Carbon group
|
Position in the periodic table
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| group | 14th |
| Main group | 4th |
| period | |
| 2 |
6 C |
| 3 |
14 Si |
| 4th |
32 Ge |
| 5 |
50 Sn |
| 6th |
82 Pb |
| 7th |
114 bottles |
Carbon group or carbon-silicon group refers to the 4th main group (" tetrele ", rarely also "tattogens" (scaffolding)) (according to the new numbering of IUPAC group 14) of the periodic table . It includes the elements carbon (C), silicon (Si), germanium (Ge), tin (Sn) and lead (Pb). A radioactive element, the Flerovium (Fl), is also represented.
C 60 - fullerene
properties
The elements of the carbon group have very different chemical and physical properties because the group is split into two parts by the dividing line between metals and non-metals . The first element of the group, carbon, is a non-metal, the following two (silicon and germanium) are semimetals and all others (tin, lead and flerovium) are metals.
Physical Properties

With increasing atomic number, atomic mass , atomic radius and ionic radius grow . The density of graphite (C) and silicon are close together (approx. 2.3 kg / dm 3 ); within the main group it increases to 11.34 kg / dm 3 up to the lead . There is also a wide range of Mohs hardness , ranging from a maximum of 10 for diamond to a minimum of 1.5 for tin. Tin has the highest electrical conductivity with 9.17 M S / m, silicon has the lowest with 25.2 mS / m. The 1st ionization energy decreases with increasing atomic number from 11.26 eV for carbon to 7.34 eV for tin. At 7.42 eV, lead again has a slightly increased value. The electronegativity tends to decrease with increasing atomic number from 2.5 (C) to 1.6 (Pb); silicon is an outlier with 1.7.
| element | Atomic mass |
Melting point ( K ) |
Boiling point (K) |
Density (kg / m 3 ) |
Mohs hardness |
Electr. Conductivity ( S / m) |
Electronegativity |
|---|---|---|---|---|---|---|---|
| carbon | 12.011 | 3823 | 5100 | 2250 to 3510 | 0.5 to 10.0 | 10 −4 ... 10 +6.5 | 2.5 |
| Silicon | 28.086 | 1683 | 2628 | 2330 | 6.5 | 2.52 · 10 −4 | 1.7 |
| Germanium | 72.59 | 1211 | 3093 | 5323 | 6th | 1.45 | 2.0 |
| tin | 118.71 | 505 | 2875 | 7310 | 1.5 | 9.17 · 10 6 | 1.96 |
| lead | 207.2 | 601 | 2022 | 11340 | 1.5 | 4.81 · 10 6 | 1.6 |
Electron configuration
The electron configuration is [ X ] y s 2 y p 2 . The X stands for the electron configuration of the noble gas , which is one period higher , and the period in which the element is located must be used for the y . From germanium there is also a (y − 1) d 10 orbital; and from lead there is also a (y − 2) f 14 orbital.
The electron configurations for the individual elements are:
- Carbon: [ He ] 2s 2 2p 2
- Silicon: [ Ne ] 3s 2 3p 2
- Germanium: [ Ar ] 3d 10 4s 2 4p 2
- Tin: [ Kr ] 4d 10 5s 2 5p 2
- Lead: [ Xe ] 4f 14 5d 10 6s 2 6p 2
- Flerovium (calculated): [ Rn ] 5f 14 6d 10 7s 2 7p 2
The oxidation states are +4 and –4, but with increasing atomic number, the oxidation state +2 becomes more important.
Chemical reaction
Due to the large differences within the group, it is difficult to state a general reaction behavior, as this varies from element to element. In the following equations, the E stands for an element from the carbon group.
- Reaction with oxygen :
- The most important reaction is the formation of the respective dioxide from the elements.
- In addition to the tetravalent oxides, the divalent oxides of all group elements are also known. The stability of the divalent oxides increases with increasing atomic number, that of the tetravalent oxides decreases somewhat. In addition, various sub- and mixed oxides are known, such as. B. the carbon suboxide C 3 O 2 or the lead (II, IV) oxide Pb 3 O 4 .
- Reaction with hydrogen (without chain formation, not spontaneous):
- Reaction with water :
- None of the group elements react with water.
- Carbon, silicon and germanium only react to form tetrachloride, with tin SnCl 4 and SnCl 2 are possible and lead only forms the dichloride PbCl 2 .
Chain formation

A special feature of the group 14 elements is their ability to form long-chain hydrogen compounds with the structure X H 3 - ( X H 2 ) n - X H 3 . All hydrogen atoms are covalently bound, but the stability of these compounds decreases with increasing atomic number of the element.
- Hydrocarbons : The group of hydrocarbons is the most extensive, as there are hardly any limits to the number of C atoms and thus also to the chain length. Another specific property of carbon is its ability to form stable double and triple bonds . Organic chemistry is concerned with hydrocarbons and their derivatives .
- Silanes : With silicon, the ability to form chains is already limited to a maximum of 15 Si-Si bonds. Double or even triple bonds are unstable in silicon and the following elements, but the silanes are not among the most stable compounds either.
- Germane : Germanium is only to a maximum of nine Ge Ge bonds capable. This of course severely limits the possibilities.
- Hydrogen tin: Only one Sn-Sn bond is possible with tin. There are therefore only two compounds in this class: SnH 4 and SnH 3 -SnH 3 .
- Hydrogen lead : Lead does not have the ability to form chains. Only PbH 4 is known, but this compound is also almost unstable.
Ring formations are also possible, the sum formula is then ( X H 2 ) n .
links
-
Oxides (IV), hydroxides and acids
- Due to the stability of the C = O double bond, carbon dioxide (CO 2 ) is a triatomic, linear and therefore non-polar molecule and is in the gaseous state of aggregation . In water it forms the inconsistent, weak carbonic acid (H 2 CO 3 ).
- In silicon dioxide (SiO 2 ) single bonds exist exclusively. It is a solid and consists of SiO 4 - tetrahedra , which are linked together via all the corners. In nature, SiO 2 occurs as quartz . Amorphous SiO 2 ( silica glass ) is the essential component of glass .
- Germanium dioxide (GeO 2 ) essentially corresponds to silicon dioxide, but can also crystallize in the rutile structure. The latter forms germanic acid (H 4 GeO 4 ) with water .
- Tin (IV) oxide (SnO 2 ) is a solid and is not soluble in water.
- Lead (IV) oxide (PbO 2 ) is also a solid which is insoluble in water.
- Hydrogen compounds: see under chain formation
- other:
- Carbon monoxide (CO) is a poisonous gas.
- Silicon monoxide (SiO) is a dark brown, amorphous solid made of polymeric (SiO) x chains.
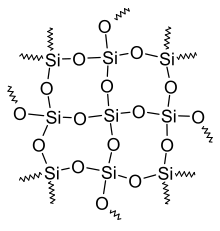
- Silicones consist of three-dimensional networks of alternating silicon and oxygen, with mostly organic residues (for example methyl groups (-CH 3 )) on the silicon atoms .
- Lead (II) oxide
- Lead (II, IV) oxide
Occurrence

The earth's crust consists of 27.7% elements of the carbon group. Of this, 99.8% is silicon, the second most common element in the earth's crust (after oxygen).
The remaining 0.2% are broken down as follows:
- 99.1% carbon
- 0.94% lead
- 0.02% tin
- 0.01% germanium
With the exception of germanium, they also occur naturally in a dignified form.
Web links
literature
- Hans Breuer: dtv-Atlas Chemie (Volume 1: General and inorganic chemistry) . Pp. 131-153 (2000), ISBN 3-423-03217-0