benzene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | benzene | |||||||||||||||
| other names |
Benzene ( IUPAC ) |
|||||||||||||||
| Molecular formula | C 6 H 6 | |||||||||||||||
| Brief description |
colorless liquid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 78.11 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.88 g cm −3 |
|||||||||||||||
| Melting point |
6 ° C |
|||||||||||||||
| boiling point |
80 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
bad in water (1.77 g l −1 ) |
|||||||||||||||
| Refractive index |
1.5011 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Benzene (according to IUPAC benzene ) is a liquid organic hydrocarbon with the empirical formula C 6 H 6 . It has a characteristic aromatic odor, is colorless, easily flammable and burns with a strongly sooty flame. It mixes with almost all organic solvents , but hardly with water. Benzene is the parent compound of aromatic hydrocarbons .
Benzene was originally obtained from coke production for the steel industry . Nowadays it is mostly obtained through refinery and petrochemical processes such as steam cracking as a by-product in gasoline, ethylene and p -xylene production . There are also specific industrial production routes for benzene such as the dealkylation of toluene .
Benzene is an important building block for the petrochemical industry ; global demand in 2013 was over 40 million tons per year. It is converted into numerous secondary products through addition , substitution and oxidation reactions. Its derivatives and secondary products such as ethylbenzene , cumene , cyclohexane , alkylbenzenes , chlorobenzenes , nitrobenzene and maleic anhydride are processed into a wide range of products such as paints , pharmaceuticals , insecticides and plastics for various industries. It is also found in petrol , although the content is limited in most countries. Benzene is carcinogenic and toxic .
nomenclature
The name benzene was first used by Justus von Liebig in 1834 . Liebig changed Eilhard Mitscherlich's name from 1833, who called benzene gasoline. In the Anglo-Saxon and French-speaking areas, however, the adapted name (French: benzène , English: benzene ) was still used by Mitscherlich.
Since the ending -ol is used for alcohols in the systematic chemical nomenclature , the historically determined term benzene most commonly used in German is misleading; the name benzene was determined by the IUPAC as the official nomenclature for this hydrocarbon.
history
In the second half of the 17th century, Johann Rudolph Glauber , who also discovered Glauber's salt (sodium sulfate), discovered benzene in the distillation of coal tar . However, the composition was unknown to him, he called it a "subtle and lovely oleum". In 1825, the English physicist Michael Faraday discovered benzene in coal gas when he isolated it from liquid residues that separated from the gas phase when whale oils were burned in London's street lamps. He therefore suggested the name “Pheno” (Greek phainein = to shine). This name is part of the term phenyl group , which in organic chemistry denotes the atomic group –C 6 H 5 .
A year later, this oil was identified as a hydrocarbon. In 1834, the German chemist Eilhard Mitscherlich obtained benzene from benzoic acid and calcium oxide , and he also converted benzene into nitrobenzene, azobenzene and benzenesulfonic acid . He called the substance "gasoline" because of its relationship to benzoic acid. In addition, he created the correct empirical formula C 6 H 6 . In the same year Justus von Liebig renamed “benzine” to benzene. In 1845 the English chemist Charles Mansfield isolated benzene from coal tar while working under the direction of August Wilhelm von Hofmann .
A long scholarly dispute smoldered about the correct structural formula of benzene. Initial ideas like those of Albert Ladenburg proposed prismane structure that the benzvalene , the Dicyclopropenyls and the Dewar benzene by James Dewar turned out to be wrong. It was not until 1861 that the Austrian chemist Johann Josef Loschmidt , then a school teacher, formulated some possible structural formulas for benzene, which the German chemist and professor of chemistry August Kekulé adopted in 1865 - possibly as a suggestion for his Kekulé structural formula. Legend has it that this idea came to Kekulé in a dream. He dreamed of a snake that bit its own tail . Kekulé describes this in his speech on the 25th anniversary of the benzene ring in 1890. The six monkeys, who alternately grabbed each other's feet with either both or one hand, thus forming the ring structure, are based on an 1886 beer evening of the German Chemical Society made joke.
Kekulé's structure was the first to take into account the experimental finding that all carbon atoms in benzene are equivalent. However, it did not explain all the peculiarities of benzene, such as its unusually low reactivity compared to olefins . The absence of an addition reaction with bromine water , as would be expected from the Kekulé structural formula, remained a mystery. The proof of the equivalence of the hydrogen in the benzene molecule could be provided between 1869 and 1874. In 1872 Kekulé formulated his "oscillation hypothesis" of a permanent change of place of single and double bonds .
Suggested structural formulas 


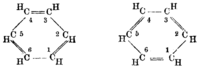


By Adolf Karl Ludwig Claus (1867) By James Dewar (1867) By Albert Ladenburg (1869) By August Kekulé (1872) By Henry Edward Armstrong (1887),
Adolf von Baeyer (1888)By Friedrich Karl Johannes Thiele (1899)
It was only in the 20th century that the phenomenon of delocalized electron clouds, which give the benzene molecule a particular stability, could be demonstrated using X-ray structure analysis. In 1925 Armit and Robinson introduced the simplified notation with the concentric circle in the formula, which is intended to express that all bonds are absolutely equivalent and that there are no localizable double bonds. A purely hypothetical form with three fixed double bonds and three single bonds would be called "1,3,5-cyclohexatriene".
The industrial production of benzene on the basis of hard coal began in 1849 . It was handled carelessly until campaigns finally cleared up the dangers of benzene over 100 years later when the toxicity of benzene became known.
Occurrence
When smoking cigarettes , small amounts of benzene vapor (10–100 µg per cigarette) are released, traces of benzene are also produced in the event of volcanic eruptions and forest fires, as well as incomplete combustion of organic material, such as inhibited smoldering fires in nature, as well as deliberately incomplete smoldering of incense sticks and incense . After two to five days, half of the benzene present in the atmosphere is broken down as it reacts with hydroxyl radicals .
Smaller amounts of benzene occur in natural gas , oil and coal tar . Benzene does not dissolve very well in water, but due to the large excess of water when petroleum is released locally, it dissolves almost completely. If there is a release in the marine environment during the extraction or transport of crude oil, benzene dissolved in water can have a toxic effect on fish larvae and other marine organisms in concentrations as low as a few parts per billion . In higher concentrations of over a part per million, benzene has a lethal effect on many aquatic organisms. Benzene exposure after a release or when handling crude oil also represents a health risk for the employees involved. The analysis of dissolved benzene in water samples from exploration wells is considered a reliable method for predicting petroleum deposits. Small amounts of benzene can also be found in soft drinks, so the Stiftung Warentest found small amounts of benzene in cherry juice in 2013. The occurrence could be explained by the fact that benzaldehyde is converted into benzene when exposed to light; this applies to all aromas containing benzaldehyde.
Benzene emissions
Benzene is mainly released through the exhaust of gasoline engines. 75 percent of emissions come from motor vehicles. As of the year 2000, the proportion of benzene in motor fuel has been limited to a maximum of one percent by volume throughout Europe in accordance with DIN EN 228; the average in 2003 was ≈ 0.7 percent by volume. The current limit in 2010 in the USA was five percent by volume. From 2013 onwards, the US average for benzene in gasoline was to be reduced to 0.62 percent, but there was still no plan to limit the maximum proportion below five percent.
The average exposure to the population is around 2 µg / m 3 air, but this value can be significantly higher depending on the environment (for example at petrol stations, in poorly ventilated garages, etc.). There was a sharp drop in benzene emissions around 1980 that continued into the 1990s. Between 1997 and 2005, benzene pollution was so significantly reduced both at the urban traffic-related measuring stations and at the urban background stations that in 2005 the limit values of 5 µg / m 3, which had only been in effect since 2010 , were largely undershot.
Manufacturing
The increased demand for benzene in the 1950s, mainly due to the growing polymer industry, made it necessary to manufacture benzene from petroleum. In 2002 around 94 percent of benzene worldwide was obtained from crude oil, of which 40 percent from pyrolysis gasoline , 54 percent from reformate gasoline including de- and transalkylation and five percent from coal and coal tar and about one percent based on C3 / C4 cuts. Around 40 million tons of benzene are produced worldwide every year.
Coal coking
For commercial use until World War II, most of the benzene was obtained as a by-product in the production of coke for the steel industry, mostly from the distillation of light oils made from coke. For those looking for possibilities of selling was in 1918 B enzol- V founded erband. It turned out to be a suitable gasoline additive to increase its anti-knock properties; so the "Super" gasoline emerged from ar omatischen and al iphatischen ingredients and led to the company's name BV Aral . This traditional method of making benzene has been complemented by a number of newer processes.
Steam cracking
In Europe, benzene is mainly obtained from the pyrolysis gasoline used in steam cracking , a process used to produce ethylene and other olefins from aliphatic hydrocarbons. Depending on the feedstock used to make the olefins, a benzene-rich liquid by-product called pyrolysis gasoline is obtained. If heavier raw materials are used, the amount of aromatics produced increases. Pyrolysis gasoline can be mixed with other hydrocarbons as a gasoline additive, used by an extraction process or by reactive distillation to obtain benzene and other aromatics.
Catalytic Reforming
Benzene is obtained by catalytic reforming of naphtha . Here, n- hexane is cyclized to cyclohexane and then dehydrogenated to benzene. In catalytic reforming, a mixture of hydrocarbons with boiling points between 60 and 200 ° C in the presence of hydrogen is passed over a bifunctional platinum / rhenium or platinum / tin chloride catalyst at 500-525 ° C and pressures in the range of 8-50 bar. A C 6 / C 8 cut is used as raw material to optimize the aromatics yield. The aromatic products of the reaction can be separated from the reaction mixture by extraction with a number of solvents, also called reformate. Benzene is separated from the other aromatics by distillation. In the United States , catalytic reforming is a major source of benzene.
Transalkylation
In transalkylation, two toluene molecules are transalkylated to form a benzene and a xylene molecule . Modified zeolites are often used as the catalyst . Since the demand for p -xylene exceeds the demand for other xylene isomers, a variant of the Toluene Disproportionation Process [TDP process and Selective TDP (STDP)] can be used. In this process, the xylene stream exiting the TDP unit is about 90% p- xylene.
Thermal dealkylation
Another method for producing benzene is the thermal dealkylation of toluene. The methyl group of the toluene used is split off at 780 ° C. and a pressure of 40 bar . The carrier gas in the reactor is 90 percent hydrogen. After cooling, gas-liquid separation and cleaning, pure benzene is obtained.
Production from renewable raw materials
Benzene can also be produced from renewable raw materials and residues together with other aromatics . Various pyrolysis reactions are known for this. Industrial production is currently being tested by various companies in pilot projects or has already been tested successfully, but there is still no large-scale production. In these projects, only biomass that does not compete with land-based food production and plastic waste are used as raw materials. This production method enables the CO 2 balance to be reduced by 70–90% compared to the petrochemical production of aromatics.
Further processes
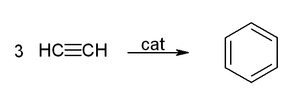
Benzene can be produced from ethyne molecules in the presence of a catalyst , which is referred to as Reppe chemistry after the German chemist Walter Reppe .
This manufacturing method has no technical relevance.
Other processes for the production of benzene are based on natural gas as a raw material via the intermediate stage methanol , for example processes such as methanol-to-aromatics with modified zeolites of the ZSM-5 type. The dehydrocyclodimerization of natural gas components such as propane and n -butane can also be used to produce benzene in the cyclar process.
properties
Physical Properties
Benzene crystallizes in an orthorhombic crystal structure with the space group Pbca (space group no. 61) and the lattice parameters a = 746, b = 967 and c = 703 pm. Benzene is a colorless, clear, with a refractive index of 1.5011 highly refractive , volatile and easily flammable liquid and burns with a luminous, strongly sooting flame. The refractive index of benzene agrees quite well with that of window glass . A glass rod immersed in benzene is therefore almost invisible. The viscosity of benzene is lower than that of water, it is thinner. It solidifies at 5.5 ° C and boils at 80.1 ° C. At room temperature (20 ° C) it has a density of 0.88 kg per liter and a vapor pressure of 100 hPa .
The coefficient of thermal expansion γ of liquid benzene is at 25 ° C; 0.00114 K −1 , the relative dielectric constant is 2.28. The specific heat capacity (liquid) is at 20 ° C; 1.738 kJ / kg · K, the thermal conductivity at 20 ° C is 0.144 W / (m · K), the dynamic viscosity at 20 ° C; 0.648 mPa · s, this gives the Prandtl number at 20 ° C of 7.8. The enthalpy of evaporation is at the evaporation point (80 ° C); 30.75 kJ / mol, this corresponds to an evaporation entropy of 87 J / (K · mol). The calorific value of benzene is 40,580 kJ / kg, the enthalpy of combustion is −3257.6 kJ / mol for liquid benzene and −3301 kJ / mol for gaseous benzene.
Molecular Properties
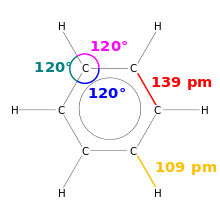
A special feature is that benzene has six bonds of equal length of 139 pm. This value lies between that for a single bond of about 147 pm and a double bond of about 135 pm and is an indicator of the aromatic character of benzene. Benzene is the simplest of the benzenoid aromatic hydrocarbons , also called arenes . The special bonding relationships of this group of substances are called aromaticity . The length of all carbon-hydrogen bonds is 109 pm.
Each carbon atom has four valence electrons , two of which connect the atom to the neighboring carbon atoms. An electron binds the associated hydrogen atom. The remaining six π - electron yield three π- formal bonds , such as those expressed in the structural formula with three double bonds. In the orbital model that is valid today , the six π electrons form a delocalized charge cloud (delocalized 6 π electron system) above and below the level of the carbon ring.
Molecular geometry and aromaticity of benzene

|
| Mesomerism of Benzene. The delocalized π electrons are energetically more favorable than the boundary structures with localized double bonds. Mesomeric energy E M (resonance energy) |
Kekulé expressed this fact of mesomerism by means of two structural formulas, which only symbolize the two extreme points of the charge cloud . Because of the mesomerism, the carbon ring is more stable than the hypothetical cyclohexa-1,3,5-triene with localized double bonds at fixed positions. In the simplified notation, the carbon ring is now represented as a hexagon and the electron cloud as an inscribed circle . However, it was only Linus Pauling who recognized the effect of mesomerism or the resonance in the benzene molecule in the 1930s.
Benzene is a planar molecule in which the carbon atoms are all sp 2 hybridized . This was reported by the British crystallographer Kathleen Lonsdale by X-ray diffraction demonstrated in 1929th The delocalization of the electrons establishes the equivalence of all CH groups in the molecule and thus the high symmetry, which is why benzene belongs to the point group D 6h , i.e. is provided with a six-fold axis. However, the influence of the π-electron system on the structure is not undisputed. In the summer of 2013, researchers at the Lawrence Berkeley National Laboratory succeeded in using an atomic force microscope to demonstrate the hexagonal molecular geometry of benzene and to represent it photographically.
Spectroscopic properties
In the 1 H-NMR spectrum , the hydrogen atoms show an unusually strong deshielding ( singlet at 7.28 ppm in CDCl 3 for all six hydrogen atoms), which is caused by the ring current induced by the magnetic field of the spectrometer .
In the 13 C-NMR spectrum , benzene in CDCl 3 shows a signal at 128.5 ppm for all six carbon atoms.
In the IR spectrum the phenyl hydrogen oscillation appears at around 3,035 cm −1 . The C – C stretching vibration appears at 1,500 to 2,000 cm −1 . The C – H deformation vibrations are located at 650 to 1,000 cm −1 .
With UV spectroscopy, benzene can still be detected in high dilution at two typical absorptions (π-π * transitions) in the range from 200 to about 250 nm.
Chemical properties
With non-polar, organic solvents such as ether , as well as with methanol , ethanol , ethyl acetate , acetone , benzene is infinitely miscible in any ratio, but only poorly with water (maximum 1.8 grams of benzene per liter). Benzene forms an azeotrope with 8.83% water , which boils at 69.25 ° C.
oxidation
Benzene burns with a yellow flame to form water and carbon dioxide , whereby the strong soot development indicates the high carbon content of the compound. Benzene has a characteristic odor. The odor threshold is very low and is 1.5 to 900 milligrams per cubic meter of air. Combustion in the engine as gasoline is one of the largest reactions of benzene in terms of quantity.
Complete oxidation (combustion) of benzene:
- Benzene reacts with oxygen to form carbon dioxide and water.
The catalytic oxidation of benzene with atmospheric oxygen using a vanadium pentoxide catalyst at around 450 ° C produces maleic anhydride . However, because of the loss of poor atom economy , methods using a C4 raw material are preferred.
Radical substitution
Compared to alkenes, there are no radical substitutions on benzene due to the stabilization through the aromaticity. The so-called Sandmeyer reaction is an exception, in which a phenyl radical is formed from diazonium salts in a homolytic splitting off of molecular nitrogen catalyzed by copper . However, this is highly reactive and continues to react immediately. An important characteristic of the aromaticity of benzene is the lack of an addition reaction with hydrobromic acid or bromine water.
Radical addition
Example of a radical addition reaction ( chlorination ):
The addition of chlorine to benzene takes place as a radical chain reaction:
- [...]
The reaction is carried out at a temperature of 15 to 20 ° C. At a conversion of 12 to 15% the reaction is terminated and the reaction mixture is worked up.
Electrophilic aromatic substitution
An example of an electrophilic aromatic substitution is the nitration of benzene. A reactive nitronium ion is formed by protonation of nitric acid with concentrated sulfuric acid :
This reacts as an electrophile with the benzene:
- Benzene reacts with nitric acid in the presence of sulfuric acid to form water and nitrobenzene.
Eilhard Mitscherlich described the conversion of benzene to nitrobenzene in 1834, and the industrial production of nitrobenzene, an important raw material for the paint industry, began as early as 1847.
Even with the Friedel-Crafts reaction is an electrophilic aromatic substitution. Under the catalytic effect of a Lewis acid such as iron (III) chloride (FeCl 3 ) or aluminum chloride (AlCl 3 ) or a strong Brønsted acid such as sulfuric acid (H 2 SO 4 ), phosphoric acid (H 3 PO 4 ) or hydrogen fluoride (HF) benzene is reacted with an alkyl halide , an alcohol , an alkene or an alkyne to form an alkyl aromatic.
The resulting alkyl aromatics are used as raw materials for detergents .
By a Friedel-Crafts acylation of benzene is also under Lewis acid - catalysis with carbonyl halides implemented. This introduces an acyl group .
Organometallic reactions
To date, many complexes have been synthesized in organometallic chemistry that contain benzene as a ligand . A known compound is the first time in 1955 by Ernst Otto Fischer and Walter Hafner shown bis (benzene) chromium , called a sandwich complex . Ernst Otto Fischer received the Nobel Prize for Chemistry in 1973 for the synthesis and investigation of the chemistry of bis (benzene) chromium and the chemistry of the organometallic sandwich compounds .
The hapticity of benzene as a ligand can change in redox reactions , for example in the reduction of the bis (benzene) ruthenium cation.
Benzene derivatives and substitution patterns
In the monoderivatization of benzene, in which one hydrogen atom has been replaced by another group, only one possible derivative is formed due to the equivalence of all hydrogen atoms. If two identical substituents are introduced, three different derivatives occur (→ disubstituted benzenes ). The relative position of the substituents is indicated by ortho-, meta- and para-. When three identical substituents are introduced, three isomers occur in turn, which are called vicinal, symmetric and asymmetric (→ trisubstituted benzenes ).
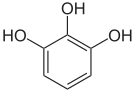
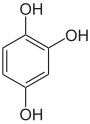

Pyrogallol ,
1,2,3-trihydroxybenzene,
vicinal -trihydroxybenzeneHydroxyhydroquinone ,
1,2,4-trihydroxybenzene,
asymmetric -trihydroxybenzenePhloroglucinol ,
1,3,5-trihydroxybenzene,
symmetrical -trihydroxybenzene
Many important chemicals are derivatives of benzene, so they have a benzene ring as a basic structure. The following is an overview of the most important and simplest derivatives of benzene, which are themselves basic materials for other chemicals:
use
In the past, benzene was used as a good solvent and cleaning agent in many areas. As a solvent for waxes , resins and oils , benzene is increasingly being replaced by less toxic substances. It was also used to decaffeinate coffee in the so-called Roselius process. In the 19th and early 20th centuries, benzene was used as an after shave because of its pleasant odor .
Because of the associated dangers, benzene and preparations containing benzene with more than 0.1 percent may not be placed on the market in Germany. Benzene may only be used in larger quantities in closed systems and for industrial or research purposes. Fuels are an exception here ; Benzene increases the knock resistance of gasoline , which is why it played an important role in the development of gasoline . Some earlier internal combustion engine locomotives used a fuel consisting primarily of benzene. Today it is only permitted as a fuel additive in a concentration of up to one percent.
Benzene is used in the petrochemical industry for the synthesis of many compounds, mainly ethylbenzene (52%), cumene (20%), cyclohexane (13%) and nitrobenzene (9%). As the figure on the right shows, these compounds are in turn starting materials for the synthesis of compounds such as styrene , phenol , acetone , cyclohexanol , cyclohexanone and aniline . Plastics such as polystyrene , styrene-butadiene rubber , polyamides (nylon) and epoxy resins are based on them . Many other products, such as active washing salts of alkylbenzenesulfonic acids , technical solvents, certain pesticides such as lindane and dyes are based on benzene or its derivatives.
Safety-related parameters
The compound has a flash point of −11 ° C. Benzene forms highly flammable vapor-air mixtures. The explosion range lies between 1.2 volume percent (39 g / m 3 ) as the lower explosion limit (LEL) and 8.6 volume percent (280 g / m 3 ) as the upper explosion limit (UEL). The limit gap width was determined to be 0.99 mm. This results in an assignment to explosion class IIA. The minimum ignition energy is 0.2 mJ. In this way, energetically weaker ignition sources such as brush discharges and grinding or impact sparks can cause vapor-air mixtures to ignite within the explosion limits. The ignition temperature is 555 ° C. The substance therefore falls into temperature class T1.
toxicology
Benzene fumes are poisonous if inhaled; the symptoms of acute poisoning only appear at relatively high concentrations. Mild poisoning manifests itself in dizziness, nausea, drowsiness and apathy . Severe poisoning leads to fever and visual disturbances up to temporary blindness and unconsciousness. The so-called benzene addiction, which can occur when breathing in benzene, leads to feelings of drunkenness and euphoria. Benzene can lead to death if the organism is exposed to it for a long time.
The poisonous effect, as well as the carcinogenic effect as a clastogenic, is due to the formation of a carcinogenic metabolite . In the body, benzene is enzymatically oxidized on the ring. The resulting highly reactive epoxide ( arene oxide ) reacts with numerous biological compounds and can damage the genetic material or form protein adducts . The arene oxide is further metabolized to phenol . The highly reactive arene oxide can react further to trans -1,2-dihydrobenzene-1,2-diol by hydrolases . In addition, the Arenoxid can reversibly to Oxepin migrate. These further reactions take place non-enzymatically. Long-term ingestion of small amounts of benzene mainly leads to damage to the internal organs and the bone marrow . The latter results in a decrease in the number of red blood cells ( anemia ), which manifests itself in palpitations, eye flickering, tiredness, dizziness , paleness and headache. Benzene is stored in the brain, bone marrow, and adipose tissue. It is only slowly excreted through the kidneys . The breakdown takes place via various conversion products such as catechol , phenol, hydroquinone and benzoquinone . The main excretion product is phenyl mercapturic acid ( N -acetyl- S- phenyl-cysteine).
With benzene in the air volume of two percent by volume, death occurs after five to ten minutes. The acute lethal dose (oral) in humans is 50 milligrams per kilogram of body weight. Benzene forms explosive mixtures between 1.4 and 8 percent air volume.
Benzene should be handled with particular care because of these hazards. Benzene must be stored at 15 ° C to 25 ° C. The TRK value was 1 milliliter per cubic meter of air (or 3.25 mg / m 3 air). Any exposure to benzene should be avoided or reduced as much as possible; obtain special instructions before using benzene. In the event of an accident or if you feel unwell, a doctor should be consulted immediately. Places where benzene leaks or could leak should be vacated immediately and only entered again in full protective suits . Benzene is very hazardous to water.
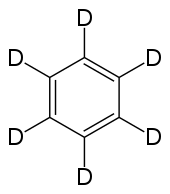
Hexadeuterobenzene
Deuterated benzene ( molecular formula: C 6 D 6 ), also called hexadeuterobenzene or benzene-d 6 , is used as a solvent in nuclear magnetic resonance spectroscopy (NMR).
The physical properties are slightly different from the non-deuterated compound:
- Refractive index: 1.497 (20 ° C)
- Melting point: 6.8 ° C
- Boiling point: 79.1 ° C
- Density: 0.950 g / ml (25 ° C)
literature
- E. Heilbronner, J. Jacques: Paul Havrez and his benzene formula. In: Chemistry in our time , 32, 1998, pp. 256–264, doi: 10.1002 / ciuz.19980320505 , with a discussion of earlier benzene formulas .
Web links
- Entry on Benzene in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST)
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q Entry on benzene in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ^ Heinz GO Becker, Werner Berger and Günter Domschke: Organikum . 22nd edition. Wiley-VCH, Weinheim 2004, ISBN 3-527-31148-3 , p. 732.
- ↑ Entry on Benzene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 71-43-2 or benzene ), accessed on September 15, 2019.
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 1: A-Cl. 8th, revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1979, ISBN 3-440-04511-0 , pp. 402-403.
- ↑ E. Mitscherlich: About the benzene and the acids of the oil and valley garden. In: Annals of Pharmacy . 1834, 9 (1), pp. 39-48, doi: 10.1002 / jlac.18340090103 .
- ↑ Karsten Strey: The world of poisons. Verlag Lehmanns, 2015, ISBN 978-3-86541-728-2 , p. 202.
- ^ Charles Blachford Mansfield: Investigation of the coal horse. In: Annals of Chemistry and Pharmacy . 69, 1849, pp. 162-180, doi: 10.1002 / jlac.18490690203 .
- ↑ Lexicon of Chemistry: Prisman .
- ↑ Lexicon of Chemistry: Dewar-Benzol .
- ^ Stephan Kekule von Stradonitz: Two chemical visions. In: Journal of Applied Chemistry . 1927, 40 (25), pp. 736-737, doi: 10.1002 / anie.19270402505 .
- ↑ a b K. Lonsdale: The Structure of the Benzene Ring. In: Nature . 122, 1928, p. 810, doi: 10.1038 / 122810c0 .
- ^ Alan R. Katritzky: Advances in heterocyclic chemistry. Volume 17, Academic Press, 1974, ISBN 0-12-020617-X , p. 7.
- ^ Criminalistics Lexicon . CF Müller, 2013, ISBN 978-3-7832-0024-9 , pp. 599 ( limited preview in Google Book search).
- ↑ ARD Buffet Household 1 × 1 even more expert knowledge . TOPP, 2014, ISBN 978-3-7358-0012-1 ( limited preview in Google book search).
- ↑ Ronald L. Smith, Jane Anne Cameron: Effect of Water Soluble Fraction of Prudhoe Bay Crude Oil on Embryonic Development of Pacific Herring. In: Transactions of the American Fisheries Society . 108, 1979, pp. 70-75, doi : 10.1577 / 1548-8659 (1979) 108 <70: EOWSFO> 2.0.CO; 2 .
- ^ Wyman Harrison, Mitchell A. Winnik, Paul TY Kwong, Donald Mackay: Crude oil spills. Disappearance of aromatic and aliphatic components from small sea-surface slicks. In: Environmental Science & Technology , 9, 1975, pp. 231-234, doi: 10.1021 / es60101a006 .
- ↑ BE Gjesteland, J. Hollund, J. Kirkeleit, P. Daling, M. Bråtveit: Oil Spill Field Trial at Sea: Measurements of Benzene Exposure. In: Annals of Work Exposures and Health . 2017, 61, 6, pp. 692–699, doi: 10.1093 / annweh / wxx036 . PMID 28595265 .
- ↑ Stephen G. Burtell, Victor T. Jones III: EXPLORATION: Benzene content of subsurface brines can indicate proximity of oil, gas. In: Oil and Gas Journal , 1996, 94, 23 (full text)
- ↑ food monitor: Benzene in cherry juice: How does it happen and how can it be avoided. In: food monitor. March 26, 2020, accessed on June 28, 2020 (German).
- ↑ James W. Weaver, Linda R. Exum, Lourdes M. Prieto: Gasoline Composition Regulations Affecting LUST Sites. (PDF; 1.1 MB) EPA publication , January 2010, p. 18.
- ↑ Safety data sheet firstfuelbank (PDF; 36 kB) Status: February 2003.
- ^ EPA: Summary and Analysis of the 2009 Gasoline Benzene Pre-Compliance Reports. (PDF; 389 kB) November 2009.
- ↑ Felicity Barringer, EPA Limits the Benzene in Gasoline by 2011. Article in the New York Times, February 10, 2007.
- ↑ Umweltbundesamt : DATA ON THE ENVIRONMENT: The state of the environment in Germany 2005 edition (PDF) accessed on December 27, 2015 (pdf).
- ^ A b D. Netzer, OJ Ghalayini: Improve benzene production from refinery sources. In: Hydrocarbon Processing . 2002, pp. 71-78.
- ^ Marcos Granda, Clara Blanco, Patricia Alvarez, John W. Patrick, Rosa Menéndez: Chemicals from Coal Coking. In: Chemical Reviews . 114, 2014, pp. 1608-1636, doi: 10.1021 / cr400256y .
- ↑ The Aral History .
- ↑ George J. Antos, Abdullah M. Aitani: Catalytic Naphtha Reforming. Marcel Dekker Inc., 2004, ISBN 0-8247-5058-6 , p. 18.
- ↑ SH Oh, KH Seong, YS Kim, S. Choi, BS Lim, JH Lee, J. Woltermann, YF Chu: Reformate upgrading to produce enriched BTX using noble metal promoted zeolite catalyst. In: Studies in Surface Science and Catalysis . Pp. 595-601, doi: 10.1016 / S0167-2991 (02) 80078-7 .
- ^ CC Zimmerman, Robert York: Thermal Demethylation of Toluene. In: Ind. Eng. Chem. Process Des. Dev. 3, 1964, pp. 254-258, doi: 10.1021 / i260011a013 .
- ↑ Chunbao Xu & Fatemeh Ferdosian: Conversion of Lignin into Bio-Based Chemicals and Materials . Springer, Berlin 2017, ISBN 978-3-662-54957-5 , pp. 13-33 .
- ↑ Patent US9453166B2 : Systems and processes for catalytic pyrolysis of biomass and hydrocarbonaceous materials for production of aromatics with optional olefin recycle, and catalysts having selected particle size for catalytic pyrolysis. Filed September 29, 2015 , published September 27, 2016 , Applicant: University of Massachusetts, Inventor: George H. Huber, Anne Mae Gaffney, Jungho Jae & Yu-Ting Cheng.
- ↑ TCat-8® Pilot Plant. Anellotech, accessed October 29, 2019 .
- ↑ The BioBTX technology. BioBTX, accessed October 29, 2019 .
- ↑ Jacobs Confirms Anellotech's Bio-TCat ™ Process Leads to Significant Carbon Emission Reductions Compared to Petro-based Aromatics. Anellotech, October 11, 2018, accessed October 29, 2019 .
- ^ Walter Reppe, Walter Joachim Schweckendiek: Cyclizing Polymerization of Acetylene. III Benzene, Benzene Derivatives and Hydroaromatic Compounds. In: Justus Liebig's Annals of Chemistry . 1948, 560 (1), pp. 104-116, doi: 10.1002 / jlac.19485600104 .
- ↑ Marco Conte, Jose A. Lopez-Sanchez, Qian He, David J. Morgan, Yulia Ryabenkova, Jonathan K. Bartley, Albert F. Carley, Stuart H. Taylor, Christopher J. Kiely, Karim Khalid, Graham J. Hutchings: Modified zeolite ZSM-5 for the methanol to aromatics reaction. In: Catal. Sci. Technol. 2, 2012, pp. 105-112, doi: 10.1039 / C1CY00299F .
- ↑ Cyclar ™ Process Produces High-Quality Aromatic Products .
- ^ A b EG Cox, DWJ Cruickshank, JAS Smith: The Crystal Structure of Benzene at −3 ° C. In: Proceedings of the Royal Society of London . Series A, Mathematical and Physical Sciences , 1958, 247, 1248, pp. 1-21, doi: 10.1038 / 173075a0 .
- ↑ Joachim Berber, Heinz Kacher, Rudolf Langer: Physics in formulas and tables. Springer, 1994, ISBN 3-519-03200-7 , p. 129.
- ^ Walter J. Moore, Dieter O. Hummel: Physikalische Chemie. 4th edition. de Gruyter, 1986, ISBN 3-11-010979-4 , p. 253.
- ^ Linus Pauling: Interatomic Distances in covalent Molecules and Resonance between two or more Lewis electronic structures. In: Proceedings of the National Academy of Sciences . 18, 1932, pp. 293-297, doi: 10.1073 / pnas.18.4.293 .
- ↑ Eric D. Glendening, Rudiger Faust, Andrew Streitwieser, K. Peter C. Vollhardt, Frank Weinhold: The Role of Delocalization in Benzene. In: Journal of the American Chemical Society . 1993, 115 (23), pp. 10952-10957, doi: 10.1021 / ja00076a061 .
- ^ E. Cox: Crystal Structure of Benzene. In: Reviews of Modern Physics . 1958, 30 (1), pp. 159-162, doi: 10.1103 / RevModPhys.30.159 .
- ↑ Philippe C. Hiberty, David Danovich, Avital Shurki, Sason Shaik: Why Does Benzene Possess a D 6h symmetry? A Quasiclassical State Approach for Probing π-Bonding and Delocalization Energies. In: Journal of the American Chemical Society . 1995, 117 (29), pp. 7760-7768, doi: 10.1021 / ja00134a022 .
- ↑ Article Photographic confirmation of the structural formula of benzene on elektronik.net, accessed on June 13, 2013.
- ↑ Article Atom by Atom, Bond by Bond, a Chemical Reaction Caught in the Act , Lawrence Berkeley National Laboratory press release, accessed June 13, 2013.
- ↑ a b c Entry on Benzene in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST) .
- ↑ a b Richard Wegler (ed.): Chemistry of pesticides and pesticides . Volume 1, Springer-Verlag, 1970, ISBN 3-642-46212-X , pp. 129-132.
- ^ EO Fischer, W. Hafner: Di-benzol-chrom. In: Journal of Nature Research B . 10, 1955, pp. 665-668, doi: 10.1515 / znb-1955-1201 .
- ^ The Nobel Prize in Chemistry 1973 .
- ^ Gottfried Huttner, Siegfried Lange, Ernst O. Fischer: Molecular Structure of Bis (hexamethylbenzene) ruthenium (0). In: Angewandte Chemie International Edition in English. 10, 1971, p. 556-, doi: 10.1002 / anie.197105561 .
- ↑ Decaffeination 101: Four Ways to decaffeinate coffee .
- ^ John McMurry: Organic Chemistry. Cengage Learning Services, 2003, ISBN 0-534-38999-6 , p. 478.
- ↑ Annex XVII No. 5 REACH regulation .
- ↑ Thomas Hillenbrand, Frank Marscheider-Weidemann, Manuel Strauch, Kerstin Heitmann, Dora Schaffrin: Emission reduction for priority and priority hazardous substances of the Water Framework Directive. (PDF; 3.7 MB) Material data sheets 29-07 of the Federal Environment Agency , Section 18.2.
- ↑ a b c d e E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ G. Eisenbrand, M. Metzler: Toxicology for Chemists. Georg Thieme Verlag, Stuttgart 1990, ISBN 3-13-127001-2 , p. 20.
- ↑ External identifiers or database links for hexadeuterobenzene : CAS number: 1076-43-3, EC number: 214-061-8, ECHA InfoCard: 100.012.784 , PubChem : 71601 , ChemSpider : 64671 , Wikidata : Q1101314 .
- ↑ Benzene-d6 data sheet from Sigma-Aldrich , accessed on March 7, 2018 ( PDF ).