vaccination
A vaccination , also known as protective vaccination , vaccination (older vaccination ) or vaccination (originally the infection with cowpox material ; from Latin vacca , 'cow') is the administration of a vaccine with the aim of protecting against a (communicable) disease. It activates the immune system against specific substances. Vaccinations were developed as a preventive measure against infectious diseases . Later, for cancer immunotherapies and cancer vaccines developed.
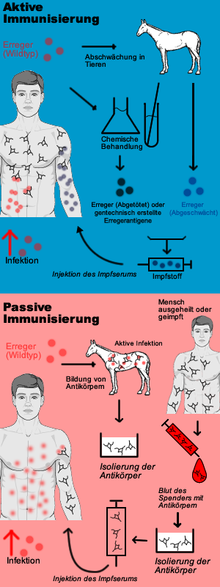
A preventive vaccination against an infectious disease is based on a specific, active immunization against the pathogen and is therefore also called active vaccination or active protective vaccination . The aim of active vaccination is to enable the body's own immune system to react to an infection with the pathogen so quickly and effectively that no or only a weakened infectious disease results. A distinction is made between live vaccines and dead vaccines ; the latter also include toxoid vaccines . In contrast, passive vaccination (also known as therapeutic vaccination ) is merely passive immunization through the administration of antibodies .
Vaccinations are currently available against a wide variety of viral and bacterial infectious diseases. Further vaccines against some major infectious diseases and against chronic infections that promote cancer are currently in development .
Mode of action and effectiveness
Administration of vaccinations
Depending on the vaccine and type of immunization (passive or active immunization), different application methods are used: Active vaccinations are carried out parenterally ("bypassing the gastrointestinal tract") with a syringe . A distinction is made between intradermal ("into the skin"), subcutaneous ("under the skin") or intramuscular ("into the muscle") injections . Intradermal vaccination can also be done with a lancet or a vaccination gun . For a few immunizations, the vaccine was or will be administered orally (in the mouth, " oral vaccination ") or nasally (in the nose), with a test patch on the skin. Most active vaccinations, however, are given intramuscularly in the upper arm ( deltoid muscle ). In children, an injection into the thigh ( vastus lateralis muscle ) is also allowed; In young children, there are fewer local reactions after certain vaccinations if they occur in the thigh. The injection of active vaccines into the middle gluteus muscle ( musculus glutaeus medius ) is considered obsolete due to its lower effectiveness and more frequent complications, according to the Standing Vaccination Commission (STIKO). Passive immunizations, on the other hand, are often given into the gluteal muscle. The powder injection is a method of vaccination under development, in which the solid vaccine at high speed in the epidermis is shot.
Active vaccination
Active vaccination is based on active immunization. The aim of active vaccination is the formation of the body's own protective substances against certain diseases. For this purpose, the immune system is stimulated to develop a pathogen-specific immune competence without having to go through the infectious disease itself. Live or dead vaccines are used for this. The live vaccine contains weakened, still reproductive pathogens, which do not trigger the disease in the immunocompetent vaccinee. In the case of a dead vaccine, however, these pathogens were killed or only fragments of the pathogen are still present. After penetration of the vaccine into the body of its proteins (are proteins ) and / or sugar molecules by circulating in the blood and / or PAMP immunocompetent white blood cells recognized as exogenous antigens. This is followed by the primary immune response through the pathogen-specific imprinting of immunocompetent lymphocytes in the form of long-lived memory cells . For the protection against a later infection it is crucial that the antigens of the vaccine are largely similar to those of the pathogen of the infectious disease. If the infection now occurs, the memory cells recognize the antigens of the previous vaccine on the invading pathogen and cause lymphocytes to differentiate into short-lived plasma cells that produce antibodies on the one hand, and T lymphocytes and NK cells , which represent the cellular defense , on the other . The vaccination should therefore bring about the pre- existence of immunity against the pathogen, so that due to the specific and rapid immune response after infection, the infectious disease does not occur. Toxoid vaccines, which only contain the biologically inactive component (toxoid) of the toxin of a pathogen (e.g. the tetanus toxoid), also belong to the dead vaccines. They do not reduce the number of pathogens in the body. In the case of infections that are transmissible, they do not interrupt the chain of infection, but prevent the vaccinated from developing an infectious disease, provided that the pathogen's toxins are not effective in them.
Different live vaccines can be administered either simultaneously or at least four weeks apart. For dead vaccines alone or in combination with live vaccines, there are no necessary intervals.
A form of therapy that is similar to the principle of active immunization, but which is not a vaccination, is desensitization . It is used, for example, for hay fever or allergies to dust mites and insects .
Passive vaccination
If a person is at risk of developing a serious infectious disease because they have had contact with the pathogen in question without being protected against it by silent feasting or vaccination, passive vaccination (possibly simultaneous vaccination - see below) is indicated: Here, the recipient is injected with immune serum containing high concentrations of antibodies against the pathogen. It is therefore not a vaccination in the medical sense, as the immune system does not produce antibodies itself, ie remains "passive", but these are produced outside of the person being vaccinated. For this purpose, monoclonal human antibodies or homologous antibodies produced by genetic engineering from cell cultures are preferably used today , or, if they are not available, extracts from the blood (convalescent serum) of people who have (unintentionally) suffered the infectious disease in question, or from the blood from animals or heterologous (alien) antibodies that were specifically infected with the pathogen. Passive immunization is therefore an emergency measure in the sense of post-exposure prophylaxis . Examples of this are injuries with soiling of the wound (suspected tetanus infection ), bites from or contact with the mucous membrane with certain wild animals (suspected rabies ) or the contact of medical staff with the blood of patients who are carriers of the pathogen of hepatitis B (in particular after needle stick injury ).
The advantage of immune serum is that protection occurs more quickly: the antibodies do not have to be formed within one to two weeks, but are available immediately after the injection of the immune serum. The disadvantage is that the protection lasts only a few weeks, after which the administered antibodies are broken down by the recipient, and his organism is endangered again by a renewed infection with the same pathogen. This is due to the fact that the immune system is not stimulated by the administration of immune serum to develop its own immune memory with regard to the pathogens via memory cells.
If the immune serum comes from animals or humans, there is a further disadvantage that, apart from the desired antibodies , it can contain traces of foreign proteins or polysaccharides from the donor. The recipient's immune system then sets in motion a cascade of immunological reactions against these components, which are perceived as exogenous antigens. This means that the antibodies accumulated in the vaccine serum are excreted more quickly and therefore remain effective for a shorter time than desired. Repeated administration of foreign serum, especially from the same animal species, can also lead to an undesirable allergic reaction on the part of the recipient in the form of serum sickness or allergic shock . Therefore, such immune sera are replaced by monoclonal antibodies whenever possible.
For example, until around 1965 there were no human antibodies against tetanus, so one had to rely on animal antibodies. The sequence horse , cattle , mutton was established here.
Introduced passive immunization in 1890 by Emil von Behring when he was a medical treatment against diphtheria developed, in which he used from horse blood isolated antibodies.
An important and widespread natural form of passive immunization against infectious diseases is mother-child immunization .
Passive immunizations that are not directed against infectious diseases include injecting anti-D immune serum into pregnant women if the newborn is at risk of developing haemolyticus neonatorum , and injecting antivenin after snake bites.
Simultaneous vaccination
If a patient with possibly or known to be inadequate immune protection is suspected of having been infected with pathogens of a dangerous infectious disease, he or she will receive passive immunization in addition to active vaccination to prevent a life-threatening infection. Such a simultaneous active and passive immunization of a patient is called simultaneous vaccination . The active and passive vaccine is injected into different parts of the body so that the injected antibodies do not immediately absorb the antigens (or toxins) of the vaccination.
Mother-child immunization
Mother-child immunization, also known as nest protection or loan immunity, is based on two possible processes: On the one hand, pregnant women who have developed a corresponding antibody titer after infections or vaccinations pass these antibodies on to the unborn child via the placenta , which is then released after birth is protected to a certain extent for a few weeks to months. On the other hand, breastfeeding mothers supply the infant with antibodies with a similar effect through breast milk. However, these antibodies absorbed by the mother do not protect against all infectious diseases. The child vaccinations recommended in general and in Germany in particular by the Standing Vaccination Commission should therefore be carried out early enough so that there is no gap in the pathogen defense.
effectiveness
| vaccine | before (year) |
after (year) |
|---|---|---|
| diphtheria | 175,885 (1922) |
1 (1998) |
| Haemophilus influenzae B | 20,000 (1982) |
54 (1998) |
| whooping cough | 147.271 (1925) |
6,279 (1998) |
| measles | 503,282 (1962) |
89 (1998) |
| mumps | 152,209 (1968) |
606 (1998) |
| smallpox | 48,164 (1904) |
0 (1998) |
| rubella | 47,745 (1968) |
345 (1998) |
No vaccination can protect one hundred percent against the disease in question. Vaccinations, however, significantly reduce the likelihood of illness. The protective effect differs depending on the vaccination. The vaccination effect ( english vaccine efficacy ) has been documented through extensive studies and confirmed by government agencies.
The respective protective effect can be assessed in a laboratory by measuring the antibody concentration formed against the pathogen or its components, the antibody titer . The decisive factor is the effectiveness in the context of clinical studies , if possible in the form of randomized controlled studies . The study participants are randomly divided into two groups. One compares either certain laboratory values ( surrogate markers , especially antibodies ) or the frequency and severity of the infectious disease in the study group , i.e. in humans or animals who received the vaccine to be assessed, with those in the control group , i.e. in humans or animals, who have not received a vaccine or a known vaccine. Proof of efficacy in which people are specifically infected with pathogens causing serious infectious diseases are prohibited for ethical reasons, since the control group and study group are exposed to an unacceptable risk. The approval of vaccines takes place in Europe according to the guidelines of the European Medicines Agency and the appropriate government authorities. It requires preclinical and clinical trials and requires further controls after the market launch. In Germany, the Paul Ehrlich Institute checks and monitors the approval of vaccines. The criteria and procedures are similar in other developed countries such as the USA and Canada.
There are vaccinations that so far have only mitigated the course of the disease and thus only protect against the worst complications. This is particularly the case when the pathogens in question frequently change their properties due to antigen shift or antigen drift , as is the case with influenza pathogens, and when the pathogens circulate in numerous antigen subtypes, such as pneumococci. Government agencies evaluate the vaccines in terms of their benefits and then issue an official recommendation. The recommendations have consequences under health insurance, liability and medical law.
Modern vaccines against tetanus, hepatitis, meningococci, pneumococci and cervical cancer contain the salt aluminum hydroxide as an enhancer to reduce the number of vaccination cycles required.
Since vaccination was introduced in the US, the number of diphtheria, mumps, whooping cough, and tetanus illnesses has decreased by more than 92% each year , while the number of deaths from these diseases has decreased by at least 99%. Poliovirus, measles and rubella virus are considered to be eradicated in the United States, and in 1980 the World Health Organization (WHO) declared the world to be free of smallpox.
Conversely, according to estimates by the WHO and the Global Alliance for Vaccines and Immunization ( GAVI ), over two million people died from infectious diseases in 2002 alone that could have been prevented by vaccination. Reducing these causes of death through vaccination programs is therefore a primary goal of the WHO. The success of these vaccination programs prove the effectiveness of vaccination. Most of the vaccines available are listed in the Recommended Vaccinations section .
On the cost-benefit ratio using the example of pneumococcal vaccination: Silvia Evers et al. from Maastricht University have calculated figures for this. The upper limit was 50,000 euros per year of life of the same quality (QALY; 1 QALY, in short, corresponds to a year of life spent in perfect health). Health economists consider measures that cost less than 50,000 euros per QALY to be cost-effective. Evers' analysis extended to ten European countries. The cost-benefit ratios were between 9239 (Denmark) and 23,657 euros per QALY (Sweden). In these calculations, Germany ranks in the middle with 17,093 euros per QALY. In Germany, the pneumococcal vaccination is recommended for the chronically ill (diabetics, asthmatics, COPD and cardiovascular patients ) and for people over 60 years of age.
Reduced effectiveness through interaction with painkillers
Vaccines are drugs, and with simultaneous impact of multiple drugs can drug interactions occur. They can consist of an increase or decrease in the effectiveness, an increase or decrease in known or new side effects. Recently there has been increasing evidence that certain drugs from the group of non-steroidal anti-inflammatory drugs (NSAIDs) or non-steroidal anti-inflammatory drugs such as acetylsalicylic acid (aspirin), but also other non-opioid analgesics such as paracetamol and substances derived from it, can reduce the effectiveness of vaccines. This is attributed to the fact that these drugs achieve their antipyretic and (in the case of NSAIDs) also anti-inflammatory effects by inhibiting certain enzymes , the cyclooxygenases (COX), which is why they are also called cyclooxygenase inhibitors . The COX involved in prostaglandin synthesis also play an important role in immune defense . Blocking the enzyme appears to have the side effect of reducing the production of protective antibodies after vaccination, since the terminal differentiation of B cells into antibody-producing plasma cells is impaired. It is therefore recommended to avoid COX-inhibiting drugs for some time before and after vaccination.
refreshing
After the primary vaccination course , regular booster vaccinations are necessary to maintain long-term immunity against most pathogens . The booster dose (as a booster vaccination or revaccination called) is different from the primary series in that it already leads to a sufficient clinical patient protection with the single administration of a lower dose of vaccine within a short time. The recommendations for the timing of such booster vaccinations are based on observational observations of individual vaccines and depend on both the type of pathogen and the vaccine: Some vaccinations can provide protection that is very likely to last a lifetime, even without a booster. In people vaccinated against smallpox, immunity could be demonstrated up to 88 years after vaccination, which was comparable to a survived illness. For the most part, people who had been vaccinated against measles, mumps and rubella still found sufficiently high antibody titers after 20 years. The same apparently applies to vaccinations against hepatitis A. According to current recommendations, other vaccines, such as the vaccine against whooping cough, require a booster vaccination every ten years, because the antibody level drops after four to twelve years. However, natural immunity from infection with whooping cough also declines after four to 20 years. In contrast, the vaccination against influenza with the previous vaccines must be repeated annually.
The booster vaccination should not be confused with the necessary partial vaccinations, which, depending on the vaccine, are necessary for a completed primary vaccination. These are used, for example, in the "2 + 1" scheme or the "3 + 1" scheme. With the 3 + 1 scheme, the partial vaccinations required for the basic immunization take place three times at four-week intervals, followed by the booster vaccination six months later. With the 2 + 1 scheme, the interval between the two basic immunizations is eight weeks, six months after the scheme ends with the booster vaccination.
Side effects
The side effects of the vaccinations that are officially recommended today are usually so minor that they are not perceived or perceived as insignificant. A distinction is made between vaccination reaction and vaccination complication .
When evaluating reactions after vaccination, it must always be borne in mind that vaccinations are carried out in healthy people and that subsequent illness is strongly felt. The expectation of severe side effects can lead to increased introspection ( nocebo effect ). As a result, randomly occurring disturbances of well-being that would normally not be taken into account can suddenly be consciously perceived and mistakenly blamed on the vaccination.
So-called vaccination reactions are short-term and temporary local and general reactions . These can appear as temporary, minor side effects such as pain, tension and swelling at the injection site, fatigue, or headache and body aches. In double-blind experiments without the action of pathogens, in which one half of the volunteers was injected with the vaccine, the other half with a saline solution or an adjuvant containing aluminum hydroxide , both groups reported quantitatively and qualitatively similar side effects for most of the officially recommended vaccines: e.g. . B. dizziness, headache, weakness, muscle pain.
| Symptom / illness | Disease complication rate |
Complication rate after vaccination |
|---|---|---|
| measles | MMR | |
| Rash | 98% | 5%, attenuated |
| fever | 98%, mostly high | 3 to 5%, very rarely high |
| Febrile seizures | 7 to 8% | ≤ 1% |
| Decrease in platelet count | 1/3000 | 1 / 30,000 to 1 / 50,000 |
| Encephalitis | 1/1000 to 1 / 10,000 | 0 |
| Lethality | 1/1000 to 1 / 20,000 | 0 |
| mumps | MMR | |
| Inflammation of the salivary gland | 98% | 0.5% |
| Pancreatitis | 2 to 5% | 0.5% |
|
Inflammation of the testicles in adolescents and adult men |
20 to 50% | 1 / 1,000,000 |
| meningitis | ~ 15% | 1 / 1,000,000 |
| deafness | 1 / 20,000 | 0 |
| rubella | MMR | |
| Joint discomfort in women |
40 to 70%, persistent | 1 / 10,000, mostly short and weak |
| Encephalitis | 1/6000 | 0 |
| Decrease in platelet count | 1/3000 | 1 / 30,000 to 1 / 50,000 |
|
Rubella embryo fetopathy in infection during pregnancy |
> 60% | 0 |
A vaccination complication , on the other hand, is a complication as a result of a vaccination that goes beyond the usual extent of a vaccination reaction. Live vaccines can rarely lead to an outbreak of the disease that was vaccinated against. For example, so-called "vaccine measles" occur in three to five percent of vaccinations against measles. The side effects of the vaccination then include the symptoms of the disease, for example a slight rash or fever, but these are usually easier than the "natural" infection. In very rare cases, allergic - anaphylactic shock can occur as a reaction to the ingredients of a vaccine dose. This was observed in 33 cases (often with pre-existing conditions such as atopy or asthma ) with 25.2 million vaccine doses administered; none of the cases was fatal. In addition to the active ingredient itself, additives such as. B. aluminum compounds, mercury compounds (thiomersal) , formaldehyde and antibiotics or substances from the production of the active ingredient such as egg white trigger such a reaction. Doctors must provide adequate information about this risk, as well as the risk of vaccination, before vaccination . Those who vaccinate must be prepared through exercise and appropriate equipment to manage possible life-threatening allergic reactions to vaccination.
Since January 1, 2001, doctors in Germany have been subject to the "obligation to report suspected health damage beyond the usual extent of a vaccination reaction" anchored in the Infection Protection Act (IfSG). According to § 6 Abs. 1, Nr. 3 IfSG there is an obligation for doctors to report to the health department if symptoms occurring after a vaccination that go beyond a vaccination reaction could be causally related to the vaccination. This reporting system is a so-called spontaneous detection system in order to identify early risk signals of side effects of vaccination that were not recorded during approval. Up to December 31, 2003, 3328 cases of possible consequences of vaccinations had been registered in all age groups (in three years, with around 30 million vaccine doses / year). Four percent of these victims suffered permanent damage and 1.6% died (mainly documented coincidences). In the majority of the suspected cases reported to the Paul Ehrlich Institute (PEI), the causal relationship between vaccination and illness was rated as improbable. In the other cases, the causal relationship with the vaccination could not be assessed due to a lack of valid scientific data. A connection between vaccination and reaction is only certain in 0.2% of all IfSG reports. In Germany there is an entitlement to benefits from the pension offices if a health disorder is possibly due to a publicly recommended vaccination. However, the patient does not need to prove that the vaccination was the cause of his illness.
A comparison of the number of possible vaccine reactions with the vaccinations carried out in the same period shows a very low risk, for example 250 IfSG reports on possible reactions of around six to eight million vaccine doses to the MMR vaccine in the same period. However, the rate of reports depends on the doctors' motivation and ability, despite the statutory reporting requirement. Therefore, spontaneous recording alone is not suitable for estimating the frequency of vaccination side effects. Active pharmacovigilance systems and studies aimed at the respective vaccination complication are used for this purpose .
"Wrong" contraindications
Often indicated vaccinations are not given (or postponed indefinitely and finally forgotten) because certain circumstances are mistakenly viewed as obstacles to vaccination. According to the Robert Koch Institute , these are in particular:
- common infections, even if they are accompanied by a slight fever (up to 38.5 ° C),
- A history of febrile seizures
- Taking antibiotics,
- Immunodeficiency (exceptions are certain live vaccines; in general, however, immunocompromised people are particularly dependent on vaccination protection) and
- chronic diseases (on the contrary, vaccination is particularly important for chronically ill patients).
Mode of action on the spread of infectious diseases
| illness | Transmission path | R 0 | Minimum proportion of immunized |
|---|---|---|---|
| measles | Droplet infection | 12-18 | 83-94% |
| mumps | Droplet infection | 4-7 | 75-86% |
| polio | fecal-oral infection | 5-7 | 80-86% |
| rubella | Droplet infection | 5-7 | 80-85% |
| smallpox | Droplet infection | 6-7 | 83-85% |
|
The base reproduction number R 0 indicates how many other people an infected person will infect if the population or subpopulation surrounding them is neither vaccinated nor otherwise protected from infections. |
|||
Vaccinations also work on the spread of infectious diseases in a population. The specialty of mathematical modeling in epidemiology studies the epidemiological behavior of infectious diseases and can calculate the effects of vaccination programs. Under certain conditions, high vaccination rates in a population can, in addition to the immunity of the vaccinated, also lead to herd immunity (collective immunity) , which also serves to protect the unvaccinated against disease, because the high proportion of immunized persons restricts the circulation of the pathogen within the population. The herd effect reduces the risk of exposure of unvaccinated persons such as infants , the elderly or immunodeficient patients to pathogens to which they are not immune themselves.
If the local outbreak of an infectious disease tries to build up herd immunity by means of a rapid vaccination campaign, this is also known as a bar vaccination .
According to the Robert Koch Institute , vaccinations are among the “most important and effective preventive measures available in medicine”. Since the middle of the 20th century, extensive vaccination programs have led to a massive reduction in various infectious diseases or even to their regional or - as in the case of smallpox - global eradication. Thus, the United States health authority, the Centers for Disease Control and Prevention (CDC), ranks vaccination among the ten outstanding achievements in medicine and public health . The vaccination is thus the most important part of the disposition prophylaxis within the general infection protection .
In the twentieth century, until the eradication of smallpox in 1978, there were an estimated 375 million deaths worldwide; further infectious diseases, which have now hardly occurred due to vaccination, caused 39 million diseases in the USA in the twentieth century. It is estimated that 1.5 million children (three per minute) continue to die each year from vaccine-preventable infections.
Vaccination programs
smallpox
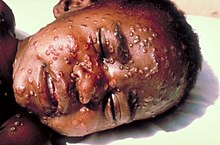
The pox , also known as smallpox, are a dangerous infectious disease. The smallpox virus can be transmitted directly from person to person through droplet infection when coughing. Symptoms are a high fever, chills and the typical blisters on almost all parts of the body that lead to pockmarks. In more severe cases, blindness , deafness , paralysis or brain damage can occur, and death in 30% of cases.
A consistent vaccination and control program by the World Health Organization (WHO) and other health organizations enabled the WHO to declare the world to be free of smallpox in 1980. The world's penultimate case was documented in Merka (Somalia) in 1977 in an unvaccinated cook. By vaccinating over 50,000 people in the area, it was ensured that no more epidemic could develop from there. The last death of the British photographer Janet Parker occurred in 1978 .
The smallpox vaccination itself is not entirely uncomplicated vaccination with a live vaccine and is no longer carried out today because there is no longer an immediate threat. However, other smallpox infections, e.g. B. laboratory accidents or bio-terrorism , not completely excluded.
poliomyelitis
The polio or poliomyelitis is transmitted by the poliovirus infectious disease. While most diseases have an uncomplicated and almost symptom-free course, 10–20% are more severe with severe headaches, neck stiffness, gastrointestinal symptoms and muscle pain. In 0.1% of all infections, the nerve cells of the spinal cord and / or the brain are still attacked directly by the virus: this is the paralytic form of poliomyelitis, in which permanent paralysis occurs. In the last major epidemics in Germany in 1952/53, 15,000 paralytic cases were known. This paralysis leads to death in 1–4% of cases. In addition to these acute consequences, up to 60% of people who previously had to be treated in hospital for acute poliomyelitis develop post-poliomyelitis symptoms years later , for example in the form of severe signs of fatigue, muscle cramps and pain.
In 1961 Austria carried out a large-scale, nationwide mass vaccination campaign on a legal basis, which had not yet existed in the West. In 1962 (in the GDR as early as 1960) the oral poliomyelitis vaccination was introduced in Germany as well as in other European countries. As early as 1965, only four years after the start of the first vaccination campaign, the number of diseases recorded in Germany had reduced to less than 50 new cases. Compared to the 4,670 new cases reported in 1961, that was a decrease of 99%. The last two domestic diseases caused by wild polio viruses occurred in Germany in 1986 and 1990; the last imported cases were recorded in 1992.
The " oral vaccination " against polio was the cause of a rare but serious vaccination complication. The attenuated vaccine viruses capable of replication were able to reverse mutate into the wild-type virus and cause vaccine-associated poliomyelitis (VAPP) with a frequency of 1: 890,000 at the first vaccination . Since the poliovirus has largely disappeared from Europe, this risk was no longer considered acceptable. That is why a dead vaccine (IPV) has been injected against polio since 1998 without this risk of side effects.
In 1980, after the official smallpox eradication, the WHO set the global eradication of poliomyelitis as a goal. Three of the six WHO regions are now designated as "polio-free" (America 1994, Western Pacific 2000, Europe 2002). Thanks to extensive vaccination programs, polio has also been largely suppressed in Africa and Asia. Only a few countries are now considered endemic to poliovirus (Nigeria, Pakistan, Afghanistan). However, as vaccination efforts decline in neighboring countries, there are repeated outbreaks of poliomyelitis through re-imports, most recently in 2006 in Namibia. Poliomyelitis has been eradicated in India since January 2014.
Since poliomyelitis has not yet been eradicated, the danger has not yet been averted for Europe either. For example, a regional polio epidemic occurred in part of the Netherlands in 1992/93 - in the middle of otherwise polio-free Europe - which resulted in dozens of lifelong paralyzes and a few deaths within a few weeks, despite the relatively small population Result. Part of the population there refuses to be vaccinated for religious reasons. Apart from that, this part of the Dutch population is just as well educated, health, food and housing etc. as the rest of the country. Analysis of the virus revealed that the particular strain of virus responsible for this outbreak likely had not been imported from outside but had survived within the unvaccinated population. If polio vaccination rates drop, polio could quickly return to Europe.
measles

In addition to poliomyelitis, the WHO has also set the global elimination of measles as a goal. This has currently been achieved on the continents of America and Australia as well as in Scandinavia. Measles vaccination has been recommended in Germany since 1973, and nowadays about 90% vaccination coverage is achieved among school beginners. The nationwide incidence of measles in 2004 was 0.15 per 100,000 inhabitants across Germany (121 reported measles cases in total). For the first time in all federal states, it was below the threshold of 1 per 100,000 inhabitants. Nevertheless, local measles outbreaks occur again and again, which particularly affect unvaccinated children, for example measles epidemics with severe complications and deaths in Hesse, Bavaria, Baden-Württemberg and North Rhine-Westphalia in the years 2005 to 2008. In 2005, Robert Koch -Institute 780 measles cases reported (0.95 cases per 100,000 inhabitants), in 2006 there were already 2242 cases by September (2.72 cases per 100,000 inhabitants). However, thanks to the high vaccination coverage, these outbreaks usually remain regionally limited.
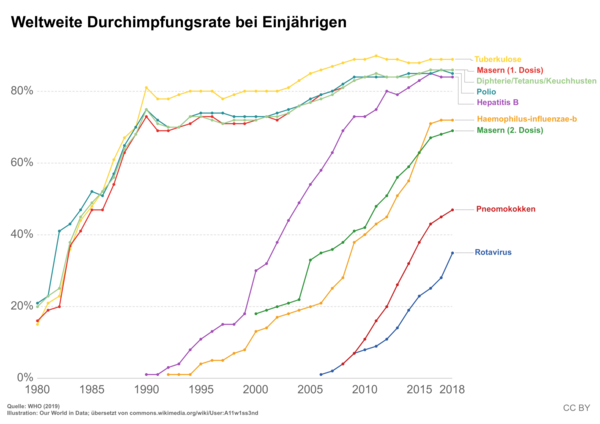
Switzerland has been affected by three measles epidemics since 1999: in 2003, from the end of 2006 to summer 2009 and in 2011. The outbreak from 2006–2011 was significant, with a total of 4,371 cases reported. Poor vaccination coverage is blamed for the large outbreak. Worldwide, the diseases reported to the WHO fell from around four million cases (vaccination coverage 13%) in 1980 to around 500,000 cases (vaccination coverage 80%) in 2003 and 139,300 cases (vaccination coverage 85%) in 2010.
Other ongoing vaccination programs
In the case of other infectious diseases such as diphtheria , tetanus , whooping cough and others, global eradication is not achievable, but the successes of vaccination achieved in German-speaking and many other countries are very impressive. For example, no case of diphtheria has been reported in Germany since 2000. Currently, 97% of children with a vaccination certificate are sufficiently vaccinated against diphtheria. In adults, only about 30% have antibodies in protective amounts because they often lack booster vaccinations, which should be carried out every 10 years. Unprotected adolescents and adults are therefore currently beneficiaries of the high population immunity among children. However , they are at risk when they come into contact with an imported case of illness, when they come into contact with infected people from epidemic or endemic areas or when traveling to endemic areas.
When fighting whooping cough (pertussis), different strategies were pursued in the old Federal Republic and in the GDR. From 1974 to 1991 vaccination in Germany was only recommended for children with a high individual risk of developing the disease because of fear of individual vaccine side effects. This led to a very low vaccination rate (less than ten percent) and a correspondingly high disease rate in infants and children. In the GDR, the pertussis vaccination had been established since 1964, vaccination rates of around 90% were achieved. Whooping cough was largely eliminated in young children and school children, while the babies who were not yet protected benefited from the vaccination of their older siblings. Immediately after reunification, there was a significant decrease in vaccination rates in the new federal states and a subsequent increase in morbidity , which can be demonstrated in the new federal states due to the continued recording through reporting of pertussis: an increase of 0.2 diseases followed per 100,000 inhabitants in 1991 to 20.4 illnesses per 100,000 inhabitants in 2000. Neither the vaccination nor the past illness with whooping cough guarantee lifelong protection against a new illness. Whooping cough is only dangerous for small children, it is stressful and tedious for everyone else. In addition, the treatment also leads to the massive use of antibiotics in the affected families with the risk of an increased development of resistance in the population. This would be avoidable if the vaccine immunity was increased (this is currently 87% in children). Attempting to largely eliminate this disease would require regular vaccinations in both adolescents and adults.
In the meantime, the pneumococcal vaccination has proven its effectiveness on an epidemiological level. It drastically reduced the number of hospitalizations due to pneumonia among those under two .
New vaccinations and developments
Vaccines against most types of enteroviruses , against hepatitis C , tuberculosis , syphilis , gonorrhea , HIV , Ebola and diseases caused by parasites such as plasmodia , such as malaria in particular, are not yet available. However, several new vaccines are being developed, approved or already launched on the market:
In Europe and the USA, two vaccines against human rotavirus , the pathogen that causes severe diarrhea in babies and young children, have been approved since 2006 . The Food and Drug Administration was in the spring / summer of 2006 in the United States a vaccine against certain papillomavirus to, in addition to genital warts and cervical cancer cause. This vaccine, which has meanwhile been approved in Germany and is already available in most European countries, is therefore, in addition to the one against the hepatitis B virus , another one that is also used to prevent certain types of cancer. In 2010, a therapeutic vaccine for the treatment of prostate cancer was approved in the United States .
Even old vaccines are constantly being further developed in the course of a vaccine design in order to improve the purity of the products, to increase the effectiveness and the response rates. Today, many vaccines are no longer produced by chemically inactivating a pathogen, but genetic engineering can be used to produce specific immunogenic parts of a pathogen. The targeted selection of antigens means that a patient's immune system is exposed to fewer antigens overall despite the larger number of available vaccines. New forms of application are also being developed, for example the nasal application of a new influenza vaccine should better imitate the natural route of infection. Immunization can be carried out ex vivo through adoptive cell transfer .
But new vaccines are also promising, for example against Helicobacter pylori , the main pathogen causing gastric and duodenal ulcers , and against the herpes simplex virus , which causes herpes simplex . Research is being carried out on various vaccines against the tropical disease malaria .
The development of vaccines against HIV , the Epstein-Barr virus (trigger of Pfeiffer's glandular fever ), many types of cancer , other diarrheal diseases and many other infectious diseases are still in an early clinical development phase.
Vaccination fatigue and countermeasures
Due to the so-called "vaccination fatigue", i. H. the neglect or conscious rejection of the recommended vaccinations, some infectious diseases occur again more frequently. If the vaccination coverage falls below the critical threshold for herd immunity , vaccination fatigue is also a life-threatening behavior for risk groups such as infants , the elderly and immunodeficient patients .
In addition to the vaccination criticism presented below, one of the causes of vaccination fatigue lies in the great effectiveness of the vaccinations themselves and the resounding success of the state vaccination programs: Numerous diseases that were widespread and feared a few generations ago have lost their horror, as they hardly exist today knows someone from personal experience. As a result, there are now large vaccination gaps in children, especially with the mumps, measles, rubella and hepatitis B vaccinations. Vaccination appointments are more likely to be missed, and adults in particular miss booster vaccinations for tetanus and diphtheria. That is why there are always local epidemics of infectious diseases, for example in North Rhine-Westphalia in 2006 there was a measles epidemic with over 2,000 people. With growing concern, epidemiologists observe a drop in vaccination rates in Germany and other industrialized nations. For example, measles in 2009 in some cases already affected less than 80 percent of the population, in Germany even only 70 percent. This is well below the vaccination coverage recommended by the WHO for highly contagious measles for at least 95 percent of the population, which would be necessary to effectively prevent outbreaks in the population.
It is therefore the declared aim of the health authorities to carry out basic immunizations and booster vaccinations across the board in order to maintain the population's herd immunity. As measures against vaccination fatigue, there are public vaccination recommendations from the health authorities ( vaccination calendar ) and the European vaccination week of the World Health Organization (WHO).
According to the planned epidemiology law, which is to be passed by the German Bundestag on June 1, 2017, parents who do not take part in the mandatory vaccination counseling for their children should in future be reported to the health department by daycare centers.
Recommended vaccinations
The vaccination calendar of the permanent vaccination commission, which gives an overview of the vaccinations recommended in Germany, provides for repeated vaccinations from infancy: children should be vaccinated against various pathogens from the age of 3 months (9 weeks) up to the age of 18. The attending physicians are obliged to explain the advantages and possible disadvantages of the vaccination. A vaccination - like other medical treatment measures - constitutes bodily harm from a legal point of view. The bodily harm is only not unlawful if the consent of the person being treated or the parents is available or a guardianship court replaces the consent of the parents. Informing the patient or the parents is the basis of this consent. The parents' responsibility relates primarily to protecting their child from serious illnesses, but also to society. Epidemic outbreaks of infectious diseases can only be prevented effectively if the highest possible percentage of the population is vaccinated. To achieve this goal, vaccination coverage rates of around 90% are required , depending on the disease and the effectiveness of the vaccine . For more information on calculating these vaccination coverage rates, see Epidemiology: Reproduction Rate , Mathematical Modeling of Epidemiology . There is no compulsory vaccination in Germany.
In Germany, the recommendations of the Standing Vaccination Commission are the basis for determining the “publicly recommended vaccinations”. The latter are definitely determined by the health authorities of the federal states. If a publicly recommended vaccination results in permanent damage (then called vaccination damage - in contrast to the vaccination reaction and vaccination complication ), there is a claim to compensation from the pension office. Since 1 April 2007 the cost unit for all vaccines that are recommended according to the Commission, the health insurance company . The patient does not have to pay anything for this. In Switzerland, the recommendation is made by the Federal Office of Public Health and the Swiss Commission on Vaccination. In Austria, the vaccination plan is issued by the Supreme Sanitary Council (Vaccination Committee) of the Federal Ministry for Health and Women.
Recommendations for Germany, Switzerland and Austria
The following vaccinations are recommended by the Standing Vaccination Commission in Germany in August 2014. These recommendations largely correspond to the vaccination plan of Switzerland and the vaccination plan of Austria.
| Vaccination against | STIKO recommendation |
|---|---|
| cholera | Only in exceptional cases: at the request of a destination or transit country when traveling |
| diphtheria | Standard for children over two months, refreshment for adults every ten years |
| TBE | People exposed to ticks in TBE risk areas (residents, forest workers, etc.) |
| Cervical cancer / HPV | Girls and young women from 9 to 17 years of age. Boys between the ages of 9 and 14, or catch-up vaccinations up to the age of 17. |
| Yellow fever | When traveling to endangered areas (tropical Africa, South America), sometimes even mandatory |
| Haemophilus influenzae type b | Standard for children from two months of age, people with anatomical or functional asplenia |
| Hepatitis A | Risk groups Endangered personnel (health service, research, sewerage, etc.) |
| Hepatitis B. | Standard for children from two months of age at risk groups at risk in the health service |
| Herpes zoster (shingles) | Vaccination with an inactivated vaccine for all people over the age of 60 and for all people over the age of 50 who have certain risk factors. |
| Influenza | People aged 60 and over People with a weakened immune system People with chronic illnesses |
| Japanese encephalitis | When traveling to endemic areas |
| measles | Standard for children from eleven months |
| Meningococcal type C | Standard for children from twelve months of age People with a weakened immune system Personnel at risk (laboratory, development cooperation , etc.) Young people with long-term stay in endangered countries |
| Meningococci type A, C, W135, Y | Vulnerable personnel (laboratory, development cooperation , etc.) Young people with long-term stays in endangered countries Travel to endemic areas |
| mumps | Standard for children from eleven months |
| Pertussis (whooping cough) | Standard for children from two months, refreshment with five to six years, and between 9 and 17 years of age. All adults, especially with possible contact with newborns and infants (couples wishing to have children, parents-to-be and grandparents, etc.) |
| Pneumococci | Standard for children from two months to two years, people over 60 years of age, people with a weakened immune system |
| poliomyelitis | Standard for children from two months / u. & Refresher |
| rubella | Standard for children from eleven months |
| Rotavirus | Oral vaccination from the sixth week of life, depending on the vaccine, two or three times with an interval of four weeks |
| tetanus | Standard for children from two months, refreshment for adults every ten years and if necessary in the event of injury |
| rabies | Persons dealing with animals in areas with wild animal rabies (veterinarians, hunters, forest staff, etc.) |
| tuberculosis | Not currently recommended |
| typhus | When traveling to endemic areas |
| chickenpox | Standard for children from eleven months of age Seronegative persons when indicated (organ transplantation, women wanting to have children, etc.) Seronegative personnel in health care |
pregnancy and breast feeding period
According to current recommendations from the Robert Koch Institute responsible for this in Germany, vaccinations with live vaccines from three months before and during the entire pregnancy are contraindicated . On the other hand, due vaccinations with dead vaccines can be given to expectant mothers in the second and third trimester of pregnancy without hesitation; In the first third , on the other hand, to rule out any risk to the child, only those dead substance vaccinations that are urgently indicated on an individual basis should be carried out . During the subsequent breastfeeding period , vaccinations are generally possible without restrictions.
Combination vaccinations
Combination vaccines are vaccines that combine different components against various infectious diseases and can thus guarantee protection against these diseases with one vaccination. Combination vaccinations with these vaccines are recommended because they simplify handling, reduce the number of injections and vaccination appointments, and thus lower costs and improve the vaccination rate for the population. The lower number of injections is more comfortable for the patient, especially with children. Further information on the individual multiple vaccines can be found in the respective articles for these vaccines.
The MMR vaccine is a combined live vaccination against measles, mumps and rubella. An MMRV vaccine has also been approved in Germany since 2006 , with an additional component against varicella (chickenpox). In September 2011, the Standing Vaccination Commission at the Robert Koch Institute recommended that for the first vaccination against measles, mumps, rubella and varicella, the combined administration of the combined measles-mumps-rubella vaccination on the one hand and a varicella vaccination on the other should be preferred and the second vaccination can then be given with the MMRV combination vaccine.
In October 2000, were first hexavalent vaccines approved in the European Union, which are designed to protect against six infectious diseases: polio , diphtheria , tetanus , pertussis , Haemophilus influenzae type B infections and hepatitis B . An alternative for infants is the five-fold vaccination with separate immunization against hepatitis B. However, this means an additional injection in each case. Infection with hepatitis B is very rare in infants, but the consequences are severe, so that the six-fold vaccination is recommended in Germany and is now established.
As further combinations one knows u. a. DTP vaccines against diphtheria, tetanus and pertussis (whooping cough), DTP-IPV vaccines against diphtheria, tetanus, pertussis (whooping cough) and poliomyelitis (polio), as well as a combination vaccine against hepatitis A and B.
Compulsory vaccination
If a vaccination is required by law, this is called mandatory vaccination. In the Federal Republic of Germany it last existed in the 1980s against smallpox for individual groups of people, in the GDR there was a comprehensive compulsory vaccination for the entire population.
In the majority of EU countries, vaccination is currently compulsory for humans. In Germany, it has been possible since 2001 to apply the compulsory vaccination at any time by means of a simple ordinance and thus to protect the population. This is regulated in the Infection Protection Act (IfSG) with the following wording: "Section 20 (6) The Federal Ministry of Health is authorized to order by ordinance with the consent of the Federal Council that threatened parts of the population have to take part in protective vaccinations or other measures of specific prophylaxis, if a communicable disease with clinically severe forms occurs and its epidemic spread is to be expected. The basic right to physical integrity (Article 2, Paragraph 2, Clause 1 of the Basic Law) can be restricted in this respect. [...] ". In Switzerland, Article 23 paragraph 2 of the Epidemics Act provides a similar option for cantons to introduce mandatory vaccinations .
There are similar regulations for animal vaccinations across Europe; for example, dogs, cats and ferrets are required to be vaccinated against rabies in cross-border traffic . In Germany, the obligation to vaccinate is regulated in the Animal Disease Act (TierSG), on the basis of which there is currently compulsory vaccination for cattle, sheep and goats against bluetongue .
Vaccination opponents
People who generally reject vaccination are called vaccination opponents . Opponents of vaccination are either religiously motivated or are afraid of alleged or possible vaccine damage (see also pharmacovigilance ). Some opponents of vaccinations suspect a conspiracy behind vaccinations , others even deny the existence or pathogenicity of viruses . The "arguments", some dogmatically put forward by opponents of vaccination, have all been scientifically refuted, the phenomenon is considered a widespread conspiracy theory and a form of science denial . The World Health Organization counts anti-vaccinations among the ten greatest threats to human health in the world.
history
Up until the 19th century, doctors in Europe were usually powerless against the widespread and recurring major diseases and epidemics . One of these widespread infectious diseases was smallpox , from which around 30% of sufferers died. Survivors were often disfigured by scars. It was recognized early on, however, that surviving smallpox once made people immune to further infection by smallpox . Thus, smallpox was the first disease in which it was attempted to immunize individuals through deliberate infection . It is believed that attempts with this technique (the variolation ) either in India or China as early as 200 BC. Began. Chinese doctors selected people with mild disease to obtain the vaccine and removed pieces of smallpox crust from those infected. The pieces were ground to a powder and inserted into the nose of the person to be vaccinated. Lady Mary Wortley Montagu reported in 1718 that the Turks similarly exposed themselves to the body fluids of those easily infected, and used this method on their own children.
The Boston smallpox epidemic in 1721 marked the first viral epidemic in North America that used vaccination to contain the disease. At the time, it was not yet known that the administration of weakened smallpox viruses would trigger an immune response , but usually no disease. In the course of the epidemic there was a heated controversy about variolation , the focus of which was the puritan preacher Cotton Mather and his opponent the doctor William Douglass .
Surviving cowpox (a cattle disease that progresses easily in humans, also known as milker's knot ) made people immune to further infections from smallpox. After six people had immunized people with cowpox lymph (including Sevel, Jensen, Benjamin Jesty 1774, Rendall, Peter Plett 1791), the English doctor Edward Jenner (1749-1823) also experimented with this knowledge and infected a boy in 1796 the cowpox. The boy was later found immune to common smallpox. Since the vaccine came from cows, Jenner called his vaccine Vaccine (from Latin vacca "cow") and the technique of artificial immunization "Vaccination" (from Latin vaccinus "from cows"). A pioneer of protective vaccination in Germany was the Hanoverian doctor and court medic Georg Friedrich Ballhorn (1770–1805), who translated Jenner's publication from 1798 into German as early as 1799, did research on it himself from spring 1799 and made the first attempts at counter vaccination from January 1800. The doctor Jean de Carro was the first to carry out the vaccination on the European continent in 1799. This first modern form of vaccination against human smallpox was quickly adopted in Europe. The German doctor Theodor Christian Eulner , who worked as a doctor in Greenland , tried to end the smallpox epidemic in Greenland with vaccinations in 1800. Vaccination became compulsory from around 1810. However, the cause of the infectious diseases was still unknown.
This changed towards the end of the 19th century. Louis Pasteur formulated the germ theory in 1864, Robert Koch provided evidence of anthrax pathogens ( Bacillus anthracis ) in 1876 and the evidence of tuberculosis bacteria ( Mycobacterium tuberculosis ) in 1881 . This discovery is considered to be the definitive proof of the existence of bacterial pathogens. Students of Koch and Pasteur expanded the concept further. Pasteur developed vaccines against anthrax together with Émile Roux in 1881 and against rabies in 1885 . Paul Ehrlich , Emil von Behring and Shibasaburo Kitasato used the knowledge in 1890 for passive immunization against diphtheria and tetanus .
With the standardization of vaccines, the first national vaccination programs began at the end of the 19th century. It started with vaccinations against smallpox , which u. a. in England (1867) and in the German Empire (1874) were introduced by law as mandatory vaccinations. While the compulsory vaccination in England was relaxed again in 1898 and 1907, it was adhered to in the German Empire. However, contamination in the early vaccines also led to accidents such as the Lübeck vaccination accident in 1930, in which 77 children died as a result of a contaminated vaccine, which sparked a discussion about state vaccination programs in the German public and among health experts. The Calmette process following this case marks the beginning of modern medical law in Germany .
Since the middle of the 20th century, numerous other vaccines against infectious diseases have been systematically developed, for example by Jonas Salk and Albert Sabin against polio and a vaccine against yellow fever by Max Theiler . During the Cold War, there was in part a “race for better immunization” between the two blocs. Above all, the GDR tried in propaganda campaigns to point out the superiority of the East German health care system and to blame the West for “picking the vaccine”. Global vaccination programs have been launched since 1967 under the auspices of the World Health Organization ( WHO ). Some examples are given in the following sections.
Chronology of the development and introduction of vaccines
| Infectious disease | trigger | vaccine | introduction | Remarks |
|---|---|---|---|---|
| smallpox | Variola virus | Smallpox vaccine | 1796 | first large-scale experiment as early as 1714 in Constantinople (via variolation ). Used in international vaccination campaigns from 1798. |
| rabies | Rabies virus | Rabies vaccine | 1885 | |
| typhus | Salmonella Typhi bacteria | Typhoid vaccine | 1896 | |
| cholera | Vibrio cholerae bacteria | Cholera vaccine | 1896 | |
| pest | Yersinia pestis | Plague vaccine | 1897 | |
| diphtheria | Corynebacterium diphtheriae bacteria | Diphtheria vaccine | 1923 | passive immunization from 1890 with the diphtheria antitoxin |
| whooping cough | Bordetella pertussis bacteria | Pertussis vaccine | 1926 | |
| tuberculosis | Mycobacteria species | Tuberculosis vaccine | 1927 | Successful vaccination with BCG uncertain, no longer recommended since 1998 |
| tetanus | Clostridium tetani bacteria | Tetanus vaccine | 1927 | |
| flu | Influenza virus | Influenza vaccine | 1936 | |
| Yellow fever | Yellow fever virus | Yellow fever vaccine | 1937 | |
| Typhus | Rickettsiae | Typhus vaccine | 1938 | |
| poliomyelitis | Poliovirus | Polio vaccine | 1955/1960 | Broad application in GDR from 1960, FRG from 1962, IPV since 1998 |
| mumps | Mumps virus | Mumps vaccine | 1967 | |
| measles | Measles virus | Measles vaccine | 1968 | |
| rubella | Rubella virus | Rubella vaccine | 1969 | |
| TBE | TBE virus | TBE vaccine | 1973 | |
| chickenpox | Varicella zoster virus | Varicella vaccine | 1974 | General vaccination for children in Germany recommended since 2004 |
| lung infection | Pneumococcal bacteria | Pneumococcal vaccine | 1977/2000 | General vaccination for children in Germany recommended since 2006 |
| Hepatitis B. | Hepatitis B virus | Hepatitis B vaccine | 1981 | General vaccination for children in Germany recommended since 1995 |
| meningitis | Meningococcal bacteria | Meningococcal vaccine | 1982/1999 | z. Currently only effective against meningococcal type C, not against the most common pathogen type B; Recommended in Germany since 2006 |
| Haemophilus influenzae type b | Haemophilus influenzae bacteria | Haemophilus vaccine | 1985 | General child vaccination recommended in Germany since 1990 |
| Hepatitis A | Hepatitis A virus | Hepatitis A vaccine | 1992 | |
| Severe diarrhea | Rotavirus | Rotavirus vaccine | 1998/2005 | for children under 6 months |
| cervical cancer | Human papillomavirus | HPV vaccine | 2006 | also cancer precursors and condylomata acuminata in the anogenital area ("genital warts") |
| Japanese encephalitis | Japanese encephalitis | Japanese encephalitis vaccine | 2009 (in Europe) | Previously another vaccine was available in Asia, this was introduced in the USA in 1992 |
| Meningococcal type B | Neisseria meningitidis type B bacteria | Meningococcal vaccine | 2013 (in Europe) | approved by EMA , available in Germany since December 2013 |
Vaccinations for animals
According to prevailing opinion, many pets can and should be vaccinated. The recommended vaccinations are administered by the veterinarian and documented in a vaccination certificate , just like for humans . In Germany, the Standing Vet. Vaccination Commission (StIKo Vet.) In the Federal Association of Practicing Veterinarians prepares vaccination recommendations for the German small animal practice . In contrast to vaccinations for humans, most vaccinations for animals are administered under the skin ( subcutaneously ) - mostly in the area of the flanks or the neck area. In 2011, a turnover of 191 million euros was achieved with animal vaccines in Germany, about half each with farm animals and pets.
Vaccination against rabies and proof in the new EU pet passport are required by law in practically all European countries for dogs, cats and ferrets to enter the country. It must be repeated (one to three years) according to the vaccine manufacturer's instructions and the last must be at least 30 days in the past for most states. After the German Rabies Vaccination Ordinance was changed, an annual refresher is no longer required since 2006 if the manufacturer specifies a longer period.
Even farm animals such as pigs, cattle or sheep can be vaccinated against various diseases. According to the manufacturer of the vaccines, this leads to improved fattening performance and higher milk yield and at the same time reduces the use of antibiotics .
For certain animal diseases , vaccination can be ordered by the state according to the Animal Disease Act . The costs for these vaccinations are reimbursed by the animal disease insurance funds , for some vaccinations there is a partial reimbursement of the costs with regard to the rehabilitation of the stocks.
However, other prophylactic vaccinations are prohibited for farm animals. Instead, the killing of sick animals is planned. There are only stocks of vaccines for emergencies.
The prophylactic vaccination against foot and mouth disease (FMD), which was already carried out in Germany, was banned in Europe by the EU in 1991. Instead, if foot-and-mouth disease occurs, all cloven-hoofed animals in the affected herd must be killed under strict safety measures and disposed of harmlessly. However, a vaccine bank has been set up on the island of Riems for emergencies . The prohibition of the FMD vaccination was particularly politically motivated (exportability of the meat, etc.) and has been very controversial since over four million animals were culled in the UK in 2001 .
dogs
Domestic dogs are usually vaccinated against canine distemper , hepatitis contagiosa canis , leptospirosis (Stuttgart dog disease), parvovirus (cat disease of dogs ), parainfluenza and rabies . More recent studies show that vaccination protection (with the exception of leptospirosis) can last for three years or more, and many manufacturers now list their vaccines with a duration of action of three years. The vaccination against Lyme borreliosis and the bacterial component of the kennel cough ( Bordetella bronchiseptica ) is less common . Furthermore, vaccinations against the death of puppies only play a role in breeding .
The vaccination against rabies and its documentation in the EU pet passport is only mandatory when traveling. If the animal bites a person, however, the attending physician may suspect that the animal is rabid. This suspicion necessarily leads to the killing of the animal and can only be refuted if the dog or cat has been vaccinated against rabies within the last three years. Otherwise, this vaccination is no longer considered necessary within Germany. If a valid vaccination cannot be proven with an EU pet passport during controls, entry to the destination country will be refused. The same can happen when entering Germany, Austria or Switzerland when returning. An annual booster vaccination for rabies is no longer required (according to Regulation (EU) No. 576/2013 for travel within the EU, except for the special provisions of the above-mentioned countries), the vaccine manufacturer's repeat vaccination dates apply.
Cats
In Germany, the STIKO Vet. Issues recommendations for the vaccination of cats.
The occurrence of vaccine-associated tumors ( fibrosarcomas ) has been documented for cats (especially with the FeLV vaccination) and to a limited extent also for dogs . This may be due to the effect enhancers ( adjuvants ) added to the vaccines , to which the organism may react by forming tumors. Other studies tend to suggest an association with hypodermic injury from a hypodermic needle , as the tumor also appeared in non-vaccine puncture sites that did not involve adjuvants. Although the incidence of this side effect is comparatively low at around 1: 10,000 in relation to the protection against diseases achieved, vaccinations in cats are preferably recommended in the area of the flank in order to leave room for surgical intervention if this locally aggressive tumor occurs.
| Infectious disease | trigger | Remarks |
|---|---|---|
| Cat flu | Different pathogens | Recommended. |
| Panleukopenia (cat disease) | Parvoviridae | Recommended. |
| rabies | Rabies virus | Outdoor cats should also be vaccinated against rabies. The rabies vaccination is recommended despite the current freedom from terrestrial rabies in German-speaking countries, because the animals are better off when they come into contact with an animal suffering from rabies. |
| Feline leukemia | Feline Leukosis Virus (FeLV) | A vaccination against the Feline Leukosis Virus (FeLV) is only recommended for cats up to eight years old. |
| Feline Infectious Peritonitis (FIP) | Feline Enteral Coronavirus (FeCV) | The vaccination against Feline Infectious Peritonitis (FIP) is only appropriate if the FeCV status is negative and does not offer reliable protection. |
Ferrets
Ferrets are usually vaccinated using combination vaccines from dogs in the absence of approved vaccines for this species. Legally, this procedure is problematic, since dog vaccines may not be used for ferrets if the pharmaceutical law is strictly interpreted . In Germany there are approved vaccines against distemper and rabies for ferrets .
Rabbits
Domestic rabbits can be vaccinated against myxomatosis and RHD ( Chinese disease ). A vaccination is also possible to protect against the contagious rabbit cold (rhinitis contagiosa) , but this is only useful in larger herds. After the primary vaccination, an annual repetition, and for most myxomatosis vaccines even a six-monthly repetition is necessary.
Horses
The StIKo Vet. Recommends vaccinations against tetanus , equine influenza , EHV-1 and EHV-4 (rhinopneumonitis, virus abortion) for horses . The equine influenza vaccination is compulsory when participating in tournaments by the FN . As noncore vaccinations, ie those that are recommended only under certain conditions that apply against strangles , rabies , equine arteritis , Equine rotavirus infections , West Nile virus infection and dermatophytosis .
Cattle and small ruminants
There are several ordinances in Germany for cattle and small ruminants (sheep, etc.) and the recommendations of the STIKO Vet. A distinction is made between milk production, suckler cow husbandry and cattle fattening when it comes to vaccination recommendations for cattle.
| Infectious disease | trigger | Remarks |
|---|---|---|
| Foot and mouth disease | Foot-and-mouth disease virus | According to Section 2 of the FMD Ordinance , vaccinations are prohibited, subject to Section 2 and Section 16. |
| Bovine Herpes Virus 1 Infection | Bovine herpes virus 1 | Vaccination, which can be ordered by the state and reimbursed by the animal disease fund. |
| Intoxicating fire | Clostridium chauvoei | Cattle and sheep are affected. In principle, vaccination is initially not allowed. In Germany, it can be ordered by the state in accordance with Section 9 of the Ordinance on Protection against Anthrax and Intoxication and is reimbursed by the Animal Disease Fund. |
| Bovine viral diarrhea | Bovine Viral Diarrhea Virus | A partial reimbursement of the costs is available for vaccinations against bovine virus diarrhea / mucosal disease in cattle (BVD / MD).
The BVD vaccine from Pfizer , which has now been withdrawn from the market in the EU, is the likely cause of bovine neonatal pancytopenia . |
Pigs
There are several ordinances for pigs in Germany and the recommendations of STIKO Vet.
| Infectious disease | trigger | Remarks |
|---|---|---|
| Aujeszky's disease | Aujeszky virus | According to Section 3 of the Ordinance on Protection against Aujeszky's Disease , vaccinations are prohibited. The vaccination can be ordered by the state and reimbursed by the animal disease fund. |
poultry
In Germany, the vaccination of chicks and pullets in chicken rearing farms against Salmonella Enteritidis is legally prescribed ( § 13 GflSalmoV). Since the revision of the Chicken Salmonella Ordinance in 2009, this regulation has been in force for businesses with more than 350 animals. Until then, a vaccination of 250 animals or more and also against Salmonella typhimurium was mandatory (Section 2 Chicken Salmonella Ordinance old version). Newcastle disease vaccination is compulsory for flocks of chickens and turkeys . Other useful vaccinations are those against Marek's disease , infectious laryngotracheitis , infectious bronchitis , avian encephalomyelitis and birdpox .
See also
literature
- Heinz Spiess, Ulrich Heininger (Ed.): Vaccination Compendium . 6th edition. Thieme, Stuttgart / New York 2005, ISBN 3-13-498906-9 .
- Volker Klippert, Ulrike Röper, Roland J. Riedl-Seifert: Vaccination and law . Zuckschwerdt, Germering 2003, ISBN 3-88603-826-2 .
- Kenneth Murphy: Janeway's Immunobiology . 7th edition. Garland Science, London 2007, ISBN 978-0-8153-4123-9 ( 5th online edition ).
- Horst Kremling : Historical considerations on preventive medicine. In: Würzburg medical history reports. No. 24, 2005, pp. 222-260.
- Ute Quast, Sigrid Ley: Vaccinations in Dialog . 2nd Edition. Kilian, Marburg 1999, ISBN 3-932091-41-8 .
- Johannes-Peter Rupp (Ed.): Hundred years of vaccination law . Exhibition catalog. University Library, Giessen 1974 ( digitized version, 1.03 MB ).
- Reiner Thomssen: Vaccinations. Basics, advantages, risks . Beck, Munich 2001, ISBN 3-406-44775-9 .
- G. Weigand: Vaccination protects. Medical advisor for long-distance travelers . J. Fink, Ostfildern 2001, ISBN 3-7718-1075-2 .
- C. Meyer, S. Reiter: Vaccination opponents and vaccination skeptics. In: Federal Health Gazette - Health Research - Health Protection . No. 47, 2004, pp. 1182-1188. doi: 10.1007 / s00103-004-0953-x ( full text as PDF file; 226 kB (PDF)).
- Burkhard Schneeweiß, Michael Pfleiderer, Brigitte Keller-Stanislawski: Vaccination safety today . In: Deutsches Ärzteblatt (Dtsch Arztebl Int) . tape 105 , no. 34–35 , 2008, pp. 590-595 ( online ).
- Axel Helmstädter: On the history of active immunization. In: Pharmacy in our time . Volume 37, No. 1, 2008, ISSN 0048-3664 , pp. 12-18.
- Iris Ritzmann : Vaccination. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. de Gruyter, Berlin 2005, ISBN 3-11-015714-4 , pp. 660–664.
- Malte Thießen: From the immunized national body to the preventive self. Vaccination as biopolitics and social practice from the German Empire to the Federal Republic. In: Vierteljahrshefte für Zeitgeschichte . Volume 61, No. 1, 2013, ISSN 0042-5702 , pp. 34-65.
- Malte Thießen: Provision as a social order: vaccination in the Federal Republic and the GDR. In: Contemporary historical research . Volume 10, Issue 3, 2013, ISSN 1612-6033 , pp. 409-432. ( online version )
- Ilse Zündorf, Angelika Vollmar, Theodor Dingermann: Immunological basics of vaccination. In: Pharmacy in our time. Volume 37, No. 1, 2008, ISSN 0048-3664 , pp. 20-27.
- M. Wiese-Posselt et al .: Vaccination recommendations for Germany . In: Deutsches Ärzteblatt (Dtsch Arztebl Int) . tape 105 , no. 34–35 , 2011, pp. 771-780 ( review ).
Broadcasts
- Mirko Smiljanic : Religious dispute about small pricks - Discussion about the advantages and disadvantages of vaccinations. In: dradio background. August 28, 2013.
- Volkart Wildermuth : Vaccinations with "added value" - some protect against several diseases at the same time. In: SWR2 knowledge. February 25, 2015.
Web links
- Vaccinations , Vaccinations - 20 Objections and Answers. of the Robert Koch Institute
- Vaccinations on kindergesundheit-info.de, the information offered by the Federal Center for Health Education (BZgA)
- Vaccination, infectious diseases, immunization, vaccines, vaccination reactions, vaccination calendar - information from the Federal Center for Health Education (BZgA)
- 'Pink Book' - standard work on vaccinations of the Centers for Disease Control and Prevention (CDC) of the USA
- Information on vaccinations for children from the professional association of paediatricians
- Recommendations for travel vaccinations from the German Society for Tropical Medicine and International Health e. V.
- Pages on vaccinations (English) of the World Health Organization (WHO)
- Vaccinations for infants and children of the Federal Office of Public Health in Switzerland
- Samantha Vanderslott, Bernadeta Dadonaite and Max Roser: Vaccination . In: Our World in Data . September 2019 (English, ourworldindata.org [accessed January 12, 2020]).
Individual evidence
- ↑ § 2 No. 9 of the Infection Protection Act
- ↑ LA Jackson, D. Peterson, JC Nelson, SM Marcy, AL Naleway, JD Nordin, JG Donahue, SJ Hambidge, C. Balsbaugh, R. Baxter, T. Marsh, L. Madziwa, E. Weintraub: Vaccination site and risk of local reactions in children 1 through 6 years of age. In: Pediatrics. Volume 131, Number 2, February 2013, pp. 283-289, doi: 10.1542 / peds.2012-2617 , PMID 23319538 .
- ↑ AF Ochsenbein, DD Pinschewer, S. Sierro, E. Horvath, H. Hengartner, RM Zinkernagel: Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. In: Proceedings of the National Academy of Sciences . Volume 97, number 24, November 2000, pp. 13263-13268, doi: 10.1073 / pnas.230417497 , PMID 11069289 , PMC 27213 (free full text).
- ↑ Antje Hüter-Becker: Investigations in Physiotherapy , p. 157.
- ↑ Andreas Hummel: Drug theory. Vincentz Network, 2004, ISBN 3-87870-482-8 , p. 544. Restricted preview in Google Book Search
- ↑ Immune protection through breast milk . ( Spektrum.de [accessed on July 31, 2018]).
- ↑ RKI - Meaning of vaccinations - Answers from the Robert Koch Institute and the Paul Ehrlich Institute to the 20 most frequent objections to vaccination. Retrieved July 31, 2018 .
- ↑ Centers for Disease Control and Prevention (CDC): Impact of Vaccination Universally Recommended for Children - United States, 1900-1998 . In: MMWR, Morbidity and Mortality Weekly Report . tape 48 , no. 12 , April 1999, p. 243-248 , PMID 10220251 .
- ^ Centers for Disease Control and Prevention; J. Hamborsky, A. Kroger, S. Wolfe (Eds.): Epidemiology and Prevention of Vaccine-Preventable Diseases - The Pink Book. 13th edition. 2nd pressure. Public Health Foundation, Washington DC 2015, online edition , accessed July 27, 2017.
- ^ Protective vaccinations - 20 objections and answers from the Robert Koch Institute and the Paul Ehrlich Institute. Robert Koch Institute , October 23, 2013, accessed January 4, 2015 .
- ^ SW Roush, TV Murphy; Vaccine-Preventable Disease Table Working Group: Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States . In: JAMA . tape 298 , no. 18 , 2007, p. 2155-2163 , PMID 18000199 .
- ↑ More benefits than costs. In: Medical Practice. August 5, 2008, p. 6.
- ^ VA Blaho, MW Buczynski, EA Dennis, CR Brown: Cyclooxygenase-1 orchestrates germinal center formation and antibody class-switch via regulation of IL-17. In: Journal of Immunology. Volume 183, number 9, November 2009, pp. 5644-5653, doi: 10.4049 / jimmunol.0901499 , PMID 19843949 , PMC 2857380 (free full text).
- ↑ R. Prymula, CA Siegrist, R. Chlibek, H. Zemlickova, M. Vackova, J. Smetana, P. Lommel, E. Kaliskova, D. Borys, L. Schuerman: Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomized controlled trials. In: The Lancet . Volume 374, number 9698, October 2009, pp. 1339-1350, doi: 10.1016 / S0140-6736 (09) 61208-3 , PMID 19837254 . (PDF) ( Memento from November 14, 2011 in the Internet Archive )
- ↑ Common Pain Relievers May Dilute Power of Flu Shots. University of Rochester Medical Center (URMC), accessed July 27, 2011 .
- ↑ Painkillers weaken vaccination protection. Spiegel online, accessed December 4, 2009 .
- ↑ MP Bernard, RP Phipp: inhibition of cyclooxygenase-2 impairs the expression of essential plasma cell transcription factors and human B-lymphocyte differentiation . In: Immunology . tape 129 , no. 1 , 2010, p. 87-96 , PMID 20050331 .
- ↑ S. Bancos, MP Bernard, DJ Topham, RP Phipps: Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells . In: Cell Immunol . tape 258 , no. 1 , 2009, p. 18-28 , PMID 19345936 .
- ^ Over-the-Counter Pain Drugs May Affect Vaccine Strength. WXXI, accessed July 27, 2011 .
- ↑ The vaccination effect can be significantly weakened by some drugs. Pulmonologist online, accessed December 4, 2009 .
- ^ Effect of Prophylactic Paracetamol Administration at Time of Vaccination on Febrile Reactions and Antibody Responses in Children F1000 Ranking: "Exceptional" and Changes Clinical Practice ". Medscape, accessed July 26, 2011 .
- ↑ DD Taub, WB Ershler, M. Janowski, A. Artz, ML Key, J. McKelvey, D. Muller, B. Moss, L. Ferrucci, PL Duffey, DL Longo: Immunity from smallpox vaccine persists for Decades: a longitudinally study. In: The American journal of medicine. Volume 121, number 12, December 2008, pp. 1058-1064, doi: 10.1016 / j.amjmed.2008.08.019 , PMID 19028201 , PMC 2610468 (free full text).
- ↑ I. Davidkin, p Jokinen, M. Broman, P. Leinikki, H. Peltola: Persistence of measles, mumps, and rubella antibodies in at MMR-vaccinated cohort: a 20-year follow-up. In: The Journal of Infectious Diseases. Volume 197, Number 7, April 2008, pp. 950-956, doi: 10.1086 / 528993 , PMID 18419470 .
- ↑ AM Wendelboe, A. Van Rie, S. Salmaso, JA Englund: Duration of immunity against pertussis after natural infection or vaccination. In: The Pediatric infectious disease journal. Volume 24, Number 5 Suppl, May 2005, pp. S58-S61, PMID 15876927 .
- ↑ What does "2 + 1 or 3 + 1 scheme" mean? In: Vaccination with Reason. August 4, 2017, accessed March 13, 2020 .
- ^ A b C. Meyer, S. Reiter: Vaccination opponents and vaccine skeptics - history, background, theses, handling . In: Federal Health Gazette - Health Research - Health Protection, Springer Medicine Verlag . tape 47 , 2004, p. 1182–1188 , doi : 10.1007 / s00103-004-0953-x ( rki.de [PDF; accessed April 30, 2015]).
- ↑ Carlo Di Pietrantonj et al .: Vaccines for measles, mumps, rubella, and varicella in children . In: The Cochrane Database of Systematic Reviews . tape 4 , April 20, 2020, p. CD004407 , doi : 10.1002 / 14651858.CD004407.pub4 , PMID 32309885 , PMC 7169657 (free full text).
- ↑ a b Beate Schumacher: Rare emergency: vaccination can trigger an anaphylactic reaction. October 20, 2016, accessed October 12, 2019 .
- ^ Obligation to report to the Paul Ehrlich Institute .
- ↑ cf. Infection Protection Act, Section 12, Section 61 Recognition of Damage to Health
- ^ B. Keller-Stanislawski et al .: Suspected cases of vaccination complications according to the IfSG and suspected cases of side effects according to the Drugs Act from January 1, 2001 to December 31, 2003. In: Bundesgesundheitsblatt 47, 2004, pp. 1151–1164 doi: 10.1007 / s00103-004-0946-9 .
- ^ STIKO: Communication from the Standing Vaccination Commission at the Robert Koch Institute (RKI). Recommendations of the Standing Vaccination Commission at the Robert Koch Institute / Status: August 2014. (PDF) In: Epidemiologisches Bulletin . No. 34, 2014.
- ^ History and Epidemiology of Global Smallpox Eradication . ( Memento from July 15, 2007 in the Internet Archive ) (PDF; 1.5 MB) From the training course Smallpox: Disease, Prevention, and Intervention . CDC and WHO . Slide 16-17.
- ↑ Vaccination. Robert Koch Institute , accessed on May 9, 2012 .
- ^ Achievements in Public Health, 1900-1999 Impact of Vaccines Universally Recommended for Children - United States, 1990-1998. Centers for Disease Control and Prevention , accessed May 9, 2012 .
- ↑ Vaccination program with unprecedented success. Doctors newspaper , accessed on May 9, 2012 .
- ^ Ten Great Public Health Achievements --- Worldwide, 2001-2010. Centers for Disease Control and Prevention , accessed May 9, 2012 .
- ^ Ten Great Public Health Achievements - United States, 1900-1999. Centers for Disease Control and Prevention , accessed May 9, 2012 .
- ^ Gary J. Nabel: Designing Tomorrow's Vaccines New England Journal of Medicine 2013; Volume 368, Issue 6 of February 7, 2013, pp. 551-560.
- ^ Colette Flight: Smallpox: eradicating the scourge . BBC. Published on February 17, 2011. Retrieved January 4, 2015.
- ↑ Hanna Ronzheimer: polio. The forgotten epidemic. On: science.orf.at from June 15, 2020.
- ^ WHO: Poliomyelitis in Namibia . Disease Outbreak News, June 6, 2006.
- ↑ India is polio-free. In: rki.de. January 20, 2014, accessed December 27, 2014 .
- ↑ PM Oostvogel, JK van Wijngaarden, HG van der Avoort, MN Mulders, MA Conyn-van Spaendonck, HC Rümke, G. van Steenis, AM van Loon: Poliomyelitis outbreak in an unvaccinated community in The Netherlands, 1992-1993. In: The Lancet . Volume 344, Number 8923, September 1994, pp. 665-670, PMID 7915354 .
- ^ A b Robert Koch Institute: Measles in 2005 and outbreaks in Baden-Württemberg and North Rhine-Westphalia in the first half of 2006. In: Epidemiologisches Bulletin. No. 27, July 7, 2006 (PDF) (PDF)
- ↑ https://survstat.rki.de/
- ^ Measles epidemiology in Switzerland: today and recently. In: BAG Bulletin. No. 17 of April 22, 2013, pp. 276-277.
- ^ BAG: New wave of the measles epidemic in early 2009: Description and measures. In: BAG Bulletin 2009, No. 27, pp. 484-491, ZDB -ID 2556348-8 .
- ↑ Global Measles and Rubella Strategic Plan 2012–2020. (PDF) World Health Organization (WHO), May 3, 2012, accessed on January 4, 2015 . ISBN 978-92-4-150339-6 .
- ^ Robert Koch Institute: Vaccine-preventable diseases in Germany up to the year 2000. In: Epidemiologisches Bulletin . No. 7 , 2002, p. 49-60 , doi : 10.25646 / 4005 .
- ^ Robert Koch Institute: On the occurrence of pertussis in the new federal states . In: Epidemiological Bulletin . No. 23 , 2005, pp. 195-202 , doi : 10.25646 / 4182 .
- ↑ Erika J. Sims, Miranda Mugford, Allan Clark, David Aitken, Jonathan McCormick, Gita Mehta, Anil Mehta, on behalf of the UK Cystic Fibrosis Database Steering Committee: Economic implications of newborn screening for cystic fibrosis: a cost of illness retrospective cohort study . In: The Lancet . tape 369 , no. 9568 , 2007, p. 1187–1195 , doi : 10.1016 / S0140-6736 (07) 60565-0 .
- ↑ Renate Leinmüller, Rüdiger Meyer: Rotavirus infections: Oral vaccines offer comprehensive protection . In: Deutsches Ärzteblatt . tape 103 , no. 40 . Deutscher Ärzte-Verlag , October 6, 2006, p. A-2650 .
- ↑ USA: Approval of the first vaccine against cervical cancer. In: aerzteblatt.de . June 9, 2006, archived from the original on December 20, 2014 ; accessed on December 27, 2014 .
- ↑ Eric von Hofe: Immunotherapy against cancer . Spectrum of Science , March 2012, ISSN 0170-2971 , p. 24–30 ( Spektrum.de [accessed on February 27, 2012]).
- ↑ RB Belshe, KM Edwards, T. Vesikari, SV Black, RE Walker, M. Hultquist, G. Kemble, EM Connor: Live attenuated versus inactivated influenza vaccine in infants and young children. In: The New England Journal of Medicine . Volume 356, Number 7, February 2007, pp. 685-696, doi: 10.1056 / NEJMoa065368 , PMID 17301299 .
- ↑ P. Graves, H. Gelband: Vaccines for preventing malaria. In: The Cochrane database of systematic reviews. Number 1, 2003, S. CD000129, ISSN 1469-493X , doi: 10.1002 / 14651858.CD000129 . PMID 12535387 . (Review).
- ^ WHO Initiative for Vaccine Research: New Vaccines against Infectious Diseases: Research and Development Status . (PDF; 85 kB). Feb 2006.
- ↑ Vaccination fatigue - the result of a deceptive feeling of security. Thieme (Reprinted from: . German medical weekly . 2005, Vol 130, No. 7l, accessed 11 May 2012 . )
- ^ Vaccine fatigue: the danger of measles. Infection Research, accessed May 11, 2012 .
- ↑ a b P. L. Lopalco, R. Martin: Measles still spreads in Europe: who is responsible for the failure to vaccinate? In: Euro Surveill . tape 15 , no. 17 , 2012, p. pii: 19557 , PMID 20460089 .
- ↑ M. Muscat, H. Bang, J. Wohlfahrt, S. Glismann, K. Mølbak: Measles in Europe: an epidemiological assessment. In: The Lancet . Volume 373, Number 9661, January 2009, pp. 383-389, doi: 10.1016 / S0140-6736 (08) 61849-8 , PMID 19131097 .
- ↑ Measles are difficult to eradicate Why measles is so dangerous
- ^ Epidemiology Act: Gröhe wants tougher sentences for parents who are tired of vaccinations , Deutsche Apotheker-Zeitung , May 26, 2017.
- ↑ Bodily harm when vaccinating a child against the mother's will? Judgment of July 13, 2007, Az .: 5 Cs 135 Js 52229/04 (241/05)
- ↑ Recommendations of the Standing Vaccination Commission at the Robert Koch Institute. August 25, 2014, accessed January 4, 2015 .
- ↑ Swiss Vaccination Plan 2017 ( Memento from November 26, 2017 in the Internet Archive )
- ^ Vaccination plan Austria
- ↑ Epidemiological Bulletin 26/2018, Scientific justification for recommending HPV vaccination for boys aged 9 to 14 years (PDF) from June 28, 2018.
- ↑ Standing Vaccination Commission: Scientific justification for recommending vaccination with the herpes zoster subunit dead vaccine . In: Robert Koch Institute (Ed.): Epidemiologisches Bulletin . No. 50 , 2018, doi : 10.25646 / 5825.2 .
- ↑ FAQ of the Robert Koch Institute: Can I be vaccinated during pregnancy? Are vaccinations possible during breastfeeding? As of November 21, 2012
- ^ Doctors newspaper, July 28, 2006.
- ↑ Epidemiological Bulletin No. 38 (PDF) of the Robert Koch Institute of September 26, 2011, p. 353.
- ↑ SR 818.101, Art. 23 Vaccinations (Federal Act on Combating Communicable Diseases in Humans). In: admin.ch. Retrieved December 27, 2014 .
- ↑ Andreas Hoffmann, Klaus Cussler: vaccination campaign to combat bluetongue. (PDF) First analysis and assessment of the vaccination reactions reported in 2008. In: Pharmacovigilance. Paul Ehrlich Institute, accessed on January 4, 2015 (copy from Deutsches Tierärzteblatt 2/2009, pp. 166–168).
- ^ Avoxa media group Deutscher Apotheker GmbH: Pharmazeutische Zeitung online: Vaccination opponents: From AIDS deniers and conspiracy theorists. Retrieved July 24, 2017 .
- ↑ Peter-Philipp Schmitt: The Swine Flu Conspiracy. In: FAZ.net . August 20, 2009, accessed December 27, 2014 .
- ↑ tagesschau.de: Does Germany need a vaccination? Retrieved July 24, 2017 .
- ^ Sven Ove Hansson: Science denial as a form of pseudoscience . In: Studies in History and Philosophy of Science . tape 63 , 2017, p. 39–47 , doi : 10.1016 / j.shpsa.2017.05.002 .
- ^ John B. Blake: The Inoculation Controversy in Boston: 1721-1722 . In: The New England Quarterly. 25, 4, 1952, pp. 489-506; JSTOR 362582 .
- ^ Sudhoff's archive. Zs. F. History of Science, Volume 90, Issue 2, 2006, pp. 219–232.
- ^ Georg Friedrich Ballhorn: Eduard Jenners of Arzneywissenschaft Doctor and member of the Königl. Society of Sciences Investigations into the causes and effects of cowpox, a disease which has been observed in some western provinces of England, especially in Gloucestershire . 2nd edition. Hahn brothers, Hanover 1799.
- ↑ Irmtraut Sahmland: Ballhorn, Georg Friedrich. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. de Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 134 f.
- ^ Georg Friedrich Ballhorn, Christian Friedrich Stromeyer : Traité de l'inoculation Vaccine avex l'exposé et les résultats des observations faites sur cet objet à Hannovre et dans les environs de cette capitale, par Mr. Ballhorn, Medecin de la cour et Mr. Stromeyer, Surgery de la cour. Rein, Leipzig 1801 ( archive.org ).
- ^ Johann Carro in the Vienna History Wiki of the City of Vienna
- ↑ Th. Chr. Eulner in Dansk Biografisk Leksikon
- ↑ In Denmark and Schleswig-Holstein, confirmands and bridal couples had to prove that they had been vaccinated against smallpox since 1811. In addition: Gerda Bonderup: The role of the clergy in the introduction of the smallpox vaccination in Denmark. In: Manfred Jakubowski-Tiessen: Geistliche Lebenswelten: on the social and mental history of the late Middle Ages and early modern times. Studies on the economic and social history of Schleswig-Holstein, Neumünster 2005, pp. 253–270.
- ↑ Malte Thießen: From the immunized national body to the preventive self. Vaccinations as biopolitics and social practice from the German Empire to the Federal Republic. In: Vierteljahrshefte für Zeitgeschichte. Volume 61, 2013, pp. 34-65, doi: 10.1524 / vfzg.2013.0002 .
- ↑ Malte Thießen: Provision as an order of the social: vaccination in the Federal Republic and the GDR. In: Contemporary historical research. 10, 2013, p. 428. zeithistorische-forschungen.de
- ↑ The great vaccination attempt of Constantinople
- ↑ a b c Iris Ritzmann: vaccination. Berlin 2005, p. 663.
- ↑ EMA approval (PDF; 1.9 MB)
- ↑ Epidemiological Bulletin 37/2015. (PDF) In: rki.de. Robert Koch Institute , 2015, accessed January 1, 2017 .
- ↑ Information on the StIKo Vet. ( Memento from March 18, 2012 in the Internet Archive )
- ↑ Moderate sales in veterinary drugs. In: Dt. TAB. 60, No. 2012, p. 849.
- ↑ Uwe Truyen: The vaccination of the dog . (PDF; 547 kB) Uwe Truyen is chairman of the StIKo Vet .;
- ↑ STIKO Vet .: Guideline for the vaccination of small animals.
- ↑ K. Wiedmann: Clinical phase I study on the neoadjuvant immune stimulating therapy of feline fibrosarcoma with interleukin-2 and interferon gamma . Vet.-med. Diss. Munich 2005, pp. 5-7. ( PDF (PDF); 7.3 MB)
- ↑ The updated vaccination guidelines for small animals and horses. In: Dt. TAB. Volume 65, Issue 4, pp. 460-461.
- ↑ The updated vaccination guidelines for small animals and horses. In: Dt. TAB. Volume 65, Issue 4, p. 462.
- ↑ Anne Posthoff: vaccination of pet rabbits. In: Small Animal Medicine. 3 / 4-2010, pp. 107-109.
- ↑ The updated vaccination guidelines for small animals and horses. In: Dt. TAB. Volume 65, Issue 4, pp. 463-464.
- ↑ STIKO Vet .: Vaccination guidelines for cattle and small ruminants.
- ↑ M. Bastian, M. Holsteg, H. Hanke-Robinson, K. Duchow, K. Cussler: Bovine Neonatal pancytopenia: is this alloimmune syndrome Caused by vaccine-induced antibodies alloreactive? In: Vaccine . Volume 29, Number 32, July 2011, pp. 5267-5275, doi: 10.1016 / j.vaccine.2011.05.012 , PMID 21605614 .
- ↑ STIKO Vet .: Pigs vaccination guidelines.
- ↑ tierseucheninfo.niedersachsen.de
- ↑ Anne Vits et al .: Information on animal disease law on vaccinations in small and hobby poultry flocks. In: Lohmann Information. 3/2005, pp. 1-2.