Ethanol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
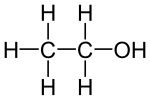
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Ethanol | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 2 H 6 O | |||||||||||||||||||||
| Brief description |
clear, colorless, spicy smelling and burning tasting, highly flammable, hygroscopic liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 46.07 g · mol -1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.7893 g cm −3 (20 ° C ) |
|||||||||||||||||||||
| Melting point |
−114.5 ° C |
|||||||||||||||||||||
| boiling point |
78.32 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| pK s value |
16 |
|||||||||||||||||||||
| solubility |
arbitrarily with water, diethyl ether , chloroform , gasoline and benzene miscible |
|||||||||||||||||||||
| Refractive index |
1.3638 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
|
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−277.6 kJ / mol (l) −234.8 kJ / mol (g) |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
The ethanol ( IUPAC ), or of ethyl alcohol , and ethanol or ethyl alcohol , in common terminology also (usually) alcohol mentioned, is an aliphatic , monovalent alcohol with the molecular formula C 2 H 6 O.
The pure substance is a colorless, easily inflammable liquid at room temperature with a burning taste and a characteristic, spicy (sweet) smell. Substances classified as toxic liver drug is in the production of luxury foods and alcoholic beverages such as wine , beer and spirits from carbohydrate-material by one of yeasts induced fermentation in large scale production .
The fermentation of sugar into ethanol is one of the oldest known biochemical reactions. Ethanol has been produced from ethene for industrial purposes since the 19th century . Ethanol is widely used as a solvent for substances that are used for medical or cosmetic purposes, such as fragrances , aromas , dyes or medicines, and as a disinfectant . The chemical industry uses it both as a solvent and as a raw material for the synthesis of other products such as carboxylic acid ethyl ester .
Ethanol is used energetically as a biofuel , for example as so-called bioethanol . For example, the ethanol fuel E85 contains an ethanol content of 85 percent by volume .
Systematics
Ethanol (C 2 H 5 OH) belongs to the linear n - alkanols . Ethanol is derived from the alkane ( saturated hydrocarbon ) ethane (C 2 H 6 ), in which a hydrogen atom has been formally replaced by the functional hydroxyl group (-OH). To name the name is Ethan , the suffix ol appended. The empirical formula for ethanol according to the Hill system is C 2 H 6 O, the frequently used notation C 2 H 5 OH is not a sum formula but a semi-structural formula .
"Alcohol" is the slang term for "ethanol" ; The technical term “ alcohols ”, on the other hand, stands for a group of organic-chemical compounds which, in addition to the hydrocarbon structure, have at least one hydroxyl group as an additional functional group , with no higher-valued substituent on the carbon atom with the hydroxyl group .
history
Ethanol is produced naturally, primarily during the fermentation of sugary fruits. The man has long been known for intoxication this naturally occurring substance. In Egyptian scrolls of the 3rd dynasty and on ancient Mesopotamian cuneiform tablets there are references to the production of beverages containing ethanol.
Beers and later wines were initially produced with the help of wild yeast . The ethanol content of such beverages was lower than it is today, as the wild yeasts stop converting sugar into ethanol above a certain ethanol concentration. Through centuries of cultivation, today's yeasts such as Saccharomyces cerevisiae tolerate higher ethanol contents. In around 900 the Persian doctor, scientist, philosopher and writer Abu Bakr Mohammad ibn Zakariya ar-Razi succeeded in extracting ethanol in concentrated form by distilling wine; on a word of the Arabic language ( Arabic الكحول), the term alcohol for 'spirit of wine' can be traced back in the 18th century . Such a distillation separation was probably already carried out in China in the early Middle Ages, was known - probably through Arab mediation - around 1100 in Salerno and was made known to a wider public in Europe by Taddeo Alderotti before 1288.
In 1796 Johann Tobias Lowitz received pure ethanol for the first time by filtering distilled alcohol over activated charcoal . At that time, the term wine spirit , which is still used today, was common for pure alcohol. Antoine Lavoisier first described ethanol as a combination of carbon , hydrogen and oxygen . In 1808, Nicolas-Théodore de Saussure determined the chemical composition of ethanol. Fifty years later, Archibald Scott Couper published the structural formula of ethanol. It was one of the first structural formulas to be determined.
Ethanol was first made synthetically in 1826 by Henry Hennel and Georges Simon Serullas . In 1828, Michael Faraday produced ethanol by acid-catalyzed hydration of ethylene , a process that is similar to industrial ethanol synthesis.
Today ethanol is mainly obtained from biomass by fermentation . In the context of biofuel production , it is called bioethanol . Agricultural alcohol is ethanol made from agricultural raw materials; in Germany, agricultural alcohol is produced in agricultural distilleries under state supervision .
Occurrence
Ethanol is a naturally occurring product of alcoholic fermentation in ripe fruits and juices . Many foods naturally contain small amounts of ethanol. Also, non-alcoholic beer still contains up to 0.5 percent by volume of ethanol. According to the German Food Book, fruit juices may have an ethanol content of around 0.38 percent by volume. Apple juice contains up to 0.016 percent by volume , grape juice up to 0.059 percent by volume. A ripe banana can contain up to 1 percent by volume, bread up to 0.3 percent by volume. Ripe kefir can contain up to 1 percent by volume of ethanol, sauerkraut up to 0.5 percent by volume. The physiological ethanol content of human blood is approximately 0.02 to 0.03 ‰.
Ethanol, along with other organic molecules such as acetaldehyde, has been detected in interstellar molecular clouds, although its mechanism of formation is unclear.
Manufacturing
Alcoholic fermentation
Ethanol is obtained through fermentation from biomass , mostly from crops containing sugar or starch or traditionally from horticultural products. This process is carried out in a controlled manner with a number of foods, such as wine made from grapes or beer made from malt and hops . Wood sugar can be used as a by-product of the sulfite to Sulfitsprit be fermented. However, due to numerous impurities, this can only be used for energy.
Before the actual fermentation, starch is usually first split into disaccharides , the glycosidic bonds of which are broken by hydrolases ; then the resulting monosaccharides are fermented by yeast or bacteria . When the ethanol concentration is close to 15%, yeast cells and bacteria begin to die, which is why fermentation cannot achieve a higher concentration. The gross equation of alcoholic fermentation is:
distillation
Ethanol can be concentrated by distillation for technical and pleasure purposes, as it already evaporates at 78 ° C.
Drinking alcohol
Drinking alcohol suitable for consumption is obtained by distillation - the so-called burning - of an alcohol-containing mash from agricultural raw materials. Depending on the distillation process, the distillate, the so-called brandy, contains flavors , fusel oils , other organic compounds and water in addition to ethanol , which determine the character and taste of the end product such as brandy , whiskey or rum . For the production of vodka , on the other hand, almost pure ethanol is used and only diluted with water. In undiluted form, pure ethanol with the sales designation ethyl alcohol of agricultural origin is used as the starting product for other alcoholic beverages, for example for most liqueurs . Alcoholic beverages that contain distilled ethanol are called spirits (colloquially also brandy or schnapps ) - in contrast to wine and beer , the ethanol of which is exclusively produced through alcoholic fermentation.
Technical purposes
On an industrial scale, pure ethanol for technical applications is produced by azeotropic rectification (entrainer rectification). The plant consists of two rectification columns. In the main separation column, the ethanol-water mixture is rectified up to the vicinity of the azeotropic point. The bottom product is water.
The auxiliary cyclohexane is added to the top product, which consists of 95.6% ethanol and 4.4% water . Previously common entrainers such as benzene in the Young process or such as trichloroethene in the Drawinol process are no longer used today. This three-substance mixture of ethanol, water and entrainer enters the auxiliary separating column. There it is separated into the pure alcohol obtained in the sump and into a cyclohexane-water mixture as the top product. Cyclohexane and water are immiscible in the liquid state and separate after condensation in a separator (decanter). The auxiliary cyclohexane is added back to the inflowing, azeotropic ethanol-water mixture at the inlet of the auxiliary separating column. It runs in a circuit in the upper area of the auxiliary material separation column and is therefore referred to as an "upside-down auxiliary material". Anhydrous ethanol is obtained on a laboratory scale by distillation over dehydrating chemicals such as calcium oxide , anhydrous calcium sulfate or molecular sieves . The process of making absolute alcohol is known as absolutization .
Technical syntheses
Ethanol is produced by chemical synthesis from water and ethene in the so-called indirect process using homogeneous catalysis with the addition of sulfuric acid. Alcohol produced in this way is also known as industrial alcohol .
The process takes place in two stages with the formation of sulfuric acid esters , which have to be hydrolyzed in a second step. The sulfuric acid must be concentrated again after hydrolysis has taken place . In the direct process , phosphoric acid applied to silica serves as a heterogeneous catalyst . At temperatures up to 300 ° C and pressures of 70 bar, ethanol is produced directly from ethene and water in the gas phase. However, the conversion per reactor passage is only 5% based on ethene. Because of the wastewater problems and corrosion problems caused by the sulfuric acid produced in the indirect process , ethanol is nowadays produced industrially using phosphoric acid catalysis. The gross equation for both processes is:
In principle, it is possible to obtain ethanol by catalytic hydrogenation of acetaldehyde . At high hydrogen pressures, acetaldehyde is converted to nickel-containing contacts:
In the Synol process, ethanol is produced by the reaction of carbon monoxide with hydrogen and can be separated from the other alcohols formed by distillation. By nuclear magnetic resonance spectroscopy synthetic ethanol from fossil fuels ethanol from renewable raw materials can be distinguished on the basis of hydrogen and carbon isotope ratios. This fact can be used to detect the splashing of wine or spirits with industrial ethanol. In the case of ethanol produced by fermentation processes, the plant origin can be determined via the deuterium distribution.
Production quantities
Worldwide, the USA and Brazil together produced over 90% of the annual output of 29 million tons in 2005. The largest European producers are Russia and France. Germany produces almost 4 million hl annually in equal parts as beverage alcohol and as alcohol for chemical-technical purposes, which corresponds to an internal requirement coverage of around 62%.
In addition to the production of neutral alcohol for beverages, food and technical purposes, around 65% of the world's fuel is produced . In the USA, the construction of new production facilities for ethanol is being particularly promoted, above all through the "Energy Policy Act" (EPACT) of 2005, which is intended to promote the expansion of renewable liquid energy sources.
Taxation and Denaturation
In Germany, ethanol is subject to alcohol tax ( spirits tax until 2018 ). It is collected by the customs administration from the person placing it on the market (liquor manufacturer, authorized recipient, liquor store owner) at the time of the departure from the warehouse. Shipping with tax suspension is possible via BVD or EVD - for example between manufacturer and wholesaler with open liquor storage and in export transactions.
For technical purposes, for example in printing , in paint production , cleaning agent production , for cosmetics and similar areas of application and as denatured alcohol, the use of ethanol is tax-free. In order to prevent this ethanol from being drunk as luxury goods or added to such without paying the tax, untaxed alcohol is denatured under customs supervision. Denaturation means that ethanol is mixed with other chemicals, such as methyl ethyl ketone (MEK) and two other marking components prescribed by spirits tax law, petroleum ether , cyclohexane , diethyl phthalate , Bitrex or the like, in order to make it unusable for human consumption. This is regulated in Germany by the Spirits Tax Ordinance (BrStV) and in Austria by the ordinance of the Federal Minister of Finance on denaturing alcohol (VO denaturing).
Bioethanol for admixture with fuel is denatured with ETBE or gasoline during production . The denaturants mentioned above, commonly used for alcohol or cosmetic purposes, for example methyl ethyl ketone (MEK), must not be used in fuels according to EN 228.
In the case of ethanol used as fuel in the form of denatured alcohol , for example for rechauds as well as camping and expedition stoves, the extremely bitter denatonium benzoate (1 gram / 100 liters) is added to the ethanol in addition to the MEK . Pyridine , which was previously used as a denaturant for denatured alcohol, has not been used by German manufacturers since 1993 due to its health concerns and has no longer been permitted since July 1, 2013. In contrast to pyridine, which has a boiling point of 115 ° C, denatonium benzoate is a solid that only melts at 163 to 170 ° C. It therefore does not evaporate when using denatured alcohol, but rather accumulates in the wicks of alcohol devices, which leads to malfunctions, for example in the case of incandescent lamps and alcohol gas stoves .
Denaturants usually have boiling points similar to ethanol, making them difficult to remove by distillation.
Ethanol is classified as “ UN 1170” as a dangerous good.
properties
Physical Properties
| Flash point | 12 ° C (information refers to
Measurement in a closed crucible.) |
| Ignition temperature | 400 ° C |
| Explosion limits | lower: 3.1 volume percent upper: 27.7 volume percent Max. Pressure: 8.4 bar |
| Speed of sound | 1180 m s −1 (20 ° C) Temp.-dependency: −3.6 m s −1 ° C −1 |
| density | 0.79 g cm −3 = 0.79 kg dm −3 |
| Energy density ( calorific value ) | 7.44 kWh kg −1 = 26.78 MJ kg −1 5.87 kWh l −1 = 21.14 MJ l −1 |
| dynamic viscosity | 1.2 · 10 −3 Pa · s (20 ° C) |
| kinematic viscosity | 1.52 10 −6 m 2 s −1 (20 ° C) |
| Surface tension | 0.02255 Nm −1 (20 ° C) |
| Refractive index | 1.3638 |
| Biodegradability | 94% ( OECD 301 E) |
| UN number | 1170 |
| Hazard number | 30 + 33 |
| Triple point | 150 ± 20 K / 0.43 mPa −123.15 ± 20 ° C / 0.43 mPa |
| Critical point | 514.0 K / 6.137 MPa / 168 cm 3 / mol 240.85 ° C / 6.137 MPa / 168 cm 3 / mol |
The outstanding feature of ethanol is its hydroxyl group . Since an oxygen atom attracts electrons more strongly than hydrogen and carbon, the result is an asymmetrical distribution of the electron density along this bond: a molecular dipole is formed . It gives ethanol its typical properties. On the one hand, the dipoles attract each other at the molecular level, so that a comparatively high boiling temperature of 78 ° C results (S p, ethane = −88.6 ° C), on the other hand, ethanol can be mixed with liquids that have similar dipole properties, for example with water and methanol. This property is known as hydrophilicity . At the same time, the molecule has an organic residue that gives it limited miscibility with purely lipophilic substances. For this reason, ethanol is an important solvent in chemistry and pharmacy. Plant extracts or other medicines are offered as alcoholic solutions, so-called " tinctures ".
At the freezing point, ethanol forms single crystals of sufficient size for determination by means of crystal structure analysis . It crystallizes in the monoclinic crystal system with the space group Pc (space group no. 7) and has the lattice parameters a = 537.7 pm, b = 688.2 pm, c = 825.5 pm and β = 102.2 ° at 87 K on and 4 formula units per unit cell . The molecules form chains via hydrogen bonds with an oxygen-oxygen distance of 271.6 pm and 273.0 pm. The conformation around the carbon-carbon bond is offset in both molecules. While the hydroxyl group in one molecule has a gauche conformation along the CC-OH axis, the other molecule has a trans conformation.
Mixtures with other solvents
Ethanol is miscible with water in any ratio . When mixing, there is a volume contraction with the development of heat . The total volume of a water / ethanol mixture is smaller than the sum of the individual volumes. Mixing 50 ml of ethanol with 50 ml of water creates 97 ml of an ethanol-water mixture (see calculation and other examples and conclusion in the main article, alcohol content ).
The melting point of aqueous ethanol solutions decreases with increasing ethanol content until a eutectic with a melting temperature of −118 ° C is reached at a content of 93.5 % by mass . At temperatures around −20 ° C, ethanol (96%) hardly evaporates and takes on more viscous properties. At −70 ° C it becomes even more viscous (cooling oil) .
Ethanol forms azeotropic mixtures with many other substances .
In organic solvents such as carbon tetrachloride , ethanol forms dimers , trimers and tetramers via hydrogen bridge formation, depending on the concentration . The enthalpy of formation can be determined using infrared spectroscopic studies . It is 92 kJ mol −1 for the tetramer , 42 kJ mol −1 for the trimer and 21 kJ mol −1 for the dimer.
Excess volume ( volume contraction ) when mixing ethanol and water
Chemical properties
The OH group of the ethanol is provided with a pK s value very slightly acidic by 16, thereby being capable of reacting with a strong base (such as the alkali metals sodium and potassium ), a proton (H + ) cleave. By reacting with alkali metals, ethanol is quantitatively converted into its deprotonated form , the ethanolate ion (CH 3 CH 2 O - ). The reaction takes place with evolution of hydrogen:
Ethanol dissolves in all proportions with water and many other organic solvents such as diethyl ether , chloroform and benzene .
Autoprotolysis
Ethanol can be used as Brønsted - acid as a Brønsted base react, making it an ampholyte :
The autoprotolysis constant is pK au = 19.5.
Nucleophilic substitution
In aprotic solvents , ethanol reacts with hydrogen halides via nucleophilic substitution to form ethyl halides. Ethanol and hydrogen chloride react to form ethyl chloride and water:
Ethanol and hydrogen bromide react to form ethyl bromide and water:
More specifically, ethyl halides can be formed by halogenating reagents such as thionyl chloride or phosphorus tribromide .
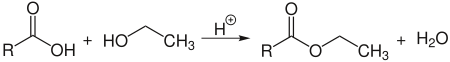
Esterification
Ethanol reacts acid-catalyzed with carboxylic acids in an equilibrium reaction to form ethyl esters :
However, since the water formed has a higher boiling point than ethanol, ethyl ester is better produced by reacting with acid anhydrides. Ethyl esters are used as additives for cosmetics as well as smells and flavors.
Dehydration
Very strong acids such as sulfuric acid catalyze the dehydration of the ethanol. It formed diethyl ether or ethylene :
In an elimination reaction, ethanol splits off water with the formation of a double bond:
Which product is formed depends on the reaction conditions such as temperature, concentrations, etc. In the case of dehydration, the highly toxic diethyl sulfate can be formed under certain reaction conditions.
oxidation
Ethanol can already be oxidized by atmospheric oxygen at room temperature via acetaldehyde to acetic acid . Such reactions are catalyzed by enzymes in biological systems, for example . In the laboratory, powerful inorganic oxidizing agents such as chromic acid or potassium permanganate are used for the oxidation to acetic acid. The partial oxidation to acetaldehyde is possible with weaker oxidizing agents such as pyridinium chlorochromate (PCC) .
The oxidation of the ethanol does not have to stop at the acetic acid level. In air, ethanol burns with a blue flame (see picture) with a calorific value of 26.8 MJ / kg to form carbon dioxide and water:
Ethanol reacts slowly with chlorine or bromine to form acetaldehyde and other halogenated oxidation products. Acetaldehyde forms hemiacetals with excess ethanol . However, the halogen addition to the enol form of acetaldehyde predominates and this leads to the formation of α-haloacetaldehyde (which is irritating to tears ). The further oxidation with chlorine ultimately leads to hemiacetals of chloral .
Disinfection due to denaturation
Corresponding to the denaturation by acids or bases , ethanol can disrupt the hydrogen bonds required in biopolymers to maintain the structure by interfering as a polar solvent. This results in conformational changes . 50 to 70 percent ethanol denatures most proteins and nucleic acids. Since membrane proteins lose their function due to the destruction of the spatial structure and the affected cells burst like a balloon due to the membrane defects, higher-percentage ethanol can be used for disinfection : Bacterial and fungal cells are irreversibly inactivated by denaturing their membrane proteins, and enveloped viruses are deprived of their protein-containing shell.
use
Ethanol is used in the three main markets of alcoholic beverages , as a raw material for the chemical industry and as an energy source . Ethanol, which comes from the fermentation of foods containing sugar and starch, is used in all areas, while synthetic ethanol is only used as a chemical raw material and energy source. The competing use of ethanol from food production as a chemical and energy resource is controversial.
Most of the ethanol produced is consumed in the form of alcoholic beverages for pleasure purposes. It is also used as a solvent both for consumer products in the household ( perfume , deodorant ) and for medical applications (solvents for medicines , disinfectants ) and in industry itself as a solvent and generally as a fuel.
Household and consumer products
Ethanol is used everywhere in the household as an excellent solvent, for example as a carrier for odorous substances such as perfume, deodorant and fragrance spray. Ethanol is also used as a cleaning agent, for example for glass ( window cleaning agent ), chrome, plastic, in car windscreen washer solutions and as a stain remover. When added to water, it serves as an antifreeze agent.
Ethanol is widely used as a food additive. For example, ethanol is added to port wines , sherry and other southern wines , the so-called Aufspritung , in order to end the fermentation process at the desired time . Due to the premature fermentation, these liqueurs and wines - with a few exceptions - have a high residual sugar content and are therefore very sweet.
Ethanol can be added to preserve other foods.


As a fuel for camping stoves as so-called denatured alcohol , ethanol is used for energy in households. By adding cellulose acetate or soap , denatured alcohol can be converted into a gel, the so-called hard alcohol .
Simple capillary thermometers with a blue or red visible liquid column are filled with colored ethanol. With a sufficiently long, graduated tube, temperatures from the melting point to close to the boiling point can be measured, which means that outside temperatures are well covered.
medicine
The effectiveness as a disinfectant or antiseptic (e.g. for hand disinfection ) depends on the concentration of the ethanol-water mixture. With an optimal alcohol content between 50 and 80%, the bacterial shell is destroyed and ethanol is deadly. All bacteria including the tubercle bacteria are killed within a minute by denaturing the bacterial cell wall ( bactericidal ). In addition, ethanol-water mixtures have a high osmotic pressure ; 70 percent ethanol has the highest osmotic pressure of all mixtures with water at 250 · 10 6 Pascal. The mixture is only effective against viruses , but not effective against bacterial endospores . It should not be used on open wounds : In addition to an unpleasant burning sensation, ethanol has a vasodilatory effect (mainly cutaneous ) , which is generally beneficial for cleaning wounds, but can drastically worsen bleeding, especially in the case of larger injuries. Solutions with over 80% alcohol have an even stronger effect, but are not used regularly due to poor skin tolerance. Anhydrous ethanol hardens the bacterial shell, so the bacteria stay alive. Drinking ethanol or alcoholic beverages does not have an antiseptic effect. Drinks with an ethanol content of less than 20% kill practically no germs. Combination with alkalis (approx. 1%) or peroxycarboxylic acids (0.2 to 0.5%) greatly improves the effectiveness against viruses and spores, among other things. Ethanol is used as a solvent for the production of iodine tincture , a mixture of iodine in ethanol for wound disinfection, to which potassium iodide is added to prevent the formation of hydrogen iodide .
95 percent or pure ethanol can be used as PEI therapy for the obliteration of "hot" thyroid nodules ( percutaneous ethanol injection therapy ) and other circumscribed tumors such as hepatocellular carcinoma (also percutaneous ethanol injection therapy ).
Liquid medicaments can contain ethanol as a solvent, cosolvent or solubilizer if the medicament (s) are poorly soluble or insoluble in water. Ethanol itself can be mixed with water as desired. It has an important function in the preservation and stabilization of liquid herbal medicines ( phytotherapeutic agents ). The drugs must be labeled in accordance with the drug warning ordinance (AMWarnV).
Rubbing the skin with a high-percentage ethanol solution ( e.g. rubbing alcohol ) promotes blood circulation. For cleaning wounds , “burnt wine” has been used regularly by German-speaking surgeons since the 12th century. In folk medicine, dilute ethanolic solutions are still used today to treat insect bites . An alcohol-soaked cloth is placed on the fresh stitch for some time. The pain relief occurs due to the cooling effect of the ethanol solution; the itching is suppressed. However, ethanol does not chemically change or inactivate the toxins. Alcoholic beverages were used as pain relievers and numb anesthetics in ancient times.
In the case of poisoning with methanol , the first measure is ethanol given intravenously , which inhibits the conversion of methanol into the poisonous methanal via the enzyme alcohol dehydrogenase . Ethanol binds to alcohol dehydrogenase about 25 times more strongly than methanol. In the case of severe alcohol addiction, an alcohol predelir with ethanol can be interrupted in order to be able to treat an acute second illness without the symptoms that otherwise occur .
Ethanol as a fuel
Ethanol is used as an ethanol fuel in the form of biogenic bioethanol as a fuel for gasoline engines , mainly mixtures with gasoline . Both fossil bioethanol and bioethanol produced from regenerative biomass can be used for this, as there is no chemical difference between the two types. Due to the availability, the production costs and political support measures, bioethanol is mainly used today, which is produced on the basis of fermentable sugar ( sugar cane and sugar beet ) and starch (especially corn and wheat starch). It is being investigated whether cellulosic ethanol can be used from wood in the future .
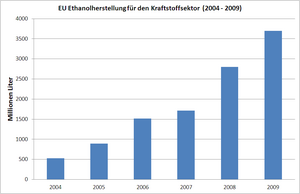
Ethanol is mainly used as an admixture to conventional fuel , for example in a concentration of 5% ethanol (E5 as an admixture in normal vehicle gasoline) or 85% ethanol (as E85 for suitable vehicles ). In connection with the Kyoto Protocol today, often on the manufacture and use of biogenic fuels (to be biofuels ) and the reduction of carbon dioxide - emissions debated per kilometer. In the European Union, the amount of ethanol produced for the fuel sector increased from 525 million liters in 2004 to 3.7 billion liters in 2009. Since 2011, ethanol production has remained the same for both fuel and other purposes.
According to a development by Wernher von Braun, ethanol was also used as a fuel for the rockets of the types A1 , A2 , A3 , A4 , A4b and A5 until the 1950s . In contrast to gasoline , the calorific value can easily be reduced by diluting it with water for test purposes in order to prevent explosions during test runs of engines, and on the other hand, ethanol was easily obtained from agricultural products during the Second World War , in contrast to the scarce gasoline.
In addition to pure ethanol, its derivatives are used in the fuel sector. Like methyl tert-butyl ether , ethyl tert -butyl ether (ETBE) is used to increase the octane number of petrol . ETBE is produced by the acid-catalyzed addition of ethanol to isobutene :
Further use of ethanol
Ethanol is an important solvent and intermediate in the chemical industry . An important secondary product is ethyl chloride , which is produced from ethanol by reacting with hydrogen chloride . Oxidation produces other secondary products such as acetaldehyde and acetic acid .
Ethanol is used in a wide variety of esterification reactions. The esters obtained have a wide range of uses as solvents and as intermediates for subsequent syntheses. An important secondary product is ethyl acrylate , a monomer that is used as a co-monomer in various polymerization processes. Ethyl acetate is used as a solvent for adhesives and nail polish and for the extraction of antibiotics . Glycol ethers such as 2-ethoxyethanol are widely used as solvents for oils, resins, fats, waxes, nitrocellulose and paints.
In reverse of the petrochemical production reaction, ethanol is converted back into ethene, which is used, for example, by the Brazilian chemical company Braskem as a raw material for the production of polyethylene . In a plant in Rio Grande, Brazil, Braskem already produces sugar cane- based polyethylene in a plant with an output of 200,000 t per year.
Liquid preparations from biology and human medicine are often fixed and preserved with ethanol-water mixtures or formalin .
Biological importance
Ethanol is absorbed throughout the digestive tract . This begins to a small extent in the oral mucosa . The ethanol absorbed there passes directly into the blood and is thus distributed over the entire body including the brain . About 20% is absorbed in the stomach, the rest in the small intestine . The ethanol absorbed in the stomach and intestines first reaches the liver with the blood , where it is partially broken down. Ethanol intake is increased by factors that increase blood flow, such as heat ( Irish coffee , grog ), sugar ( liquor ) and carbon dioxide ( sparkling wine ). In contrast, fat slows down absorption. This does not lead to a lower absorption of the alcohol overall, but only to a time extension.
About 2 to 10% of the ingested ethanol is released unchanged via urine, sweat and breath. Partial degradation already takes place in the stomach; a sigma alcohol dehydrogenase found there shows an activity approximately 200 times higher than the isoenzymes localized in the liver. The share in the total degradation of ethanol is only about 5%.
As other water-soluble toxins - - In the liver, the major part of the ethanol is by the enzyme alcohol dehydrogenase (ADH) and catalase and the MEOS system to ethanal ( acetaldehyde , H 3 C-CHO) degraded to continue by acetaldehyde to acetic acid is oxidized to be . Acetic acid is breathed into CO 2 via the citric acid cycle and the respiratory chain in all cells of the body while generating energy . The liver can adjust its breakdown activity to a small extent with significantly increased, regular consumption. The intermediate product ethanal is partly responsible for the so-called " hangover " symptoms such as headache, nausea and vomiting. The breakdown of ethanol is inhibited by sugar , so the hangover is particularly intense with sweet alcoholic drinks, especially liqueurs, punch bowls , fruit wines and some types of sparkling wine.
The rate of degradation by alcohol dehydrogenase is constant within certain limits. It is around 0.1 for men and 0.085 grams per hour and kilogram of body weight for women. The precisely measured degradation rates for men were between 0.088 and 0.146 grams per hour and kilogram of body weight. In men there is a slightly increased activity of gastric alcohol dehydrogenase in the stomach, resulting in a slight acceleration of the breakdown of alcohol. In some people, high doses of fructose can lead to a faster metabolism by supporting the catalase-ethanol breakdown. With higher alcohol concentrations - from around 50 g ethanol intake per day - or with chronic drinkers, the alcohol is also broken down via the microsomal ethanol oxidizing system (MEOS) . Ethanol in the smooth ER of the liver cells is oxidized to ethanal by cytochrome P450 (CYP2E1) with consumption of oxygen. Depending on the situation, ethanol causes anesthesia, stimulation or a change of mood. It leads to an expansion of the peripheral blood vessels in particular.
toxicology

Pathologists consider ethanol to be one of the "obligatory hepatotoxic substances", that is to say, one of the liver toxins. A "direct toxic effect of alcohol on erythropoiesis ", the formation of red blood cells, is considered to be certain. Pediatricians call it a " teratogenic noxious agent", ie a poison that damages the fetus, and pharmacologists and toxicologists speak of "acute poisoning" above a certain threshold dose and of "chronic poisoning" in alcoholism .
Ingestion leads to typical acute intoxication symptoms such as dizziness , nausea , disorientation, talkativeness and increased aggressiveness - from around 0.5–1 per mille ethanol concentration in the blood . The lethal dose (LD) is around 3.0 to 4.0 per thousand for inexperienced drinkers. However, values above 7 per thousand have already been measured. The LD 50 for the rat is 7060 mg / kg for oral administration. In acute ethanol poisoning , the alcohol still in the stomach can be partially removed by inducing vomiting or by pumping out the stomach contents . Alcohol psychoses have been described.
proof
Ethanol can be detected as p -nitrobenzoic acid ester or 3,5-dinitrobenzoic acid ester by esterification . The reaction takes place by reaction with the corresponding acid chloride . Ethanol can be detected non-specifically by the iodoform sample . Ethanol can be quantified using chromatographic methods such as gas chromatography . Wet chemical quantitative detection is possible by oxidation with an excess of potassium dichromate , whereby the unreacted potassium dichromate can be determined iodometrically.
In food analysis, the difference in density between water and ethanol is used. The ethanol content is separated off in a (steam) distillation and determined pycnometrically. Alternatively, the density can also be measured in a flexural oscillator. Both methods are evaluated using tabular values.
In the proton resonance spectrum , ethanol has a triplet structure at room temperature due to the coupling of the protons of the hydroxy group with the methylene protons . This indicates a fixation of the hydroxyl group with respect to the methylene protons. With increasing temperatures, the splitting becomes smaller and finally disappears completely due to the increasing rotation of the hydroxyl group.
The ethanol concentration during the manufacturing process, for example in breweries, can be monitored using infrared spectroscopy by measuring the intensity of the oscillation frequency of the CH band at 2900 cm −1 . The infrared spectrum for ethanol shows a CH, an OH and a CO stretching vibration as well as various bending vibrations. The OH stretching vibration appears as a broad band at around 3300–3500 cm −1 , the CH stretching vibration at around 3000 cm −1 .
1 H-NMR spectrum of ethanol
See also
literature
- Allinger , Cava , de Jongh , Johnson , Lebel , Stevens : Organic Chemistry. 1st edition, Walter de Gruyter, Berlin 1980, ISBN 3-11-004594-X , pp. 125-127.
- Beyer , Walter : Textbook of Organic Chemistry. 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 115-117.
- Morrison , Boyd : Textbook of Organic Chemistry. 3rd edition, VCH, Weinheim 1986, ISBN 3-527-26067-6 , pp. 526-527.
- Beilstein: Handbook of Organic Chemistry. Volume 1, pp. 292-314 ( ext. Link ).
Web links
- What actually is ethanol? Article from May 18, 2020 at Espresso
- How does alcohol work in the brain? Post of 10 February 2004 & Quarks Co .
- "Alcohol" ethanol. An article on the subject by Peter Bützer (PDF file; 966 kB).
Individual evidence
- ↑ Entry on ALCOHOL in the CosIng database of the EU Commission, accessed on December 28, 2019.
- ↑ a b c d e f g h Entry on ethanol. In: Römpp Online . Georg Thieme Verlag, accessed on March 22, 2015.
- ↑ a b Gerhard Eisenbrand (Ed.), Peter Schreier (Ed.): RÖMPP Lexikon Lebensmittelchemie. 2nd edition, Thieme Verlag, Stuttgart 2006, p. 322.
- ↑ a b c d e f g h i j Entry on ethanol in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d e f g h Entry on ethanol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Entry on ethanol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 64-17-5 or ethanol ), accessed on September 13, 2019.
- ^ A b G. Stuart Wiberg, H. Locksley Trenholm, Blake B. Coldwell: Increased ethanol toxicity in old rats: Changes in LD50, in vivo and in vitro metabolism, and liver alcohol dehydrogenase activity. In: Toxicology and Applied Pharmacology . Vol. 16, 1970, pp. 718-727, doi: 10.1016 / 0041-008X (70) 90077-3 .
- ↑ Gigiena i Sanitariya. For English translation, see HYSAAV , 1967, Vol. 32 (3), p. 31.
- ↑ Raw Material Data Handbook. Vol. 1: Organic Solvents, 1974, p. 44.
- ↑ Matti Välimäki, Matti Härkönen, Reino Ylikahri: Acute Effects of Alcohol on Female Sex Hormones. In: Alcoholism: Clinical and Experimental Research . Vol. 7, 1983, pp. 289-293, doi: 10.1111 / j.1530-0277.1983.tb05462.x .
- ^ SJ Baker, GJ Chrzan, CN Park, JH Saunders: Behavioral effects of 0 and 0.05% blood alcohol in male volunteers. In: Neurobehavioral Toxicology and Teratology . Vol. 8, 1986, pp. 77-81, PMID 3703098 .
- ↑ M. Yamagishi, T. Iwasaki: Acute alcohol intoxication in a two-month-old baby. In: Journal of UOEH . Vol. 9, 1987, pp. 53-59, PMID 3576010 .
- ↑ CRC Handbook, pp. 5–22 ( Memento from April 26, 2015 in the Internet Archive ).
- ↑ Chemical technical terms, cf. Entry of ethanol or ethyl alcohol in duden-online; accessed on May 13, 2018.
- ↑ See entry ethanol in duden-online; accessed on May 14, 2018.
- ↑ See entry ethyl alcohol in duden-online; accessed on May 14, 2018.
- ↑ Robert Dudley: Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. In: Integrative and Comparative Biology . Vol. 44, No. 4, 2004, pp. 315-323, doi: 10.1093 / icb / 44.4.315 .
- ^ J. Westermeyer: Cross-cultural studies on alcoholism. In: HW Goedde: Alcoholism: Biomedical and genetic aspects. Pergamon Press, New York 1989, pp. 305-311.
- ^ A b Diana von Cranach: Drugs in Ancient Egypt. In: G. Völger, K. Welck: Intoxication and Reality: Drugs in a Cultural Comparison. Vol. 2, Rowohlt, Reinbek 1982, ISBN 3-499-34006-2 , pp. 480-487.
- ↑ Helmut Hans Dittrich: Microbiology of Wine. Ulmer Eugen Verlag, 2005, ISBN 3-8001-4470-0 , p. 89.
- ↑ Claus Priesner, Karin Figala: Alchemy: Lexicon of a Hermetic Science. CH Beck, 1998, ISBN 3-406-44106-8 , p. 146.
- ↑ Lu Gwei-Djen, Joseph Needham and Dorothy Needham: "The coming of ardent water". In: Ambix 19, 1972, pp. 69-112
- ↑ Edmund O. von Lippmann and Karl Sudhoff : Thaddäus Florentinus (Taddeo Alderotti) about the spirit of wine. In: Sudhoffs Archiv 7, 1914, pp. 379–389
- ↑ Gundolf Keil : Ipokras. Personal authoritative legitimation in medieval medicine. In: Origin and Origin. Historical and mythical forms of legitimation. Edited by Peter Wunderli, Jan Thorbecke, Sigmaringen 1994, pp. 157–177; here: p. 170
- ^ Taddeo Alderotti: I "Consiglia". Publicati a cura di Giuseppe Michele Nardi, Turin 1937, pp. 235-242
- ↑ Paul Braun: The Weißenauer alcohol recipe from the 13th century. In: Contributions to Württemberg pharmacy history V (1960–1962), No. 3, 1961, p. 78 f.
- ^ Leo Jules van de Wiele: De eerste publikatie in het Nederlands over alcohol. In: Pharm. Tschr. Belg. Volume 41, 1964, pp. 65-80.
- ↑ Ram B. Gupta: Gasoline, Diesel and Ethanol Biofuels from Grasses and Plants. Cambridge Univ. Press, 2010, ISBN 0-521-76399-1 , p. 74.
- ↑ Label fraud - alcohol-free beer does contain alcohol. welt.de, March 28, 2012, accessed on March 22, 2015 .
- ↑ Principles for fruit juices. (PDF) Federal Ministry of Food, Agriculture and Consumer Protection, November 27, 2002, accessed on March 22, 2015 .
- ↑ Annex I, No. 8 of Regulation (EC) No. 1439/1999.
- ↑ Peter Bützer: "Alcohol" Ethanol. (PDF; 1000 kB) St.Gallen University of Education, February 2015, accessed on March 22, 2015 .
- ↑ DT Halfen, AJ Apponi, N. Woolf, R. Polt, and LM Ziurys: A Systematic Study of Glycolaldehyde in Sagittarius B2 (N) at 2 and 3 mm: Criteria for Detecting Large Interstellar Molecules. In: The Astrophysical Journal . Vol. 639, No. 1, 2006, pp. 237-245, doi: 10.1086 / 499225 .
- ↑ a b H. G. Hirschberg: Handbook of process engineering and plant construction. Chemistry, technology and business administration. Springer, Berlin 1999, ISBN 3-540-60623-8 , pp. 350-355.
- ↑ Beyer-Walter, Textbook of Organic Chemistry, 23rd Edition, S. Hirzel Verlag 1998 ISBN 3-7776-0808-4
- ^ A b c d W. Keim , A. Behr , G. Schmitt: Fundamentals of industrial chemistry. Salle-Sauerländer Verlag, 1986, ISBN 3-7935-5490-2 , pp. 183-184.
- ↑ E. Breitmaier, G. Jung: Organic chemistry. Basics, substance classes, reactions, concepts, molecular structure. Thieme, Stuttgart 2005, ISBN 3-13-541505-8 , p. 214.
- ^ C. Bauer-Christoph, N. Christoph, M. Rupp: Spirituosenanalytik. Behr, 2009, ISBN 3-89947-440-6 , p. 313.
- ↑ A. Rapp, A. Markowetz: NMR spectroscopy in wine analysis. In: Chemistry in Our Time . 27. Year 1993, No. 3, pp. 149-155, doi: 10.1002 / ciuz.19930270307 .
- ^ C. Ford Runge, Benjamin Senauer: How Biofuels Could Starve the Poor. Council on Foreign Affairs, May / June 2007. Retrieved March 22, 2015.
- ↑ Evelyn Boos, Thomas Priermeier: Profit Opportunity Climate Change: Investment Opportunities and Investment Strategies . Linde Verlag, Vienna 2008, ISBN 978-3-7093-0216-3 , p. 81.
- ↑ § 50 BrStV denaturation .
- ↑ RIS - Denaturation of Alcohol (VO denaturation) - Consolidated federal law, version from 07.01.2019 .
- ↑ Implementing Regulation (EU) No. 162/2013 of the Commission of February 21, 2013. (PDF; 749 kB)
- ^ Karl-Ludwig Haken: Fundamentals of automotive engineering. Hanser Verlag, 2007, ISBN 978-3-446-22812-2 , p. 23.
- ↑ a b Entry on ethanol (phase change data). In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed November 17, 2019.
- ↑ CRC , pp. 6–54, accessed on March 22, 2015.
- ^ A b Per-Gunnar Jönsson: Hydrogen Bond Studies. CXIII The Crystall Structure of Ethanol at 87 K. In: Acta Cryst. Vol. 32, 1976, pp. 232-235, doi: 10.1107 / S0567740876002653 .
- ^ EW Flick: Industrial Solvents Handbook. Fifth Edition, Noyes Data Corporation (ndc), Westwood, NJ / USA 1998, ISBN 0-8155-1413-1 , p. 252.
- ^ WC Coburn Jr., E. Grunwald: Infrared Measurements of the Association of Ethanol in Carbon Tetrachloride. In: J. Am. Chem. Soc. Vol. 80, No. 6, 1958, pp. 1318-1322, doi: 10.1021 / ja01539a010 .
- ↑ George Brink, Leslie Glasser: Studies in hydrogen bonding: the enthalpy of hydrogen bond formation of ethanol in carbon tetrachloride solutions. In: Journal of Molecular Structure . Vol. 145, 1986, pp. 219-224, doi: 10.1016 / 0022-2860 (86) 85026-8 .
- ^ Entry on diethyl sulfate in the GESTIS substance database of the IFA , accessed on March 22, 2015(JavaScript required) .
- ↑ Series of lessons on protein. WWU Münster, Seminar: School-Oriented Experimentation. WS 06/07 (PDF; 355 kB). Retrieved March 22, 2015.
- ↑ Standard instructions for hygienic hand disinfection. ( Memento from June 1, 2010 in the Internet Archive ) Institute for Hygiene and Environmental Medicine, University of Greifswald (PDF; 127 kB). Retrieved March 22, 2015.
- ↑ WIGL teaching aids: Alcoholic beverages: Spirits. (doc; 47 kB). Retrieved March 22, 2015.
- ↑ Wolfgang Staudt: 50 Simple Things You Should Know About Wine. Westend, 2007, ISBN 978-3-938060-04-9 , p. 37.
- ↑ Nagl-Netzreport: Preservation of food. ( Memento of October 8, 2007 in the Internet Archive ) (PDF; 202 kB). Retrieved March 22, 2015.
- ↑ Hard alcohol. At: Spektrum.de. Entry in the lexicon of chemistry. Retrieved March 22, 2015.
- ↑ a b H.-H. Frey, FR Althaus: Textbook of pharmacology and toxicology for veterinary medicine. Georg Thieme Verlag, 2007, ISBN 978-3-8304-1070-6 , p. 469.
- ↑ H. Hof, R. Dörries: Medical Microbiology. 3rd edition, Georg Thieme Verlag, Stuttgart 2005, p. 686.
- ↑ Percutaneous alcohol injection (PEI) of the thyroid. At: madeasy.de. Retrieved March 22, 2015.
- ↑ P. Janowitz, S. Ackmann: Long-term results of ultrasound-guided alcohol instillation in patients with focal thyroid autonomy and hyperthyroidism. In: Medical Clinic . 96, 2001, p. 451, doi: 10.1007 / PL00002227 .
- ^ W. Caspary, U. Leuschner, S. Zeuzem: Therapy of liver and gallbladder diseases. Springer, 2001, ISBN 3-540-67390-3 , p. 365.
- ↑ Lynn Thorndike and Francis S. Benjamin Jr. (Eds.): The herbal of Rufinus. Chicago 1945 (= Corpus of mediaeval scientific texts , 1), p. 119
- ↑ Volker Zimmermann: The two Harburg syphilis tracts. In: Würzburg medical history reports. Volume 7, 1989, pp. 71-81, here: p. 76.
- ^ Rudolf Frey , Otto Mayrhofer , with the support of Thomas E. Keys and John S. Lundy: Important data from the history of anesthesia. In: R. Frey, Werner Hügin , O. Mayrhofer (Ed.): Textbook of anesthesiology and resuscitation. Springer, Heidelberg / Basel / Vienna 1955; 2nd, revised and expanded edition. With the collaboration of H. Benzer. Springer-Verlag, Berlin / Heidelberg / New York 1971. ISBN 3-540-05196-1 , pp. 13-16, here: p. 13.
- ↑ Alcohol dehydrogenase. Technische Universität Darmstadt, Institute for Inorganic Chemistry, archived from the original on February 24, 2008 ; accessed on March 22, 2015 .
- ↑ a b Renewable ethanol: driving jobs, growth and innovation throughout Europe. State of the Industry. Report 2014. (PDF) 2014, archived from the original on June 16, 2015 ; accessed on March 22, 2015 .
- ^ Braskem Ethanol-to-Ethylene Plant, Brazil. At: chemicals-technology.com. Retrieved March 22, 2015.
- ↑ Entry on conservation. In: Römpp Online . Georg Thieme Verlag, accessed on March 25, 2015.
- ↑ H. Lüllmann, L. Hein, K. Mohr, M. Wehling: Pharmakologie und Toxikologie. 16th edition, Georg Thieme Verlag, 2006, ISBN 978-3-13-368516-0 , p. 521.
- ↑ K. Roth : The chemistry of the hangover: alcohol and its consequences. In: Chemistry in Our Time . Vol. 41, 2007, pp. 46-55, doi: 10.1002 / ciuz.200700409 .
- ^ W. Gerok, C. Huber, T. Meinertz, H. Henning Zeidler (eds.): The internal medicine: reference work for the specialist. 11th edition, Schattauer Verlag, 2006, ISBN 978-3-7945-2222-4 , pp. 644-646.
- ↑ a b c H.-K. Biesalski, O. Adam: Nutritional medicine: According to the nutritional medicine curriculum of the German Medical Association. 3rd edition, Georg Thieme Verlag, 2004, ISBN 978-3-13-100293-8 , pp. 520-528.
- ^ A b P. Schauder, G. Ollenschläger: Nutritional medicine: prevention and therapy. Elsevier Germany, 2006, ISBN 978-3-437-22921-3 , p. 162.
- ^ Heinrich Kasper: Nutritional medicine and dietetics. 10th edition, Elsevier, Urban & Fischer Verlag, 2004, ISBN 978-3-437-42011-5 , p. 70.
- ^ Eduard Burgis: Intensive course in general and special pharmacology. 4th edition, Elsevier, Urban & Fischer Verlag, 2008, ISBN 978-3-437-42613-1 , p. 520.
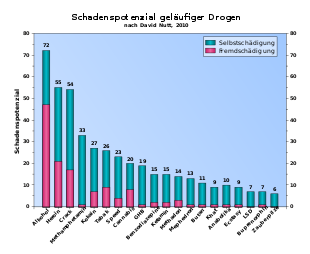
- ↑ David J. Nutt, Leslie A. King, Lawrence D. Phillips: Drug harms in the UK: a multicriteria decision analysis . In: The Lancet . tape 376 , no. 9752 , November 6, 2010, p. 1558-1565 , doi : 10.1016 / S0140-6736 (10) 61462-6 , PMID 21036393 .
- ^ Robert Gable: Drug Toxicity. Retrieved February 17, 2011 .
- ↑ RS Gable: Acute toxicity of drugs versus regulatory status. In: JM Fish (Ed.): Drugs and Society. US Public Policy. Rowman & Littlefield Publishers, Lanham, MD 2006, ISBN 0-7425-4244-0 , pp. 149-162.
- ↑ Ekkehard Grundmann (Ed.): Special Pathology. Textbook. Bgr. v. Franz Büchner, 7th, revised. Ed., Munich / Vienna / Baltmimore 1986, ISBN 3-541-00467-3 , p. 258.
- ↑ E. Grundmann (Ed.): Special Pathology. Textbook. Bgr. v. Franz Büchner, 7th, revised. Ed., Munich / Vienna / Baltmimore 1986, ISBN 3-541-00467-3 , p. 75.
- ↑ K.-H. Niessen (Ed.): Pediatrics. 3rd, revised edition, Weinheim / Basel / Cambridge / New York 1993, ISBN 3-527-15517-1 , p. 64.
- ↑ W. Forth et al. a. (Ed.): General and special pharmacology and toxicology. For students of medicine, veterinary medicine, pharmacy, chemistry, biology as well as for doctors, veterinarians and pharmacists. 6., completely reworked. Ed., Mannheim / Leipzig / Vienna / Zurich 1992, ISBN 3-411-15026-2 , p. 798.
- ↑ Man found with just under 7.7 per mille. At: Tagesspiegel.de. November 11, 2008, accessed March 22, 2015.
- ^ A. Chandrakumar, A. Bhardwaj, GW 't Jong: Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis. In: Journal of basic and clinical physiology and pharmacology. Volume 30, Number 2, October 2018, pp. 153–162, doi : 10.1515 / jbcpp-2018-0075 , PMID 30281514 .
- ^ Holly A. Stankewicz: Alcohol Related Psychosis. In: ncbi.nlm.nih.gov. December 23, 2018, accessed April 21, 2019 .
- ^ WJ Moore, DO Hummel: Physical chemistry. Walter de Gruyter, Berlin / New York 1983, ISBN 978-3-11-008554-9 , p. 958.
- ↑ M. Hesse, H. Meier, B. Zeeh: Spectroscopic methods in organic chemistry. Thieme, Stuttgart 2005, ISBN 3-13-576107-X , pp. 40-44.