in the universe. Their frequencies are determined by the various mechanisms of nucleosynthesis .
In the solar system, hydrogen and helium are mainly found in the sun and gas planets . In contrast, the heavy elements predominate on earth. The most common elements here are oxygen , iron , silicon and magnesium . The earth's core consists primarily of iron, while oxygen and silicon predominate in the earth's crust.
Components of the atom
The two main components of an atom are the nucleus and the atomic shell. The shell consists of electrons. It contributes less than 0.06 percent to the mass of the atom, but determines its size and its behavior towards other atoms when they come close to each other. The nucleus consists of protons and neutrons, is ten to one hundred thousand times smaller in diameter than the shell, but contains more than 99.9 percent of the mass of the atom.
Atomic nucleus
construction

The protons and neutrons present in an atom , also known collectively as nucleons , are bound to one another and form the atomic nucleus. The nucleons belong to the hadrons . The proton is positively charged, the neutron is electrically neutral. Proton and neutron have a diameter of about 1.6 fm ( femtometer ) and are not themselves elementary particles, but are built up from point-like quarks according to the standard model of elementary particle physics . Three quarks each bind to a nucleon through the strong interaction that is mediated by gluons . The strong interaction is also responsible for the cohesion of the nucleons in the atomic nucleus, in particular the attraction up to a distance of about 2.5 fm is significantly stronger than the mutual electrical repulsion of the protons. Below about 1.6 fm, however, the strong interaction of the hadrons becomes strongly repulsive. To put it clearly, the nucleons in the nucleus behave somewhat like hard spheres that stick to one another. Therefore, the volume of the nucleus increases proportionally to the number of nucleons (mass number) . Its radius is about fm.
The lightest atomic nucleus consists of only one proton. Several protons repel each other according to electrostatics , but together with a suitable number of neutrons can form a stable system. However, even with small deviations from the energetically most favorable numerical ratio, the nucleus is unstable and spontaneously transforms by turning a neutron into a proton or vice versa and releasing the energy and charge released as beta radiation . Nuclei with up to about 20 protons are only stable when the number of neutrons is approximately the same. In addition, the ratio of neutrons to protons in the stable atomic nuclei increases from 1: 1 to around 1.5: 1, because with larger numbers of protons the number of neutrons must grow faster than that of protons due to their electrostatic repulsion (for details see droplet model ). The binding energy in stable nuclei (apart from the lightest) is above 7 MeV per nucleon (see illustration) and thus exceeds the binding energy of the outer electrons of the atomic shell or the chemical binding energy in stable molecules by about 10 6 times. Nuclei with certain numbers of nucleons, which are called magic numbers , for example helium -4, oxygen -16 or lead -208, are particularly stable, which can be explained with the shell model of the atomic nucleus .
All nuclei are unstable above a number of 82 protons (i.e. beyond lead). They are transformed into lighter nuclei by ejecting a He-4 nucleus ( alpha radiation ). This is repeated, together with beta radiation, until a stable core is reached; several decay stages form a decay series . There is also no stable nucleus for the proton numbers 43 ( technetium ) and 61 ( promethium ). Therefore there can only be a total of 80 different stable chemical elements, all others are radioactive. They only occur naturally on earth if they themselves or one of their parent substances have a sufficiently long half-life.
Dimensions
Since most of the atomic mass comes from the neutrons and protons and these are roughly the same weight, the total number of these particles in an atom is called the mass number . The exact mass of an atom is often given in the atomic mass unit u; their numerical value is then roughly equal to the mass number. Smaller deviations arise from the mass defect of the atomic nuclei. The atomic mass unit results from the definition of the SI unit of the mole in such a way that an atom of the carbon isotope 12 C (in the ground state including its shell electrons) has a mass of exactly 12 u. This means that 1 u is equal to 1.6053904 · 10 −27 kg. An atom of the lightest isotope of hydrogen has a mass of 1.007825 u. The heaviest stable nuclide is the lead isotope 208 Pb with a mass of 207.9766521 u.
Since macroscopic amounts of substance contain so many atoms that specifying their number as a natural number would be unwieldy, the amount of substance was given its own unit, the mole . One mole is about 6.022 · 10 23 atoms (or molecules or other particles; the type of particle under consideration must always be mentioned). The mass of 1 mol of atoms with atomic mass X u is therefore exactly X g. It is therefore common in chemistry to specify atomic masses indirectly in g / mol instead of in u.
Formation and decay
The way in which an unstable atomic nucleus decays is typical for the respective radionuclide. In the case of some nuclides, the nuclei (which are completely identical to one another) can also decay in different ways, so that several decay channels with specific proportions are involved. The main radioactive decays are
- Alpha decay , in which a helium atomic nucleus is formed from two protons and two neutrons of the nucleus due to the strong interaction , which is ejected,
- Beta decay , in which a neutron of the nucleus is converted into a proton or vice versa by means of the weak interaction and an electron and an antineutrino or a positron and a neutrino are emitted,
- Gamma decay , in which an excited nucleus generates gamma radiation through electromagnetic interaction and reaches a lower energy level with the same number of protons and neutrons.

The energies of the radiation are characteristic of the respective nuclide, as is the half-life , which indicates how long it takes for half of a sample of the nuclide to decay.
The addition of a neutron can transform a nucleus into the next heavier isotope of the same element. When bombarded with neutrons or other atomic nuclei, a large atomic nucleus can be split into several smaller nuclei . Some heavy nuclides can split spontaneously without any external influence .
Larger atomic nuclei can be formed from smaller nuclei. This process is called nuclear fusion . For a fusion, atomic nuclei have to come very close. This approach is countered by the electrostatic repulsion of both nuclei, the so-called Coulomb wall . For this reason, nuclear fusion (except in certain experiments) is only possible at very high temperatures of several million degrees and high pressures such as those found inside stars. Nuclear fusion is an exothermic reaction with nuclides up to nickel-62 , so that it can largely take place in a self-sustaining manner. It is the energy source of the stars. For atomic nuclei beyond nickel, the binding energy per nucleon decreases; the fusion of heavier atomic nuclei is therefore endothermic and therefore not a self-sustaining process. Nuclear fusion in stars comes to a standstill when the light atomic nuclei are used up.
Atomic shell
Build-up and binding energy
The atomic shell consists of electrons that are bound to the positive atomic nucleus due to their negative charge. It is often referred to as the electron shell. In the case of a neutral atom, the average binding energy of the electrons in the shell is approximately . It therefore increases considerably with increasing particle number, in contrast to the average binding energy per nucleon in the nucleus. To explain it, it is stated that only short-range binding forces act between nucleons, which barely extend beyond the neighboring particles, while the shell is bound by the electrostatic attraction force, which as a long-range interaction decreases comparatively weakly with greater distance from the core.
Apart from the mass, which is more than 99.9 percent concentrated in the atomic nucleus, the atomic shell is responsible for practically all external properties of the atom. The term atomic model therefore mostly only refers to the shell in the narrower sense (see list of atomic models ). A simple atomic model is the shell model , according to which the electrons are arranged in certain shells around the nucleus, in each of which there is space for a certain number of electrons. However, these shells neither have a specific radius nor a specific thickness, but rather overlap and partially penetrate one another.
Essential properties of the shell are shown above under quantum mechanical atomic models and explanation of basic atomic properties . More details are given in the following sections.
Interpretation of basic atomic properties in the context of the shell model
The atomic shell determines the strength and distance dependence of the forces between two atoms. In the distance range of several atomic diameters, the entire atomic shells are mutually polarized, so that attractive forces, the van der Waals forces , arise through electrostatic attraction . Above all, they cause the gases to condense to form liquids , i.e. a change in the physical states .
The (approximate) incompressibility of liquids and solids, on the other hand, is based on the fact that all atoms strongly repel each other when they approach each other as soon as their shells noticeably overlap in space and therefore have to deform. Except in the case of two hydrogen atoms, each with only one electron in their shell, the electrostatic repulsion of the two atomic nuclei plays only a minor role.
In a middle distance range between the predominance of the weakly attractive van der Waals forces and the strong repulsion, a particularly strong attraction occurs between two or more matching atomic shells, the chemical bond . In the case of atoms of certain elements, this attraction can lead to a stable molecule made up of atoms in a precisely defined number and spatial arrangement. The molecules are the smallest units of matter in chemical compounds, i.e. homogeneous materials in all their diversity. Molecules also attract each other through the shells of their atoms. A solid body is created when many molecules bind to each other and, because it is energetically favorable, maintain a fixed arrangement. If this arrangement is regular, a crystal lattice is formed . As a result of this bond, the solid body is not only largely incompressible like a liquid, but, in contrast to this, can also be subjected to tensile loads and is significantly less easily deformed. If atoms of metallic elements connect with one another, their number is not fixed and any size and shape can be formed. In particular, chemically pure metals then usually show a high degree of ductility . Compounds of different metals are called alloys . The way metal atoms are bonded explains why electrons can move almost freely through the crystal lattice, which is what causes the great electrical and thermal conductivity of metals. In summary, the mechanical stability and many other properties of the macroscopic materials result from the interaction of the atomic shells with one another.
Due to the fuzzy edge of the atomic shell, the size of the atoms is not clearly established. The values tabulated as atomic radii are obtained from the bond length , which is the energetically most favorable distance between the atomic nuclei in a chemical bond. Overall, with increasing atomic number, there is an approximately periodic variation in the atomic size, which agrees well with the periodic variation in the chemical behavior. In the periodic table of the elements, the general rule is that within a period, i.e. one line of the system, a certain bowl is filled. The size of the atoms decreases from left to right because the nuclear charge increases and therefore all shells are more strongly attracted. If a certain shell is filled with the strongly bound electrons, the atom belongs to the noble gases . With the next electron, the shell begins to be filled with the next higher energy, which is associated with a larger radius. Within a group, i.e. a column of the periodic table, the size therefore increases from top to bottom. Accordingly, the smallest atom is the helium atom at the end of the first period with a radius of 32 pm, while one of the largest atoms is the cesium atom, the first atom of the 5th period. It has a radius of 225 pm.
Explanation of the atomic properties in the context of the orbital model
The electron shells on which the shell model is based result from the quantization of the electron energies in the force field of the atomic nucleus according to the rules of quantum mechanics . Various atomic orbitals are formed around the nucleus , these are fuzzy probability distributions for possible spatial states of the electrons. Due to the Pauli principle, each orbital can be filled with a maximum of two electrons, the electron pair . The orbitals, which would theoretically have the same energy if the mutual repulsion of the electrons and the fine structure were neglected , form a shell. The shells are numbered consecutively with the main quantum number or consecutively designated with the letters K, L, M, .... More precise measurements show that from the second shell onwards, not all electrons in a shell have the same energy. If necessary, a certain subshell is identified by the secondary quantum number or angular momentum quantum number .
If the orbitals, starting with the lowest energy level, are occupied with electrons to such an extent that the total number of electrons is equal to the number of protons in the nucleus, the atom is neutral and is in the ground state. If one or more electrons in an atom are moved into energetically higher orbitals, the atom is in an excited state . The energies of the excited states have well-defined values for each atom, which form its term scheme . An excited atom can give off its excess energy through collisions with other atoms, through the emission of one of the electrons ( Auger effect ) or through the emission of a photon , i.e. through the generation of light or X-rays. At very high temperatures or in gas discharges , the atoms can lose electrons through collisions (see ionization energy ). A plasma is created , e.g. B. in a hot flame or in a star.
Since the energies of the quanta of the emitted radiation differ depending on the atom or molecule and the states involved, the source can generally be clearly identified by spectroscopy of this radiation. For example, the individual atoms show their element-specific optical line spectrum . The sodium D line is known, for example , a double line in the yellow spectral range at 588.99 nm and 589.59 nm, which is also referred to as D-1 in the figure opposite. Their lighting up indicates the presence of excited sodium atoms, be it in the sun or over the stove flame in the presence of sodium or its salts. Since this radiation can also supply the same energy to an atom through absorption, the spectral lines of the elements can be observed in both absorption and emission spectra. These spectral lines can also be used to measure frequencies very precisely, for example for atomic clocks .
Although electrons electrostatically repel each other, up to two additional electrons can be bound if there are orbitals with further free places at the highest electron energy (see electron affinity ). Chemical reactions , d. H. The connection of several atoms to a molecule or of a large number of atoms to a solid are explained by the fact that one or two electrons from one of the outer orbitals of an atom ( valence electrons ) move over to a free space in an orbital of a neighboring atom ( ionic bond ) or there is a certain likelihood of staying there ( covalent bond through a bonding pair of electrons ). The electronegativity of the elements determines at which atom the electrons are more likely to be. As a rule, chemical bonds are formed in such a way that the atoms receive the electron configuration of a noble gas ( noble gas rule ). The shape and occupation of its orbitals are decisive for the chemical behavior of the atom. Since these are determined solely by the number of protons, all atoms with the same number of protons, i.e. the isotopes of an element, show almost the same chemical behavior.
If two atoms get closer to each other beyond the chemical bond, the electrons of one atom have to move to free but energetically unfavorable orbitals of the other atom due to the Pauli principle, which results in an increased energy requirement and thus a repulsive force.
Interaction between core and shell
The interaction between core and shell is described with great accuracy by the simple approach in which the core represents a point source of an electrostatic field according to Coulomb's law . All of the atomic models mentioned are based on this. Due to additional effects that are dealt with in extended models, only extremely small corrections are necessary, which are summarized under the name hyperfine structure . There are three effects to consider here: firstly, the finite expansion that each nucleus has, secondly, a magnetic dipole interaction when both the nucleus and shell have an angular momentum quantum number of at least ½, and thirdly, an electrical quadrupole interaction when both angular momentum quantum numbers are at least 1.
The finite expansion of the nucleus - compared to a theoretical point charge - causes a weaker attraction of those electrons, the probability of which extends into the nucleus. Only s orbitals ( orbital angular momentum zero) are affected . For atoms of medium atomic number, the correction is in the order of 1 percent. The magnetic dipole or electrical quadrupole moments of the shell and core cause a coupling with the result that the total energy of a free atom is split extremely slightly depending on the quantum number of its total angular momentum . In the H atom, the splitting is about one millionth of the binding energy of the electron (see 21 cm line ). In clear terms, the energy depends on the angle at which the axes of the magnetic dipole moment or electrical quadrupole moment of the core and shell are to one another.
In the case of atoms in liquids and solids, these interactions occur in a correspondingly modified form. Despite the small size of the effects caused by them, they have played a major role in atomic and nuclear research and in special cases are also important in modern applications.
observation
Indirect observation
Indirect ways of recognizing atoms are based on observing the radiation emitted by them. For example, the elemental composition of distant stars can be determined from atomic spectra. The different elements can be identified by characteristic spectral lines that go back to emission or absorption by atoms of the corresponding element in the star's atmosphere. Gas discharge lamps that contain the same element show these lines as emission lines. In this way, z. B. In 1868 helium was detected in the spectrum of the sun - over 10 years before it was discovered on earth.
An atom can be ionized by removing one of its electrons. The electrical charge ensures that the trajectory of an ion is deflected by a magnetic field. Light ions are deflected more strongly than heavy ions. The mass spectrometer uses this principle to determine the mass-to-charge ratio of ions and thus the atomic mass .
The electron energy loss spectroscopy measures the energy loss of an electron beam in the interaction with a sample in a transmission electron microscope .
Observation of single atoms

A direct image that allows individual atoms to be recognized was first achieved in 1951 with the field ion microscope (or field emission microscope ). On a spherical screen, in the center of which there is an extremely fine needle point, an image that has been enlarged approximately a million times appears. The top atoms, which form the tip, can be seen next to each other as individual points of light. Today this can also be demonstrated in physics class at school. The image is created in real time and allows z. B. the consideration of the heat movement of individual foreign atoms on the tip.
The scanning tunneling microscope is also a device that makes individual atoms on the surface of a body visible. It uses the tunnel effect , which allows particles to pass an energy barrier that, according to classical physics, they could not overcome. In this device, electrons tunnel between an electrically conductive tip and the electrically conductive sample. When moving sideways to scan the sample, the height of the tip is readjusted so that the same current always flows. The movement of the tip depicts the topography and electronic structure of the sample surface. Since the tunnel current is very dependent on the distance, the lateral resolution is much finer than the tip radius, sometimes atomic.
A tomographic atom probe creates a three-dimensional image with a resolution below a nanometer and can assign individual atoms to their chemical element.
Building on an atom-light interface developed around 2010, it was possible in 2020 to take photos of individual atoms that hover less than a thousandth of a millimeter above a light-conducting glass fiber. This means that under laboratory conditions it is now possible to investigate effects such as the absorption and emission of light in a more controlled manner than before. This can help in the development of novel fiber optic networks.
literature
- Hans-Werner Kirchhoff: Concepts of the atom 1800-1934 . Aulis Verlag Deubner, 2001, ISBN 3-7614-2300-4 .
- Richard Feynman , Robert B. Leighton, Matthew Sands: Lectures on Physics. Volume I-III . Oldenbourg, 1991.
- Wolfgang Demtröder : Atoms, molecules and solids . 3. Edition. Springer, 2005, ISBN 3-540-21473-9 .
- Richard Feynman: Six Easy Pieces . The Penguin Group, 1995, ISBN 0-14-027666-1 .
- Oskar Höfling , Pedro Waloschek : The world of the smallest particles . Rowohlt, 1984, ISBN 3-498-02862-6 .
- Jeremy I. Pfeffer, Shlomo Nir: Modern Physics: An Introductory Text . Imperial College Press, 2000, ISBN 1-86094-250-4 (English).
- Robert Siegfried: From Elements to Atoms: A History of Chemical Composition . In: Transactions of the Americal Philosophical Society . tape 92 , no. 4 . American Philosophical Society, 2002, ISBN 0-87169-924-9 .
- Werner Kutzelnigg : Introduction to Theoretical Chemistry . Wiley Chemie, 2002, ISBN 3-527-30609-9 .
- Dick Teresi: Lost Discoveries: The Ancient Roots of Modern Science-from the Babylonians to the Maya . Simon & Schuster, 2003, ISBN 0-7432-4379-X , p. 213-214 .
Web links
- HydrogenLab: What does an atom look like?
- Overview of the different atomic models
- Historical overview of the concept of the atom from the perspective of natural philosophy by Brigitte Falkenburg in the online lexicon of basic concepts of natural philosophy.
Individual evidence
- ↑ Dick Teresi: Lost Discoveries: The Ancient Roots of Modern Science - from the Babylonians to the Maya . Simon & Schuster, 2003, ISBN 0-7432-4379-X , p. 213-214 .
- ^ Leonid I. Ponomarev: The Quantum Dice . 2nd Edition. Inst. Of Physics Pub, 1993, ISBN 0-7503-0251-8 , pp. 14-15 .
- ↑ a b Jörn Bleck-Neuhaus: Elementary Particles . From the atoms to the standard model to the Higgs boson. 2nd, revised edition. Springer, 2013, ISBN 978-3-642-32578-6 , ISSN 0937-7433 , Chapter 1, doi : 10.1007 / 978-3-642-32579-3 .
- ^ Robert Siegfried: From Elements to Atoms: A History of Chemical Composition . In: Transactions of the Americal Philosophical Society . tape 92 , no. 4 . American Philosophical Society, 2002, ISBN 0-87169-924-9 , pp. 42-55 .
- ↑ Charles Kittel: Introduction to Solid State Physics. 7th edition 1988, Verlag R. Oldenbourg (Munich), p. 16.
- ^ Lavoisier's Elements of Chemistry. In: Elements and Atoms. Le Moyne College, Department of Chemistry, accessed March 2, 2014 .
- ^ Charles Adolphe Wurtz: The Atomic Theory . D. Appleton and company, New York 1881, p. 1-2 .
- ^ J. Dalton: A New System of Chemical Philosophy, Part 1 . S. Russell, London / Manchester 1808.
- ^ F. Dannemann: The natural sciences in their development and in their context. Vol. 3, Verlag W. Engelmann 1922, p. 198.
- ↑ Loschmidt: On the size of air molecules. In: Meeting reports of the Imperial Academy of Sciences in Vienna. Volume 52, 1866, Abt. II, pp. 395-413.
- ↑ Albert Einstein: A new determination of the molecular dimensions . Bern 1905 ( online [PDF; accessed on March 25, 2014]).
- ↑ Albert Einstein: About the motion of particles suspended in liquids at rest, required by the molecular kinetic theory of heat . In: Annals of Physics . tape 322 , no. 8 , 1905, pp. 549-560 , doi : 10.1002 / andp.19053220806 ( PDF ( memento of March 18, 2006 in the Internet Archive ) [accessed on February 4, 2007]). About the movement of particles suspended in liquids at rest as required by the molecular kinetic theory of heat ( Memento from March 18, 2006 in the Internet Archive )
- ^ Robert M. Mazo: Brownian Motion: Flucuations, Dynamics, and Applications . In: The International Series of Monographs on Physics . tape 112 . Oxford University Press, 2002, ISBN 0-19-851567-7 , pp. 1-7 .
- ^ YK Lee, Kelvin Hoon: Brownian Motion. (No longer available online.) Imperial College, London, 1995, archived from the original on December 18, 2007 ; accessed on March 2, 2014 (English).
- ↑ Christian Wiener: Explanation of the atomistic nature of the drip liquid body state and confirmation of the same by the so-called molecular movements . In: Poggendorff's annals . tape 118 , 1863, p. 79-94 .
- ^ G. Patterson: Jean Perrin and the triumph of the atomic doctrine . In: Endeavor . tape 31 , no. 2 , 2007, p. 50-53 , doi : 10.1016 / j.endeavor.2007.05.003 .
- ^ The Nobel Foundation: JJ Thomson. Nobelprize.org, 1906, accessed March 2, 2014 .
- ^ E. Rutherford: The Scattering of α and β Particles by Matter and the Structure of the Atom . In: Philosophical Magazine . tape 21 , 1911, pp. 669-688 ( scans [accessed March 2, 2014]).
- ↑ Frederick Soddy, The Nobel Prize in Chemistry 1921. Nobel Foundation, accessed March 2, 2014 .
- ^ Nagel, Miriam C .: Frederick Soddy: From Alchemy to Isotopes . In: Journal of Chemical Education . tape 59 , no. 9 , 1982, pp. 739-740 , doi : 10.1021 / ed059p739 .
- ^ Joseph John Thomson: Bakerian Lecture: Rays of Positive Electricity . In: Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character . tape 89 , no. 607 , 1913, pp. 1–20 ( royalsocietypublishing.org [PDF; accessed March 2, 2014]).
- ^ Francis W. Aston: The constitution of atmospheric neon . In: Philosophical Magazine . tape 39 , no. 6 , 1920, p. 449-455 .
- ↑ James Chadwick: Nobel Lecture: The Neutron and Its Properties. Nobel Foundation, December 12, 1935, accessed March 2, 2014 .
- ↑ David P. Stern: The Atomic Nucleus and Bohr's Early Model of the Atom. NASA Goddard Space Flight Center, May 16, 2005, accessed March 2, 2014 .
- ^ Niels Bohr, The Nobel Prize in Physics 1922, Nobel Lecture. The Nobel Foundation, December 11, 1922, accessed March 2, 2014 .
- ^ Gilbert N. Lewis: The Atom and the Molecule . In: Journal of the American Chemical Society . tape 38 , no. 4 , April 1916, p. 762-786 , doi : 10.1021 / ja02261a002 .
- ↑ Walther Kossel: About molecular formation as a question of atomic structure. Annalen der Physik Vol. 49, 1916, pp. 229-362, doi: 10.1002 / andp.19163540302 .
- ↑ Niels Bohr: Atomic structure . In: Nature . tape 107 , 1921, pp. 104-107 , doi : 10.1038 / 107104a0 .
- ↑ Kevin Brown: The Hydrogen Atom. MathPages, 2007, accessed March 2, 2014 .
- ^ David M. Harrison: The Development of Quantum Mechanics. University of Toronto, March 2000, accessed March 2, 2014 .
- ^ Lise Meitner, Otto Robert Frisch: Disintegration of uranium by neutrons: a new type of nuclear reaction . In: Nature . tape 143 , 1939, pp. 239 .
- ↑ Manfred Schroeder: Lise Meitner - For the 125th anniversary of your birthday . ( Online [accessed March 2, 2014]). Online ( Memento from July 19, 2011 in the Internet Archive )
- ^ Sven Kullander: Accelerators and Nobel Laureates. The Nobel Foundation, August 28, 2001, accessed March 2, 2014 .
- ↑ Staff: The Nobel Prize in Physics 1990. The Nobel Foundation, October 17, 1990, accessed on March 2, 2014 .
- ↑ P. Domokos, J. Janszky, P. Adam: Single-atom interference method for generating Fock states . In: Physical Review . tape 50 , 1994, pp. 3340-3344 , doi : 10.1103 / PhysRevA.50.3340 .
- ^ The Nobel Prize in Physics 1997. Nobel Foundation, October 15, 1997, accessed March 2, 2014 .
- ↑ a b Marilyn Jacox, J. William Gadzuk: Scanning Tunneling Microscope. National Institute of Standards and Technology, November 1997, accessed March 2, 2014 .
- ^ A b The Nobel Prize in Physics 1986. The Nobel Foundation, accessed on January 11, 2008 (English, in particular the Nobel Prize lecture by G. Binnig and H. Rohrer).
- ↑ Jiwoong Park, et al. : Coulomb blockade and the Kondo effect in single-atom transistors . In: Nature . tape 417 , no. 6890 , 2002, p. 722-725 , doi : 10.1038 / nature00791 .
- ↑ G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties . In: Nuclear Physics . A 729, 2003, p. 3–128 , doi : 10.1016 / j.nuclphysa.2003.11.001 (English, in2p3.fr [PDF; accessed on March 2, 2014]).
- ↑ Entry on Isotopes. In: Römpp Online . Georg Thieme Verlag, accessed on February 2, 2014.
- ↑ Roger Barrett, Daphne Jackson , Habatwa Mweene: The Strange World of the Exotic Atom . In: New Scientist . No. 1728 , 1990, pp. 77–115 ( online [accessed March 2, 2014]).
- ^ Paul Indelicato: Exotic Atoms . In: Physica Scripta . T112, 2004, p. 20-26 , doi : 10.1238 / Physica.Topical.112a00020 .
- ↑ Barrett H. Ripin: Recent Experiments on Exotic Atoms . American Physical Society, July 1998 ( online [accessed March 2, 2014]).
- ↑ G. Baur et al .: Production of antihydrogen. In: Physics Letters B . 368, No. 3, 1996, pp. 251-258, doi: 10.1016 / 0370-2693 (96) 00005-6 ; Preprint online .
- ↑ Craig J. Copi, David N. Schramm, Michael S Turner: Big-Bang Nucleosynthesis and the Baryon Density of the Universe . In: Science . tape 267 , 1995, pp. 192-199 , doi : 10.1126 / science.7809624 , PMID 7809624 .
- ^ Brian Abbott: Microwave (WMAP) All-Sky Survey. Hayden Planetarium, May 30, 2007, archived from the original on September 5, 2008 ; accessed on March 2, 2014 (English).
- ↑ DC Knauth, SR Federman, David L. Lambert, P. Crane: Newly synthesized lithium in the interstellar medium . In: Nature . tape 405 , 2000, pp. 656-658 , doi : 10.1038 / 35015028 .
- ↑ Michael Banks: Planck reveals 'almost perfect' universe. March 21, 2013, accessed January 20, 2014 .
- ^ Masataka Fukugita, James Peebles: The Cosmic Energy Inventory . August 18, 2004, arxiv : astro-ph / 0406095 (English).
- ↑ Michael Richmond: The Interstellar Medium: Gas. Retrieved March 12, 2014 .
- ^ Arthur F. Davidsen: Far-Ultraviolet Astronomy on the Astro-1 Space Shuttle Mission . In: Science . tape 259 , no. 5093 , 1993, pp. 327-334 , doi : 10.1126 / science.259.5093.327 , PMID 17832344 .
- ^ AGW Cameron: Abundances of the elements in the solar system . In: Space Science Reviews . tape 15 , 1970, pp. 121-146 .
- ↑ Jeremy I. Pfeffer: Modern Physics: An Introductory Text . Imperial College Press, 2000, ISBN 1-86094-250-4 , pp. 330-336 .
- ^ Tatjana Jevremovic: Nuclear Principles in Engineering . Springer, 2005, ISBN 0-387-23284-2 , pp. 63 .
- ↑ a b E.R. Cohen, et al. : Quantities, Units and Symbols in Physical Chemistry . 3. Edition. IUPAC & RSC Publishing, 2008, ISBN 978-0-85404-433-7 , pp. 88, 92 (English, online [PDF; accessed April 28, 2014]). Online ( Memento from February 11, 2014 in the Internet Archive )
- ^ G. Audi, AH Wapstra, C. Thibault: The Ame2003 atomic mass evaluation (II) . In: Nuclear Physics . A729, 2003, p. 337-676 (English, online [accessed March 2, 2014]).
- ↑ Wolfgang Demtröder: Experimentalphysik Vol. 4: Nuclear, Particle and Astrophysics . 3. Edition. Springer, 2010, ISBN 978-3-642-01597-7 , ISSN 0937-7433 , p. 366-367 , doi : 10.1007 / 978-3-642-01598-4 .
- ^ Julian Schwinger: Thomas-Fermi model: The leading correction . In: Phys. Rev. A . tape 22 , 1980, pp. 1827-1832 , doi : 10.1103 / PhysRevA.22.1827 .
- ^ Mark Winter: Covalent radius. Retrieved March 12, 2014 .
- ↑ Yu. Ralchenko, AE Kramida, J. Reader: NIST Atomic Spectra Database. National Institute of Standards and Technology, Gaithersburg, MD, 2008, accessed March 2, 2014 (Version 5).
- ↑ Jim Lochner, Meredith Gibb, Phil Newman: What Do Spectra Tell Us? NASA / Goddard Space Flight Center, April 30, 2007, accessed March 2, 2014 .
- ↑ Mark Winter: Helium. WebElements, 2007, accessed March 2, 2014 .
- ↑ Erwin W. Müller, John A. Panitz, S. Brooks McLane: The Atom-Probe Field Ion Microscope . In: Review of Scientific Instruments . tape 39 , no. 1 , 1968, ISSN 0034-6748 , p. 83-86 , doi : 10.1063 / 1.1683116 .
- ↑ Atoms during a photo shoot. In: press release. Humboldt-Universität zu Berlin, August 3, 2020, accessed on August 3, 2020 .












![1 {,} 07 \ sqrt [3] {A}](https://wikimedia.org/api/rest_v1/media/math/render/svg/dd4a17faeeb01b512f441e3c77acc1cb4101b337)


![13 {,} 6 \; Z ^ {{4/3}} \ left [1 + {\ tfrac 12} (1-Z ^ {{- 1/3}}) ^ {2} \ right] \, { \ mathrm {eV}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/56bb872c43eea5e000d52260d1f9d6f6fa5cf8d1)
