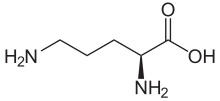
Proteus (bacteria)
| Proteus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Electron microscope image of Proteus penneri , the bar corresponds to 200 nm. |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Proteus | ||||||||||||
|
Hauser 1885 emend. Hyun et al. 2016 |
Proteus is a genus of bacteria and is named after the Greek god of the sea, Proteus , who is characterized in Homer's Odyssey as being extremely changeable on the outside. This also applies to the bacteria: The length and function of cells of Proteus - species is variable; so-called "swarm cells" can be formed, which cause the "swarm phenomenon".
Bacteria of the genus Proteus are widespread in various habitats such as soil and water . They are able to utilize dead biomass and thus count among the saprobionts . They have numerous enzymes at their disposal , for example urease . They are also present in the intestinal tract of animals and humans, as well as in feces . Some representatives of the genus Proteus can also have a pathogenic effect, they are considered to be facultative pathogenic . For example, they can cause urinary tract infections .
An infection with Proteus has to be differentiated from Proteus syndrome , a very rare severe congenital growth disorder that has only the name in common with bacteria.
features
Appearance

The cells of representatives of the genus Proteus are rod-shaped with a diameter of 0.4 to 0.8 µm and very variable length. They are flagellated with flagella ( peritrich ) distributed evenly over the body surface and thus actively mobile ( motile ). The Gram test is negative, so Proteus belongs to the group of gram-negative bacteria.
Swarm phenomenon

Proteus shows a clear swarming behavior, which can be explained by dimorphism ("two forms") of the cells. In liquid nutrient media , the cells are in the form of short rods with a length of 1 to 2 µm, which can move through their perithric flagella (the flagella are evenly distributed over the cell surface). They are called "swimming cells". Long (20 to 80 µm), particularly densely flagellated cells ("swarm cells") are formed from this on solid nutrient media, which can move on the surface of the nutrient medium in the very thin layer of liquid formed by syneresis ("swarming"). As a result, the initially narrower colony spreads quickly over the surface of the nutrient medium. Swarming and local reproduction alternate with a shorter morphotype (consolidation). As a result, the surface is covered by a large colony that is terraced.

The development of swim cells to swarm cells occurs when the rotation of the flagella is inhibited by contact with a surface. The addition of antibodies directed against the flagella also leads to this result. In this antigen-antibody reaction , the flagella are referred to as H antigens (see section on chemotaxonomy ). The lipopolysaccharides in the outer membrane of the cells are also important for the swarm phenomenon. The polysaccharide part of the lipopolysaccharides, known as the O6 antigen, is a prerequisite for the formation of swarm cells. Mutants of Proteus mirabilis that produce a lipopolysaccharide that consists only of lipid A and the core region, without the O-specific polysaccharide, are unable to swarm. The replication of the DNA takes place without the formation of new cell walls and septation of the cells, which leads to very long polyploid cells. The exact molecular background is still unclear, but so-called SulA proteins , which are known from Escherichia coli , are probably involved.
The swarm cells are converted back to swim cells when the local cell density is low. To do this, the cells must be able to measure the population density via chemical communication ; this is called quorum sensing . The search for the autoinductors used as signaling molecules is still ongoing. Autoinducer-1 (AI-12) is not produced by the cells and autoinducer-2 (AI-2) plays no role in the swarm phenomenon. In contrast, oleic acid , glutamine and putrescine have been recognized as signal molecules without the mechanism of action being fully understood. In addition to the inhibition of flagella rotation, the formation of swarm cells is initiated by the accumulation of extracellular putrescins and interactions of O-antigens with a surface. A key event in the process of swarm cell formation is the upregulation of the transcriptional regulator gene FlhD2C2, which activates the flagellar regulon and additional genes.
Growth and metabolism
The representatives of the genus Proteus are facultatively anaerobic , i. H. they can grow with or without oxygen . The catalase test is positive for them, the oxidase test negative. Furthermore, their metabolism of substances and energy is to be characterized as chemoorganotrophic and heterotrophic , they use organic compounds as an energy source and also to build up the cell's own substances. They live saprophytically and utilize dead biomass .
Bacteria of the genus Proteus are mesophilic , i. H. They prefer medium temperatures, bacterial growth occurs in the temperature range of 10–37 ° C, optimally around 30 ° C. With regard to the pH value , they are to be labeled as neutrophil , they grow at a pH value of 5 to 9, optimally at a pH value of 7 to 8. They tolerate a mass fraction of 0–10% of sodium chloride (NaCl ) in the nutrient medium, 1–2% NaCl is optimal.
They use various carbohydrates as carbon and energy sources , e.g. B. glucose , maltose and sucrose , often with acid and gas formation, while they carry out fermentations under anoxic conditions (without oxygen) , for example mixed acid fermentation . They cannot use the disaccharide lactose (milk sugar) because they lack the enzyme β-galactosidase . They also reduce nitrate to nitrite .
Proteus is also characterized by the ability to hydrolysis of gelatin (gelatin-liquefaction), the hydrogen sulphide formation from sulfur-containing amino acids and Proteus can with the existing enzyme urease urea in ammonia and carbon dioxide column, which leads to an increase of the pH value in the medium . Most members of the genus have a lipase that the lipids of corn oil splits hydrolysis. Further information can be found in the Evidence section .
The genus Proteus is very similar to the genera Providencia and Morganella , they have been part of the Morganellaceae family since 2016 . All three genera produce the enzyme phenylalanine deaminase but no arginine dihydrolase (ADH), cannot metabolize malonic acid and do not form acid when metabolizing dulcitol , D - sorbitol and L - arabinose . In contrast to the two genera, representatives of Proteus can produce hydrogen sulfide and lipases, hydrolyze gelatin , but they do not produce mannose .
Chemotaxonomy

Components of the bacterial cell act as antigens : The so-called somatic O-antigens occur once and then the H-antigens caused by the flagella. The somatic O-antigens are based on the lipopolysaccharides in the outer membrane of the cells.
The designation and cellular assignment of the antigens is described in the Kauffmann-White scheme used for Salmonella . The differentiation of serotypes is not used in routine diagnostics. The O and H antigens are involved in the swarm phenomenon.
Proteus - species have in their membrane lipids v. a. the following fatty acid chains: 28–30% palmitic acid (a saturated fatty acid with the abbreviation C 16: 0 ), 15–36% of a mixture of cis - palmitoleic acid (a monounsaturated omega-n fatty acid - in in this case omega-7 fatty acid - with the abbreviation C 16: 1 (ω − 7) c ) and / or the isomeric omega-6 fatty acid with the abbreviation C 16: 1 (ω − 6) c ( systematic name : (10 Z ) -hexadec-10-enoic acid), as well as 16–19% of a mixture of cis - vaccenic acid (a monounsaturated omega-7 fatty acid with the abbreviation C 18: 1 (ω − 7) that cannot be separated using the analytical method used ) c ) and / or the isomeric omega-6 fatty acid with the abbreviation C 18: 1 (ω − 6) c (systematic name: (12 Z ) -octadec-12-enoic acid). The main ubiquinones that occur are Q-8 and Q-10.
genetics
The genome of several Proteus species has been sequenced, including eleven strains of Proteus mirabilis and three strains of Proteus vulgaris . For example, the genome of the Proteus mirabilis HI4320 strain has a size of 4.06 megabase pairs (Mbp), which is roughly comparable to the genome size of Escherichia coli . There are 3562 proteins annotated . The sequenced plasmid is 0.04 megabase pairs in size and contains 48 genes . The results of the sequencing show a GC content (the proportion of the nucleobases guanine and cytosine ) in the bacterial DNA of 38.9%.
Pathogenicity
Some representatives of the genus Proteus can have a pathogenic effect on people with impaired health, so they are facultative pathogenic (opportunistic) pathogens . They are often found in the large intestine , even in healthy people , and do not necessarily cause disease. Correspondingly immunocompromised people can suffer from the following symptoms caused by these bacteria: urinary tract infection , wound infection and sepsis . The species Proteus mirabilis and Proteus vulgaris are most frequently isolated. Urinary tract infections, it is believed that the occurring in bacteria urease as a virulence factor acts and by increasing the pH of the bacterial growth is made possible. It can also promote the formation of kidney stones .
Proteus hauseri , P. mirabilis , P. penneri and P. vulgaris are assigned to risk group 2by the Biological Agents Ordinance in conjunction with the TRBA ( Technical Rules for Biological Agents) 466. Other Proteus species belong to risk group 1 (they are regarded as non-pathogenic) or have not yet been assigned.
proof
To isolate the bacteria from samples, e.g. B. urine , selective nutrient media are usually used, which are suitable for the isolation and differentiation of representatives of the enterobacteria , for example eosin methylene blue agar (EMB agar), MacConkey agar or VRB agar . Bacteria of the genus Proteus typically grow as lactose-negative and possibly sucrose-positive colonies (if the nutrient medium contains sucrose, such as EMB agar).
Biochemical evidence
Biochemical features, such as the enzymes present and the resulting metabolic properties, can be used in a colorful series to identify Proteus species or to distinguish them from other representatives of the Morganellaceae, in particular Providencia and Morganella , as these have similar metabolic properties. A systematic overview from the year 2000 of representatives of the three genera is the basis for the results in the following table.
| Test method, feature | Proteus | Providencia | Morganella |
|---|---|---|---|
| "Swarm phenomenon" | + ( 1 ) ( 2 ) | - ( 1 ) | - |
| Methyl red sample | + | (+) ( 1 ) | (+) |
| Citrate utilization | d ( 1 ) | + | - |
| H 2 S formation | d | - | d |
| Urease | + | d | + |
| Hydrolysis of gelatine (22 ° C) | d | - | - |
| Lipase (corn oil) | d | - | - |
| Arginine dihydrolase (ADH) | - | - | - |
| Lysine decarboxylase (LDC) | - | - | d |
| Ornithine decarboxylase (ODC) | d | - | d |
| Phenylalanine deaminase | + | + | + |
| Acid formation from L - adonitol ( 3 ) | - | d | - |
| Acid formation from L - arabinose | - | - | - |
| Acid formation from dulcitol | - | - | - |
| Acid formation from myo - inositol | - | d | - |
| Acid formation from lactose | - | - | - |
| Acid formation from D - mannose | - | + | + |
| Acid formation from D - sorbitol | - | - | - |
Remarks:
- + stands for a positive, - for a negative result; if put in brackets, it applies that most representatives of the genus (> 75%), but not all, show this result; the indication d (for different) means "variable", d. that is, there are strains that can produce a positive result as well as strains that do not; or the positive reaction is too weak to be seen clearly.
- Reactions that are suitable for differentiation, at least in combination with others, are contrasted in color.
- "Acid formation from ...": These are reactions in which the utilization of carbohydrates is checked; monosaccharides , disaccharides and sugar alcohols are listed . When carbohydrates are used, a pH indicator is used to check whether acids are formed during the breakdown.
The following are the results of some biochemical tests of individual Proteus species, which - at least in combination with others - are suitable for differentiation. The species first described in 2016 and later are sorted in the right part of the table. For the details +, - etc. see comments above. The specification n. means "not checked".
| Test method, feature | P. hauseri | P. mirabilis | P. penneri | P. vulgaris | P. alimentorum | P. cibarius | P. columbae | P. terrae |
|---|---|---|---|---|---|---|---|---|
| Indole formation | + | - | - | + | + | + | + | + |
| Citrate utilization | - | d | - | d | - | - | - | - |
| H 2 S formation | d | + | (-) | (+) | + | n. o. | + | n. o. |
| Hydrolysis of gelatine (22 ° C) | + | + | d | d | + | + | + | n. o. |
| Lipase (corn oil) | - | + | d | (-) | n. o. | n. o. | n. o. | n. o. |
| Ornithine decarboxylase (ODC) | - | + | - | - | - | - | - | - |
| Aesculin splitting | - | - | - | + | + | - | - | - |
| DNase (25 ° C) | - | d | (-) | + | n. o. | n. o. | n. o. | n. o. |
| Acid formation from D - fructose | + | + | + | + | n. o. | + | - | + |
| Acid formation from maltose | + | - | + | + | + | + | + | + |
| Acid formation from L - rhamnose | - | - | - | - | - | - | - | + |
| Acid formation from sucrose | + | - | + | + | + | + | + | + |
| Acid formation from salicin | - | - | - | + | + | - | - | - |
| Acid formation from trehalose | - | + | d | - | n. o. | + | + | + |
These examinations can be used for miniaturized test systems (e.g. the API-20E system). The results can be viewed in the freely accessible database BacDive of the DSMZ ( German Collection of Microorganisms and Cell Cultures ), for example for Proteus vulgaris . Systems that are automated in terms of equipment (e.g. the Vitek system) are also based on metabolic properties.
Further evidence
Identification using the MALDI-TOF method in combination with mass spectrometry (MS) is becoming increasingly important , especially in laboratories with a high sample throughput. Here, too, the first step is usually to isolate the bacteria from the test material by culturing them on nutrient media. For the identification databases are needed that contain bacteria spectra of structures by certain recognition pattern (engl. Pattern ) the assignment is made to a genus or species. Biomarkers are used for the mass spectra in order to improve the specificity of the identification, particularly in the case of medically relevant bacteria . Certain proteins within the bacterial cells, especially ribosomal proteins, serve as biomarkers , as they are abundant in the sample material used and are coded in a highly conserved manner by genes in the bacterial chromosome .
In 2017, the identification results were compared using MALDI-TOF MS using biomarkers and conventional biochemical methods (Vitek 2). In the case of 222 gram-negative bacteria from a total of 383 isolates, MALDI-TOF MS resulted in a correct identification of the genus of 97.6% and a correct identification of the species of 97.4%. These values are better compared to conventional identification with 95.7% and 88.0%, respectively. In the 383 isolates there were 14 representatives of the genus Proteus , all of which were correctly identified at the genus level using MALDI-TOF MS (100%). Among them there are seven correct identifications of P. mirabilis (100%) and seven correct identifications of P. penneri / vulgaris (100%). The specificity of identification by conventional methods at the genus level is also 100%, but only six out of seven cases correctly identify P. penneri / vulgaris . These results also show that the procedures routinely used in laboratories do not allow any distinction between P. penneri and P. vulgaris .
In order to further minimize the examination time, there are currently approaches to use the examination material directly for identification by means of MALDI-TOF MS - without prior cultivation and thus isolation of the bacteria. In a study from 2019, urine samples with a volume of 30 ml each , which presumably contained pathogens from urinary tract infections, were used directly for MALDI-TOF MS after enrichment through centrifugation and washing steps. Of the 1638 urine samples, 265 contained mainly one species of bacteria, 184 of which were Gram-negative, of which 163 (88.6%) were correctly identified at the species level. P. mirabilis was contained in seven enriched isolates ; there are six correct identifications at the species level (85.7%), in one case only at the genus level. If the bacteria were previously isolated by cultivation, all seven isolates were correctly identified as P. mirabilis . The direct examination by means of MALDI-TOF MS of the bacteria accumulated from the urine samples takes less than two hours, if the bacteria are cultivated beforehand, the procedure takes a total of 18-48 hours.
Systematics and taxonomy
External system
As early as the 1960s, the close relationship of the genera Proteus , Providencia and Morganella known at the time was shown on the basis of phenotypic characteristics. They were listed in the Proteeae tribe . Since the establishment of the Enterobacterales order in 2016, they belong to the Morganellaceae family along with other genera. The Enterobacterales belong to the class of Gammaproteobacteria , which in turn belongs to the strain of Proteobacteria .
Internal system
Currently (September 2020) nine named species and a number of unnamed genotypes are known. Proteus vulgaris is the type species of the genus Proteus Hauser 1885 (Approved Lists 1980) emend. Hyun et al. 2016. The species are (as of September 2020):
- Proteus alimentorum Dai et al. 2018
- Proteus cibarius Hyun et al. 2016
- Proteus cibi Dai et al. 2019
- Proteus columbae Dai et al. 2018
- Proteus hauseri O'Hara et al. 2000
- Proteus mirabilis Hauser 1885
- Proteus penneri Hickman et al. 1983
- Proteus terrae Behrendt et al. 2016
- Proteus vulgaris Hauser 1885 (Approved Lists 1980) emend. Judicial Commission 1999.
Some synonyms and changes
| Taxonomy of the Proteus vulgaris group | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
| Changes in the taxon Proteus vulgaris according to O'Hara et al. (2000) |
The previously listed species Proteus myxofaciens is genetically quite different from the other species of the genus, so it was separated out as Cosenzaea myxofaciens into its own genus Cosenzaea , which continues to be part of the Morganellaceae family.
The classification of the genera Proteus , Providencia and Morganella in the tribe Proteeae in the 1960s shows the initial difficulty in assigning certain taxa to a genus with certainty. Accordingly, there are numerous synonyms . In Morganella morganii ( Winslow et al. 1919) Fulton 1943 and Proteus morganii ( Winslow et al. 1919) Yale 1939, in Providencia rettgeri ( Hadley et al. 1918) Brenner et al. 1978 and Proteus rettgeri ( Hadley et al. 1918) Rustigian and Stuart 1943 and Providencia alcalifaciens ( de Salles Gomes 1944) Ewing 1962 and Proteus inconstans ( Ornstein 1920) Shaw and Clarke 1955 are both homotypical synonyms , since both species have the have the same type trunk.
The first use of the generic name "Proteus" in bacteriological nomenclature comes from Gustav Hauser in 1885, who described two types of these organisms, which he isolated from rotten meat , Proteus mirabilis and Proteus vulgaris . So this organism has a long history in microbiology . In the past few decades the taxon Proteus , particularly Proteus vulgaris , has undergone some changes in taxonomy . In 1982 Proteus vulgaris was separated into three groups (English biogroups ) on the basis of indole production , the utilization of salicin and the aesculin cleavage . The first group (biogroup 1) behaves negatively in all three reactions and was separated as a new species ( Proteus penneri ). The second group (biogroup 2) behaved positively in all three reactions and remained as Proteus vulgaris . The third group (biogroup 3) is positive in the indole test , but negative for the utilization of salicin and the aesculin breakdown. Genetic investigations in 1995 with the help of DNA hybridization led to the subdivision of biogroup 3 into four taxa (genome species 3 to 6), which were initially not described as species until they were better characterized; one of them (genome species 3) was described in 2000 as Proteus hauseri . Hyun et al. published an extended description (emendation) of the genus Proteus in 2016 with the first description of P. cibarius .
Occurrence
Bacteria of the genus Proteus are widespread as saprophytes in various habitats such as soils and bodies of water that contain organic material such as dead biomass and excretions from living beings . These bacteria are also present in the intestinal tract of animals and humans as well as in faeces .
In an article published in the scientific journal Microbial Ecology in 2016 , over 150 scientific articles on the topic “Importance and functions of Proteus spp. Bacteria in the natural environment ”: They occur in the entire gastrointestinal tract of healthy people. In a Brazilian study with as many women as men, the bacteria were found in the corresponding part of the gastrointestinal tract as follows: stomach 8%, duodenum (duodenum) 45%, jejunum (empty intestine) 45%, ileum (ileum) 20%, appendix (caecum ) 12%, large intestine : ascending colon 33%, transverse colon 37%, descending colon 25%, sigmoid colon 35% and rectum 30%. The highest concentrations of bacteria, expressed as colony-forming units (CFU) per milliliter, are found in the ileum with 10 6 , in the various areas of the large intestine with 10 5 to 10 7 and in the rectum with 10 7 CFU per milliliter.
Bacteria of the Proteus genus are also found in the intestines of numerous animals - both wild and domesticated - including gorillas , raccoon dogs , rats , flying foxes , birds , snakes , alligators , turtles , amphibians , fish , insects , " seafood " such as mussels and shrimp or crabs ; Domestic dogs , domestic cats , domestic pigs , domestic horses , domestic donkeys , domestic cattle and poultry . The Proteus species is not specified in all documented finds ; if this is the case, information is provided on P. mirabilis , P. vulgaris group (including P. hauseri , which was later separated as a separate species, and the genomic species) and P. penneri . Proteus mirabilis was more likely to be isolated from domestic dogs, domestic cattle and birds, while Proteus vulgaris et al. was found in domestic pigs and cold-blooded animals .
The species first described in 2016 and later were isolated from food ( P. alimentorum from pork and lobster , P. cibarius from Jeotgal , a salted, fermented dish made from seafood, which is eponymous for the members of the gram-positive genus Jeotgalicoccus , P. columbae from pigeon meat ), P. terrae was found in a soil sample ( peat soil from a bog area ).
In medical samples such as urine and wounds was P. mirabilis demonstrated rare, other members of the genus, even if an exact identification of the kind took place. About 80-90% of those caused by Proteus spp. infections caused are due to P. mirabilis . A comparative study of faecal samples from healthy people and patients suffering from diarrhea showed that P. mirabilis could be isolated more frequently from samples from sick people than from healthy people. One possible explanation for this is that the diarrhea is caused by other pathogens, but that it gives P. mirabilis the opportunity to reproduce opportunistically. In contrast , with P. vulgaris no difference was found in terms of occurrence in the two groups of people.
importance
ecology
As saprophytes, bacteria of the genus Proteus are involved in the breakdown of organic compounds (dead biomass) in the environment. The presence of various enzymes enables them to break down urea (through urease), hydrolyze proteins (through proteolytic enzymes ) and oxidative deamination of amino acids . They act as destructors in the nitrogen cycle and convert nitrogen-containing organic compounds (proteins, amino acids, urea) into inorganic substances and the amino nitrogen they contain into ammonium ions, whereby these products can again be used as nutrients by plants .
In the rhizosphere , they can have a positive effect on the growth of certain plants. A strain of P. vulgaris from the soil of a tea plantation produces siderophores , which improve the bioavailability of iron ions for the tea plant ( Camellia sinensis ). Also antifungal substances produced by this strain in the test against the pathogenic fungus Fusarium moniliformae act and thus the growth of the so infected legumes promote. In soils and waters that are polluted with heavy metals , representatives of the genus (including P. mirabilis and P. vulgaris ) can be found that are resistant to copper , chromium , cobalt , cadmium , zinc and mercury . In some cases it can even be proven that the bacteria can reduce the content of chromium (VI) compounds in the water.
The ecological relationship between bacteria of the genus Proteus and the animals they colonize is still unclear in many cases, but examples of opportunistic infections, commensalism and symbiosis can be found. P. mirabilis strains can cause urinary tract infections in domestic cats, dogs, and house urchins. Proteus bacteria are part of the normal flora of animals; this applies to numerous birds, including poultry as farm animals , for example sparrows , blackbirds , ravens and crows ( Corvus spp.), White storks ( Ciconia ciconia ) and domestic geese . The bacteria are found in the cloaca , in the excrement and sometimes in the beak .
In numerous representatives of the flies (including the housefly Musca domestica , the common biting fly Stomoxys calcitrans and various blowflies Lucilia spp.) These bacteria are a main component of the microbiome in the digestive system as well as the body surface. The flies act as a vector through which the bacteria are transmitted to other living things. One hypothesis concerns messengers , the " kingdom -übergreifend" act (Engl. Inter-kingdom signaling ) - in terms of taxa animals and bacteria - and is based on the ecological relationship of P. mirabilis and gold fly Lucilia sericata as a host . The signal substances are volatile compounds such as putrescine , which are produced by P. mirabilis during the breakdown of dead biomass containing nitrogen. Putrescine contributes to the smell of putrefaction and attracts the gold fly to the carcass . At the same time, putrescine is an extracellular messenger substance that is necessary for the swarming phenomenon of bacterial cells.
Strains of P. mirabilis and P. vulgaris belong to the symbiotic intestinal flora of the Indian giant bat ( Pteropus giganteus ) and, together with other enterobacteria, play an important role in its digestion . The microorganisms produce enzymes ( cellulases and xylanases ), which break down the cellulose or xylans contained in the vegetable food , whereby the metabolites can be used by the host animal.
Medical importance
Proteus bacteria are part of the normal intestinal flora in humans; some representatives can, however, have a pathogenic effect on people who are already weakened, so they are among the opportunistic pathogens. You can here z. B. urinary tract infection , renal pelvic inflammation , cystitis and prostatitis . A gastroenteritis can occur if highly contaminated foods were consumed. Infections with Proteus bacteria occur relatively frequently during a hospital stay ( nosocomial ) and can then lead to wound infections (e.g. in the case of burns) or sepsis . Urinary tract infections often occur with urinary obstruction (obstructive uropathies), after surgical interventions on the urinary tract or with long-term use of urinary catheters . The species P. mirabilis , P. vulgaris and P. penneri are most commonly isolated from medical specimens from human patients.
A problem with urinary tract infections, especially in patients with catheters, is the formation of a biofilm due to the rapid growth. To make matters worse, in biofilms dominated by Proteus , crystals form through biomineralization of the urea , which leads to crust formation and ultimately clogging of the catheter.
In a 2016 report by the ECDC ( European Center for Disease Prevention and Control ) on urinary tract infections (UTI) acquired in intensive care units , bacteria of the genus Proteus are responsible for 5.4% of cases. Considered only for Germany, the rate is higher at 7.9%.
Antibiotic resistance and effective antibiotics
The Proteus species P. mirabilis , P. penneri and P. vulgaris are naturally resistant to the antibiotics tetracyclines , tigecycline , colistin and nitrofurantoin . P. mirabilis strains are generally more sensitive to antibiotics than P. vulgaris , P. penneri, and P. hauseri strains . Thus, P. mirabilis in principle susceptible to ampicillin and cephalosporins cefazolin and cefuroxime (lead compounds of cephalosporins 1st and 2nd generation), while P. penneri and P. vulgaris however, have a natural resistance. At least in P. vulgaris , the formation of β-lactamases is seen as the cause. However, the resistance of the bacterial strains can vary with time and region. Therefore, at least in the case of serious infections or therapy failure, it is advisable to check the resistance to certain antibiotics, i.e. to carry out an antibiogram , in order to identify an effective antibiotic for therapy.
P. mirabilis is fundamentally sensitive to aminopenicillins (ampicillin and amoxicillin ) and ureidopenicillins ( piperacillin ), cephalosporins , for example cefazolin, cefoxitin (1st generation); Cefuroxime (2nd generation); Cefotaxime , ceftazidime , ceftizoxime , ceftriaxone (3rd generation) and cefepime (4th generation), aminoglycoside antibiotics ( amikacin , gentamicin and tobramycin ), imipenem from the group of carbapenems , ciprofloxacin from the group of fluoroquinolones and cotrimoxazolones . Resistance to ciprofloxacin has been observed in infections with P. mirabilis occurring during hospitalization (nosocomial) . As early as 1979, some acquired resistances of individual strains of P. mirabilis were reported, among other things. the antibiotics ampicillin, cefalotin , chloramphenicol , carbenicillin , colistin , cotrimoxazole and numerous aminoglycoside antibiotics (including streptomycin and gentamicin).
P. penneri and P. vulgaris are generally sensitive to cefoxitin and broad-spectrum cephalosporins (cefotaxime, ceftazidime, ceftizoxime, ceftriaxone, cefepime), aztreonam , aminoglycoside antibiotics, ciprofloxacin and imipenem. In addition, β-lactamase inhibitors such as tazobactam can be used. Possible resistances concern the cephalosporins cefazolin, cefprozil, cefuroxime, cefamandol , cefdinir , cefoperazon , loracarbef and the ureidopenicillins and ampicillins belonging to the penicillins .
Most Proteus species are sensitive to quinolone antibiotics and broad-spectrum 2nd and 3rd generation cephalosporins. For the treatment of uncomplicated urinary tract infection with Proteus TYPES is occasionally cotrimoxazole recommended.
swell
literature
- Caroline Mohr O'Hara, Frances W. Brenner, J. Michael Miller: Classification, Identification, and Clinical Significance of Proteus, Providencia, and Morganella . In: Clinical Microbiology Reviews . tape 13 , no. 4 . American Society for Microbiology, Washington October 2000, p. 534-546 , doi : 10.1128 / cmr.13.4.534-546.2000 , PMID 11023955 , PMC 88947 (free full text).
- Dominika Drzewiecka: Significance and Roles of Proteus spp. Bacteria in Natural Environments . In: Microbial Ecology . tape 72 , no. 4 , January 2016, p. 741-758 , doi : 10.1007 / s00248-015-0720-6 , PMID 26748500 , PMC 5080321 (free full text).
Individual evidence
- ↑ a b c d e f g h i j k l m C. M. O'Hara, FW Brenner, JM Miller: Classification, identification, and clinical significance of Proteus, Providencia, and Morganella . In: Clinical Microbiology Reviews . tape 13 , no. 4 , October 2000, p. 534-546 , doi : 10.1128 / cmr.13.4.534-546.2000 , PMID 11023955 , PMC 88947 (free full text).
- ↑ a b c d e f g Antoni Różalski, Agnieszka Torzewska, Magdalena Moryl, Iwona Kwil, Agnieszka Maszewska, Kinga Ostrowska, Dominika Drzewiecka, Agnieszka Zabłotni, Agata Palusiak, Małgorzata Siwińska sp. - an opportunistic bacterial pathogen - classification, swarming growth, clinical significance and virulence factors . In: Folia Biologica et Oecologica . tape 8 , no. 1 , December 2012, p. 1-17 ( sciendo.com ).
- ↑ George M. Garrity: Bergey's manual of systematic bacteriology. 2nd Edition. Springer, New York, 2005, Volume 2: The Proteobacteria , Part B: The Gammaproteobacteria ISBN 0-387-24144-2
- ↑ a b JN Schaffer, MM Pearson MM .: Proteus mirabilis and urinary tract infections. In: Microbiol Spectrum . tape 3 , no. 5 , 2015, p. UTI-0017-2013 , doi : 10.1128 / microbiolspec.UTI-0017-2013 .
- ↑ a b c J. Manos, R. Belas: The Genera Proteus, Providencia, and Morganella . In: Prokaryotes . tape 6 , 2006, p. 245-269 , doi : 10.1007 / 0-387-30746-x_12 .
- ↑ Randy M. Morgenstein, Bree Szostek & Philip N. Rather: Regulation of gene expression during swarmer cell differentiation in Proteus mirabilis . In: FEMS Microbiol Rev. Volume 34 , 2010, p. 753-763 , doi : 10.1111 / j.1574-6976.2010.00229.x .
- ↑ Chelsie E. Armbruster and Harry LT Mobley: Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis . In: Nat. Rev. Microbiol. tape 10 , no. 11 , 2012, p. 743-754 , doi : 10.1038 / nrmicro2890 .
- ↑ a b c d e f g h Dong-Wook Hyun, Mi-Ja Jung, Min-Soo Kim, Na-Ri Shin, Pil Soo Kim, Tae Woong Whon, Jin-Woo Bae: Proteus cibarius sp. nov., a swarming bacterium from Jeotgal, a traditional Korean fermented seafood, and emended description of the genus Proteus . In: International Journal of Systematic and Evolutionary Microbiology . tape 66 , no. 6 , June 2016, p. 2158-2164 , doi : 10.1099 / ijsem.0.001002 .
- ↑ a b c d e f g h i j Dominika Drzewiecka: Significance and Roles of Proteus spp. Bacteria in Natural Environments . In: Microbial Ecology . tape 72 , no. 4 , January 2016, p. 741-758 , doi : 10.1007 / s00248-015-0720-6 , PMID 26748500 , PMC 5080321 (free full text).
- ↑ Proteus. In: National Center for Biotechnology Information (NCBI) Genome website . Retrieved February 8, 2021 .
- ↑ a b Enterobacteria, Proteus . In: Helmut Hahn, Stefan HE Kaufmann, Thomas F. Schulz, Sebastian Suerbaum (eds.): Medical microbiology and infectious diseases . 6th edition. Springer Verlag, Heidelberg 2009, ISBN 978-3-540-46359-7 , p. 237, 250 .
- ↑ TRBA (Technical Rules for Biological Agents) 466: Classification of prokaryotes (bacteria and archaea) into risk groups. In: Website of the Federal Institute for Occupational Safety and Health (BAuA). August 25, 2015, p. 346 , accessed on December 27, 2019 (last change on August 14, 2019).
- ↑ EMB agar. In: Merck Millipore website . Retrieved December 26, 2019 .
- ↑ a b c d e C. M. O'Hara, FW Brenner, JM Miller, B. Holmes, PA Grimont, JL Penner, AG Steigerwalt, DJ Brenner, BC Hill, PM Hawkey: Classification of Proteus vulgaris biogroup 3 with recognition of Proteus hauseri sp. nov., nom. rev. and unnamed Proteus genomospecies 4, 5 and 6. In: International Journal of Systematic and Evolutionary Microbiology . tape 50 , no. 5 , September 2000, pp. 1869-1875 , doi : 10.1099 / 00207713-50-5-1869 .
- ↑ a b c d Hang Dai, Yonglu Wang, Yujie Fang, Zhenzhou Huang, Biao Kan, Duochun Wang: Proteus alimentorum sp. nov., isolated from pork and lobster in Ma'anshan city, China . In: International Journal of Systematic and Evolutionary Microbiology . tape 68 , no. 4 , April 2018, p. 1390-1395 , doi : 10.1099 / ijsem.0.002689 .
- ↑ a b c d Hang Dai, Yonglu Wang, Yujie Fang, Tao Xiao, Zhenzhou Huang, Biao Kan, Duochun Wang: Proteus columbae sp. nov., isolated from a pigeon in Ma'anshan, China . In: International Journal of Systematic and Evolutionary Microbiology . tape 68 , no. 2 , February 2018, p. 552-557 , doi : 10.1099 / ijsem.0.002541 .
- ↑ German Collection of Microorganisms and Cell Cultures (DSMZ): Proteus vulgaris, Type Strain. In: Website BacDive . Retrieved December 23, 2019 .
- ↑ a b Ali Kassim, Valentin Pflüger, Zul Premji, Claudia Daubenberger, Gunturu Revathi: Comparison of biomarker based Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) and conventional methods in the identification of clinically relevant bacteria and yeast . In: BMC Microbiology . tape May 17 , 2017, p. 128 (online) , doi : 10.1186 / s12866-017-1037-z , PMID 28545528 , PMC 5445374 (free full text).
- ↑ Wei Li, Enhua Sun, Ying Wang, Hongwei Pan, Y. i. Zhang, Yong Li, Xin Zhang, Chen Li, Lutao Du, Chuanxin Wang: Rapid Identification and Antimicrobial Susceptibility Testing for Urinary Tract Pathogens by Direct Analysis of Urine Samples Using a MALDI-TOF MS-Based Combined Protocol . In: Frontiers in Microbiology . tape June 10 , 2019, p. 1182 (online) , doi : 10.3389 / fmicb.2019.01182 , PMID 31231323 , PMC 6560049 (free full text).
- ↑ a b c d Jean Euzéby, Aidan C. Parte: Genus Proteus. In: List of Prokaryotic names with Standing in Nomenclature, Systematics of Bacteria (LPSN) . Retrieved September 17, 2020 .
- ↑ a b Taxonomy Browser Proteus. In: National Center for Biotechnology Information (NCBI) website . Retrieved December 27, 2019 .
- ↑ a b U. Behrendt, J. Augustin, C. Spröer, J. Gelbrecht, P. Schumann, A. Ulrich: Taxonomic characterization of Proteus terrae sp. nov., a N 2 O-producing, nitrate-ammonifying soil bacterium . In: Antonie van Leeuwenhoek . tape 108 , no. 6 , December 2015, p. 1457-1468 , doi : 10.1007 / s10482-015-0601-5 .
- ↑ Giovanni M. Giammanco, Patrick AD Grimont, Francine Grimont, Martine Lefevre, Giuseppe Giammanco, Sarina Pignato: Phylogenetic analysis of the genera Proteus, Morganella and Providencia by comparison of rpoB gene sequences of type and clinical strains suggests the reclassification of Proteus myxofaciens in a new genus, Cosenzaea gen. nov., as Cosenzaea myxofaciens comb. nov .. In: International Journal of Systematic and Evolutionary Microbiology . Volume 61, July 2011, pp. 1638-1644. doi : 10.1099 / ijs.0.021964-0 .
- ^ A b c Herbert Hof, Rüdiger Dörries: Dual series: Medical microbiology . 3. Edition. Thieme Verlag, Stuttgart 2005, ISBN 978-3-13-125313-2 , p. 11-12, 289-296, 399-400 .
- ^ Reham Wasfi, Samira M. Hamed, Mai A. Amer & Lamiaa Ismail Fahmy: Proteus mirabilis Biofilm: Development and Therapeutic Strategies. In: Front. Cell. Infect. Microbiol. tape 10 , 2020, p. 414 , doi : 10.3389 / fcimb.2020.00414 .
- ↑ Healthcare-associated infections acquired in intensive care units - Annual Epidemiological Report for 2016. (PDF; 962 KB) ECDC Surveillance Report. European Center for Disease Prevention and Control (ECDC), May 2018, pp. 1–11 , accessed May 24, 2018 .
- ↑ a b c R. Leclercq et al .: EUCAST expert rules in antimicrobial susceptibility testing . In: Clinical Microbiology and Infection . tape 19 , no. 2 . Wiley-Blackwell, February 2013, ISSN 1469-0691 , pp. 141-160 , doi : 10.1111 / j.1469-0691.2011.03703.x , PMID 22117544 ( wiley.com ).
- ↑ Journal of chemotherapy 4-2006 ( Memento of 12 March 2014 Internet Archive )
- ↑ "Active ingredient updates", issue 2/2012 ( Memento from November 7, 2014 in the Internet Archive )