carbon dioxide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | carbon dioxide | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | CO 2 | |||||||||||||||||||||
| Brief description |
colorless, odorless gas |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 44.01 g mol −1 | |||||||||||||||||||||
| Physical state |
gaseous |
|||||||||||||||||||||
| density |
1.98 kg m −3 (0 ° C and 1013 hPa) |
|||||||||||||||||||||
| Melting point |
no melting point (triple point at −56.6 ° C and 5.19 bar) |
|||||||||||||||||||||
| Sublimation point |
−78.5 ° C / 1013 mbar |
|||||||||||||||||||||
| Vapor pressure |
5.73 MPa (20 ° C) |
|||||||||||||||||||||
| solubility |
in water: 3.3 g l −1 at 0 ° C, 1.7 g l −1 at 20 ° C, both at 1013 hPa |
|||||||||||||||||||||
| Dipole moment |
0 |
|||||||||||||||||||||
| Refractive index |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
|
|||||||||||||||||||||
| Global warming potential |
1 (by definition) |
|||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−393.5 kJ mol −1 (g) |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Carbon dioxide or carbon dioxide is a chemical compound of carbon and oxygen with the empirical formula CO 2 , an incombustible, acidic and colorless gas . It dissolves well in water: colloquially it is often incorrectly called " carbonic acid ", especially in connection with beverages containing carbon dioxide . With basic metal oxides or - hydroxides it forms two types of salts , the carbonates and bicarbonates are called.
CO 2 is an important part of the global carbon cycle and, as a natural component of the air, an important greenhouse gas in the earth's atmosphere : Human activities, above all the burning of fossil fuels , increased the proportion in the earth's atmosphere from approx. 280 parts per million (ppm, parts per million ) at the beginning of industrialization to 407.8 ppm in 2018. In May 2019, a monthly average of around 415 ppm was measured at the NOAA monitoring station Mauna Loa in Hawaii, and the trend is rising. This increase increases the greenhouse effect , which in turn is the cause of current global warming . Around 100 million tons of carbon dioxide are released into the atmosphere every day through human activities (as of 2020).
With an adequate supply of oxygen , CO 2 is produced both when carbon-containing substances are burned and in the organism of living beings as a product of cellular respiration . Plants , algae and some bacteria and archaea convert CO 2 into biomass through fixation ( carbon dioxide assimilation ) . During photosynthesis , glucose is created from inorganic CO 2 and water .
CO 2 can be toxic. The concentrations in the air or the amounts due to the consumption of lemonade , for example, are far from sufficient for this. CO 2 has a broad technical spectrum of applications in the chemical industry for. B. it is used to extract urea . In solid form as dry ice , it is used as a coolant , while supercritical carbon dioxide is used as a solvent and extraction agent .
story
CO 2 was one of the first gases to be given a name. The Flemish chemist Johan Baptista van Helmont (1580–1644) observed that the mass of charcoal decreased during combustion because the mass of the remaining ash was less than that of the charcoal used. His interpretation was that the rest of the charcoal had turned into an invisible substance that he called gas or spiritus sylvestre ("forest spirit").
The Scottish doctor Joseph Black (1728–1799) studied the properties of CO 2 more thoroughly. In 1754 he found out that when calcium carbonate solutions are mixed with acids, a gas is released which he called fixed air . He realized that this was heavier than air and did not support combustion processes. When this gas was introduced into a solution of calcium hydroxide , it was able to generate a precipitate . With this phenomenon he showed that carbon dioxide occurs in the breath of mammals and is released through microbiological fermentation. His work proved that gases can be involved in chemical reactions and contributed to the case of the phlogiston theory .
Joseph Priestley succeeded in making the first soda water in 1772 by adding sulfuric acid to a calcareous solution and dissolving the resulting carbon dioxide in a beaker of water. William Brownrigg had recognized the connection between carbon dioxide and carbonic acid earlier. In 1823, Humphry Davy and Michael Faraday liquefied carbon dioxide by increasing the pressure. Henry Hill Hickman operated animals from 1820, which was achieved painlessly after inhaling carbon dioxide to achieve anesthesia . He also described the physiological processes during anesthesia. The first description of solid carbon dioxide comes from Adrien Thilorier , who in 1834 opened a pressurized container of liquid carbon dioxide and found that the spontaneous evaporation takes place with cooling, which leads to solid CO 2 .
Occurrence
Carbon dioxide is found in the atmosphere , the hydrosphere , the lithosphere, and the biosphere . The carbon exchange between these earth spheres takes place largely through carbon dioxide. Around 2015 there were around 830 gigatons (830 billion tons ) of carbon in the atmosphere in the form of carbon dioxide. The hydrosphere contains around 38,000 gigatons of carbon in the form of physically dissolved carbon dioxide as well as dissolved hydrogen carbonates and carbonates . The lithosphere contains by far the largest proportion of chemically bound carbon dioxide. Carbonate rocks such as calcite and dolomite contain around 60,000,000 gigatons of carbon. In addition, large amounts of carbon are stored in permafrost areas such as the tundras of the Arctic and Antarctic polar regions, in boreal coniferous forests or high mountains and in bogs .
Occurrence in the atmosphere and man-made climate change
- For the influence of humans see also specifically anthropogenic increase in CO2 concentration and Keeling curve .

Carbon dioxide is a naturally occurring trace gas in the earth's atmosphere that has an impact on the climate , but its concentration increases, in particular due to the burning of fossil fuels . Ice core data showed that atmospheric CO 2 levels fluctuated between 190 ppm during the climax of the ice ages and 280 ppm during the warm periods over the past 420,000 years up to the start of industrialization in the mid-18th century .
With industrialization , there was a sharp increase in the amount of carbon dioxide in the atmosphere as a result of human activities, which continues. Between 1750 and 1958 (the beginning of systematic measurements by Charles David Keeling ), the CO 2 value initially rose moderately to 315 ppm, and then increased to 401 ppm by 2015. On May 9, 2013, the local daily mean concentration exceeded the threshold of 400 ppm (0.04% by volume of the total gas envelope on earth), as measured by the National Agency for Ocean and Atmospheric Research (NOAA) of the United States on the Mauna Loa (Hawaii) revealed. The monthly global mean value measured by NOAA exceeded the 400 ppm limit for the first time in March 2015; in February 2018 this value was 408 ppm (provisional status, as the data from the previous year is still being checked). Finally, the data for 2017 show a new record high of 405.5 ppm, which is 46 percent above the pre-industrial value. In 2018, a new record high was reached again at 407.8 ppm. The main sources are the burning of fossil fuels for energy production and in the industrial sector. The release of carbon dioxide stored in soils and forests through changes in land use, for example through clearing of forests, also contributes to the increase to a much lesser extent. In 2014, energy use and industrial use of fossil fuels as well as land use accounted for 70% and 5%, respectively, of total man-made greenhouse gas emissions (measured in carbon dioxide equivalents ).
The total mass of carbon dioxide in the atmosphere is around 3000 gigatons or around 800 Gt carbon (the ratio of the molar masses of CO 2 to C is rounded 44:12). The concentration varies seasonally and locally, especially near the ground. In urban areas, the concentration is generally higher; indoors, the concentration can be up to ten times the average value.
Carbon dioxide absorbs part of the thermal radiation ( infrared radiation ), while the shorter-wave part of the solar radiation can pass through almost unhindered. An absorbent body also emits according to its temperature. These properties make carbon dioxide a so-called greenhouse gas . After water vapor , carbon dioxide is the second most effective of the greenhouse gases in terms of its proportion, although the specific effectiveness of methane and ozone are higher. All greenhouse gases together increase the mean temperature on the earth's surface from around −18 ° C to +15 ° C due to the natural greenhouse effect . Carbon dioxide has a relatively large share in the overall effect and thus contributes to the earth-friendly climate.
The proportion of carbon dioxide in the earth's atmosphere has been subject to considerable fluctuations over the course of the earth's history, which have various biological, chemical and physical causes. 500 million years ago, the concentration of carbon dioxide was at least ten times higher than it is today. As a result, the CO 2 concentration steadily decreased and was around 300 million years ago during the Permocarbon Ice Age , at the transition from the Carboniferous to the Permian , at an average of around 300 ppm and fell briefly in the early Permian to a low of probably 100 ppm during the In the Mesozoic era , the CO 2 level was mostly between 1,000 and 2,000 ppm, only to fall well below 1,000 ppm in the New Earth Age , after a climatic optimum in the early Eocene , up to the beginning of the Cenozoic Ice Age about 34 million years ago.
For at least 800,000 years, the carbon dioxide content has always been below 300 ppm. The carbon dioxide concentration has remained relatively constant at 300 ppm over the past 10,000 years. The balance of the carbon dioxide cycle was thus balanced during this time. With the beginning of industrialization in the 19th century, the amount of carbon dioxide in the atmosphere increased. The current concentration is likely the highest in 15 to 20 million years. In the period from 1960 to 2005, the carbon dioxide content rose by an average of 1.4 ppm per year. In 2017, the 10-year average increase was a good 2 ppm per year.
The anthropogenic , i.e. man-made carbon dioxide emissions amount to around 36.3 gigatons per year and are only a small proportion of the carbon dioxide, which comes mainly from natural sources, of around 550 gigatons per year. However, since the natural carbon sinks absorb the same amount of CO 2 again, the carbon dioxide concentration remained relatively constant before industrialization. About half of the additional carbon dioxide is absorbed by the biosphere and the oceans (this leads to their acidification ), so that these now absorb more carbon dioxide than they give off. As a result, since 1982 there has been a "greening" of the earth (Leaf Area Index), as has been proven by satellite data from NASA. However, more recent data suggest that this greening, which was observed until the late 20th century, subsequently stopped and, as a result of a larger saturation deficit (more drought), an opposite trend developed; H. the earth is currently losing vegetation. The other half of the carbon dioxide emitted remains in the atmosphere and leads to a measurable increase in concentration, which Charles Keeling was able to demonstrate for the first time in the early 1960s with the Keeling curve named after him .
It is widely recognized scientifically that there is a statistically significant human impact on climate that is the primary cause of global warming . This warming is most likely largely due to the anthropogenic amplification of the natural greenhouse effect through the emission of greenhouse gases. The additional carbon dioxide produced contributes around 60% to the intensification of the greenhouse effect.
Calculated per capita, Luxembourg , Belgium and Switzerland have the largest CO 2 footprint in all of Europe. The consequences of global warming should be reduced through climate protection .
Occurrence in oceans
The water of the oceans contains carbon dioxide in dissolved form and as carbonic acid in equilibrium with hydrogen carbonates and carbonates. The amount dissolved changes with the season, as it depends on the temperature and salinity of the water: Cold water dissolves more carbon dioxide. Since cold water has a higher density, the carbon dioxide-rich water sinks into deeper layers. Only at pressures above 300 bar and temperatures above 120 ° C (393 K) is it the other way round, for example in the vicinity of deep geothermal vents .
The oceans contain around 50 times as much carbon as the atmosphere. The ocean acts as a large carbon dioxide sink and absorbs around a third of the amount of carbon dioxide released by human activities. In the upper layers of the oceans, it is partially bound by photosynthesis. As the solution of carbon dioxide increases, the alkalinity of the salt water decreases , which is known as acidification of the oceans and which is very likely to have negative consequences for the ecosystems of the oceans. Many marine life are sensitive to fluctuations in the acidity of the oceans; Acidification events in the history of the earth led to mass extinctions and a sharp decline in biodiversity in the world's oceans. Organisms that build up calcium carbonate structures are particularly affected , as this dissolves as the acidity of the oceans increases. Corals , mussels and echinoderms such as starfish and sea urchins are particularly vulnerable .
Among other things, it is feared that this will have a negative effect on the formation of mussel shells, among other things . These effects are already visible in coral reefs and certain oyster farms; with increasing acidification, stronger ecological consequences are expected. On the other hand, there is evidence that an increased concentration of carbon dioxide stimulates some species to produce more mussel shells.
Occurrence in fresh water
Aerobic bacteria and animals living in the (under) water consume oxygen and exhale CO 2 . If there is sufficient contact with the atmosphere, this gas can be released into the air and oxygen can be absorbed at the same time. A surface that freely adjoins the air and is free of ice or oil is beneficial, as are wave movements, turbulence with air, i.e. the formation of foam and spray, water currents that also include deeper layers and wind. Without sufficient gas exchange, a body of water can become oxygen-poor and CO 2 -rich on the surface . They say "it tips over".
Due to special geological conditions, fresh water can be loaded with considerable amounts of carbon dioxide from volcanic sources, such as water from mineral springs or in lakes on extinct volcanoes, so-called maars . Under the pressure of great water depth, CO 2 can be dissolved in a much higher mass concentration than under atmospheric pressure at the surface of the water. If a lake is not (sufficiently) flowed through by water or is mixed by wind and / or heat convection currents and if more CO 2 is brought in from below than mixing and diffusion can transport upwards , then CO 2 -rich deep water forms , which has the potential to increase a catastrophic CO 2 release into the air. A local outgassing once triggered locally under water leads to the rise of a mass of water, the relief of hydrostatic pressure that takes place in the process intensifies the outgassing. This self-reinforcing process can lead to the release of large amounts of CO 2 , which can kill people and animals near the lake.
One of these natural disasters occurred in 1986 at Lake Nyos in Cameroon . The lake is located in an old volcanic crater in the Oku volcanic area. A magma chamber feeds the lake with carbon dioxide and thus saturates its water. Probably triggered by a landslide in 1986, large amounts of carbon dioxide were released from the lake, killing around 1,700 residents and 3,500 livestock in the surrounding villages. Another catastrophe occurred in 1984 at Lake Manoun , the water of which is saturated with carbon dioxide by a similar mechanism. 37 people were killed in this carbon dioxide release. The Kiwu Lake in Central Africa also has high concentrations of dissolved gases in its deep water. It is estimated that around 250 km³ of carbon dioxide is dissolved in this lake.
Extraterrestrial occurrence
The atmosphere of Venus consists of 96.5% carbon dioxide, has about 90 times the mass of the earth's atmosphere and a pressure of about 90 bar. The high proportion of carbon dioxide is one of the causes of the strong greenhouse effect. In addition, the distance from the sun is on average 41 million kilometers shorter than the earth, which leads to a surface temperature of around 480 ° C. With a share of 95%, carbon dioxide also makes up the main part of the Martian atmosphere . At the Mars poles, atmospheric carbon dioxide is partially bound as dry ice. Due to the low atmospheric pressure of about seven millibars, the greenhouse effect only leads to an increase of about 5 K despite the high carbon dioxide content. The atmospheres of the outer planets and their satellites contain carbon dioxide, the origin of which is impacts from comets such as Shoemaker-Levy 9 and cosmic dust is attributed. Using the instruments of the Hubble Space Telescope , NASA found carbon dioxide on extrasolar planets like HD 189733 b .
Carbon dioxide is found both in interstellar space and in protoplanetary disks around young stars. The formation takes place through surface reactions of carbon monoxide and oxygen on water ice particles at temperatures around −123 ° C (150 K). When the ice evaporates, the carbon dioxide is released. The concentration in free interstellar space is relatively low, as reactions with atomic and molecular hydrogen form water and carbon monoxide.
Extraction and presentation
Carbon dioxide is produced by burning carbonaceous fuels, especially fossil fuels . Around 36 gigatons (billion tons) of carbon dioxide are produced around the world each year and are released into the atmosphere. Processes for separating carbon dioxide and storing it in deep rock layers are currently (2016) at the beginning of their development and are not yet ready for series production; their effectiveness and profitability, especially in sustainable energy systems, are critically assessed.
Carbon dioxide is produced when carbon reacts with oxygen :
Technically, carbon dioxide is produced when coke is burned with excess air. In coal gasification and the steam reforming of natural gas , carbon dioxide is produced, among other things, as a product of the water-gas shift reaction in synthesis gas production.
For use in ammonia synthesis and in methanol production , for example, the synthesis gas is washed using the Rectisol process , which means that large amounts of carbon dioxide are produced in a very pure form. Carbon dioxide is a by-product of lime burning . Subsequent purification via the formation of potassium carbonate to hydrogen carbonate and subsequent release by heating, around 530 million tons per year are obtained.
In the laboratory, carbon dioxide can be released from calcium carbonate and hydrochloric acid , for example in a Kipp apparatus . The device was previously used in laboratories. The method is rarely used any more, as carbon dioxide is available in gas bottles or as dry ice .
Carbon dioxide is also extracted from the air using the direct air capture (DAC) process.
properties

Physical Properties

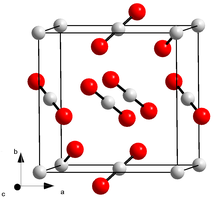
At normal pressure below −78.5 ° C, carbon dioxide is present as a solid, known as dry ice . If this is heated, it does not melt, but rather sublimates , i.e. it changes directly into the gaseous state of aggregation. It therefore has no melting point and no boiling point under these conditions.
The triple point at which the three phases solid, liquid and gaseous are in thermodynamic equilibrium is at a temperature of −56.6 ° C and a pressure of 5.19 bar .
The critical temperature is 31.0 ° C, the critical pressure 73.8 bar and the critical density 0.468 g / cm³. Below the critical temperature, gaseous carbon dioxide can be compressed to a colorless liquid by increasing the pressure. A pressure of around 60 bar is required for this at room temperature.
Solid carbon dioxide crystallizes in the cubic crystal system in the space group Pa 3 (space group no. 205) with the lattice parameter a = 562.4 pm .
Carbon dioxide absorbs electromagnetic radiation mainly in the spectral range of infrared radiation and is stimulated to vibrate molecules . Its effect as a greenhouse gas is based on this property .
The solubility in water is comparatively high. At 20 ° C under normal pressure, the saturation is in equilibrium with the pure carbon dioxide phase at 1688 mg / l. For comparison, the solubility of oxygen or nitrogen is shown below : with a pure oxygen phase, saturation is reached at 44 mg / l and with a pure nitrogen phase at 19 mg / l. Under standard conditions the density of carbon dioxide is 1.98 kg / m³.
Molecular Properties
The carbon dioxide molecule is linear, all three atoms are in a straight line. The carbon is bound to the two oxygen atoms with double bonds , with both oxygen atoms having two lone pairs of electrons . The carbon-oxygen distance is 116.32 pm. The carbon-oxygen bonds are polarized by the different electronegativities of carbon and oxygen ; the electrical dipole moments cancel each other out due to the molecular symmetry , so that the molecule does not have an electrical dipole moment. The (bending) vibration mode of the molecule, in which the carbon atom moves perpendicular to the axis and the oxygen atoms in the opposite direction (and vice versa), corresponds to an infrared wavelength of 15 μm . This 15 μm radiation is the main component the effect of carbon dioxide as a greenhouse gas .
Chemical properties
Carbon dioxide is an incombustible, acidic and colorless gas; At low concentrations it is odorless, at high concentrations one perceives a sharp to sour odor, although here too (similar to, for example, hydrogen cyanide ) there are people who cannot perceive this odor. Carbon dioxide dissolved in water forms carbonic acid (H 2 CO 3 ), whereby more than 99% of the carbon dioxide is only physically dissolved; the aqueous solution therefore reacts slightly acidic. The carbonic acid as such and the dissolved carbon dioxide are in equilibrium with their dissociation products ( species ) hydrogen carbonate ( bicarbonate , HCO 3 - ) and carbonate (CO 3 2− ), which are in a proportion to each other depending on the pH value . In water, this equilibrium is predominantly on the side of carbon dioxide and hydrogen carbonate ions are only formed to a small extent. If the oxonium ions (H 3 O + ) formed during dissociation are intercepted with hydroxide ions (OH - ) by adding a lye , the quantitative ratio shifts in favor of carbonate.
Carbon dioxide is a very weak oxidizing agent . Base metals such as magnesium , which act as strong reducing agents , react with carbon dioxide to form carbon and metal oxides according to:
Due to the positive partial charge on carbon, carbon dioxide reacts as an electrophile in the carboxylation of carbon nucleophiles such as metal alkynylidene or alkyl magnesium compounds to form a carbon-carbon bond. Carbon dioxide reacts with phenolates to form phenol carboxylic acids .
use
In industry, carbon dioxide is used in a variety of ways. It is inexpensive, non-flammable and is used physically as a compressed gas, in liquid form, solid as dry ice or in supercritical phase. The chemical industry uses carbon dioxide as a raw material for chemical synthesis. This CO 2 comes from z. B. from fertilizer production, where it is very pure, or from exhaust gases that require downstream cleaning to remove unwanted accompanying substances.
Use in food technology

Carbon dioxide contained in beverages stimulates the taste sensory cells when drinking, which has a refreshing effect. In drinks such as beer or sparkling wine , it is produced through alcoholic fermentation , in others, such as lemonade or soda water , it is added artificially or natural mineral water containing carbon dioxide is used. During production, carbon dioxide is pumped into the drink under high pressure, about 0.2% of which reacts with water to form carbonic acid, while the majority of it dissolves as a gas in the water. As a food additive , it bears the designation E 290. In private households, carbon dioxide from pressure cartridges is passed through the drink to be enriched with soda makers .
Baker's yeast develops carbon dioxide through the fermentation of sugar and is used as a leavening agent in the production of yeast dough . Baking powder , a mixture of sodium hydrogen carbonate and an acid salt, releases carbon dioxide when heated and is also used as a leavening agent.
In winemaking, dry ice is used as a coolant to cool freshly picked grapes without diluting them with water, thus avoiding spontaneous fermentation. The winemakers in Beaujolais use carbonic acid maceration to produce Beaujolais Primeur .
In addition to the temperature, the composition of the atmosphere plays an important role in the storage of fruit and vegetables. In the warehouses of fruit producers and retailers, apples have been stored in controlled atmospheres for many decades. The realization that ripening fruit consumes oxygen and gives off carbon dioxide and that an atmosphere without oxygen brings ripening to a standstill goes back to the early 19th century. In the 1930s, a warehouse was first set up in Great Britain with the ability to regulate the levels of oxygen and carbon dioxide in the air. The economic importance of precisely adapted controlled atmospheres in fruit storage is considerable. By adding carbon dioxide to the atmosphere, the shelf life can be extended by months, thus reducing dependence on imports from warmer regions for part of winter and spring. On the other hand, an improper addition of carbon dioxide can cause defects in the pulp and render an entire inventory or container shipment worthless. The biochemical processes that lead to the delayed maturity have not yet been deciphered. It is currently assumed that both the slowing down of the maturation process and the formation of the various types of damage are controlled by stress reactions at the cellular level.
Fruit, vegetables and mushrooms packaged in foil for retail purposes, unprocessed or cut, are provided with a protective atmosphere in order to extend their shelf life and not to lose the impression of freshness on the way to the consumer. Today, meat, fish and seafood , pasta, baked goods and dairy products are also offered in this way. The proportion of carbon dioxide in the protective atmosphere is significantly higher for packaged products that are not intended to be stored for months than for stored fruit and vegetables (1–5%, rarely up to 20%), for which carbon dioxide can cause damage. Typical proportions are 20% carbon dioxide for beef, 50% for veal, pork and pasta , 60% for baked goods and 80% for fish. Packaging under pure carbon dioxide is avoided, however, as it would promote the development of pathogenic, anaerobic germs and in many cases would impair the color and taste of the products. Determining the optimal protective atmosphere for a product is the subject of intensive research in the food industry .
Supercritical carbon dioxide has a high solubility for non-polar substances and can replace toxic organic solvents . It is used as an extraction agent, for example for the extraction of natural substances such as caffeine in the production of decaffeinated coffee through decaffeination .
Technical use
Carbon dioxide is due to its oxygen-displacing properties fire-fighting purposes , especially in portable fire extinguishers and automatic fire extinguishing systems, as extinguishing agent used. CO 2 extinguishing systems flood the entire room with carbon dioxide to protect silos or storage halls for flammable liquids. This led to repeated accidents, some of which resulted in death from suffocation. A study by the US Environmental Protection Agency (EPA) identified 51 accidents between 1975 and 1997 with 72 deaths and 145 injuries.
As a refrigerant , carbon dioxide is used under the designation R744 in vehicle and stationary air conditioning systems, in industrial refrigeration technology, supermarket and transport refrigeration and in drinks machines. It has a large volumetric cooling capacity and therefore a higher efficiency for a given volume. Carbon dioxide is more environmentally friendly because its global warming potential is only a fraction of that of synthetic refrigerants. In contrast to these, it does not contribute to ozone depletion. Carbon dioxide is also used in air conditioning systems for vehicles. In gas-cooled nuclear reactors of the AGR type , carbon dioxide is used as a coolant .
Carbon dioxide is used as a protective gas in welding technology, either in its pure form or as an additive to argon or helium . At high temperatures it is thermodynamically unstable, which is why it is not called an inert gas , but an active gas .
With the carbon dioxide laser, laser gas, a mixture of nitrogen , helium and carbon dioxide, flows continuously through the discharge tube. In addition to the solid-state lasers , these gas lasers are among the most powerful lasers in industrial use with outputs between 10 watts and 80 kilowatts. The efficiency is around 10 to 20%.
In liquid form, carbon dioxide is traded in pressurized gas cylinders . There are two types: ascending pipe bottles for withdrawing liquids and bottles without a rising pipe for withdrawing gaseous carbon dioxide. Both must be vertical for removal. The riser cylinder is always operated without, the other with a pressure reducing valve . As long as there is still liquid carbon dioxide in the pressure bottle, the internal pressure is only dependent on the temperature. A measurement of the fill level is therefore only possible with both types of bottle using a weighing system. The removal speed is limited by the fact that the liquid carbon dioxide has to evaporate again in the bottle due to the absorption of heat from the environment in order to build up the pressure corresponding to the temperature again.
During the sublimation of dry ice , a white mist is created from the cold carbon dioxide-air mixture and condensing humidity, which serves as a stage effect. There are also fog cooling attachments for evaporator fog machines that are operated with liquid carbon dioxide.
Increasingly, carbon dioxide is used in conjunction with an automatable blasting process to produce high-purity surfaces. With its combination of mechanical, thermal and chemical properties, carbon dioxide snow , for example, can loosen and remove various types of surface contamination without leaving any residue.
Supercritical carbon dioxide is used as a solvent for cleaning and degreasing, for example wafers in the semiconductor industry and textiles in dry cleaning . Supercritical carbon dioxide is used as a reaction medium for the production of fine chemicals, for example for the production of flavorings, since isolated enzymes often remain active in them and, in contrast to organic solvents, no solvent residues remain in the products.
In tertiary oil production , supercritical carbon dioxide is used to flood oil reservoirs in order to flush oil to the surface from greater depths.
Heat pipes filled with carbon dioxide are used to provide geothermal energy and are more energy efficient than brine cycles.
Use as a chemical raw material
In the chemical industry, carbon dioxide is used primarily for the production of urea through conversion with ammonia . In the first step, ammonia and carbon dioxide react to form ammonium carbamate , which in the second step further reacts to form urea and water.
Formamide is obtained by reduction with hydrogen . By reaction with amines such as dimethylamine is dimethylformamide obtained.
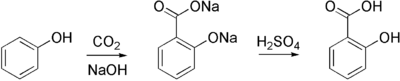
By reacting carbon dioxide with sodium phenolate, the Kolbe-Schmitt reaction produces salicylic acid.
By reaction with ethylene oxide is ethylene produced. In the OMEGA process, this is converted into monoethylene glycol in a highly selective manner with water .
The reaction of carbon dioxide with a Grignard reagent leads to carboxylic acids , e.g. B .:
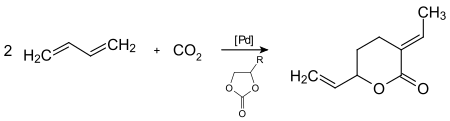
The telomerization of carbon dioxide with two molecules of 1,3-butadiene under homogeneous palladium - catalysis leads to fine chemicals such as lactones under mild reaction conditions.
In the Solvay process , carbon dioxide is used to produce soda (sodium carbonate). Some metal carbonates such as lead carbonate , which are obtained, for example, by reacting the metal hydroxides with carbon dioxide, are important as pigments .
With a high oil price and low electricity prices for renewable energies, for example from wind power and solar systems , it could be worthwhile in the future to use carbon dioxide for other applications such as methane production in power-to-gas systems ( Sabatier process ) and methanol production ( Power -to-Liquid ) with hydrogen from electrolysis . Further potential fields of application would be the production of formic acid and synthesis gases for the production of fuels ( power-to-fuel ) and chemical raw materials ( power-to-chemicals ). This can be done via a Fischer-Tropsch synthesis or the direct use together with ethylene oxide or propylene oxide for the production of polyols and polymers such as polyurethanes or polycarbonates. For thermodynamic reasons , however, the use of carbon dioxide is currently mostly uneconomical.
Carbon dioxide recycling
In addition to the separation and storage of carbon dioxide , research is aimed at converting the carbon dioxide that arises from the combustion of fossil fuels into usable compounds and, if possible, back into energy sources. Compounds such as methanol and formic acid can already be produced via reduction .
The synthesis of urea is also possible. A French research team is investigating the organocatalytic conversion to formamide or its derivatives. Since the process energy has to be supplied, these processes are not suitable for the economical production of energy carriers. Scientists at RWTH Aachen University developed a homogeneous catalytic process for the production of methanol from carbon dioxide and hydrogen under pressure with a special ruthenium-phosphine complex in which the catalyst and starting materials are in solution. Likewise, a continuous process for the production of formic acid with an organometallic ruthenium complex has been developed, in which carbon dioxide has the double role both as a reactant and in supercritical form as an extractive phase for the formic acid formed. In another variant developed by a Spanish research group, carbon dioxide can be converted via an iridium-catalyzed hydrosilylation and captured in the form of a silyl formate from which formic acid can be easily separated. This reaction, which could already be carried out on a gram scale, takes place under very mild reaction conditions, is very selective and has a high conversion.
In the “ Coal Innovation Center ”, RWE and Brain AG are researching how microorganisms convert CO 2 .
Other uses
Carbon dioxide was routinely used as an anesthetic in humans until the 1950s, particularly in the United States, and was found to be very satisfactory. This method is no longer used in traditional anesthesia for humans, as more effective, inhalable anesthetics have been introduced.
This method is still used today for stunning animals for slaughter . Pigs are lowered into a pit in groups with an elevator system, the atmosphere of which contains at least 80% carbon dioxide, and lose consciousness in it. This process is controversial and is subject to intensive efforts to improve animal welfare . Fish are anesthetized by injecting gaseous carbon dioxide or adding carbonated water. In Germany, stunning slaughter animals with carbon dioxide is only permitted for pigs, turkeys , day-old chicks and salmon fish .
In the context of animal euthanasia , carbon dioxide is used for killing. In Germany, the application is limited to small laboratory animals , also for purposes such as the procurement of feed animals in animal husbandry. However, the legality of such animal killings without prior stunning has been questioned. For officially ordered killing of livestock, the club , carbon dioxide may also be used to kill other animals if a special permit is available. The Veterinary Association for Animal Welfare (DVT) describes this method as suitable for poultry.
Carbon dioxide is used as a laxative in suppositories . The reaction of sodium dihydrogen phosphate and sodium hydrogen carbonate during the dissolution of the suppository releases carbon dioxide and stretches the bowel, which in turn triggers the stool reflex.
In carbon dioxide fertilization , it is used as a fertilizer in greenhouses . The reason is the carbon dioxide deficiency caused by photosynthetic consumption when there is insufficient fresh air, especially in winter when the ventilation is closed. The carbon dioxide is introduced either directly as a pure gas or as a combustion product from propane or natural gas. This achieves a coupling of fertilization and heating. The possible increase in yield depends on how strong the lack of carbon dioxide is and how strong the light is available for the plants. Carbon dioxide is used in aquaristics as a fertilizer for aquatic plants (CO 2 diffuser). By adding organic matter, the carbon dioxide content in the water can be increased through inhalation at the expense of the oxygen content.
The gas is used to catch blood-sucking insects and vectors that use the carbon dioxide found in the breath to find hosts , such as mosquitoes . It is released from dry ice , from gas cylinders or from the combustion of propane or butane and attracts insects near the intake opening of special traps. The gas is also used in the cultivation of microorganisms , especially for obligate (strictly) anaerobic bacteria, which can only grow under anoxic conditions. They can be incubated in a CO 2 incubator that is supplied by a gas bottle. In addition to strictly anaerobic bacteria, there are also so-called capnophilic bacteria, which require a proportion of 5–10 percent by volume of carbon dioxide in the surrounding atmosphere for growth. They are often cultivated in a closable anaerobic pot into which a commercially available reagent carrier is placed, the chambers of which are filled with sodium hydrogen carbonate and tartaric acid or citric acid . Moistening releases CO 2 , similar to the principle of baking powder .
Physiological effects and hazards
Effect on animals and people
Too high a proportion of carbon dioxide in the air we breathe has harmful effects on animals and humans. These are not only based on the displacement of oxygen in the air. DIN EN 13779 divides the room air into four quality levels depending on the carbon dioxide concentration. At values below 800 ppm, the indoor air quality is considered to be good, values between 800 and 1000 ppm (0.08 to 0.1% by volume) are considered to be medium, and values of 1000 to 1400 ppm are considered to be of moderate quality. At values above 1400 ppm, the indoor air quality is considered to be low. For comparison: the global mean the CO 2 content of the air is around 400 ppm by volume; however, it fluctuates strongly depending on the region, time of day and season.
The maximum workplace concentration for a daily exposure of eight hours per day is 5000 ppm. At a concentration of 1.5% (15,000 ppm) the respiratory time volume increases by more than 40%.
According to studies, significantly increased CO 2 concentrations and / or lack of ventilation in rooms with comparatively clean ambient air can lead to a strong and avoidable impairment of brain performance - especially in decision-making and complex strategic thinking - in rooms such as classrooms.
Carbon dioxide dissolved in the blood activates the breathing center of the brain in a physiological and slightly increased concentration .
In significantly higher concentrations, it leads to a reduction or elimination of the reflex breathing stimulus, initially to respiratory depression and finally to respiratory arrest . From about 5% carbon dioxide in the inhaled air headaches and dizziness occur, with higher concentrations accelerated heartbeat ( tachycardia ), rise in blood pressure, shortness of breath and unconsciousness , the so-called carbon dioxide anesthesia . Carbon dioxide concentrations of 8% lead to death within 30 to 60 minutes. An accumulation of carbon dioxide in the blood is called hypercapnia .
Accidents occur again and again in wine cellars, feed silos, wells and cesspools due to high carbon dioxide concentrations. Fermentation processes there produce considerable amounts of carbon dioxide; when fermenting one liter of must, for example, around 50 liters of fermentation gas. Often several people fall victim to fermentation gas poisoning because the helpers inhale carbon dioxide themselves during the rescue attempt and become unconscious. Rescuing an injured person from suspected carbon dioxide situations is only possible by professional emergency responders with self- contained breathing apparatus .
If adequate ventilation is not provided, natural carbon dioxide sources in caves and mine tunnels can create high concentrations of the gas. These are then close to the ground, so that smaller animals in particular can suffocate. For example, the dog grotto in Italy has a carbon dioxide concentration of around 70%. Around 1700 people died in a CO 2 outbreak in Lake Nyos in 1986.
The carbon dioxide concentration in the blood influences its pH value and thus has an indirect effect on the oxygen balance. The carbonic acid-bicarbonate system , a carbonic acid - bicarbonate buffer, represents around 50% of the total buffer capacity of the blood, which is catalyzed by the enzyme carbonic anhydratase.
At a lower pH value, the oxygen-binding capacity of the red blood pigment hemoglobin is reduced . With the same oxygen content in the air, hemoglobin therefore transports less oxygen. The Bohr effect and the Haldane effect describe this fact.
Effect on plants
On plants, a slightly increased carbon dioxide concentration has the effect of carbon dioxide fertilization , since the plants need CO 2 for carbon dioxide assimilation during photosynthesis . However, excessively high concentrations are also harmful to plants. For C3 plants , the optimum is usually between 800 and 1000 ppm, but for C4 plants it is only just over 400 ppm. The C4 plant maize as an indicator plant showed streaks on its leaves at 10,000 ppm CO 2 after a six-day exposure period. In rice changes were noted in the nutrient composition (proteins, micronutrients and vitamins). Protein, iron, zinc, vitamins B1, B2, B5 and B9 decrease as the CO 2 concentration rises excessively , whereas vitamin E increases. Such a reduction in the quality of plant foods would exacerbate the problem of global malnutrition .
Ecological importance
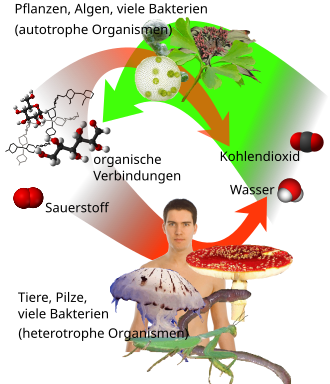
Plants and photosynthetic bacteria absorb carbon dioxide from the atmosphere and convert it into carbohydrates such as glucose through photosynthesis under the action of light and absorption of water .
- simplified net reaction equation for oxygenic photosynthesis
This process simultaneously releases oxygen from the decomposition of water. The resulting carbohydrates serve as an energy source and building material for all other biochemical substances such as polysaccharides , nucleic acids and proteins . Carbon dioxide thus provides the raw material for the formation of all biomass in the primary production of ecosystems .
The breakdown of biomass through aerobic respiration is, in reverse to the process of photosynthesis, connected again with the formation of carbon dioxide and the consumption of oxygen.
- simplified net reaction equation for aerobic respiration
All organisms in an ecosystem breathe continuously, while photosynthesis is tied to the availability of light. This leads to a cyclical increase and decrease of carbon dioxide in the daily and seasonal rhythm depending on the different light intensities.
In water bodies, the carbon dioxide concentration also fluctuates according to the daily and seasonal rhythms mentioned. Carbon dioxide is in a chemical equilibrium with the other dissolved carbonic acid species, which essentially determines the pH value in the water. The chemical equilibrium of the dissociations of ammonium / ammonia , nitrite / nitrous acid , sulphide / hydrogen sulphide and other acid-base pairs, which are noticeable through the toxicity for the organisms in the water, depend on the pH value .
If the supply of carbon dioxide in a body of water is exhausted through photosynthesis, which is noticeable by a pH value close to 8.3, some types of algae and aquatic plants are able to obtain the required carbon dioxide from the dissolved hydrogen carbonate, whereby they release hydroxide ions , so that the pH value becomes more and more alkaline. In nutrient-rich waters such as carp ponds, the pH value can then rise to 12, with the corresponding health consequences for the fish, for example carp gill necrosis .
In 2012, scientists from the Biodiversity and Climate Research Center calculated for the first time in a joint study with other institutions that cryptogamous layers of lichen, algae and moss bind around 14 billion tons of carbon dioxide annually in addition to nitrogen. They bind as much carbon dioxide as is released each year by forest fires and the burning of biomass worldwide. Fighting climate change with the help of the cryptogamous layers is not possible, however, because the extensive vegetation stores the greenhouse gas carbon dioxide for only a few years.
The storage and release of carbon dioxide in soils is important . The extent to which the release of soil organic carbon is influenced by the respective environmental conditions and other factors is currently largely unknown. However, the release is accelerated by warming, which has been shown in recent studies, and could have an impact on the climate. A study published in 2019 shows that if the CO 2 concentration exceeds 1,200 ppm, stratocumulus clouds break up into scattered clouds, which could further fuel global warming.
By specifying the CO 2 emissions, various processes can be compared in terms of energy and ecology. For this purpose, the release of carbon dioxide when burning fossil fuels is converted.
Detection and quantitative determination
A simple detection of carbon dioxide is possible with an aqueous calcium hydroxide solution , the so-called lime water sample . For this purpose, the gas to be examined is introduced into the solution. If the gas contains carbon dioxide, it reacts with calcium hydroxide to form water and calcium carbonate (lime), which precipitates as a whitish solid and makes the solution cloudy.
With barium water , an aqueous barium hydroxide solution , the detection is more sensitive, since barium carbonate is less soluble than calcium carbonate.
In aqueous solution, carbon dioxide is determined by titration with 0.1 N sodium hydroxide solution up to pH 8.3, the color change of the phenolphthalein indicator . The measurement of the acid binding capacity (SBV) , the pH value and the electrical conductivity or the ionic strength enables the calculation of the carbon dioxide content from these parameters according to the dissociation equilibrium of the carbonic acid. The Severinghaus electrode , a pH electrode with a buffer solution made from sodium hydrogen carbonate, determines the carbon dioxide concentration of a solution by measuring the change in pH value.
Carbon dioxide can be detected using infrared or Raman spectroscopy , whereby the asymmetrical stretching vibrations and tilting vibrations are infrared-active, while the symmetrical stretching vibrations at a wave number of 1480 cm −1 are Raman-active. The measuring device used for this is called a non-dispersive infrared sensor .
literature
- Hans-Otto Pörtner : Effects of CO 2 input and temperature increase on the marine biosphere. (PDF; 1.3 MB).
- Jens Soentgen , Armin Reller : CO 2 - the elixir of life and climate killer. Oekom-Verlag, Munich 2009, ISBN 978-3-86581-118-9 .
- James Newton Butler: Carbon Dioxide Equilibria and Their Applications. Lewis Publishers, 1991, ISBN 0-87371-624-8 .
- Martin M. Halmann, Meyer Steinberg: Greenhouse Gas Carbon Dioxide Mitigation: Science and Technology. CRC Press , 1998, ISBN 1-56670-284-4 .
- Martin M. Halmann: Chemical Fixation of Carbon Dioxide Methods Recycling: Methods of Recycling CO 2 into Useful Products. CRC Press, 1993, ISBN 0-8493-4428-X .
Web links
- co2online.de
- Federal Environment Agency , German Emissions Trading Authority (DEHSt): dehst.de
- US Department of Energy : cdiac.ornl.gov Carbon Dioxide Information Analysis Center
Individual evidence
- ↑ Entry on E 290: Carbon dioxide in the European database on food additives, accessed on July 1, 2020.
- ↑ Entry on CARBON DIOXIDE in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ Entry on carbon dioxide. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b c d e f g h i Entry on carbon dioxide in the GESTIS substance database of the IFA , accessed on February 1, 2016. (JavaScript required)
- ↑ Carbon Dioxide Solubility in Water ( Memento from March 27, 2010 in the Internet Archive )
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Permittivity (Dielectric Constant) of Gases, pp. 6-188.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Gases, pp. 10-254.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Inorganic Liquids, pp. 4-140.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 124-38-9 or carbon dioxide ), accessed on November 2, 2015.
- ^ United Nations Framework Convention on Climate Change: Global Warming Potentials .
- ↑ Entry on carbon dioxide . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed March 22, 2010.
- ↑ Greenhouse gas concentrations in atmosphere reach yet another high. WMO, November 25, 2019, accessed November 27, 2019 .
- ↑ Verena Kern: Greenhouse gas concentration reaches new record. In: Klimareporter. November 25, 2019, accessed November 27, 2019 .
- ↑ Florian Rötzer on Telepolis: CO2 emissions in the atmosphere continue to rise exponentially. June 7, 2019, accessed July 7, 2019 .
- ↑ Carbon Dioxide Levels Hit Record Peak in May. June 3, 2019, accessed on July 7, 2019 .
- ↑ a b World Meteorological Organization : Greenhouse gas concentrations in atmosphere reach yet another high. November 25, 2019, accessed November 25, 2019 .
- ↑ Jochem Marotzke : "Predictions are difficult ..." Possibilities and limits of climate models. In: Ders., Martin Stratmann (Ed.): The future of the climate. New insights, new challenges. A report by the Max Planck Society . Beck, Munich 2015, ISBN 978-3-406-66968-2 , pp. 9-22, here p. 22.
- ↑ Corinne Le Quéré et al .: Temporary reduction in daily global CO 2 emissions during the COVID-19 forced confinement . In: Nature Climate Change . tape 10 , 2020, p. 647-653 , doi : 10.1038 / s41558-020-0797-x .
- ^ William Emerson Brock, B. Kleidt, H. Voelker: Viewegs Geschichte der Chemie , Springer-Verlag, ISBN 3-540-67033-5 , p. 35.
- ^ William Emerson Brock, B. Kleidt, H. Voelker: Viewegs Geschichte der Chemie , Springer-Verlag, ISBN 3-540-67033-5 , p. 50.
- ^ William Emerson Brock, B. Kleidt, H. Voelker: Viewegs history of chemistry. Springer-Verlag, ISBN 3-540-67033-5 , p. 72.
- ↑ J. Priestley, W. Hey: Observations on Different Kinds of Air. By Joseph Priestley, L LDFRS. In: Philosophical Transactions of the Royal Society of London . 62 (1772), pp. 147-264, doi: 10.1098 / rstl.1772.0021 .
- ^ Humphry Davy: On the Applicatin of Liquids Formed by the Condensation of Gases as Mechanical Agents. In: Philosophical Transactions of the Royal Society of London . 113 (1823), pp. 199-205, doi: 10.1098 / rstl.1823.0020 .
- ↑ Ludwig Brandt, Karl-Heinz Krauskopf: "A discovery in surgery". 150 years of anesthesia. In: The anesthesiologist. Volume 45, 1996, pp. 970-975, here: p. 973.
- ^ Joost Mertens, You côté d'un chimiste nommé Thilorier. Balthazar Claës modèle d'Adrien Thilorier , L'Année balzacienne, 1, 2003, no.4
- ↑ Markus Reichstein: Universally and Everywhere. The terrestrial carbon cycle in the climate system . In: Jochem Marotzke , Martin Stratmann (ed.): The future of the climate. New insights, new challenges. A report from the Max Planck Society . Beck, Munich 2015, ISBN 978-3-406-66968-2 , pp. 123-136, especially p. 125.
- ↑ Martin Kappas: Climatology. Spektrum Akademischer Verlag, Heidelberg 2009, ISBN 978-3-8274-1827-2 , p. 159.
- ↑ Andrea Rehmsmeier : On Thin Ice Deutschlandfunk - Science in Focus, August 7, 2016.
- ↑ ipa.arcticportal.org: International Permafrost Association (November 5, 2016).
- ↑ Naturschutzbund Deutschland - Information about peatlands as carbon stores and their related importance for climate protection
- ^ Stefan Rahmstorf , Hans Joachim Schellnhuber : The climate change . CH Beck, 7th edition 2012, p. 23.
- ^ Hans Günter Brauch : Historical Times and Turning Points in a Turbulent Century: 1914, 1945, 1989 and 2014? , in: Ders., Ursula Oswald Spring, Juliet Bennett, Serena Eréndira Serrano Oswald (Eds.) Addressing Global Environmental Challenges from a Peace Ecology Perspective . Cham 2016, 11–68, pp. 29–31.
- ^ J. Ewald: Carbon Dioxide at NOAA's Mauna Loa Observatory reaches new milestone: Tops 400 ppm. In: NOAA Research News. May 10, 2013, accessed June 4, 2018 .
- ↑ Stern.de , May 7, 2015, CO2 concentration in the atmosphere reaches record level ( Memento of May 8, 2015 in the Internet Archive ) (May 9, 2015).
- ↑ NOAA Earth System Research Laboratory: Trends in Atmospheric Carbon Dioxide - Recent Global CO 2 . May 6, 2018, accessed June 2, 2018 .
- ↑ Christian Speicher: The CO 2 concentration climbs to a new record. In: nzz.ch . November 22, 2018, accessed June 13, 2021 .
- ^ Ottmar Edenhofer , Michael Jakob: Climate Policy. Goals, conflicts, solutions . Munich 2017, p. 20.
- ↑ Indoor air quality : carbon dioxide (CO 2 ), temperature and humidity in school classrooms. In: Lower Saxony Ministry for the Environment, Energy and Climate Protection . September 25, 2013. Retrieved May 19, 2013 .
- ↑ Karsten Schwanke, Nadja Podbregar, Dieter Lohmann, Harald Frater: Natural catastrophes. Hurricanes, quakes, volcanic eruptions - unleashed violence and its consequences. Springer Verlag, Berlin / Heidelberg 2009, ISBN 978-3-540-88684-6 , p. 119.
- ^ Carbon Dioxide through Geologic Time. In: Geoscience Research Division at Scripps Institution of Oceanography . Retrieved December 21, 2013 .
- ↑ Isabel P. Montañez, Jennifer C. McElwain, Christopher J. Poulsen, Joseph D. White, William A. DiMichele, Jonathan P. Wilson, Galen Griggs, Michael T. Hren: Climate, pCO 2 and terrestrial carbon cycle linkages during late Palaeozoic glacial – interglacial cycles . (PDF) In: Nature Geoscience . 9, No. 11, November 2016, pp. 824–828. doi : 10.1038 / ngeo2822 .
- ^ Georg Feulner: Formation of most of our coal brought Earth close to global glaciation . In: PNAS . 114, No. 43, October 2017, pp. 11333–11337. doi : 10.1073 / pnas.1712062114 .
- ↑ KJ Meissner, TJ Bralower, K. Alexander, T. Dunkley Jones, W. Sijp, M. Ward: The Paleocene-Eocene Thermal Maximum: How much carbon is enough? . In: Paleoceanography . 29, No. 10, October 2014, pp. 946–963. doi : 10.1002 / 2014PA002650 .
- ↑ Mark Pagani, Matthew Huber, Zhonghui Liu, Steven M. Bohaty, Jorijntje Henderiks, Willem Sijp, Srinath Krishnan, Robert M. DeConton: The Role of Carbon Dioxide During the Onset of Antarctic Glaciation Archived from the original on March 4, 2016. ( PDF) In: Science . 334, No. 6060, December 2011, pp. 1261-1264. doi : 10.1126 / science.1203909 . Retrieved January 12, 2019.
- ↑ Dieter Lüthi, Martine Le Floch, Bernhard Bereiter, Thomas Blunier, Jean-Marc Barnola, Urs Siegenthaler, Dominique Raynaud, Jean Jouzel, Hubertus Fischer, Kenji Kawamura, Thomas F. Stocker : High-resolution carbon dioxide concentration record 650,000–800,000 years before present . In: Nature . 453, No. 7193, May 15, 2008, p. 379. doi : 10.1038 / nature06949 .
- ^ U. Siegenthaler: Stable Carbon Cycle-Climate Relationship During the Late Pleistocene . In: Science . 310, No. 5752, November 25, 2005, p. 1313. doi : 10.1126 / science.1120130 .
- ^ Iain Colin Prentice et al .: The Carbon Cycle and Atmospheric Carbon Dioxide. In: IPCC Third Assessment Report . 2001, p. 185, accessed December 21, 2013.
- ↑ a b J. G. Canadell, C. Le Cross, MR Raupach, CB Field, ET Buitenhuis, P. Ciais, TJ Conway, NP Gillett, RA Houghton, G. Marland: Contributions to accelerating atmospheric CO 2 growth from economic activity, carbon intensity , and efficiency of natural sinks. In: Proceedings of the National Academy of Sciences . 104, 2007, pp. 18866-18870, doi: 10.1073 / pnas.0702737104 .
- ↑ Walter Roedel, Thomas Wagner. Physics of our environment: the atmosphere . 5th edition, Berlin 2017, p. 440.
- ↑ GRID-Arendal: Vital Climate Graphics: The present carbon cycle . In: Grid Arendal . Retrieved December 21, 2013 .
- ↑ AP Ballantyne, CB Alden, JB Miller, PP Tans, JWC White: Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years. In: Nature . 488, 2012, pp. 70-72, doi: 10.1038 / nature11299 .
- ↑ Karl Hille: Carbon Dioxide Fertilization Greening Earth, Study Finds. April 25, 2016, accessed December 10, 2019 .
- ^ Nate G. McDowell et al .: Pervasive shifts in forest dynamics in a changing world . In: Science . tape 368 , no. 964 , 2020, doi : 10.1126 / science.aaz9463 .
- ↑ SA Montzka, EJ Dlugokencky, JH Butler: Non-CO 2 greenhouse gases and climate change. In: Nature . 476, 2011, pp. 43-50, doi: 10.1038 / nature10322 .
- ↑ Gerald A. Meehl, Warren M. Washington, Caspar M. Ammann, Julie M. Arblaster, TML Wigley, Claudia Tebaldi: Combinations of Natural and Anthropogenic Forcings in Twentieth-Century Climate. In: Journal of Climate . 17, 2004, pp. 3721-3727, doi : 10.1175 / 1520-0442 (2004) 017 <3721: CONAAF> 2.0.CO; 2 .
- ↑ James Hansen , Makiko Sato, Reto Ruedy, Larissa Nazarenko, Andrew Lacis, Gavin A. Schmidt , Gary Russell et al .: Efficacy of climate forcings. In: Journal of Geophysical Research . Vol. 110, Issue D18, September 27, 2005, doi: 10.1029 / 2005JD005776 .
- ↑ Billions against climate change - “Switzerland has the third largest footprint in all of Europe”. In: srf.ch . September 29, 2019, accessed October 1, 2019 .
- ↑ Zhenhao Duan, Rui Sun: An improved model calculating CO 2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. In: Chemical Geology . 193, 2003, pp. 257-271, doi: 10.1016 / S0009-2541 (02) 00263-2 .
- ^ Naomi M. Levine, Scott C. Doney: How Long Can the Ocean Slow Global Warming? In: Woods Hole Oceanographic Institution . November 29, 2006, accessed December 21, 2013 .
- ↑ a b Stefan Rahmstorf , Katherine Richardson : How threatened are the oceans? In: Klaus Wiegandt (Ed.): Courage for Sustainability. 12 ways into the future. Frankfurt am Main 2016, 113–146, p. 128.
- ↑ Gabriela Negrete-García, Nicole S. Lovenduski, Claudine Hauri, Kristen M. Krumhardt, Siv K. Lauvset: Sudden emergence of a shallow aragonite saturation horizon in the Southern Ocean. In: Nature Climate Change. 9, 2019, p. 313, doi: 10.1038 / s41558-019-0418-8 .
- ↑ Tom Garrison: Oceanography: an invitation to marine science , 2005, Thomson Brooks / Cole, Belmont, CA, ISBN 0-534-40887-7 , p. 505.
- ↑ JB Ries, AL Cohen, DC McCorkle: Marine calcifiers exhibit mixed responses to CO 2 -induced ocean acidification. In: Geology . 37, 2009, pp. 1131-1134, doi: 10.1130 / G30210A.1 .
- ↑ Killer Lakes Silent Death from the Deep. In: Der Spiegel . March 7, 2008, accessed December 21, 2012 .
- ↑ Michel Halbwachs, Klaus Tietze, Andreas Lorke, Clément Mudaheranwa: Investigations in Lake Kivu after the Nyiragongo Eruption of January 2002. (PDF; 2.5 MB) In: The Water Research Institute of the ETH Domain. March 9, 2002, accessed December 21, 2012 .
- ^ The Atmosphere of Venus. In: Department of Physics and Astronomy Georgie State University . Retrieved March 22, 2010 .
- ↑ Carbon dioxide (almost) pure ... atmosphere and climate of the red planet. In: Scinexx, the knowledge magazine . December 20, 2003, accessed March 22, 2010 .
- ↑ E. Lellouch, B. Bezard, JI Moses, GR Davis, P. Drossart, H. Feuchtgruber, EA Bergin, R. Moreno, T. Encrenaz: The Origin of Water Vapor and Carbon Dioxide in Jupiter's Stratosphere. In: Icarus . 159, 2002, pp. 112-131, doi: 10.1006 / icar.2002.6929 .
- ↑ Dale P. Cruikshank, Allan W. Meyer, Robert H. Brown, Roger N. Clark, Ralf Jaumann, Katrin Stephan, Charles A. Hibbitts, Scott A. Sandford, Rachel ME Mastrapa, Gianrico Filacchione, Cristina M. Dalle Ore, Philip D. Nicholson, Bonnie J. Buratti, Thomas B. McCord, Robert M. Nelson, J. Brad Dalton, Kevin H. Baines, Dennis L. Matson: Carbon dioxide on the satellites of Saturn: Results from the Cassini VIMS investigation and revisions to the VIMS wavelength scale. In: Icarus . 206, 2010, pp. 561-572, doi: 10.1016 / j.icarus.2009.07.012 .
- ↑ Hubble finds carbon dioxide on an extrasolar planet. In: Inoovations-Report, forum for science, industry and economy . December 10, 2008, accessed March 22, 2010 .
- ↑ LB d'Hendecourt, M. Jourdain de Muizon: The discovery of interstellar carbon dioxide. In: Astronomy and Astrophysics , 223 (1989), pp. L5-L8 ( full text ).
- ↑ Rebecca L. Rawls: Interstellar Chemistry . In: Chemical & Engineering News . tape 80 , no. 28 , 2002, pp. 31-37 ( acs.org [accessed January 9, 2017]).
- ↑ D. Talbi, E. Herbst: The gas-phase destruction of interstellar carbon dioxide: Calculations on the reactions between CO 2 and H 2 and between CO 2 and H. In: Astronomy and Astrophysics 386, 2002, pp. 1139–1142 , doi: 10.1051 / 0004-6361: 20020312 .
- ^ Henrik Lund , Brian Vad Mathiesen : The role of Carbon Capture and Storage in a future sustainable energy system . In: Energy 44, 2012, pp. 469-476, doi: 10.1016 / j.energy.2012.06.002 .
- ↑ oA: Schülerduden Chemie , Bibliografisches Institut & FA Brockhaus AG, Mannheim 2007, ISBN 978-3-411-05386-5 , p. 195.
- ↑ G. Hochgesand: Application of absorption processes for the removal of CO 2 from natural and synthesis gases. In: Chemical Engineer Technology . 40, 1968, pp. 432-440, doi: 10.1002 / cite.330400904 .
- ↑ Birgit Kessler, Jörg Von Eysmondt, Heinrich Merten: Use of CO 2 from flue gases for chemical syntheses. In: Chemical Engineer Technology . 64, 1992, pp. 1075-1083, doi: 10.1002 / cite.330641207 .
- ↑ H. Kolbe: Antiseptic properties of carbonic acid. In: Journal for practical chemistry . 26, 1882, pp. 249-255, doi: 10.1002 / prac.18820260116 .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 860.
- ↑ A. Simon and K. Peters: Single-crystal refinement of the structure of carbon dioxide. In: Acta Crystallographica B . 1980, B36, pp. 2750-2751, doi: 10.1107 / S0567740880009879 .
- ↑ Frank Wisotzky: Applied groundwater chemistry, hydrogeology and hydrogeochemical modeling: Fundamentals, applications and problem solutions. Springer Verlag, 2011, ISBN 978-3-642-17812-2 , p. 65.
- ↑ a b Roland Benedix: Construction Chemistry . Vieweg + Teubner Verlag, ISBN 978-3-8348-0584-3 , p. 113 ( limited preview in Google book search).
- ↑ JT Kiehl, KE Trenberth: Earth's annual global mean energy budget. In: American Meteorological Society . Volume 78, 1997, pp. 197–208 ( PDF , 221 kB)
- ↑ Airproducts.com: Carbon Dioxide - Product Stewardship Summary , accessed July 19, 2017.
- ^ Hans-Dieter Barke: Chemiedidaktik today . Springer-Verlag, Berlin 2001, ISBN 3-540-41725-7 , p. 30 ( limited preview in Google book search).
- ↑ Carbon dioxide from the chimney: How a Thurgau company turns exhaust gas into CO2 for the tap . In: St. Galler Tagblatt , July 8, 2018. Accessed July 8, 2018.
- ↑ Additive Admissions Ordinance : Appendix 3 (to Section 5, Paragraph 1 and Section 7) Generally permitted additives .
- ↑ Information brochure - Baking with yeast. In: Adler-Mühle . Retrieved March 22, 2010 .
- ↑ Hugh Johnson, Steven Brook: The Great Johnson. The encyclopedia of the wines, wine-growing regions and wine producers of the world , Verlag Gräfe und Unzer GmbH, 2009, ISBN 3-8338-1621-X , p. 135.
- ^ A b A. Keith Thompson: Fruit and Vegetables Harvesting, Handling and Storage. Blackwell Publishing, Oxford 2003, ISBN 1-4051-0619-0 , pp. 61-70.
- ↑ Hannah James and Jenny Jobling: The Flesh Browning Disorder of 'Pink Lady' Apples. (PDF; 608 kB) In: New York Fruit Quarterly Volume 16, No. 2. 2008, pp. 23–28 , accessed on June 4, 2018 .
- ↑ Irene Palacios et al .: Use of Modified Atmosphere Packaging to Preserve Mushroom Quality during Storage. In: Recent Patents on Food, Nutrition & Agriculture , Volume 3, Issue 3, 2012, pp. 196-203, doi: 10.2174 / 2212798411103030196 .
- ^ Anne Emblem: Predicting packaging characteristics to improve shelf-life. In: David Kilcast, Persis Subramaniam (ed.): The stability and shelf-life of food, 2000, Woodhead Publishing, Cambridge (UK), ISBN 1-85573-500-8 , pp. 145-169.
- ↑ Joseph P. Kerry (Ed.): Advances in meat, poultry and seafood packaging, 2012, Woodhead Publishing, Cambridge (UK), ISBN 978-1-84569-751-8 .
- ↑ Saul Norman Katz: Method for decaffeinating coffee with a supercritical liquid. December 22, 1988. Retrieved December 21, 2013 .
- ↑ Fire brigade on gas accident: "No operational errors". In: Rheinische Post. August 20, 2008, accessed December 21, 2013 .
- ^ Carbon Dioxide as a Fire Suppressant: Examining the Risks. In: US Environmental Protection Agency . August 19, 2010, accessed March 22, 2010 .
- ↑ Carbon dioxide - special features and opportunities for use as a refrigerant. In: German Climate and Refrigeration Association . Retrieved March 22, 2010 .
- ↑ Natural refrigerant for car air conditioning systems. In: Federal Environment Agency . June 9, 2008, accessed March 22, 2010 .
- ↑ Metal active gas welding (MAG / 135). (PDF; 42 kB) In: German Association for Welding and Allied Processes . Retrieved March 22, 2010 .
- ↑ Douglas A. Skoog, James J. Leary, S. Hoffstetter-Kuhn: Instrumental Analytics: Fundamentals - Devices - Applications . Springer-Verlag, ISBN 3-540-60450-2 , p. 285 ( limited preview in Google book search).
- ↑ Compressed gases for supplying beverage dispensing systems. (PDF) In: Berufsgenossenschaft Nahrungsmittel und Gastgewerbe . February 1, 2012, accessed March 22, 2010 .
- ↑ Leaflet - Use of fog machines. (PDF) Retrieved March 22, 2010 .
- ↑ Mark Krieg: Dry ice blasting - with snow or with pellets? In: Journal für Oberflächentechnik , 45.6, 2005, pp. 50–55.
- ↑ N. Dahmen, P. Griesheimer, A. Hebach: Cleaning and surface treatment with compressed carbon dioxide. In: Galvanotechnik , 98, 2007, pp. 1111–1120.
- ↑ FM Orr, JJ Taber: Use of Carbon Dioxide in Enhanced Oil Recovery. In: Science . 224, 1984, pp. 563-569, doi: 10.1126 / science.224.4649.563 .
- ^ A b Arno Behr and Stefan Neuberg: Catalytic carbon dioxide chemistry. In: Current newsreel of the GDCh . May 13, 2008, accessed June 4, 2018 .
- ↑ A. Behr, P. Ebbinghaus, F. Naendrup: Process concepts for the transition metal-catalyzed syntheses of formic acid and dimethylformamide on the basis of carbon dioxide. In: Chemical Engineer Technology . 75, 2003, pp. 877-883, doi: 10.1002 / cite.200303221 .
- ↑ H. Kolbe: About synthesis of salicylic acid. In: Liebigs Ann. , 113, 1860, pp. 125-127, doi: 10.1002 / jlac.18601130120 .
- ↑ Alexis Bazzanella, Dennis Krämer, Martina Peters: CO 2 as a raw material. In: News from chemistry . 58, 2010, pp. 1226-1230, doi: 10.1002 / nadc.201075752 .
- ↑ Rudolf-Werner Dreier: Green gasoline from carbon dioxide. Albert-Ludwigs-Universität Freiburg im Breisgau, press release from June 13, 2012 from the Informationsdienst Wissenschaft (idw-online.de), accessed on August 24, 2015.
- ↑ Sebastian Matthes, Susanne Donner: The climate killer as raw material. In: Wirtschaftswoche online. September 16, 2012, accessed December 21, 2013 .
- ↑ Renate Hoer: Carbon dioxide recycling? Gesellschaft Deutscher Chemiker, press release from November 8, 2011 at the Science Information Service (idw-online.de), accessed on August 24, 2015.
- ↑ Christophe Das Neves Gomes, Olivier Jacquet, Claude Villiers, Pierre Thury, Michel Ephritikhine, Thibault Cantat: A Diagonal Approach to Chemical Recycling of Carbon Dioxide: Organocatalytic Transformation for the Reductive Functionalization of CO 2 . In: Angewandte Chemie . 124, 2012, pp. 191–194, doi: 10.1002 / anie.201105516 .
- ↑ Sebastian Wesselbaum, Thorsten vom Stein, Jürgen Klankermayer, Walter Leitner: Hydrogenation of Carbon Dioxide to Methanol by Using a Homogeneous Ruthenium-Phosphine Catalyst. In: Angewandte Chemie . 124, 2012, pp. 7617-7620, doi: 10.1002 / anie.201202320 .
- ↑ Sebastian Wesselbaum, Ulrich Hintermair, Walter Leitner: Continuous-Flow Hydrogenation of Carbon Dioxide to Pure Formic Acid using an Integrated scCO 2 Process with Immobilized Catalyst and Base. In: Angewandte Chemie . 124, 2012, pp. 8713-8716, doi: 10.1002 / anie.201203185 .
- ↑ Ralte Lalrempuia, Manuel Iglesias, Victor Polo, Pablo J. Sanz Miguel, Francisco J. Fernández-Alvarez, Jesús J. Pérez-Torrente, Luis A. Oro: Effective Fixation of CO 2 by Iridium-Catalyzed Hydrosilylation. In: Angewandte Chemie . 124, 2012, pp. 12996-12999, doi: 10.1002 / anie.201206165 .
- ↑ Stefan Pelzer: Tailor-made microorganisms. In: Biology in Our Time . 42, 2012, pp. 98-106, doi: 10.1002 / biuz.201210472 .
- ^ AA LaVerne: Rapid coma technique of carbon dioxide inhalation therapy. In: Diseases of the nervous system , 14.5 (1953), p. 141.
- ↑ B. Nowak, TV Mueffling, J. Hartung: Effect of different carbon dioxide concentrations and exposure times in stunning of slaughter pigs: Impact on animal welfare and meat quality. In: Meat Science . 75, 2007, pp. 290-298, doi: 10.1016 / j.meatsci.2006.07.014 .
- ↑ Roswitha Nitzsche: Improving animal welfare during pig slaughter by redesigning the access to and into the CO 2 stunning system, final report, BLE - research project 05UM012 / W , o. J. (2008), Max Rubner - Institute, Institute for Safety and Quality at Fleisch, Technology Division, Kulmbach Online PDF , 1.3 MB. Retrieved December 22, 2013.
- ↑ without author: Problems inherent in the system during slaughter. As of August 21, 2012, Deutscher Tierschutzbund e. V., Bonn 2012 PDF , 78 kB. Retrieved December 22, 2013.
- ↑ Veterinary Association for Animal Welfare (Ed.): Animal welfare-friendly slaughter of cattle, pigs, sheep and goats. Leaflet No. 89. Self-published, Bramsche 2007. Accessed on December 22, 2013.
- ↑ Lindsay G. Ross, Barbara Ross (Ed.): Anesthetic and Sedative Techniques for Aquatic Animals. Third edition. Blackwell Publishing, Oxford 2008, ISBN 978-1-4051-4938-9 , chap. 9: Anesthesia of Fish: II. Inhalation Anesthesia Using Gases , pp. 127-135.
- ↑ Almuth Hirt, Christoph Maisack, Johanna Moritz: Ordinance on the protection of animals in connection with slaughter or killing (Animal Welfare Slaughter Ordinance - TierSchlV) . In: Almuth Hirt, Christoph Maisack, Johanna Moritz: Tierschutzgesetz. 2nd Edition. Verlag Franz Vahlen, Munich 2007, ISBN 978-3-8006-3230-5 , pp. 757-804 (legal specialist commentary) , here pp. 778-779 (pigs), p. 782 (turkeys), pp. 793-796 (Day-old chicks), pp. 784–785 (fish).
- ↑ Veterinary Association for Animal Welfare (Ed.): Recommendations for killing small mammals for feed purposes , opinion of April 19, 2011, Bramsche. Retrieved December 22, 2013.
- ^ Almuth Hirt, Christoph Maisack, Johanna Moritz: Tierschutzgesetz . 2nd Edition. Verlag Franz Vahlen, Munich 2007, ISBN 978-3-8006-3230-5 (legal commentary) , pp. 217-218.
- ↑ Almuth Hirt, Christoph Maisack, Johanna Moritz: Ordinance on the protection of animals in connection with slaughter or killing (Animal Welfare Slaughter Ordinance - TierSchlV) . In: Almuth Hirt, Christoph Maisack, Johanna Moritz: Tierschutzgesetz. 2nd Edition. Verlag Franz Vahlen, Munich 2007, ISBN 978-3-8006-3230-5 , pp. 757-804 (legal commentary) , here pp. 787-789.
- ↑ Veterinary Association for Animal Welfare (ed.): Killing larger groups of animals in the event of an epidemic (pigs, cattle, sheep, poultry). Leaflet No. 84. Self-published, Bramsche 2011. Accessed on December 22, 2013.
- ↑ T. Frieling: Diagnostics in anorectal diseases. In: Praxis 96.7, 2007, pp. 243–247.
- ^ SC Wong: Elevated atmospheric partial pressure of CO 2 and plant growth. In: Oecologia . 44, 1979, pp. 68-74, doi: 10.1007 / BF00346400 .
- ↑ Plant care in aquariums. In: Mongabay.com . Retrieved December 22, 2013 .
- ↑ YT Qiu, J. Spitzen, RS Smallegange, BGJ Knols: Monitor systems for adult insect pests and disease vectors. In: W. Takken, BGJ Knols (Ed.): Ecology and control of vector-borne diseases , volume 1: Emerging pests and vector-borne diseases in Europe. Wageningen Academic Publishers, ISBN 978-90-8686-053-1 , pp. 329-353.
- ↑ Eckhard Bast: Microbiological Methods: An Introduction to Basic Working Techniques . 2nd Edition. Spectrum Akademischer Verlag GmbH, Heidelberg / Berlin 2001, ISBN 978-3-8274-1072-6 , p. 55, 132-135 .
- ↑ UBA, Announcement of the Federal Environment Agency: Health assessment of carbon dioxide in indoor air. In: Federal Health Gazette - Health Research - Health Protection Volume 51, 2008, pp. 1358-1369.
- ↑ Safety data sheet - carbon dioxide (cryogenic liquefied). (PDF) (No longer available online.) In: Tyczka Kohlensäure . Archived from the original on December 2, 2013 ; Retrieved December 21, 2013 .
- ↑ Rising carbon dioxide levels will make us stupider . In: Nature . 580, No. 7805, April 20, 2020, p. 567. bibcode : 2020Natur.580Q.567. . doi : 10.1038 / d41586-020-01134-w . PMID 32317783 .
- ↑ Kristopher B. Karnauskas, Shelly L. Miller, Anna C. Schapiro: Fossil Fuel Combustion Is Driving Indoor CO 2 Toward Levels Harmful to Human Cognition . In: GeoHealth . 4, No. 5, 2020, p. E2019GH000237. doi : 10.1029 / 2019GH000237 . PMID 32426622 . PMC 7229519 (free full text).
- ↑ Smoke poisoning / poisoning by gases. (No longer available online.) In: Techniker Krankenkasse . Archived from the original on June 4, 2010 ; Retrieved December 21, 2013 .
- ↑ First aid in case of poisoning by carbon dioxide. In: German Red Cross . Retrieved June 4, 2018 .
- ^ Carbon Dioxide - Life and Death. (PDF) (No longer available online.) In: Sensair . Archived from the original on May 22, 2013 ; Retrieved December 21, 2013 .
- ↑ Bodo Gorgaß, Friedrich W. Ahnefeld, Rolando Rossi: Paramedic and paramedic. Springer Verlag, 1997, ISBN 3-540-21487-9 , pp. 305-314.
- ↑ ester Majo: I fenomeni vulcanici della Grotta del Cane (Campi Flegrei) in rapporto all variazioni atmosferiche. In: Bulletin Volcanologique . 4, 1927, pp. 84-92, doi: 10.1007 / BF02719519 .
- ↑ David W. Christianson, Carol A. Fierke: Carbonic anhydrase: Evolution of the zinc binding site by nature and by design. In: Accounts of Chemical Research 29.7, 1996, pp. 331-339, doi: 10.1021 / ar9501232 .
- ^ Peter Karlson: Karlsons Biochemie and Pathobiochemie . Thieme-Verlag, Stuttgart 2005, ISBN 3-13-357815-4 , p. 38 ( excerpt in the Google book search).
- ↑ M. Schwarz: Carbon toxicity in plants. In: International Symposium on Growing Media and Hydroponics , 1997, p. 481 ( abstract ).
- ↑ Chunwu Zhu, Kazuhiko Kobayashi, Irakli Loladze, Jianguo Zhu, Qian Jiang, Xi Xu, Gang Liu, Saman Seneweera, Kristie L. Ebi, Adam Drewnowski, Naomi K. Fukagawa and Lewis H. Ziska: Carbon dioxide (CO 2 ) levels This century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries . In: Science Advances , 2018, doi: 10.1126 / sciadv.aaq1012 .
- ↑ Spectrum of Science Verlagsgesellschaft mbH: Photosynthesis
- ^ Heinz-Gerhard Franck, Jürgen W. Stadelhofer: Oxygen and Carbon Dioxide? Key links of life. In: The natural sciences . Volume 75, 1988, pp. 585-590, doi: 10.1007 / BF00366470 .
- ↑ Carbon cycle. In: Spectrum Academic Publishing House, Heidelberg: Lexicon of Biology . 1999, accessed March 16, 2017 .
- ↑ Ulrich Helmich: Breathing, Dissimilation
- ↑ Christian Schwägerl: Researchers demand acid limit for oceans. In: Der Spiegel . December 14, 2009, accessed December 21, 2013 .
- ↑ Kurt Bauer: On the importance of carbonic acid in carp ponds . In: Österreichs Fischerei , Volume 44, 1991, pp. 49-64.
- ↑ W. Elbert, B. Weber, S. Burrows et al .: Contribution of cryptogamic covers to the global cycles of carbon and nitrogen . In: Nature Geoscience . tape 5 , no. 7 , June 3, 2012, p. 459-462 , doi : 10.1038 / ngeo1486 .
- ↑ Sabine Wendler: Inconspicuous and yet powerful: Lichen, algae and moss are large storage facilities for nitrogen and CO 2 . Senckenberg Research Institute and Nature Museums, press release from June 4, 2012 from the Science Information Service (idw-online.de), accessed on August 24, 2015.
- ↑ Eberhard Fritz: Global warming can intensify itself through the release of CO 2 from forest soils. Max Planck Institute for Biogeochemistry, press release from June 11, 2012 from Informationsdienst Wissenschaft (idw-online.de), accessed on August 24, 2015.
- ^ Tapio Schneider, Colleen M. Kaul, Kyle G. Pressel: Possible climate transitions from breakup of stratocumulus decks under greenhouse warming. In: Nature Geoscience. 12, 2019, p. 163, doi: 10.1038 / s41561-019-0310-1 .
- ↑ Adrianus Kleinleugenmors: apparatus for measuring the partial pressure of carbon dioxide. In: European patent. December 4, 2006, accessed December 21, 2013 .
- ↑ Arthur Adel, David Dennison: The Infrared Spectrum of Carbon Dioxide. Part I. In: Physical Review . 43, 1933, pp. 716-723, doi: 10.1103 / PhysRev.43.716 .