water


Water (H 2 O) is a chemical compound made up of the elements oxygen (O) and hydrogen (H). As a liquid , water is transparent, largely colorless, odorless and tasteless. It occurs in two isomers (para- and ortho-water), which differ in the nuclear spin of the two hydrogen atoms.
Water is the only chemical compound on earth that occurs naturally as a liquid, solid and gas . The term water is used for the liquid state of aggregation . In the solid state one speaks of ice , in the gaseous state of water vapor . Water is the basis of life on earth. In nature, water rarely occurs in pure form, but mostly contains dissolved components of salts, gases and organic compounds.
Designations
etymology
The word “water” is derived from the old high German waȥȥar , “the moist, flowing”. The Indo-European names * wódr̥ and * wédōr are already used in Hittite texts of the 2nd millennium BC. Occupied. Related words can also be found in other Indo-European languages, e.g. B.
- Germanic: German water ; engl. water ; isl. vatn
- Celtic: Scottish uisge (cf. whiskey ); ir. uisce
- Slavic: Russian вода ( voda , cf. vodka ); pole. voda ; upper sorb. voda
- Baltic: lit. vanduo ; lett. ūdens
The ancient Greek word ὕδωρ, hydor , "water", from which all foreign words with the word component hydr (o) - are derived, belongs to this family.
The Arabic root “DRR” with the meaning “to flow” is similar.
Alternative chemical names
Other names for water - permitted according to the chemical nomenclature - are:
- Hydrogen oxide: There are, however, other oxides of hydrogen (see hydrogen oxides ).
- Diwasserstoffmonoxid , Wasserstoffhydroxid , Dihydrogeniumoxid , Hydrogeniumoxid , Hydrogeniumhydroxid , oxane , Oxidan ( IUPAC ) or Dihydrogen (DHMO) .
Properties of water
with all chemical and physical data in the info box , use as a chemical and density anomaly of the water .
Water molecule

Water consists of molecules , each made up of two hydrogen atoms and one oxygen atom.
On the Pauling scale, oxygen has a higher electronegativity with 3.5 than hydrogen with 2.1. The water molecule has pronounced partial charges , with a negative polarity on the side of the oxygen and a positive one on the side of the two hydrogen atoms. The result is a dipole whose dipole moment in the gas phase is 1.84 Debye .
If water occurs as a ligand in a complex bond, then water is a monodentate ligand.
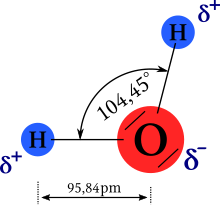
The water molecule is geometrically angled, with the two hydrogen atoms and the two electron pairs pointing into the corners of an imaginary tetrahedron . The angle enclosed by the two OH bonds is 104.45 °. It deviates from the ideal tetrahedral angle (~ 109.47 °) due to the increased space required by the lone electron pairs. The bond length of the OH bonds is 95.84 pm in each case .
Because of the nuclear spin of the hydrogen atoms, water molecules occur in two isomers (para and ortho water, para water reacts 25% faster) with almost identical physical properties. It is possible to separate the two forms and study the different chemical reactivities.
Because water molecules are dipoles , they have pronounced intermolecular forces of attraction and can assemble into clusters through hydrogen bonds . These are not permanent, fixed chains. The connection via hydrogen bonds only lasts for a fraction of a second, after which the individual molecules detach themselves from the connection and chain themselves again - with other water molecules - in an equally short period of time. This process repeats itself over and over again and ultimately leads to the formation of variable clusters. These processes cause the special properties of water:
Has water
- a density of around 1000 kg / m³ (originally the definition of the kilogram), more precisely: 999.975 kg / m³ at 3.98 ° C. As density anomaly is referred to based on the hydrogen bond property of water at this temperature, the highest density has and continuously during cooling below this temperature and even abruptly increases in volume during freezing, floats so loses its density so that ice water,
- the highest specific heat capacity of all liquids at room temperature (75.366 J mol −1 K −1 corresponding to 4.18 kJ kg −1 K −1 at 20 ° C),
- the greatest surface tension of all liquids after mercury ; with water it is 72 mN / m in moist air at +20 ° C, so that the droplet formation is facilitated,
- the largest specific enthalpy of vaporization of all liquids (44.2 kJ / mol corresponding to 2453 kJ / kg at 20 ° C; hence the cooling effect during transpiration ) and the high melting enthalpy (6.01 kJ / mol corresponding to 333 kJ / kg; so that salt water shows only a slight depression of the freezing point compared to pure water)
- a low thermal conductivity (0.6 W / (m K) at 20 ° C).
Depending on the isotopic composition of the water molecule to normal "light water" is different (two atoms of hydrogen : H 2 O), " Medium Heavy water " (an atomic hydrogen and atomic deuterium : HDO), " heavy water " (two atoms of deuterium: D 2 O ) and “ super-heavy water ” (two atoms of tritium : T 2 O), with HTO and DTO other molecules with mixed isotopes occurring.
Under high voltage, water can form a water bridge between two glass vessels.
Synthesis, electrolysis and chemical uses
Water as a chemical compound has been synthesized for the first time when Henry Cavendish in the 18th century, a mixture of hydrogen and air to the explosion brought (see detonating gas reaction).
Hydrogen is considered to be the energy carrier of the future.
Like electrical energy, hydrogen is not a primary energy, but has to be produced from primary energy , analogous to electricity generation .
For the demonstration, water is broken down into its components in Hofmann's water decomposition apparatus . Reaction scheme :
proof
Detection reaction : water turns white, crystalline water- free copper sulfate light blue, blue and cobalt (II) chloride is paper colored red by water.
In analysis, water in small quantities ( moisture or dryness ) is mainly quantified by means of Karl Fischer titration (according to Karl Fischer ). Monographs in pharmacopoeias for the quantitative detection of water are mainly based on the Karl Fischer titration.
Formation of the bubbles in the boiling water
Exposure to heat causes the water molecules to move faster. If 100 ° C is reached at the point where the heat is applied, there (depending on the germ with more or less delayed boiling ) it changes from the liquid to the gaseous state of aggregation (steam), the volume of which is around 1600 times higher (see water vapor ) and which, due to its lower density in relation to the surrounding water, rises as more or less large bubbles: The water begins to boil , whereby the vapor bubbles of layers of water that are not yet so hot are cooled and condense again to liquid water. When the entire amount of water finally reaches a temperature of 100 ° C, the now large steam bubbles reach the surface: the water boils.
Pressure and temperature are the determining factors for the solubility of gases in water. Gas bubbles that become visible even if they are slightly heated do not consist of water vapor, but of dissolved gases. The reason is the lower water solubility of gases when heated. Water that has been in a pressurized pipe or bottle for a while has often dissolved excess gases. Removing the external pressure is therefore sufficient for gas bubbles to separate out - preferably on germs on the wall - and to adhere up to a size of 1-2 mm.
Water and people
History of water use
The history of human use of water, and thus that of hydrology , water management and especially hydraulic engineering , is characterized by a comparatively small number of basic motifs. From the first settled people to the advanced civilizations of antiquity through the Middle Ages to modern times, the focus was always on a conflict between too much and too little water. You were almost always at the mercy of him, whether the harvest came in due to drought or floods threatened life and property. It also became the subject of mythology and natural philosophy . Even today, water has a special position in most of the world's religions, especially where the question of survival depends on the solution of the numerous water problems.
The aim was to meet all usage requirements and to guarantee everyone the amount of water they are entitled to. The water law served as one of the first legal forms to co-found the first centralized civilizations of Mesopotamia and Egypt as well as those that emerged in the river valleys of China and India.
The long history of water use, like human history as a whole, does not show itself as a continuous development path. It was mainly characterized by individual centers of high water management standards as well as by recurring breaks, in addition to phases of stagnation that often lasted for centuries. As impressive as the early hydraulic engineering systems were, however great the innovative strength and creativity of our ancestors were, ultimately one was and is still dependent on nature, which, however, has only really started to be understood relatively recently.
Water in ancient science and philosophy
Because of the great importance of water, it was no coincidence that the earliest philosophers counted it among the four primordial elements . Thales of Miletus even saw the primordial substance of all being in water. In the four-element theory introduced by Empedocles and then mainly represented by Aristotle , water is an element alongside fire , air and earth .
Water is represented in the Taoist five-element teaching (alongside wood, fire, earth, and metal ). The term elements is a bit misleading here, however, as these are different phases of a cyclical process. Water has different orientations which leads to different (symbolic) structures.
In ancient Greece , the icosahedron was assigned to the element water as one of the five Platonic solids .
Water in religion
Water is central to the mythologies and religions of most cultures. With the pre-Socratics about 2500 years ago occidental thinking began as a philosophy of water. In many religions of antiquity, waters in general and especially springs were revered as sanctuaries. The unborn children were thought to be hidden in springs, wells or ponds, from which the nannies ( midwives ) brought them (children's belief).
Water is the epitome of life. It has a high priority in the religions. The purifying power of water is often invoked, for example in Islam in the form of the ritual ablution before entering a mosque, or in Hindu beliefs during a ritual bath in the Ganges .
Almost every community in Judaism has a mikveh , a ritual bath with running pure water, which often comes from a deep groundwater well when spring water is not available. Only those who are completely submerged are ritually cleansed. This is necessary for converts to Judaism, for women after menstruation or childbirth, and for Orthodox Jews before the Sabbath and other holidays.

In Christianity , baptism is carried out partly by immersion or dousing with water as a whole-body baptism , in the western church today mostly by dousing with water. In the Catholic Church, the Orthodox Churches and the Anglican Church, the blessing with holy water plays a special role.
Water in esotericism
In esotericism , water plays a role, places of power are often sought at springs or rivers.
Water in legends and winged words
In many legends and fairy tales , water plays a role, for example as the water of life . The meaning of water can be found in the winged word No water can cloud .
Human health
The human body consists of over 70% water. A lack of water therefore leads to serious health problems ( dehydration , desiccosis ) in humans , as the functions of the body that rely on water are restricted. Quote from the German Nutrition Society (DGE) : If this (the water supply) is insufficient, it can lead to dizziness, circulatory disorders, vomiting and muscle cramps, since the supply of oxygen and nutrients to the muscle cells is restricted when water is lost.
How high the daily minimum requirement is is unclear. Recommendations of 1.5 liters and more per day for a healthy, adult person cannot be scientifically supported. With an average daily consumption of 2 liters, over 55,000 liters of water will be drunk in 80 years. The water requirement can be greater at higher temperatures.
Drinking excessive amounts of water in excess of 20 L / day can also damage your health. " Water poisoning " can occur or, more precisely, a lack of salts, i. H. lead to hyponatremia with permanent neurological damage or death.
In medicine, water (in the form of isotonic solutions) is mainly used for infusions and injections . In the case of inhalation , aerosolized water is used to heal a cough, for example.
Water, applied externally, has very beneficial effects on health and hygiene. See also : bathing , balneology , Kneipp therapy , sauna , swimming , washing . For these reasons, the ancient Romans maintained a "water culture" in the thermal baths.
Importance for cultivation, economy and development

Water is a basic requirement for life: without rain there is no drinking water supply, no agriculture, no bodies of water with fish for consumption, no rivers for transporting goods, no industry. The latter requires a lot of water for all production processes, which is clarified and returned to the cycle. Because of its high heat of vaporization, water is used in the form of steam to drive steam engines and steam turbines and to heat chemical production plants. Because of its high heat capacity and evaporation heat, water is used as a circulating or evaporating coolant; In 1991 in Germany alone, 29 billion m 3 were used as cooling water in power plants . Water can also be used as a refrigerant (R-718) in refrigeration machines. In salt mining , water is used as a solvent for leaching, transport, brine and cleaning.
Water as drinking water, product and commodity

The water supply uses different water resources as drinking water , but also partly for industrial water purposes : precipitation water, surface water in rivers , lakes , reservoirs , groundwater, mineral water and spring water . In Germany, the use of water is regulated in the Water Management Act. In Central Europe there is a reliable, largely cost-covering and high-quality drinking water supply. This is usually guaranteed by public providers (municipal suppliers) who take on ecological responsibility and make it available as tap water . The global water market is growing like no other industry. This is why private providers are very interested in defining water as a commodity in order to take over this market.
Where drinking water is not a direct commodity, the term virtual water was introduced in order to take into account the invisible water content of the products or the sometimes high water requirements that arise in direct connection with the production of a product.
Water consumption
The amount of water consumed by humans is referred to as water consumption. The colloquial term is - like "energy consumption" - incorrect, since nowhere is water "destroyed": its total amount on earth remains constant; “Water demand” would be more appropriate. This includes direct human consumption (drinking water and cooking ) as well as the needs for everyday life ( washing , flushing toilets, etc.) as well as the requirements for agriculture , trade and industry (see industrial water ). This is therefore not only a parameter for the amount of water demanded, but mostly also for the disposal or reprocessing of the wastewater that is generated with most water uses ( sewerage , sewage treatment plant ). The amount of water taken from the supply line is measured by a water meter and used to calculate costs.
Worldwide freshwater demand is estimated at 4,370 km³ (2015), whereby the limit of sustainable use is given at 4,000 km³ ( see also World Exhaustion Day ). A factor that has so far been underestimated is the evaporation of water used or reserved for use, for example by plants (" evapotranspiration "), which, according to the new data analysis, is assumed to be around 20% of total consumption.
In Germany in 1991 the water requirement was 47.9 billion cubic meters, of which 29 billion cubic meters were used as cooling water in power plants. Around eleven billion cubic meters were used directly by industry, 1.6 billion cubic meters by agriculture. Only 6.5 billion cubic meters were used for drinking water supply. The average water requirement (excluding industry) is around 130 liters per inhabitant and day, of which around 1–2 liters in food and beverages including the water contained in ready-made beverages.
Water supply
Supplying mankind with clean water poses a major logistical problem, not only in developing countries. Only 0.3% of the world's water supplies are available as drinking water, that is 3.6 million cubic kilometers out of a total of approximately 1.38 billion cubic kilometers.
The scarcity of water can develop into a water crisis in countries with little precipitation . Adapted technologies are particularly suitable for alleviating water scarcity . However, ideas that appeared unusual were also considered. For example, it was proposed to drag icebergs across the sea into tropical regions, which would only melt slightly on the way, in order to obtain drinking water from them at their destination.
See also: water distribution system , water treatment , water treatment plant , urban water management in Germany , water pollution control
Water availability
Around the world, around 4 billion people or two thirds of the world's population do not have sufficient water available for at least one month a year. 1.8 to 2.9 billion people suffer from severe water scarcity for 4 to 6 months a year, approx. 0.5 billion people all year round. The urbanization aggravated the water scarcity in rural areas and increased competition between cities and agriculture for water. During the drought and heat in Europe in 2018 , harvests fell massively in some cases.
Water as a human right
At the request of Bolivia, the UN General Assembly declared access to clean drinking water and basic sanitation to human rights on July 28, 2010 with the votes of 122 countries and without dissenting votes . 41 countries abstained, including the USA, Canada and 18 EU countries. Since resolutions of the UN General Assembly are not binding under international law, there are initially no legal consequences. However, the new resolution could now support the view that clean water and sanitation are part of an "adequate" standard of living and can thus be sued on the basis of the internationally binding International Covenant on Economic, Social and Cultural Rights , which contains the right to an adequate standard of living . Some countries like South Africa or Ecuador have incorporated the right to water into their constitution.
Legal basis and authorities
In Germany, the water law fundamentals of water management and the public handling of water resources are formed by the Water Resources Act and the European Water Framework Directive . Important authorities and institutions are:
- the upper and lower water authorities (at district level, different depending on the federal state in Germany)
- Waterways and Shipping Office
- LAWA (working group)
Water in the sciences
Water plays a central role in many sciences and areas of application. The science that deals with the spatial and temporal distribution of water and its properties is called hydrology . In particular, oceanology studies the water of the world's seas , limnology the water of inland waters , hydrogeology the groundwater and aquifers , meteorology the water vapor of the atmosphere and glaciology the frozen water of our planet. So far, water has only been detected in liquid form on earth. Areas of environmental economics deal with water as a resource ( water economics ).
Water chemistry
Water chemistry deals with the properties of water, its constituents and the transformations that take place in the water or are caused by the water, as well as with the material balance of the water. It deals with reactions and effects related to the origin and nature of the different types of water. It deals with all areas of the water cycle and thus takes into account the atmosphere and the soil. Among other things, she deals with the analysis of substances dissolved in water , the properties of water, its use, its behavior in various contexts.
Water is a solvent for many substances, for ionic compounds, but also for hydrophilic gases and hydrophilic organic compounds. Even compounds commonly considered insoluble in water are contained in traces in water. Hence, nowhere on earth is water in a pure state. Depending on its origin, it has dissolved a wide variety of substances in more or less large concentrations.
In water analysis, a distinction is made between the following types of water:
- Drinking water
- Mineral water
- Medicinal water
- Table water
- Fresh water / sea water / salt water / brackish water
- Ultrapure water
- Demineralised water
- Distilled water
- De-iced water
- Process water
- Industrial water
- Wastewater (household wastewater, agricultural wastewater, industrial wastewater)
- Rainwater
- Groundwater
- Surface water (flowing and standing water),
But water analysis is also used for the aqueous leaching (eluates) of sediments , sludge, solids, waste and soils.
Molecular dynamics simulation can also be useful in order to clarify the properties of water and any substances dissolved in it, or solid phases in contact with it .
Water in geosciences
In the geosciences, sciences have developed that are particularly concerned with water: hydrogeology , hydrology , glaciology , limnology , meteorology and oceanography . What is particularly interesting for geosciences is how water changes the appearance of the landscape (from small changes over a large period of time to catastrophes in which water destroys entire areas of land within a few hours), this happens, for example, in the following ways:
- Rivers or seas pull earth masses with them and give them up again in other places ( erosion ).
- Whole landscapes are reshaped by moving glaciers .
- Water is stored in stones, freezes in them and splits the stones apart because it expands when it freezes ( frost weathering ).
- Natural ecosystems are strongly influenced by droughts .
Water is not only a major factor in mechanical and chemical erosion of rocks, but also in clastic and chemical sedimentation of rocks. This creates, among other things, aquifers.
Geoscientists are also interested in predicting weather and especially rain events ( meteorology ).
See also: bodies of water , permafrost , inland sea , inland lake , pond , sea , ocean , stream , floodplain .
Water in hydrodynamics
The various fluidic properties and wave types on the microscopic and macroscopic level are intensively investigated, with the following questions being the focus:
- Optimization of boat hulls and exposed structures ( e.g. weirs ) - minimization of flow resistance
- Optimization of the efficiency of water-powered turbine wheels
- Investigation of flow phenomena ( tsunami , monster waves )
Water and nature
Occurrence on earth
Distribution and availability
Most of the earth's surface (71%) is covered by water, especially the southern hemisphere and, as an extreme, the water hemisphere . The earth's water resources amount to around 1.4 billion cubic kilometers (corresponds to the volume of a cube with an edge length of 1120 km), of which the vast majority is the salt water of the world's oceans . Only 48 million cubic kilometers (3.5%) of the earth's water is available as fresh water . With 24.4 million cubic kilometers (1.77%), most of the fresh water is bound to the poles , glaciers and permafrost as ice and is therefore not available for immediate use. Another important part is the groundwater with 23.4 million cubic kilometers. The water of rivers and lakes (190,000 km³), the atmosphere (13,000 km³), the soil (16,500 km³) and living beings (1,100 km³) is quite insignificant in purely quantitative terms. However, only a small part of the fresh water is also available as drinking water. A total of 98.233% of the water is in liquid, 1.766% in solid and 0.001% in gaseous form. In its different forms, the water has specific retention times and is constantly circulating in the global water cycle . However, these proportions can only be determined approximately and have changed significantly in the course of climate history , with an increase in the proportion of water vapor being assumed in the course of global warming .
Deep water in geological layers that are already significantly warmer is used directly or via heat exchange as a heat-energy source, with natural thermal springs and geysers on the surface as well as being drilled by humans. Due to the mountain pressure, water remains liquid in the depths even at temperatures above the boiling point at normal pressure of 100 ° C. New findings suggest that water is also present in liquid form at a depth of around 500 km, in the area between the upper and lower mantle.
The so far missing or inadequate supply of a large part of the world population with hygienic and toxicologically safe drinking water , as well as with a sufficient amount of usable water , represents one of the greatest challenges of mankind in the next decades. Since 1990 around 2.6 billion more people have Get access to a safe water supply, for example with the help of pump wells or a pipe system. But 663 million people still drink water every day, which is polluted and can make people sick.
Origin of terrestrial water
The origin of water on earth , in particular the question of why there is significantly more water on earth than on the other inner planets, has not yet been satisfactorily clarified. Part of the water undoubtedly got into the atmosphere through the outgassing of magma , so it ultimately comes from the interior of the earth . It is highly doubted whether this can explain the amount of water. The element hydrogen is the most abundant element in the universe, and oxygen is also found in large quantities, but usually in silicates and metal oxides; For example, Mars is covered with large amounts of ferric oxide , which gives it its red color. On the other hand, water can only be found there in small quantities compared to the earth.
Occurrence in the universe
Outside the earth there is also water. For example, water ice has been detected in comets , on Mars , some moons of the outer planets and the exoplanet OGLE-2005-BLG-390Lb . The rings of Saturn alone contain roughly 20 to 30 times as much water as occurs on earth. There are indications of the presence of water ice in near-pole meteorite craters at the Earth's moon and even at Mercury , the planet closest to the sun. It is suspected to be liquid water under the icy surfaces of Europe , Enceladus , a few other moons as well as OGLE-2005-BLG-390Lb. Alien liquid water has so far only been directly photographed, but only a few salty mud droplets on Mars. Extra-terrestrial water vapor could be detected in the atmosphere of Mars and Titan , the higher atmospheric layers of red giant stars , in interstellar nebulae and even in the light of distant quasars .
climate
Water decisively influences our climate and is the basis of almost all weather phenomena, especially due to its high mobility and thermal capacity . Radiant solar energy is stored in the oceans. This regionally different warming leads to different concentrations of the dissolved substances due to evaporation, since these do not also evaporate (especially salinity (salt content)). This concentration gradient generated global ocean currents that transport huge amounts of energy (heat) (z. B. Gulf Stream , Humboldt Current , the equatorial current , along with their counter-currents). Without the Gulf Stream, Central Europe would have an arctic climate.
In connection with the greenhouse effect , the oceans are the most effective CO 2 sink, as gases such as carbon dioxide are dissolved in water ( carbon cycle ). The rise in temperature of the world's oceans associated with global warming leads to a lower holding capacity for gases and thus to an increase in CO 2 in the atmosphere. Water vapor is an effective greenhouse gas in the atmosphere (see greenhouse effect )
When it is heated, water evaporates, resulting in evaporative cooling . As "dry" steam (non-condensing) and as "wet" steam (condensing: clouds , fog ) it contains and transports latent heat , which is decisive for all weather phenomena ( see also humidity , thunderstorms , foehn ). In the vicinity of large bodies of water, the heat capacity of water and the phenomena of evaporative cold and latent heat ensure moderate climates with low temperature fluctuations in the course of the year and day. Clouds also reduce the radiation from the sun and the warming of the earth's surface through reflection .
The precipitation falling from clouds and the water vapor (combing out and photosynthesis or respiration) irrigate the terrestrial ecotopes . In this way, bodies of water or ice can arise on the land masses, which also have meso- and microclimatic effects. The ratio of evapotranspiration (total evaporation of an area) to precipitation decides whether dry ( arid , steppes , deserts ) or humid ( humid , forests , forest-steppes ) climates form. On the land masses, the water balance of the vegetation is also a climatic variable.
The importance of water for life
Water is believed to be the origin of life and one of its conditions. In organisms and inanimate components of the ecosphere it plays as the predominant medium in almost all metabolic processes or geological and ecological elementary processes a decisive role. The Earth's surface is covered approximately 72% of water, oceans thereto carry the largest portion. Fresh water reserves only make up 2.53% of the earth's water and only 0.3% can be used as drinking water (Dyck 1995). Through the role of water in terms of weather and climate , as landscaping gestalter in the course of erosion and its economic importance, including in the areas of agriculture , forestry and energy industry , it is also in many ways with history , economy and Culture connected to human civilization . The importance of water for life has always been the subject of natural philosophy .
Basic building block of life
The life originated in water according to present knowledge ( see Evolution ). Autotrophic sulfur bacteria ( prokaryotes ) produce organic carbon compounds and water from hydrogen sulfide and carbon dioxide with the addition of light energy:
As successors, blue bacteria ( cyanobacteria ) and all later autotrophic eukaryotes used the high redox potential of water: With the addition of light, they produce glucose and oxygen from water and carbon dioxide:
As a result of this process, more and more oxygen was enriched in the water and in the atmosphere. This made it possible to generate energy through cellular respiration ( dissimilation ):
The prerequisite for the ability to deal with the toxic oxygen (oxidation of the sensitive biomolecules) were enzymes such as catalase , which is structurally similar to the oxygen-transporting hemoglobin . Aerobic purple bacteria were perhaps the first to use poisonous oxygen to break down organic substances to provide energy. According to the endosymbiont theory , anaerobic eukaryotes took in the aerobic prokaryotes (probably purple bacteria).
Water thus became the medium of fundamental biochemical processes ( metabolism ) for energy generation and storage:
Because of the dipole moment , water is suitable as a solvent for polar substances and because of the resulting viscosity and density as a means of transport. Water transports nutrients, breakdown products, messenger substances and heat within organisms (e.g. blood , lymph , xylem ) and cells. The properties of water are manifold in plants and animals (including humans). B. used for temperature regulation, in the form of guttation , sweating, etc., or z. B. as a basis for antibacterial protective films in toads and fish .
The turgor pressure of the water gives shape and strength to plants and animals without a skeleton . They can also move through turgor changes (for example leaf movement in plants).
The echinoderms , to which the sea urchins , starfish and sea whales belong, have a system of hydraulically working vessels ( ambulacral system ) instead of a solid skeleton . They move through targeted changes in pressure in this vascular system.
Water content in some foods:
- Butter 18 percent
- Bread 40 percent
- Cheese 30 to 60 percent
- Yogurt, milk 87.5 percent
- Meat 60-75 percent
- Apple, pear 85 percent
- Watermelon 90 percent
- Carrots 94 percent
- Cucumbers, tomatoes 98 percent
Water and ecosystems
In terrestrial ecosystems , water is the limiting factor for productivity. It is essential for the metabolism of living things ( biosphere ) as well as for the formation and shaping of their locations ( pedosphere , earth's atmosphere / climate ). Precipitation feeds bodies of water and groundwater as a resource for plant growth and as drinking water for animals.
Most of the biomass and greatest productivity is found in aquatic ecosystems, especially in oceans , in which the limiting production factor is the amount of nutrients dissolved in the water, i.e. mainly phosphate, nitrogen compounds (ammonium, nitrate) and CO 2 ( carbon dioxide ). The properties of water are used with high efficiency, e.g. B. in the surface tension of insects , arachnids , in the density and the optical properties of plankton etc.
The temperature dependence of the water density leads to temperature stratification , thermoclines and balancing currents, which are particularly characteristic of limnic (freshwater) biotopes ( see the lake ecosystem ), but can also be found and used in marine ecosystems ( e.g. whales use . the sound reflections on thermoclines to improve their communication). The density anomaly of the water also enables the survival of living things in winter, since stagnant water does not freeze through to the bottom (with the exception of shallow water and “ frost dryness ”). In addition, the density anomaly in deeper lakes of the temperate zones in spring and autumn, when a uniform temperature is reached, causes the water to circulate and thus an exchange of surface and deep water, which is essential for the nutrient and oxygen cycle.
Even if aquatic ecosystems represent very stable habitats due to the heat capacity of water, lower temperature fluctuations also have significant consequences (see ecosystem lake ). The rise in ocean temperatures will result in changes in marine ecosystems.
Ecological status of waters
In the European Union (EU) according to Directive 2000/60 / EC (EU Water Framework Directive, WFD), the ecological status of rivers and surface waters (such as groundwater ) is analyzed according to various criteria and classified according to five grades: “very good “,“ Good ”,“ moderate ”,“ unsatisfactory ”,“ bad ”.
Water in technology
Water has various uses in technology. Heat transfer for heating or cooling are among the most important. The generation of cold through evaporation, for example in cooling towers. The operation of refrigeration machines based on the adsorption of ammonia in water or water vapor in (aqueous) lithium bromide solution.
Water is used cold and warm for cleaning washing (possibly with detergents or alkalis or acids), dissolving (leaching of salt deposits), separation via chromatography or extraction (infusion beverages), recrystallization (setting of gypsum, cement, (together with carbon dioxide :) lime; but also cleaning of soluble substances in the chemistry laboratory). As a pressure jet for rinsing, showering, high-pressure cleaning , possibly with an abrasive additive, and for water jet cutting also in the hygiene-sensitive area of the food industry.
In the form of gel as a sound transmission medium from the sensor head to the human body in ultrasound diagnostics . Water is the sound transmission medium in the echo sounder .
As a medium with high surface tension and good evaporation rate for slidable clapping of labeling foil on shop windows, car bodies and other smooth surfaces to be concealed. Also as a lubricant and sealant for suction cups. The surface tension of water, in combination with soap, allows soap bubbles and the building of layers from molecular thickness and fine membranes for physical experiments. The water strider can run through dents in the surface, biofilms can spread, but also oily substances can spread .
Original hydraulics used water as a pressure transmission medium, as fountains in fountains and water features, which also enable evaporative cooling and lighting effects. The breaking up of geological layers during fracking is also a high pressure application.
The weight of water masses is used in the various mills and hydroelectric power stations to generate mechanical or electrical energy. When pumping water hammer water ram the low compressibility comes in addition of water to it. Buoyancy created by water allows ships, buoys and living things to swim. Ballast tanks help stabilize unloaded or unevenly loaded ships and submarines to surface and submerge. There are cable cars and lifts that are pulled or lifted in return by water ballast tanks.
Water as a dissociation medium is used for electrolysis, electroplating, rechargeable batteries and battery technology, and in old power plants as an electricity control tank. It is also used as a solvent for all aqueous chemistry, whether in the micro-process of the spot plate, the graphic development of photographic plates and films or the large-scale production of nitramoncal from ammonia and nitric acid.
In medicine, water is used as a dissolving medium to inject or infuse substances into the body, to correct the body's water balance, to soften hard skin or nails, or to flush the intestines. Reversible swelling of the scalp hair with water and shaping it into waves and curls is a hairdressing trade .
Willow branches , rattan cane etc. are placed in water and made flexible for braiding . Hardwood formed into bentwood furniture under steam .
Water can filter out infrared radiation from incandescent lamp light and absorbs ionizing radiation in the fountain ponds of nuclear power plants .
In water cannons , water, with and without chemical additives, is used as ammunition .
Exhibitions and events related to water
- From 2005 to 2014, has the UN for the International Decade for Action, "Water - Source of Life" called
- World Water Forum
- World water day
- WasserForum - Museum of water supply and extraction of the HWW ( Hamburger Wasserwerke / Hamburg Wasser )
- Good quality - project on the EU Water Framework Directive in Hanover.
- Wasser (Musical) - Musical by Siegfried Faderl and Ewald Mayrbäurl.
See also
literature
- Ole Pollem: Regulators for the water sector in low-income countries. A comparative study of the regulators in Ghana, Zambia, Mozambique and Mali . Publishing house Dr. Kovac, Hamburg 2009, ISBN 978-3-8300-4473-4 .
General content
- Sibylle Selbmann: The myth of water, symbolism and cultural history. Badenia, Karlsruhe 1995, ISBN 3-7617-0309-0 .
- Philip Ball : H 2 O - Biography of Water. Piper, Munich 2001, ISBN 3-492-04156-6 .
- Siegfried Dyck, Gerd Peschke: Fundamentals of hydrology. 3. Edition. Verlag für Bauwesen, Berlin 1995, ISBN 3-345-00586-7 .
- Dieter Gerten: Water. Scarcity, climate change, world food. CH Beck, Munich 2018, ISBN 978-3-406-68133-2 .
- Vollrath Hopp: Water Crisis? Water, nature, people, technology and economy. Wiley-VCH, Weinheim 2004, ISBN 3-527-31193-9 .
- Ernst Schmidt (Ed.): Properties of Water and Steam in SI Units. Springer, Berlin 1981, ISBN 3-540-09601-9 . ("Thermodynamic properties of water and water vapor, 0–800 ° C, 0–1000 bar")
- Helmut Lehn, Oliver Parodi: Water - elementary and strategic resource of the 21st century. I. An inventory. In: Environmental sciences and pollutant research . Volume 21, No. 3, 2009, pp. 272-281.
- Wolfram Mauser: How long will the resource water last? How to deal with blue gold . Fischer-Taschenbuch, Frankfurt am Main 2007, ISBN 978-3-596-17273-3 .
- Érik Orsenna : The future of water: a journey around our world (original title: L 'avenir de l'eau, translated by Caroline Vollmann). Beck, Munich 2010, ISBN 978-3-406-59898-2 ; as paperback: dtv, Munich 2012, ISBN 978-3-423-34690-0 .
- Helge Bergmann: Water myths, markets, molecules. Wiley-VCH, Weinheim 2011, ISBN 978-3-527-32959-5 .
- Leopold Schua: Habitat Water. Secrets in an unknown world. (= Kosmos Library. Volume 268). Stuttgart 1970, ISBN 3-440-00268-3 ( pdf; 23 MB ).
Water chemistry
- Heinrich Sontheimer, Paul Spindler, Ulrich Rohmann: Water chemistry for engineers . DVGW research center at the Engler-Bunte-Institute of the University of Karlsruhe. ZfGW-Verlag, Frankfurt 1980, ISBN 3-922671-00-4 .
- Bernd Naumann: Chemical studies of the basis of life water. (= Suggestions for ecological education. Vol. 2). State Institute for Teacher Training, Teacher Training and Teaching Research of Saxony-Anhalt (LISA), Halle 1994.
- Günter Wieland: water chemistry. 12th edition. Vulkan-Verlag, Essen 1999, ISBN 3-8027-2542-5 .
- Karl Höll, Andreas Grohmann u. a .: water. Use in the cycle. Hygiene, analysis and evaluation. 8th edition. Walter de Gruyter, Berlin 2002, ISBN 3-11-012931-0 . (Standard work on water research).
- Leonhard A. Hütter: Water and water investigation - methodology, theory and Practice of chemical, chemical-physical, biological and similar bacteriological test methods. Sauerländer, Frankfurt am Main 1994, ISBN 3-7935-5075-3 .
Use and protection
- Christian Opp (Ed.): Water resources. Use and protection; Contributions to the International Year of Freshwater 2003. Marburg Geographical Society, Marburg / Lahn 2004, ISBN 3-88353-049-2 .
- Christian Leibundgut, Franz-Josef Kern: Water in Germany - Deficiency or Abundance? In: Geographical Rundschau . Volume 58, No. 2, 2006, pp. 12-19.
Conflicts over water
- Aboubacry Athie: The political implications of water availability in sub-Saharan Africa illustrated using the example of the Sahel countries of West Africa. Wissenschaftlicher Verlag, Berlin 2002, ISBN 3-936846-05-7 .
- Hans Huber Abendroth: The "water war" of Cochabamba. On the dispute about the privatization of a water supply in Bolivia. Federal Chamber for Workers and Salaried Employees, Vienna 2004, ISBN 3-7062-0081-3 .
- Detlef Müller-Mahn: Water conflicts in the Middle East - a question of power. In: Geographical Rundschau . Volume 58, No. 2, 2006, pp. 40-48.
- Lisa Stadler, Uwe Hoering: The water monopoly. Of a common good and its privatization. Rotpunktverlag, Zurich 2003, ISBN 3-85869-264-6 .
- Karo Katzmann: Black Book Water - Waste, Pollution, Threatened Future. Molden, Vienna 2007, ISBN 978-3-85485-196-7 .
- Andreas Hoppe: Water in the Middle East - a reason for war? In: Naturwissenschaftliche Rundschau . Volume 59, No. 5, 2006, pp. 241-247.
Religious meaning
- Claudia Sticher: Water. Symbol of life and faith . With a contribution by Norbert Lohfink . Verlag Katholisches Bibelwerk, Stuttgart 2014, ISBN 978-3-460-27174-6 .
Web links
- Water lexicon of the University of Bremen
- Graphic showing the global distribution of water in all forms
- All physical data of water , accessed on November 25, 2011
- The absorption spectrum of liquid water from the UV to the IR (English)
- Graphic showing what it would look like if all the water on or near the surface of the earth formed a large sphere
- Where does our water come from? from the alpha-Centauri television series(approx. 15 minutes). First broadcast on Aug 4, 2002.
- Is water magical? from the alpha-Centauri television series(approx. 15 minutes). First broadcast on Oct 27, 2002.
- Marko Pauli: Water - an enigmatic liquid. In: Deutschlandfunk Kultur - Feature. January 16, 2020, accessed January 18, 2020 .
- Subject of water at the Federal Office for the Environment (Switzerland)
Individual evidence
- ^ The dictionary of origin (= Der Duden in twelve volumes . Volume 7 ). 5th edition. Dudenverlag, Berlin 2014, p. 915 ( google.de ). See also DWDS ( "water" ) and Friedrich Kluge : Etymological dictionary of the German language . 7th edition. Trübner, Strasbourg 1910, p. 484 ( Digitale-sammlungen.de ).
- ↑ Not all water is created equal: Separation and investigation of the isomers of water (para and ortho water, para water reacts 25% faster with diazenylium ions «protonated nitrogen»), chemie.de, May 31, 2018.
- ↑ Not all water is created equal. University of Basel, May 29, 2018.
- ↑ Thomas Kramar: Physics: A bridge of H 2 O . In: The press. November 8, 2007.
- ↑ Hydrogen as the energy carrier of the future ( memento of October 26, 2012 in the Internet Archive ), VDE, accessed on August 3, 2011.
- ↑ Creating structures .
- ^ Sibylle Selbmann: Myth of water. Symbolism and cultural history. Badenia Verlag, Karlsruhe 1995, ISBN 3-7617-0309-0 .
- ↑ German Society for Nutrition e. V .: Drink a lot in the heat of summer. Dge.de, July 28, 2006, accessed July 6, 2010 .
- ↑ Health Marketing - Waterlogged? by Margaret McCartney, doi: 10.1136 / bmj.d4280 .
- ↑ Linda F. Fried, Paul M. Palevsky: Hyponatremia and Hypernatremia. In: Medical Clinics of North America. Vol. 81, No. 3, May 1, 1997, pp. 585-609. doi: 10.1016 / S0025-7125 (05) 70535-6 .
- ↑ The water chiller , BINE information service.
- ↑ Dagmar Röhrlich : Resources tighter than expected. Deutschlandfunk.de , Research News. December 3, 2015, accessed on December 3, 2015. From: F. Jaramillo, G. Destouni: Local flow regulation and irrigation raise global human water consumption and footprint . In: Science . tape 350 , no. 6265 , December 4, 2015, p. 1248–1251 , doi : 10.1126 / science.aad1010 ( sciencemag.org [accessed May 29, 2019]).
- ^ Mesfin M. Mekonnen, Arjen Y. Hoekstra: Four billion people facing severe water scarcity . In: Science . 2016, doi : 10.1126 / sciadv.1500323 .
- ↑ Dustin Garrick, Lucia De Stefano et al. a .: Rural water for thirsty cities: a systematic review of water reallocation from rural to urban regions. In: Environmental Research Letters . Volume 14, No. 4, 2019, p. 043003, doi: 10.1088 / 1748-9326 / ab0db7 .
-
↑ Water is a human right. In: Red Globe. July 29, 2010. Retrieved July 29, 2010 . Right to water not enforceable. In: the standard. July 29, 2010. Retrieved July 29, 2010 .
- ↑ Wolfgang Baumjohann: Then I prefer to go to the mountains. Interview with Tiz Schaffer. In: Falter . 04/15, January 21, 2015. Retrieved May 3, 2015.
- ↑ Access to clean drinking water. Retrieved July 26, 2017 .
- ↑ Environmental goals - the good condition for our waters, bmnt.gv.at. Retrieved April 4, 2018 .
- ^ Sibylle Wilke: Ecological condition of flowing waters . In: Federal Environment Agency . October 18, 2013 ( Umweltbundesamt.de [accessed April 4, 2018]).