Erythropoietin
| Erythropoietin | ||
|---|---|---|
|
||
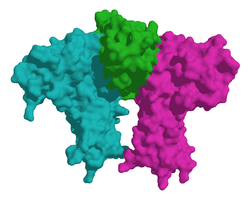
| Surface model of erythropoietin (center) in complex with its receptor, according to PDB 1EER | ||
| Properties of human protein | ||
| Mass / length primary structure | 165 amino acids ; 34 kDa | |
| Secondary to quaternary structure | Glycoprotein | |
| Precursor | Prepro-EPO protein | |
| Identifier | ||
| Gene names | EPO ; EP; MGC138142 | |
| External IDs | ||
| Drug information | ||
| ATC code |
B03 XA01 B03 XA02 |
|
| DrugBank | DB00016 | |
| Drug class | hormone | |
| Occurrence | ||
| Homology family | Erythropoietin-2 | |
| Parent taxon | Euteleostomi | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 2056 | 13856 |
| Ensemble | ENSG00000130427 | ENSMUSG00000029711 |
| UniProt | P01588 | P07321 |
| Refseq (mRNA) | NM_000799 | NM_001312875 |
| Refseq (protein) | NP_000790 | NP_001299804 |
| Gene locus | Chr 7: 100.72 - 100.72 Mb | Chr 5: 137.48 - 137.49 Mb |
| PubMed search | 2056 |
13856
|
Erythropoietin [ eˌʁyːtʁoˌpoːetiːn ] (from ancient Greek ἐρυθρός erythros , red 'and ποιεῖν poiein , make'; Synonyms : erythropoietin , epoetin , EPO or Epo , historically also hematopoietin , erythropoietic factor (kidney) , Erythropoiesestimulierender factor (ESF) ) is a glycoprotein - Hormone that is important as a growth factor for the formation of red blood cells during blood formation , especially after major blood loss and when there is a higher need for red blood cells when ascending to high altitudes with reduced oxygen partial pressure . Erythropoietin is one of the so-called " Erythropoiesis Stimulating Agents " (short form: ESA). Biotechnologically produced erythropoietin is used as a therapeutic agent primarily in the treatment of anemia in dialysis patients in whom blood formation is impaired as a result of kidney failure , and after aggressive chemotherapy cycles. Erythropoietin became well known through numerous doping cases in professional sports .
biosynthesis
When the oxygen content of the blood is reduced, the biosynthesis of EPO is triggered by transcription factors . This takes place mainly in the kidneys . The hormone produced reaches its place of action via the bloodstream.
In humans, EPO is around 90% in the kidney by the renal tubules lying fibroblasts produced the renal cortex. The oxygen supply to the kidney tissue acts as a regulator of EPO production. Around 10% are formed in the hepatocytes of the liver . In addition, a physiologically insignificant synthesis activity in the brain , in the uterus , in the testes , in the spleen and also in hair follicle cells could be detected.
The EPO gene in humans is located on chromosome 7 (position 7q21-7q22). The synthesis is stimulated by a reduced oxygen saturation ( hypoxia ) of the blood. This leads to the shift of the α-subunit of the “ hypoxia-induced factor ” (HIF for short) from the cytoplasm to the nucleus of EPO- expressing cells. There HIF-α binds to the corresponding β-subunit (HIF-β), whereby the finished heterodimer HIF-1 is created. This in turn binds to the “cAMP response element-binding protein” ( CREB for short ) and another transcription factor (p300). The resulting protein complex, which now consists of three elements, then initiates transcription into the associated mRNA by binding to the 3 'end of the EPO gene . This is to the ribosomes in the protein erythropoietin translated .
Biological function

red arrows: erythropoiesis ;
TGF = transforming growth factor ;
MIP = Macrophage Inflammatory Protein;
IL = interleukin ;
G-CSF = Granulocyte Colony Stimulating Factor ;
SCF = stem cell factor ;
IGF = insulin-like growth factor;
FLT-3 / FLK-2 = receptor tyrosine kinase ;
BFU-E = Erythroid Burst Forming Unit;
CFU-E = Erythroid Colony Forming Unit
Erythropoietin works everywhere in the body where the so-called EPO receptor is formed on the surface of the cells. These are in particular the stem cells in the bone marrow and also in other tissues , from which new blood cells continuously emerge. In particular, EPO causes erythrocytes (red blood cells) to develop from these stem cells.
Formation of erythrocytes
On the formation and development of red blood cells see also the main article Erythropoiesis .
The serum concentration of the hormone in healthy humans is between 6 and 32 mU / mL and the plasma half-life between 2 and 13 hours. In erythropoiesis , EPO binds in the bone marrow to the transmembrane erythropoietin receptor of the precursor cells of the BFU-E ( Erythroid Burst Forming Unit ) type, which first differentiate into the more mature CFU-E ( Erythroid Colony Forming Unit ) precursor cells and finally into erythrocytes .

The receptor (EpoR) belongs to the family of cytokine receptors, the structural commonalities of which consist of two or more immunoglobulin- like domains , four identically arranged cysteine residues and the extracellular sequence WSXWS ( Trp - Ser - variable amino acid - Trp - Ser ). The binding of EPO leads to a homodimerization of the receptor, which in turn via Trans phosphorylation the receptor-coupled enzyme Janus kinase activated -2. Specific, intracellular receptor-associated tyrosine residues are phosphorylated and serve as a coupling station for the signal transduction protein STAT5 , which sets various signal transduction cascades in motion. A total of 94 proteins are involved.
Around 200 billion erythrocytes are formed every day. In addition to the actual erythropoiesis, EPO acts as an apoptosis inhibitor in the differentiation of the precursor cells and to a small extent also stimulates the formation of megakaryocytes . Acute and chronic insufficiencies as a result of degenerative diseases of the kidneys lead to reduced EPO formation and thus to renal anemia .
More functions
The role of EPO in the organism is not limited to the formation of new erythrocytes. Immunocytochemical hybridization studies have shown that EpoR can be found in a wide variety of somatic cells. These include neurons , astrocytes , microglial and heart muscle cells . EPO / EpoR interactions have been demonstrated in a wide variety of non- hematopoietic tissues in connection with cell division processes , chemotaxis , angiogenesis , activation of intracellular calcium and apoptosis inhibition. Specific EPO binding sites have been detected in nerve cells, especially in the hippocampus , a brain region that is particularly susceptible to damage caused by a lack of oxygen . In the mouse model it was shown that the targeted administration of EPO increases the nerve activity in the hippocampus and thus improved learning and memory performance can be observed in the animals, regardless of the blood-forming properties of the hormone. A protective effect of EPO has been demonstrated in several animal models of cerebral infarction and oxygen deficiency . These findings could offer new therapeutic approaches for chronic diseases (multiple sclerosis, schizophrenia) as well as acute neurological diseases (stroke) (see indications for therapy with EPO ).
In the mouse model it could be shown that the cytoprotective property of EPO in heart muscle cells is based on the action of the enzyme heme oxygenase-1 , the expression of which is triggered by EPO via the p38 MAPK signal transduction cascade .
Structural properties

Asn24 : N-glycosylated, tri-antennary, di-sialylated.
Asn38 : N-glycosylated, tetra-antennary, tetra-sialylated.
Asn83 : N-glycosylated, tetra-antennary, tri-sialylated.
Ser126 : O-glycosylated, mono-antennary, di-sialylated.
Phylogenetically, EPO belongs to a cytokine family which, in addition to EPO, also includes somatropin , prolactin , interleukins 2–7 and the so-called "Colony Stimulating Factors" ( G-CSF , M-CSF and GM-CSF ).
Amino acid sequence of EPO (one letter code )
- green : N-terminal signal peptide
- red : C-terminal arginine residue
|
10 20 30 40 50 60
MGVHECPAWL WLLLSLLSLP LGLPVLG APP RLICDSRVLE RYLLEAKEAE NITTGCAEHC |
|
70 80 90 100 110 120
SLNENITVPD TKVNFYAWKR MEVGQQAVEV WQGLALLSEA VLRGQALLVN SSQPWEPLQL |
|
130 140 150 160 170 180
HVDKAVSGLR SLTTLLRALG AQKEAISPPD AASAAPLRTI TADTFRKLFR VYSNFLRGKL |
|
190 KLYTGEACRT GD R |

The EPO gene (5.4 kb, 5 exons and 4 introns ) codes for a prepro EPO protein with 193 amino acid residues. In the case of post-translational modification , a signal peptide with 27 amino acid residues at the N -terminal and an arginine residue at the C -terminal is split off by an intracellular carboxypeptidase .
Chemically, human EPO is an acidic, unbranched polypeptide consisting of 165 amino acids with a molecular mass of about 34 kDa . The secondary structure consists of four anti-parallel α-helices including adjacent loops. The carbohydrate content, which is about 40% of the molecular mass, consists of one O -glycosidic ( Ser 126) and three N -glycosidic ( Asn 24, Asn 38 and Asn 83) sugar side chains. The side chains in turn are composed of the monosaccharides mannose , galactose , fucose , N -acetylglucosamine , N -acetylgalactosamine and N -acetylneuraminic acid . The N -glycosidically bound side chains have several branches, which are also known as “ antennas ”. In contrast to the constant amino acid sequence of the EPO molecule, the sugar structures are variable. In this context, one speaks of the microheterogeneity of the EPO molecule, which occurs both in natural (native) and in recombinant EPO . This is characterized on the one hand by variable sequences of the monosaccharides in the sugar side chains, on the other hand by the variable number of terminal N- acetylneuraminic acids. These, also known under the common name sialic acids , are decisive for the biological activity of the glycoprotein : the higher the degree of sialylation, the higher the activity and the serum half-life of the hormone. It is noteworthy that highly sialylated isoforms show a lower affinity for the EPO receptor in in vitro experiments . This in turn explains why the asialylated isoforms, in which the terminal sialic acids have been removed, are depleted directly in the liver by the parenchymal cells ( hepatocytes ) that carry the EPO receptor due to the high receptor affinity and are therefore ineffective. Functional isoforms, on the other hand, are gradually broken down by other body cells that carry the EPO receptor. During degradation, the EPO molecules are internalized in lysosomes by a receptor-mediated endocytosis and broken down there. In further studies with EPO-like molecules without receptor affinity it could be shown that the endocytosis mediated via the EPO receptor only partially contributes to the depletion of EPO from the bloodstream. Rather, degradation pathways via the stroma tissue and the lymphatic system seem to be decisive. Apparently macrophages and neutrophils are also involved.
The sugar side chains also influence the stability of the EPO molecule and thereby exercise a protective function. Deglycosylated EPO, which has no sugar side chains, is significantly more sensitive to pH and temperature-induced denaturation than natural, glycosylated EPO.
An optional special feature of the EPO molecule is the sulfation of N -glycosidic sugar side chains. The exact function of sulfation, which can be detected both in the native and in the recombinant molecule , is so far unknown.
The cytoprotective properties of EPO (see chapter Biological function ) are apparently determined by the peptide sequences of the α-helix B in the EPO molecule. This has been shown by in-vitro and in-vivo studies with synthetic sequence-homologous peptides. In contrast, said sequences have no erythropoietic properties.
EPO as a drug
Research history
The history of research into erythropoietin is naturally closely linked to the knowledge gained about the formation and function of blood. The importance of blood for human vitality has been known since early history . In many cultures blood is at the center of ritual ceremonies. Often the blood of a strong animal or a slain enemy was administered in order to transfer its strength and courage to the recipient. Its meaning is written in the Bible . In 3rd book of Moses , chapter 17, verse 11 it says: "For the life of the body [soul] is in the blood (...)" .
The first successful blood transfusion to treat anemia was made by Jean-Baptiste Denis , personal physician to Louis XIV, and the surgeon Paul Emmerez († 1690) on June 15, 1667 in Paris . They gave the patient, whose condition improved significantly after the transfusion, the blood of a lamb. The English gynecologist and obstetrician James Blundell (1791-1878) carried out the first successful homologous transfusion on humans in 1825, in which a patient with profuse bleeding received her husband's blood. However, the physicians in charge of the treatment remained concealed from the precise background for the effectiveness of their therapies. It was not until the middle of the 19th century that Felix Hoppe-Seyler, with the discovery of hemoglobin, and Ernst Neumann, through his work on the bone marrow as a place of blood formation, provided the first insights into the origin and function of blood.
In 1863, the French doctor Denis Jourdanet indirectly recognized the connection between reduced oxygen partial pressure and an increase in the number of erythrocytes when he carried out hematocritical examinations on people who had been at high altitudes for a long time. Jourdanet found that the blood of his subjects was thicker than that of his “normal” patients. Friedrich Miescher established the direct connection in 1893. Miescher described the formation of erythrocytes as the result of a reduced oxygen supply to the bone marrow. On this basis, efforts were made to treat anemia by means of specifically induced hypoxia.
In 1906 the Frenchman Paul Carnot (1869–1957) and his colleague Catherine Deflandre first hypothesized that a humoral factor regulates blood formation. Their hypothesis was based on experiments in which the blood serum of rabbits that had previously been made anemic by bloodletting , after injection into healthy rabbits, markedly increased the number of red blood cells. Numerous attempts by other researchers to reproduce the results of Carnot and Deflandre have failed. It was only through the use of phenylhydrazine, a hemolytic chemical to induce anemia, that other researchers, such as, for example, Camillo Gibelli from the University of Genoa , in the experimental set-up of Carnot and Deflandre, were able to maintain their hypothesis. Further evidence for the correctness of the hypothesis of a humoral factor was provided by experiments in which blood formation in normal animals could be increased by serum from animals which were kept under hypoxic conditions. In particular, Georges Sandor (1906–1997) from the Pasteur Institute was able to achieve significant success in the 1930s. The two Finnish nephrologists Eva Bonsdorff (* 1918) and Eeva Jalavisto (1909–1966) finally gave this factor the name erythropoietin , or EPO for short , in 1948 .
The real “discoverer” is generally considered to be Allan Jacob Erslev , who in 1953 published the first well-founded scientific publications in which the existence of erythropoietin was unequivocally proven. However, Eugene Goldwasser became the key figure in further EPO research . In 1954 he and his research group from the University of Chicago confirmed Erslev's work with their own results. Goldwasser and his colleague Leon Orris Jacobson were initially able to indirectly demonstrate in 1957 that EPO is formed in the kidneys, and in 1977 they were able to isolate human EPO from urine on a milligram scale for the first time . In 1983, Fu-Kuen Lin , an employee at Amgen , succeeded in identifying the human EPO gene. In 1984 Sylvia Lee-Huang from the New York University Medical Center reported for the first time on the successful cloning and expression of recombinant human EPO (rhEPO) in Escherichia coli , which was then also carried out in mammalian cells in 1985. This made the large-scale production of recombinant EPO possible in suitable quantities.
Indications for therapy with EPO
Of the growth factors currently used clinically, EPO has the largest spectrum of indications . Classic EPO therapy aims to initiate or support the formation of red blood cells in patients with renal anemia , tumor anemia and anemia as a result of chemotherapy . In addition, it has now been established that the response rate of hypoxic tumors to radio or chemotherapy can be increased by increasing tumor oxygenation after EPO application.
Haematological diseases
In renal anemia, EPO is usually administered to patients during hemodialysis . A US short-term study indicated that there are different population-typical requirements for the use of EPO. In this study, dialysis patients of black African descent required an average of 12% higher EPO doses than whites to raise the hemoglobin level to a physiological range.
In a further, retrospective study it was found that the survival rate of dialysis patients with end-stage renal insufficiency increases after administration of EPO if these patients live in alpine altitudes. In many cases, EPO therapy can be supported by the simultaneous administration of iron preparations for blood formation. The molecular pathomechanism of tumor anemia, which can be eliminated by adding EPO, is based on impaired iron utilization . Since these mechanisms can also be demonstrated in chronic infections (such as Crohn's disease , ulcerative colitis ) or sepsis , the use of EPO as a therapy-supporting measure has been investigated in clinical studies for several years . Furthermore, EPO forms of therapy for fatigue , myelodysplastic syndrome , aplastic anemia , osteomyelofibrosis and HIV infections are discussed.
So-called infantile pycnocytosis , a special form of hereditary poikilocytosis , is a rare disease in newborns that is characterized by deformed erythrocytes and is accompanied by severe anemia. In the past, frequent red cell transfusions were required to treat this disease. In September 2008, an Italian research group reported for the first time on successful EPO therapy cases in which subsequently erythrocyte transfusions could be completely dispensed with.
Experimental approaches to treating neurological diseases
Its cytoprotective properties in cell culture and animal models also make EPO an interesting candidate for the treatment of acute neurological diseases such as stroke . While animal models of stroke and an initial pilot study in humans showed promise, the results of a large randomized clinical trial on the treatment of stroke patients remained sobering.
Based on experimental work and small clinical studies, a role in the treatment of chronic diseases of the central nervous system has also been postulated. Based on a study carried out on eight patients, it was speculated whether high-dose EPO could possibly be therapeutic in the treatment of chronically progressive multiple sclerosis . In a study carried out on 12 patients with Friedreich's ataxia , a reduction in lymphocytic frataxin concentrations was observed after administration of EPO . In the mouse model, EPO showed a retarding effect in the development of amyotrophic lateral sclerosis (ALS) . In the rat model, EPO evidently promotes axonal growth of severed nerve fibers.
Experimental approaches to treatment for psychiatric illness
According to a pilot study published in 2006, EPO as an adjunct therapeutic agent in the treatment of schizophrenic patients could potentially cause a slight improvement in cognitive abilities . The authors assume that the observed effect could be based on the protective properties of EPO against neurodegenerative mechanisms, but the results have not yet been confirmed by other research groups. In a further, individual neuropsychological study , mood-enhancing effects with a simultaneous improvement in cognitive abilities were observed by the administration of EPO in patients with anxiety states and depression .
Further experimental treatment approaches
The cytoprotective properties of EPO are not limited to neuronal tissue alone. After treatment with EPO, cardiac muscle cells are also significantly less sensitive to otherwise lethal stress factors such as those caused by e.g. B. occur in a heart attack due to insufficient oxygen supply ( hypoxia ). In this way, EPO could be administered preventively in patients at risk. However, even after an ischemic infarct has occurred, the use of EPO can be helpful, as the heart muscle cells are protected from the otherwise usual further damage during reperfusion of the organ. In a study in Switzerland it could be shown that this protective effect is based on the EPO-mediated production of nitrogen monoxide in the coronary endothelial cells . The vasodilation caused by nitric oxide apparently leads to a higher blood flow and thus to an improved oxygen supply to the tissue. In a first study with 138 patients on the treatment of myocardial infarction with EPO, however, no benefit from the administration of the cytokine could be observed. The same applies to the treatment of heart failure that is accompanied by anemia.
In the mouse model, it could be shown that EPO has a positive influence on wound healing processes : A high single dose of the cytokine EPO accelerates, among other things, the epithelialization and differentiation of the microvascular blood vessel system . The transferability of the results to humans is currently being investigated as part of a multicenter study.
In a long-term study at the Children's Hospital auf der Bult , Hanover, it was possible to show that EPO can protect against cerebral hemorrhage in extremely premature babies .
The Max Planck Institute for Experimental Medicine in Göttingen planned a randomized study in 2020 on the use of EPO to improve symptoms in the course of COVID-19 .
Name and properties of EPO preparations

In 1989, the World Health Organization ( WHO ) introduced an INN nomenclature for recombinant EPO variants . After that, all substances with the same mechanism of action as erythropoietin are given the root word “-poetin”. "Epoetin" is an active ingredient that has the same amino acid sequence including disulfide bridges and glycosylation sites as natural human erythropoietin. However, all recombinant EPO variants differ from the native, endogenous molecule in the composition of the sugar structures ( glycosylation pattern ). There are also differences between the recombinant variants. To distinguish the variants, a Greek letter is added to the term “Epoetin”. The following EPO variants are currently listed according to the INN nomenclature at the WHO: Epoetin alpha (epoetin α), epoetin beta (epoetin β), epoetin gamma (epoetin γ), epoetin delta (epoetin δ), epoetin epsilon (epoetin ε), Epoetin zeta (epoetin ζ), epoetin theta (epoetin θ), epoetin kappa (epoetin κ) and epoetin omega (epoetin ω).
The recombinant expression vehicle for the production of the variants epoetin α and β is each a genetically modified subclone of an ovarian cell line of the Chinese striped hamster ( Cricetulus griseus ), a so-called CHO cell line ( Chinese Hamster Ovary ). A genetically modified and subcloned cell line from the kidney of a young Syrian golden hamster ( Mesocricetus auratus auratus ) is used to produce the variant epoetin ω ( BHK cells , baby hamster kidney ).
Compared to epoetin α, epoetin β has a slightly higher molecular mass, a broader spectrum of basic isoforms and thus a slightly lower degree of sialylation. However, the proportion of tetra-sialylated side chains is more than twice as high in epoetin β than in epoetin α. After desialylation, epoetin β showed a 20 percent higher pharmacological activity compared to epoetin α in the mouse model . Epoetin ω, due to the different expression cell lines, differs structurally from the α and β variants in the sequence of the sugar monomers and the number of branches in the sugar side chains (antennae).
Epoetin γ is expressed by a recombinant murine fibroblast cell line, epoetin ε by a BHK line (compare epoetin ω). Both variants, like the variant epoetin κ, apparently have no clinical relevance.
Epoetin ζ ( Silapo or Retacrit ) from Stada / Hospira and Epoetin α from Hexal / Sandoz ( Epoetin alfa Hexal , Binocrit ) are copycat products of the epoetin α preparation Erypo / Eprex from Janssen Cilag. Compared to Erypo / Eprex , the copycat products contain fewer O-glycans and fewer of the undesirable sialic acid derivatives N-glycolylneuraminic acid and O-acetylneuraminic acid.
The amount of EPO is given in International Units (IU) rather than grams or moles , as native or recombinant EPO are mixtures of isoforms of different biological activity . By definition, an EPO unit in the rodent model has the same erythropoietic effect as 5 micromoles of cobalt chloride . Human EPO isolated from urine was initially used as reference material. In 1992 the WHO developed its own reference standard for recombinant EPO. The European Directorate for the Quality of Medicinal Products has again established a separate reference standard for therapeutic recombinant EPO (so-called BRP standard, BRP = biological reference preparation ). This is a 1: 1 mixture of epoetin α and epoetin β.
First generation EPO compounds
In contrast to insulin , which came from the pancreas of pigs before the use of recombinant insulin preparations (see Organon ), there was no such “archaic” origin for EPO. It was only through the isolation of the EPO gene and its cloning and expression in mammalian cells that it was possible with the help of biotechnological manufacturing processes to produce the hormone in sufficient quantities for therapy.
- The US biotechnology company Amgen brought the first recombinant EPO preparation ( Epogen , Epoetin α) onto the market in 1989 . In clinical studies of phases I and II from 1986 onwards at the University of Washington in Seattle it could be shown that the therapy of anemia with recombinant EPO in cancer and kidney patients has significantly fewer side effects than treatments with blood transfusions . The patent situation allowed Amgen to exclusively market EPO preparations in the USA until 2015 (according to another source, the Amgen patent expired in 2011). Amgen's licensee in Japan is the brewery group Kirin , whose pharmaceutical division has been selling the epoetin α variant under the trade name ESPO since 2001 . In October 2004, Kirin announced that it would end its cooperation with the Japanese pharmaceutical company Daiichi Sankyo in the distribution of ESPO on the Asian market in March 2005.
- The US pharmaceutical company Johnson & Johnson developed an epoetin α under the Amgen license, which is available under the trade name Procrit in and Eprex outside the USA. In Germany and Austria, the preparation is sold under the trade name Erypo by Janssen Cilag ( Ortho Biotech ), a subsidiary of Johnson & Johnson. Further trade names for sales in Italy are Epoxitin and Globuren . In Spain and Portugal, Eprex is also on the market under the name Epopen by the company Esteve (Laboratorios Pensa) . In Poland, Russia and the Ukraine the preparation is marketed by Jelfa Pharmaceuticals under the name Epoglobin . Also in Poland, the preparation Epox on the drug distributor Genexo on the market. In Bolivia, a preparation called Eritrogen produced by Laboratories Bagó is available.
- In 1990 Boehringer Mannheim launched an epoetin-β preparation under the name NeoRecormon . In 1997, when Boehringer Mannheim was bought by Hoffmann-La Roche , the pharmaceutical company received approval from the European Medicines Agency for Europe-wide introduction. In Japan, Chugai , a pharmaceutical company that has been part of Hoffmann-La Roche since 2002, has also been manufacturing an epoetin-β preparation under the trade name Epogin since 1990 .
Next generation EPO compounds
The enormous success of the first EPO preparations meant that (like no other recombinantly produced growth factor) numerous strategies were developed to increase the biological activity of the EPO molecule, to facilitate its use and to improve its tolerance. One focus was on structural modifications of the starting molecule (keywords: protein engineering , protein design). In addition, new fields of therapy could be defined through new findings from basic medical research. The most recent developments in this area include EPO analogs (also referred to as "mimetics" in English), gene therapy approaches to increase the EPO availability in the organism and combination preparations that are intended to be used, for example, to treat neurodegenerative diseases.
Modifications of the EPO molecule
- In 2001, Amgen generated a genetically modified erythropoietin under the trade name Aranesp (darbepoetin α). By exchanging five amino acids, this contains additional sugar side chains, which increases the proportion of terminal sialic acids and thus the serum half-life by a factor of three. It is the first of the next-generation EPO compounds to be therapeutically approved. The licensee for Amgens Darbepoetin α in Italy is Dompe Biotec , which sells the product under the name Nespo . Darbepoetin α is produced in CHO cells. In 2004, Amgen started a Phase I study of a hyperglycosylated Aranesp analog with the identification “AMG114” in the treatment of chemotherapy-induced anemia. In June 2006, at the 43rd Congress of the American Society of Clinical Oncology (ASCO) , an international team of researchers presented the results of a phase III multicenter study, according to which "AMG114" with a serum half-life of 131 hours appears to be more suitable than chemotherapy at the same time Tumor forms ( breast cancer , colon cancer , non-Hodgkin lymphoma ) to be applied. However, further studies have shown that the molecule has too little affinity for the EPO receptor. Therefore, all clinical studies with "AMG114" were terminated.
- Under the aspect of a longer duration of action, Hoffmann-La Roche developed the EPO derivative CERA ( Continuous Erythropoiesis Receptor Activator , internal Roche identifier: Ro 50-3821), in which the EPO molecule (the epoetin β known from the preparation NeoRecormon ) is linked to a methoxypolyethylene glycol polymer on the N-terminal alanine (ALA 1) or on one of the lysine residues (LYS 45 or LYS 52) (so-called PEGylation ). Due to the polymer linkage, CERA has a molecular mass of 66 kDa and is therefore almost twice as large as native EPO. According to studies from clinical phase II, the serum half-life after intravenous administration is around 133 hours and is therefore more than five times longer than with darbepoetin α. According to pharmacokinetic studies, the effect of CERA is determined by the weaker binding of the molecule to the erythropoietin receptor. After binding, CERA also releases faster from the EPO receptor. CERA is currently also in a clinical study (phase III) in the treatment of non-Hodgkin lymphoma . In April 2006, an application was submitted to the European Medicines Agency to market the preparation under the trade name Mircera . In July 2007, it was approved by the European Commission for the treatment of anemia in chronic kidney disease (CKD); safety and efficacy have not been proven in other indications. Two controlled clinical studies in which Mircera was used in patients with a variety of cancers including head and neck cancer and breast cancer showed an unexplained increase in mortality. In November 2007, the FDA approved Mircera in the United States for the treatment of renal anemia with a once-monthly maintenance dose.
- Other companies such as Bolder Biotechnology (with BBT-009), PolyTherics (with Epo TheraPEG), Prolong Pharmaceuticals (with EPEG), Neose (with NE-180 ) are also developing pegylated EPO preparations, which are still in the preclinical test stages = pegylated EPO from insect cells ), Lipoxen (ErepoXen, polysialic acid instead of polyethylene glycol as a pegylation polymer) and the Heidelberg-based company Complex Biosystems (reversible PEGylation for the controlled release of the active ingredient). In February 2008, Neose announced that activities related to its product NE-180 would be discontinued due to ongoing safety discussions about the use of erythropoietic substances and the resulting lack of market prospects. In April 2008, Lipoxen announced the successful completion of a Phase I study with ErepoXen in India and announced the start of a second Phase I study in Canada in June 2008. A first phase II study with the preparation is to be started in India in the second quarter of 2009. The company expects ErepoXen to be launched in Russia in 2011.
- In which, by the US firm CoGenesys developed preparation Albupoetin the EPO molecule is linked to a human albumin molecule. As with PEGylation, this modification increases the duration of action, as the EPO is more slowly depleted from the bloodstream via the kidneys. According to company information , albupoetin has shown its effectiveness in numerous in vitro and in vivo studies. The technique of linking albumin is also used by CoGenesys for other therapeutic agents (e.g. somatropin , G-CSF , BNP and insulin ). In January 2008, CoGenesys was taken over by the Israeli generics manufacturer Teva .
- The Florida-based company DNAPrint Genomics is currently working on preclinical studies under the identification “PT-401” on an EPO dimer preparation that is said to have a significantly higher affinity for the EPO receptor than the native EPO. In February 2008, DNAPrint Genomics was taken over by the US pharmaceutical company Nanobac Pharmaceuticals .
- The US company Syntonix is working on the development of an inhalation preparation based on its patented Transceptor technology . In this case, the EPO molecule (functional unit) is linked to the crystalline fragment (Fc) of an antibody (transport unit) to form a fusion protein (so-called Epo-Fc). Since the lung epithelium has a high density of receptors that interact with the Fc fragment (so-called FcRn), Epo-Fc, administered as an inhalation spray, is quickly absorbed in the lungs and transported into the bloodstream . The Fc unit of the fusion protein also ensures that the serum half-life is significantly longer than that of the “naked” EPO molecule. On the one hand, this is due to the increased molecular size (see CERA from Hoffmann-La Roche), which prevents it from being discharged via the kidneys. On the other hand, after endocytosis , Epo-Fc is released back into the bloodstream by the erythroblasts via the endosomal recycling route and is thus available again. Epo-Fc is in the clinical trial phase (clinical phase I). On February 1, 2007, Syntonix became a subsidiary of the biotech group Biogen Idec .
Syntonix's competitor in this area is Bolder Biotechnology , which has also developed an Epo-Fc (so-called ImmunoFusion Protein, identifier: BBT-021). - The US biotechnology company Warren Pharmaceuticals has worked with the Danish pharmaceutical company H. Lundbeck A / S to develop an EPO derivative that is intended to help treat neurodegenerative diseases . In the preparation CEPO (short for carbamylated EPO ), a carbamyl residue was coupled to all lysine monomers of the EPO molecule, which increases its affinity for specific neuronal receptors. In contrast to the native EPO molecule, CEPO has no erythropoietic properties. The effect of the preparation is based on anti-apoptotic effects, which prevent the death of myocardial and neuronal tissue. The first successes in the treatment of ischemic strokes and encephalitis were achieved in the mouse and rat models . The same applies to the therapy of myocardial infarction in the rat model. In October 2007, CEPO was used for the first time in clinical phase I.
- The Israeli pharmaceutical company Modigene (renamed PROLOR Biotech in June 2009 ) has developed an EPO preparation (MOD-7023) in which the EPO molecule is coupled to a carboxy-terminal peptide of human chorionic gonadotropin . MOD-7023 showed a longer serum half-life and higher pharmacological activity compared to standard preparations. The company also applies this technique to the structural modification of other therapeutic hormones ( somatotropin , interferon-β ).
"Natural" EPO variants
- A joint venture of the companies Sanofi-Aventis and the US company Transkaryotic Therapies (since 2005 by the British pharmaceutical manufacturer Shire Pharmaceuticals acquired) marketed one by gene activation via transfection of a viral promoter ( CMV) (of transformed human cell line HT-1080, isolated from an acetabular fibrosarcoma ) produced EPO variant under the brand name DynEpo (Epoetin δ). Shire first published the results of successful Phase III studies in September 2006. On March 15, 2007 DynEpo was launched on the German market. Other European countries followed in 2007. On July 31, 2008, Shire announced that it would cease production of DynEpo by the end of 2008.
- The French biotech company GenOdyssee has discovered a natural EPO variant characterized by a so-called SNP , which in in vitro experiments shows an activity that is 30–50% higher than that of native EPO. The variant known as “GO-EPO” shows a change in configuration near the EPO receptor binding site simply by exchanging a singular amino acid in the tertiary structure , which significantly increases the affinity of the molecule to the receptor.
- The US company GlycoFi has succeeded in generating a humanized EPO in yeasts of the genus Pichia , in particular Pichia pastoris . By introducing genetic knock-out elements and human-specific gene sequences into the yeast cells, it was possible to prevent yeast- specific glycosylation during the post-translational modification and, in return, to introduce human-specific glycosylation steps. In May 2006, GlycoFi was taken over by the US pharmaceutical company MSD Sharp & Dohme . The use of a pegylated form of humanized EPO (ID: MK2578) was investigated in phase II clinical studies. However, development was stopped in 2010.
- For more than a decade there have been efforts to produce EPO with the help of transgenic animals (cattle, pigs, goats, sheep). Japanese researchers from the University of Nagoya succeeded for the first time in the production of human EPO with the help of transgenic chickens. The hormone is isolated from the animals' eggs. The biological activity of the EPO obtained in this way in vitro is comparable to that of ordinary recombinant EPOs from CHO cells. However, the glycosylation is incomplete - the terminal sialic acids are often missing.
EPO mimetics
- In San Francisco -based biopharmaceutical company Gryphon Therapeutics (formerly Gryphon Sciences ), the first S ynthetische E rythropoese- P rotein ( Sept. developed). SEP is a fully synthetic macromolecule, consisting of a polypeptide backbone with 166 amino acid monomers, which has a high sequence homology to the native EPO molecule. This polypeptide contains two non-natural lysine monomers (Lys 24 (Nε-levulinyl) and Lys 126 (Nε-levulinyl)), via which it is chemically linked to a negatively charged polymer of a defined length. The activity of SEP in vitro is comparable to that of EPO. In contrast, the serum half-life is about 2.5 times longer. Hoffmann-La Roche acquired the license to use the protein in classic EPO therapy fields as early as 2002 .
- The US company Affymax has developed an EPO-analogous preparation under the name Hematide ™ ( INN Peginesatide, new trade name: Omontys ™). The active ingredient is a short-chain, cyclic polypeptide with a disulfide bridge , the mode of action of which corresponds to that of native EPO, but whose amino acid sequence is not homologous to the native EPO molecule. To avoid rapid excretion via the kidneys and to stabilize the structure, the peptide is also PEGylated . In March 2012, Omontys ™ received US approval for the treatment of renal anemia. In February 2013, the manufacturer announced a recall of the product, as some patients had anaphylactic reaction , some of which were fatal .
- The Canadian company ProMetic Biosciences has developed “PBI-1402”, a low-molecular EPO analogue that has shown stimulating and anti-apoptotic effects on the formation of erythrocytes and granulocytes in phase I clinical studies . The substance is now being investigated in phase II clinical trials in patients with anemia caused by chemotherapy drugs. The first results of these studies were published at the 13th Congress of the European Society of Hematology in Copenhagen in June 2008.
- The German biopharmaceutical company AplaGen Biopharmaceuticals from Baesweiler near Aachen has developed an EPO mimic , HemoMer ™, in which the functional peptide is linked to a polysaccharide-based macromolecule. As in the case of PEGylates, increasing the size of the molecules is intended to delay excretion via the kidneys. The so-called supravalence principle also ensures that in contrast to PEGylation, on the one hand, the effectiveness is increased and, on the other hand, the active ingredient carrier is also broken down in the body. The preparation is currently in preclinical studies and can so far be used intravenously as well as parenterally . The company is also working on other cytokine mimetics and alternative dosage forms. In 2010 the company had to file for bankruptcy.
- The company Abbott Laboratories has developed a therapeutic humanized antibodies (ABT007) which causes in preclinical studies in a mouse model by binding to the EPO receptor maturation of progenitor cells into erythrocytes and thus an increase in hematocrit. Due to the special binding properties of the antibody, administration is less frequent than with standard EPO preparations.
- The binding of EPO to its associated receptor (EpoR) can be prevented by certain substances, which in turn bind to the receptor instead of EPO (see competitive inhibition ). The US pharmaceutical company Merck has identified such a substance (N-3- [2- (4-biphenyl) -6-chloro-5-methyl] indolyl-acetyl-L-lysine methyl ester) by means of a competitive screening process which in the cell culture model as an octamer molecule (star-shaped connection of eight individual molecules via a central "core molecule", designation: "Compound 5") causes a receptor response identical to EPO (homodimerization and subsequent signal transduction cascades). "Compound 5" is fully synthetic and is the only ESA to date whose effect is mediated directly via the EPO receptor, without an amino acid backbone . This would be in contrast z. For example, peroral administration is also conceivable in addition to the EPO standard preparations (see also the section on dosage forms ). Further studies in the preclinical or clinical use of "Compound 5" have not yet been published.
- The US biotechnology company Centocor has developed an EPO-mimetic antibody fusion protein with the identification number "CTNO 528" without any structural similarity to erythropoietin. In the rat model, “CTNO 528” was successful in the treatment of erythrocyte aplasia . In a first phase I study on humans, the preparation increased the number of reticulocytes and the hemoglobin concentration in a dose-dependent manner .
- The US pharmaceutical company Ligand Pharmaceuticals is working on the development of a non-peptide, orally administered EPO mimetic.
Gene therapy
- The British company Oxford BioMedica is pursuing a gene therapy approach with its preparation Repoxygen in the pre-clinical phase. The drug is given intramuscularly and contains adenoviral gene shuttles , with the help of which the EPO gene is transferred into the muscle cells. The expression of the EPO gene is controlled by an oxygen-sensitive transcription factor. In this way, EPO is only formed in the transfected muscle cells when the oxygen saturation in the blood falls below a critical value. As part of the proceedings against track and field trainer Thomas Springstein on suspicion of gene doping in January 2006, company founder Alan Kingsman announced that Oxford BioMedica had ceased production of the active ingredient until further notice.
- The US pharmaceutical company Medgenics is working on the development of a so-called "biopump". Under local anesthesia, subdermal tissue , a so-called “microorgan”, is removed from the patient using a minimally invasive biopsy . The microorgan obtained in this way is then transfected with the EPO gene using adenoviral vectors. The cells genetically modified in this way then produce the desired protein (erythropoietin). After a few intermediate steps to remove excess adenoviruses and for functional testing, the microorgan is transplanted back into the patient (so-called autologous transplantation ). According to information provided by Medgenics, the function of this biopump will be maintained for a period of 6 months. In March 2009, Medgenics reported the successful results of a phase I / II study of their EPODURE therapy. According to this, one patient had been living with three EPODURE transplants for 11 months without any external EPO supply.
- In 5 to 10% of those dialysis patients in whom erythropoiesis does not respond despite treatment with high-dose EPO preparations (so-called EPO hyporesponsiveness), the reason for this is an increased expression of the protein SHP-1. SHP-1 is a protein phosphatase that prevents the JAK-STAT signal transduction cascade from occurring in hematopoietic precursor cells of the BFU-E type by dephosphorylation of the Janus kinase 2 enzyme after EPO binds to its receptor and thus prevents the Prevents precursor cells to erythrocytes (see chapter Biological function ). A Japanese research group was able to show that the introduction of antisense RNA into precursor cells of the BFU-E type, which had previously been isolated from EPO-hyporesponsive dialysis patients, prevents the protein biosynthesis of SHP-1 through complementary binding to the associated mRNA . The progenitor cells treated in this way continue the maturation process controlled by EPO. Instead of such a gene therapy approach, however, the authors suggest identifying substances that inhibit the activity of SHP-1. These substances could possibly include 4-hydroxynonenal , the inhibiting effect of which on SHP-1 in physiological concentrations has already been described.
EPO synthesis inducers
- The US company FibroGen is working on the development of a drug called "FG-2216". The substance inhibits the function of the enzyme prolyl hydroxylase , which is responsible for the breakdown of the so-called "hypoxia-induced factor" (HIF for short, see chapter on biosynthesis ). The HIF stabilization achieved in this way overexpresses the EPO gene. The preparation “FG-4592”, which was also developed by FibroGen and is to be used in the treatment of the so-called ACD syndrome (English Anemia of Chronic Disease ) , also has a corresponding mode of action . In addition, both substances seem to promote the expression of other genes that are important for erythropoiesis (EPO receptor, transferrin , transferrin receptor , ferroportin ). In April 2006, the Japanese pharmaceutical company Astellas acquired the rights to sell both products outside the USA.
- The preparation "AKB-6548" from the US company Akebia Therapeutics is also an inhibitor of prolyl hydroxylase. In September 2009 Akebia announced a Phase I study of oral administration in patients with chronic kidney disease and pre-dialysis patients.
- The South Korean pharmaceutical company CrystalGenomics is also working in competition with its American counterparts on the development of therapeutics that stabilize the effect of the HIF protein. Palkon Inc. , a joint venture between CrystalGenomics and the venture capital company ProQuest Investment , announced in June 2009 the start of preclinical studies with preparations for HIF stabilization.
- With the participation of the drug manufacturer Kowa Pharmaceutical , work is being carried out in Japan on a preparation called “K-11706”. The effect of the preparation is based on the inhibition of the transcription factor GATA2, which prevents the expression of erythropoietin by binding to the EPO promoter. K-11706 is intended to be used therapeutically for the treatment of the ACD syndrome (see above), in which inflammatory cytokines such as interleukin 1-β and TNF-α promote the DNA binding of GATA2. Initial successes were achieved in the mouse model after oral administration.
Chimeric EPO Proteins and Combination Therapies
- In 1999 the Italian pharmaceutical company Menarini patented the production of a fusion protein in CHO cells, which is composed of EPO and the “granulocyte-macrophage colony-stimulating factor” (GM-CSF for short) (US patent 5,916,773). The fusion protein with the designation “MEN 11303” achieved a significant improvement in the expansion of erythroid progenitor cells in in-vitro studies compared to equimolar doses of the individual factors. The possibility of the preparation in the ex vivo multiplication of human stem cells is currently being investigated.
- With NTx-265, the Canadian company Stem Cell Therapeutics has developed a treatment method in which the combinatorial administration of hCG ( human chorionic gonadotropin ) and EPO in an animal model were successful in treating strokes. A successful phase II study in patients was reported in February 2008.
- Scientists at the University Hospital Lausanne (CHUV) have found out in a mouse model that the protein Gas6 has a positive effect on the formation of red blood cells. In healthy mice given EPO, certain erythrocyte precursor cells (called erythroblasts ) produced the protein in question. Gas6, in turn, resulted in an improved response rate in the mice to EPO in terms of the formation of new red blood cells. In acutely and chronically anemic mice that did not respond to EPO alone, the addition of Gas6 increased the hematocrit. On the basis of these results in animal experiments, the authors assume that in the future Gas6 can be used alone or in conjunction with EPO in anemia therapies for patients in whom the administration of EPO alone has not previously achieved any success.
Imitation drugs (biogenerics, biosimilars, follow-on biologicals)
The development and use of biopharmaceuticals in medicine since the 1980s have led to significant advances in the therapy of serious diseases such as metabolic disorders as well as cancer and autoimmune diseases. However, biopharmaceuticals are very expensive and can cost 25 times as much as a conventional drug, which can place significant burdens on the healthcare system. The expiry of the patents for some biopharmaceuticals (including EPO) since 2004 and the guidelines issued by the European Medicines Agency for similar bio-medical products in general and the guidelines for similar bio-medical products containing recombinant erythropoietin in particular to permitted generic drug manufacturers in enter the business with EPO and other biopharmaceuticals (see chapter Market data for EPO preparations ). Due to the high demands on know-how and the high development costs, only a few generic manufacturers can do this. In some countries outside the European Union, as well as in Asia, Africa and South America, EPO generics ( biosimilars ) were available early on. In many cases, it would make more sense to speak of EPO plagiarism for products outside the European Union , since corresponding EPO preparations have been in circulation for many years and since patents and licenses were not taken into account in their manufacture and distribution. In the USA, Amgen currently has exclusive distribution rights due to the patent situation. Strict and standardized approval guidelines for the introduction of generic drugs, such as those issued by the European Medicines Agency, were announced by the FDA in 2003 , but have not yet been implemented. Following the change of government in the USA and President Barack Obama's declared goal of drastically reducing drug costs, the first step towards the introduction of generic drugs was presented to the US Congress in March 2009 by the so-called Biosimilar Act . FDA hearings were held in November 2010. The first EPO biosimilars were approved within the EU in August 2007.
So far, no uniform term has been established for copycat products of highly complex proteins. However, the term biosimilar is most commonly used in the scientific literature . The name refers to the high similarity between the biosimilar and its reference product (similar = English for "similar"), which is commonly referred to as the original or original preparation. The fact that the original preparation and biosimilar cannot be one hundred percent identical is due to the special nature of the manufacturing process. Since the active ingredient is produced biotechnologically, the replica depends crucially on the specifications of the manufacturing process. This includes, among other things, the selection of the cell line, the choice of the production system, the composition of the nutrient substance as well as temperature and pressure conditions during production. All biopharmaceuticals of an active ingredient group differ slightly. This applies not only to biosimilars and their reference product, but also to the original products with one another. Because they are obtained from living cells, there are always slight differences, for example between batches from a single manufacturer or between manufacturers of the same active ingredient.
In Europe, the manufacturing process is closely monitored in order to guarantee the greatest possible similarity between the biosimilar and its reference product. It is subject to the same strict quality guidelines that apply to the original preparations. Before a biosimilar comes onto the European market, the manufacturers of biosimilars have to conduct a comprehensive study program. The type and execution of the biosimilars studies are prescribed by the European Medicines Agency (EMA) and the results are checked as part of the approval process. Approval is a prerequisite for therapeutic use. The term "biogeneric" (plural: "biogenerics") is used occasionally and is an inadequate characterization of this regulatory drug class (American term: "follow-on biologics").
Asia
- Since 2000, numerous Indian pharmaceutical companies have been entering the domestic market with their own preparations. These include the companies Emcure with the preparations Vintor and Epofer, Wockhardt with Wepox, Zydus Biogen with Zyrop, Ranbaxy with the preparation Ceriton, Shantha Biotechnics with Shanpoietin and Intas Pharmaceuticals with the preparations Epofit and Erykine, Claris Lifesciences with Epotin and Zuventus with Eporise. The largest production facility for the production of recombinant proteins (including EPO) of the Bangalore-based biotech company Biocon was put into operation in April 2006. Biocon now sells the EPO preparation ERYPRO . In June 2009, Biocon entered into a strategic partnership with the US pharmaceutical company Mylan for sales in the USA. The drug manufacturer Reliance Life Sciences , which emerged from the takeover of the British company GeneMedix by the Indian company Reliance Industries , has been selling the EPO preparation ReliPoietin since 2008 .
- The Vancouver-based Canadian pharmaceutical company Dragon Biotech has been producing a generic EPO in a facility in Nanjing (China) since 2004 and sells it in China, India, Egypt, Brazil, Peru, Ecuador, Trinidad & Tobago as well as in the Dominican Republic and Kosovo. The company also announces the development of a new EPO product for the European market.
- In addition to Dragon Biotech , other companies are represented on the Chinese market with EPO preparations. These include the Hong Kong-based companies Refinex Medical and Medichem , as well as SciProgen (preparation: SEPO ), Beijing Four Rings Biopharmaceuticals , Shandong Kexing Bioproducts (preparation: EPOSINO ), Kelun Biopharmaceuticals , Chengdu Diao , Shanghai Ke-hua , Shangdong Ahua , Shenzhen Xinpeng , Shanghai Sanwei and 3SBio Shenyang Sunshine Pharmaceuticals (short: SSP ). The company PlasmaSelect from Munich intends to market the EPO preparation EPIAO distributed by SSP in Europe, which has a market share of around 40% in China. The Shijiazhuang-based pharmaceutical company North China Pharmaceutical Group Corporation (NCPC), China's largest producer of antibiotics , sells an EPO compound produced by its GeneTech Biotechnology joint venture under the trade name GerEpo .
- In Vietnam, the Ho Chi Minh City- based company Nanogenpharma produces an EPO-α preparation under the name Bioetin .
- The EPO preparation Epokine (EPO α) from the biopharmaceutical company CJ Corp is on the market in South Korea . Epokine is also available in other Asian countries ( e.g. Pakistan and Philippines) and South America ( e.g. Chile) through local distributors. The preparation Eporon is distributed by CJ Corps' domestic competitor Dong-A Pharmaceutical . In the South American and Pacific region, Eporon is on the market under the name Eritina through the Colombian company Chalver Laboratorios . A third company is LG Lifescience with Espogen , which is also marketed in India by the subsidiary LG Lifescience India . A cooperation agreement between LG Lifescience and the Swiss biogenerics developer Biopartners has been in place since 2000 for the planned introduction of Espogen and other biopharmaceuticals in the European Union.
- On February 5, 2007, according to the head of the Pasteur Institute of Iran , Abdolhossein Rouholamini Najafabadi, the largest production facility for recombinant proteins (including erythropoietin) in Southwest Asia was inaugurated in the presence of Iranian President Mahmud Ahmadineschad . In this plant, the Iranian pharmaceutical company Pooyesh Darou Pharmaceuticals produces, among other things, the EPO preparation PDpoetin .
- The Iranian biotechnology company Cinnagen produces the EPO preparation Erytrex (epoetin β) in cooperation with the drug manufacturer Zahravi Pharmaceuticals .
- In Indonesia, the pharmaceutical companies Novell Pharmaceutical Laboratories and Kalbe Farma are represented with the products Epotrex and Hemapo, respectively.
- The largest pharmaceutical company in the Philippines, United Laboratories Inc. , sells through its subsidiary Biomedis Inc. , the preparation Renogen .
USA, Central and South America
- In Brazil, the pharmaceutical company Cristália has developed a generic EPO that can be obtained without a prescription in cooperation with the semi-public research institute Instituto Butantan . The pharmaceutical company Blausiegel is also represented in Brazil with the preparations Eritromax and AlfaEpoetina .
- In Argentina (in addition to the Hemax preparation , see above) the Epoyet and Hypercrit preparations are produced by the pharmaceutical company Bio Sidus .
- In Cuba, under the leadership of the state-owned Centro de Ingeniería Genética y Biotecnología, a generic α variant in CHO cells was developed, which is sold for the domestic market by the pharmaceutical company Heber Biotec based in Havana under the trade name Heberitro . Heber Biotec's local co-provider is CIMAB SA with the EPOCIM product .
- The US biopharmaceutical service company Protein Sciences has developed a process for the production of an EPO biosimilar in insect cells and offers this process as a licensor. According to company information, the EPO generated in insect cells that are transfected with baculoviruses has a biological activity that is roughly twice that of the standard EPO preparation ( Epogen ).
- The AXXO GmbH , a company based in Hamburg company, recently acquired the Mexican company Nedder Farmaceuticos that as a subsidiary under the name Axxo Mexico changed its name and produces, among other things, a recombinant EPO for the Latin American market. The domestic competitors are the pharmaceutical companies Probiomed with BIOYETIN ™, Pisa with EXETIN-A and Laboratorios Cryopharma with EPOMAX .
Africa and Middle East
- An EPO preparation under the trade name Repotin (EPO α) has been manufactured in South Africa since 1997 by the company Bioclones from Johannesburg .
- At least four companies in Egypt manufacture EPO preparations for the domestic market: EIPICO with Epoform , Amoun Pharmaceuticals with Erypoietin , Sedico with Epoetinv and T3A Pharma with Pronivel . In Argentina Pronivel is marketed by the pharmaceutical company Laboratorio Elea .
- InSight Biopharmaceuticals is the only manufacturer of generic EPO in bulk in Israel . The company Prospec TechnoGene also produces α and β variants of EPO in CHO cells, but only for laboratory purposes.
- The pharmaceutical company Julphar (Gulf Pharmaceuticals Industries) , based in the Emirate of Ra's al- Khaimah, manufactures an EPO-α variant under the trade name Epotin .
Europe
- In June 2005 the Croatian pharmaceutical company Pliva received permission from the responsible local regulatory authority to market an EPO generic ( Epoetal ) in Croatia. An expansion of the distribution rights for the pan-European market was sought in cooperation with the Australian company Mayne Pharma , but according to a press release of February 22, 2006 it was discontinued. The reason for this decision may be the massive violations of the guidelines of good manufacturing practice in Pliva's production facility in Zagreb that the FDA found during an inspection in January / February 2006 . After a takeover by the Icelandic generics manufacturer Actavis failed, the US pharmaceutical company Barr Pharmaceuticals has been trying to acquire Pliva since June 2006 . As a result of a takeover process that was completed on July 18, 2008, Barr Pharmaceuticals and thus also Pliva belong to the Israeli pharmaceutical company Teva Pharmaceutical Industries .
- In Ukraine, the company Biopharma produces an EPO preparation under the product name Epocrin (Епокрин) for the domestic and Russian markets. The manufacturer of the Epocrin variant (Эпокрин) in Russia is the pharmaceutical company Sotex .
- In England, the generic drug manufacturer GeneMedix announced the market launch of an EPO preparation with the product name Epostim in May 2005 . In the meantime, the target date has been postponed to the third quarter of 2007. On March 31, 2008, GeneMedix announced that it had obtained authorization to manufacture Epostim at its Tullamore, Ireland facility and to conduct clinical trials in the European Union. In the meantime, GeneMedix has been taken over by the Indian company Reliance Industries .
- The board of directors of Stada Arzneimittel stated in a press release of March 30, 2006 that the submission of the approval documents to the European Medicines Agency for the production and sale of an EPO generic was planned in the second quarter of 2006 and that the market launch would take place in late 2006 or early 2007 will be expected. On June 30, 2006, STADA announced that the company had submitted the approval documents to the European Medicines Agency for the production of an erythropoietin zeta on the same day. The cooperation partner for the production of the biosimilar for the clinical study is the Bielefeld- based biotech company Bibitec . The US company Hospira acquired the distribution rights for erythropoietin zeta in November 2006 for marketing in the European Community and in Canada / USA. On October 18, 2007, STADA and Hospira received a positive decision from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency for the market launch of the products Silapo and Retacrit . STADA received the final approval notice for the market launch of both products for the first quarter of 2008 on December 19, 2007. Hospira , part of the US pharmaceutical company Pfizer since 2015 , was the first company to receive FDA approval for the US on May 15, 2018 Market.
- The British generics manufacturer Therapeutic Proteins announced in a press release on May 12, 2006 that it would submit registration documents to the European Medicines Agency for the production and sale of an EPO generic under the trade name TheraPoietin and for two other biosimilars. All three biosimilars are to be produced in collaboration with the British contract manufacturer Angel Biotechnology .
- In 2007, the generics manufacturer HEXAL was the first to receive approval from the EU Commission for an epoetin biosimilar under the trade name Epoetin alfa Hexal . The preparation was also approved under the brand names Binocrit ( Sandoz ) and Abseamed ( Medice ). All three preparations are made by the Rentschler Biotechnologie company in Laupheim on a contract basis . In January 2008, Sandoz agreed with the medical technology company Gambro to develop a joint sales structure for the preparation Binocrit in Germany.
- In July 2009, the generics manufacturer Ratiopharm received the approval recommendation from the Human Medicine Committee (CHMP) of the European Medicines Agency for an EPO biosimilar it had developed. The final marketing authorization of the preparation Eporatio (Epoetin θ), which is also sold by the Berlin CT Arzneimittel under the trade name Biopoin , took place in December 2009.
The "Eprex" case
From 1998 onwards there were severe side effects when using the EPO agent Eprex / Erypo . At the instigation of the competent authority, all human protein components had to be removed from the formulation of pharmaceuticals because of the possible risk of contamination from HIV or the pathogen causing Creutzfeldt-Jakob disease . The manufacturer Ortho Biotech then used the stabilizer polysorbate 80 instead of human serum albumin . A study conducted by Johnson & Johnson found that the addition of polysorbate fatally caused plasticizers to dissolve out of the rubber stoppers of the Epo syringes. These triggered immune reactions and an erythroblastopenia ( Pure Red Cell Aplasia = PRCA ) in at least 250 patients treated with Erypo . This incident raised the question worldwide to what extent changed amino acid sequences, modified glycostructures or impurities in the production of therapeutic proteins and their derivatives (e.g. biosimilars) can lead to such side effects. The Brazilian regulatory authority Agência Nacional de Vigilância Sanitária (short: ANVISA) imposed an import ban on two EPO preparations in the same year. In a study by the University of Utrecht on eight preparations that are sold outside the EU and the USA, serious deficiencies in terms of effectiveness, purity and formulation consistency were found. These results were confirmed by a new study with preparations from Korea, China and India. However, recent studies at the University of Utrecht on the Epo-biosimilars approved in Europe according to European guidelines show that they are of at least equivalent quality to the original preparations.
Dosage forms
The usual galenic form of the EPO preparations currently approved by the responsible authorities is that of an injection solution with different concentrations of active substances (around 500 to 30,000 IU ). In addition to EPO, the solution based on water for injection purposes also contains auxiliary substances (such as urea , polysorbate 20 , various amino acids and sodium salts) that serve to stabilize the active substance. The injection solutions are administered either subcutaneously or intravenously . Depending on the application, active ingredient concentration, indication and duration of action or serum half-life of the preparation, several injections per week or just a single injection per month are required. The DDD value for the first generation preparations is 1000 IU, in the case of the Aranesp and Mircera preparations it is 4.5 micrograms each.
Work is being carried out on alternative forms of administration, particularly in connection with the development of new erythropoietic drugs (e.g. intrapulmonary administration of the EPO-Fc preparation from Syntonix and intramuscular administration of the preparation Repoxygen from Oxford BioMedica , see the next-generation EPO preparations section ). In the case of the standard preparations (e.g. Procrit from Johnson & Johnson ), sustained release formulations have been studied, e.g. B. via so-called encapsulation in biodegradable microspheres . The main goal was to lengthen the intervals between the individual doses and to improve tolerability. A serious problem with encapsulation is the formation of EPO aggregates, which excludes use on patients. At the end of the 1990s, the American company Alkermes was able to circumvent this problem with its patented ProLease technology. However, the microspheres are potential antigenic adjuvants that can trigger undesirable immune reactions in the patient. This may explain why clinical studies of these formulations have not yet occurred. A Japanese team could show contrast in mice that an embedding of EPO in gelatin - hydrogel microspheres successfully in the treatment of circulatory disorders in the lower extremities can be used. Research was
also carried out on oral forms of administration in which the problem of acid denaturation by gastric juice had to be overcome. The British company Provalis (formerly Cortecs International ) worked on oral formulations in cooperation with Johnson & Johnson . However, the results of this were never published. With the bankruptcy of Provalis in 2006, these activities came to a standstill. The US company Access Pharmaceuticals is working on a new approach to the oral administration of EPO . The natural absorption route of vitamin B 12 is used. By coating EPO with the vitamin B 12 derivative cyanocobalamin , nanoparticles are created which, in conjunction with the haptocorrins contained in the saliva and the intrinsic factor in the stomach, form a complex that is protected from destruction by the acid attack in the stomach is introduced into the bloodstream via receptors in the small intestine . The development of such a preparation is currently still in preclinical experimental stages.
The Australian nanotechnology company Nanotechnology Victoria is working on techniques for the intrapulmonary administration of EPO . For this purpose, an inhalation device was developed which, on the basis of the surface acoustic wave, enables the generation of nanoparticulate droplets of high-molecular therapeutics.
The US pharmaceutical company Zosano claims to have developed a microinjection technology that enables the transdermal administration of therapeutic proteins. The application of this technique with EPO is currently in the preclinical experimental stages.
Side effects and contraindications
Since EPO receptors are formed on the surface of a wide variety of tumor cells, there is fundamentally the possibility that the administration of EPO preparations can stimulate the growth of malignancies of all kinds. Two controlled clinical trials in which patients with various types of cancer including head and neck cancer and breast cancer were treated with recombinant EPO showed an unexplained increase in mortality . There has been good experience in the treatment of anemia for multiple myeloma , non-Hodgkin lymphoma and chronic lymphocytic leukemia . Due to the nature of the side effects, special care should be taken in hypertensive patients. Abuse of healthy people (e.g. for doping purposes) can lead to an excessive increase in the hematocrit value . This is associated with the risk of life-threatening complications of the cardiovascular system ( risk of thrombosis due to haemoconcentration in polyglobulia ).
In the spring of 2007, the US FDA published a warning regarding the use of erythropoiesis-stimulating substances as a result of the results of four clinical studies in which previously untested treatment regimens had life-threatening side effects. Hemoglobin levels above 12 g / dL, which were adjusted in the affected patients by means of EPO preparations, led to a significant increase in the mortality rate. As a result, the FDA ordered the amendment of the previous warnings on the package inserts for the Aranesp , Epogen and Procrit products .
In another multicenter study on the use of epoetin β in anemia in breast cancer patients who underwent chemotherapy, however, no increase in mortality was found. In this study, EPO was administered even when the hemoglobin level fell below 12.9 g / dL. Obviously, the mortality with EPO therapy is not directly dependent on the hemoglobin level set. Rather, it increases in cancer patients when they are not receiving chemotherapy.
A meta-analysis of 53 clinical studies with almost 14,000 patients came to the conclusion in May 2009 that the mortality of cancer patients after administration of EPO preparations is increased by a factor of 1.17 compared to those who have not undergone any EPO therapy . In patients who were receiving chemotherapy at the same time, the factor was 1.10.
The risk of cancer patients with EPO therapy is not limited to tumor progression that can be caused by EPO. The risk of venous thromboembolism increases significantly with EPO therapy in patients with solid tumors. The German Society for Hematology and Oncology therefore recommends using EPO in cancer medicine only in adult patients with chemotherapy-induced anemia if they have symptoms. Furthermore, the hemoglobin level should be increased to a maximum of 12 g / dl. Therapy should be stopped as soon as the target hemoglobin value of a maximum of 12 g / dl is reached or four weeks after the end of chemotherapy. When prescribing drugs with "blood-forming" (erythropoiesis-stimulating) active ingredients (ESAs) for the treatment of symptomatic anemia in the case of chronic kidney failure, binding therapeutic instructions apply in Germany. The corresponding resolution of the Federal Joint Committee (G-BA) of June 23, 2011 came into force on September 22, 2011 after publication in the Federal Gazette.
Market data for EPO supplements
As a therapeutic agent, EPO was one of the ten most successful drugs in the world until 2004; among the biopharmaceuticals it is one of the outstanding blockbusters . In the meantime, EPO products accounted for more than 30% of sales of therapeutic recombinant proteins. Johnson & Johnson's Eprex / Procrit grossed $ 3.6 billion in 2004, Amgen's Epogen $ 2.6 billion and Roche's NeoRecormon $ 1.7 billion. Aranesp , the first approved next-generation EPO, has had an average growth rate of around $ 800 million annually since its inception. In 2006, Amgen's sales of Aranesp totaled 4.1 billion US dollars, surpassing sales of previous standard drugs for the first time. The successor drugs DynEpo and Mircera were expected to have initial sales rates of $ 300 million and $ 900 million, respectively. However, these prognoses were not confirmed. In 2010 sales with Mircera were 285 million US dollars, DynEpo was taken off the market at the end of 2008. In 1999, around 350,000 patients worldwide received recombinant EPO. Since the sales figures for EPO preparations more than tripled between 1999 and 2005, the number of patients treated with EPO is likely to have increased proportionally in the corresponding period.
In 2007, in the course of the market launch of the first copycat products in the European Union, the development of new EPO products ( DynEpo , Mircera ) and the safety debate surrounding the use of EPO for the treatment of tumor anemia, sales figures for standard products fell for the first time. In 2007, sales of US $ 11.8 billion were made with the standard preparations, a decrease of US $ 100 million from 2006. In 2010, sales were now just $ 8.2 billion, down to 2002 levels.
In 2007, around 470 million US dollars were sold in Germany with EPO preparations. According to officially available data, this corresponds to around 4.5% of the global sales result achieved in the same period. The introduction of generic drugs in Germany has led to a drop in prices. Biosimilars offer a significant price advantage compared to the fixed price of the reference product. In order to reduce drug costs in Germany, for example, the Association of Statutory Health Insurance Physicians in Berlin planned to increase the proportion of prescriptions for EPO biosimilars to 50% in 2008. At the beginning of 2009, the market share of EPO biosimilars was now 53%, while the share of original preparations and their re-imports fell to 38% and 9% respectively. According to estimates, health insurers would be able to save around eight billion euros by 2020 through the use of biosimilars. In China, 14 different EPO preparations are officially represented on the market, with total sales in 2006 of around 50 million US dollars. In India, sales of EPO products were US $ 22 million in 2006, with annual growth rates of 20–30% until then.
EPO doping
The more red blood cells there are in the human bloodstream , the more efficiently the entire organism works, because the cells have a correspondingly large amount of oxygen available. For this reason, EPO has been misused to improve performance since the late 1980s. Endurance athletes in particular benefit from the effect; however, the increased proportion of erythrocytes in the blood increases the risk of blood clots . EPO (and subsequently all other derivatives such as darbepoetin) has been on the doping list of the international anti-doping organization (WADA) since 1990 , so its use in competitive sports is prohibited. A practicable detection method for non-endogenous EPO has also been used in urine samples since 2000 . However, since the detection method is only effective within the first four days after administration and the significant performance-enhancing effect decreases continuously, but lasts for up to 17 days, the 2000 Olympic Games were still EPO games.
According to calculations by the Italian sports scientist Prof. Alessandro Donati from 2007, 500,000 people around the world are doping with EPO. According to Donati's research, the amount of EPO produced annually exceeds the actual therapeutic need by five to six times.
See also
- Hematocrit (abbreviation: Hct, Hkt or Hk), in medicine denotes the proportion of cellular components, mostly red blood cells (erythrocytes) , in the volume of the blood and is a measure of the viscosity of the blood. Normal values for men are between 40 and 53 percent.
- In the sedimentation reaction - abbreviated BSR, BKS, blood sedimentation; also: erythrocyte sedimentation rate (ESR), it is a non-specific, simple search method for inflammatory diseases. The cellular components of the blood are compared with the length of the cell-free column of blood plasma.
Trade names
- Epoetin alfa: Abseamed (D), Binocrit (D), Epoetin alfa HEXAL (D), Erypo (D)
- Epoetin beta: NeoRecormon (D)
- Epoetin zeta: Retacrit (D), Silapo (D)
- Epoetin theta: Biopoin (D), Eporatio (D)
literature
Biosynthesis and Biological Function
- PC Watkins et al .: Regional assignment of the erythropoietin gene to human chromosome region 7pter-q22 . In: Cytogenet Cell Genet , 1986, 42, pp. 214-218 PMID 2875851
- GL Wang, Semenza GL : General involvement of hypoxia-inducible factor 1 in transcriptional response of hypoxia . In: Proc Natl Acad Sci USA , 1993, 90, pp. 4304-4308. PMID 8387214
- SS Watowich: Activation of erythropoietin signaling by receptor dimerization . In: Int J Biochem Cell Biol , 1999, 31, pp. 1075-1088. PMID 10498627
- TR Lappin et al .: EPO's alter ego: erythropoietin has multiple actions . In: Stem Cells , 2002, 20, pp. 485-492. PMID 12456956
Structural properties
- MA Recny et al .: Structural characterization of natural human urinary and recombinant DNA-derived erythropoietin . In: J Biol Chem , 1987, 262, pp. 17156-17163 PMID 3680293
- H Sasaki. et al .: Carbohydrate structure of erythropoietin expressed in chinese hamster ovary cells by a human erythropoietin cDNA . In: J Biol Chem , 1987, 262, pp. 12059-12076. PMID 3624248
- N Kawasaki. et al .: Structural analysis of sulfated N-linked oligosaccharides in erythropoietin . In: Glycobiology , 2001, 11, pp. 1043-1049. PMID 11805077
- AW Gross, HF Lodish: Cellular trafficking and degradation of erythropoietin and novel erythropoietin stimulating protein (NESP) . In: J Biol Chem , 2006, 281, pp. 2024-2032. PMID 16286456
Research history
- D. Jourdanet: De l'anemie des altitudes et de l'anemie en general dans ses reports avec la pression del l'atmosphere . Balliere, Paris 1863.
- P. Carnot, C. Deflandre: Sur l'activité hémopoiétique du sérum au cours de la régénération du sang . In: CR Acad Sci , 1906, 143, pp. 384-386; doi: 10.1161 / 01.RES.24.3.488 .
- C. Gibelli: About the value of the serum anemic animals in the regeneration of the blood . In: Naunyn-Schmiedeberg's Archives of Pharmacology , 1911, 65, pp. 284-302 doi: 10.1007 / BF01841822 .
- G. Sandor: About the hematopoietic effect of the serum from animals kept in dilute air . In: Z. Entire Exp. Med. , 1932, 82, pp. 633-646.
- E. Bonsdorff, E. Jalavisto: A humoral mechanism in anoxic erythrocytosis . In: Acta Physiol Scand , 1948, 16, pp. 150-170; doi: 10.1111 / j.1748-1716.1948.tb00535.x .
- A. Erslev: Humoral regulation of red cell production . In: Blood , 1953, 8, pp. 349-357. PMID 13032205
- Maluf NS: History of blood transfusion . In: J Hist Med Allied Sci , 1954, 9, pp. 59-107. PMID 13118144
- LO Jacobson et al .: Role of the kidney in erythropoiesis . In: Nature , 1957, 179, pp. 633-634. PMID 13418752
- T. Miyake, CK Kung, E. Goldwasser: Purification of human erythropoietin . In: J Biol Chem , 1977, 252, pp. 5558-5564. PMID 18467
- Lee-Huang S .: Cloning and expression of human erythropoietin cDNA in Escherichia coli . In: Proc. Nat. Acad. Sci. USA , 1984, 81, pp. 2708-2712. PMID 6371819
- FK Lin et al .: Cloning and expression of the human erythropoietin gene . In: Proc Natl Acad Sci USA , 1985, 82, pp. 7580-7584. PMID 3865178
- K. Jacobs et al .: Isolation and characterization of genomic and cDNA clones of human erythropoietin . In: Nature , 1985, 313, pp. 806-810. PMID 3838366
- Patent US4703008 : DNA sequences encoding erythropoietin. Inventor: FK Lin.
- W. Jelkmann: Erythropoietin after a century of research: younger than ever . In: Eur J Haematol , 2007, 78, pp. 183-205. PMID 17253966
Indication for therapy with EPO
- S. Schreiber et al .: Recombinant erythropoietin for the treatment of anemia in inflammatory bowel disease . In: N Engl J Med , 1996, 334, pp. 619-623. PMID 8592524
- GD Demetri et al .: Quality-of-life benefit in chemotherapy patients treated with epoietin alpha is independent of disease response or tumor type: results from a prospective community oncology study . In: J Clin Oncol , 1998, 10, pp. 3412-3425. PMID 9779721
- K. Nishii et al .: Successful treatment of aplastic anemia with G-CSF and high dose erythropoietin . In: Leuk Lymphoma , 1998, 30, pp. 211-214. PMID 9669693
- DH Henry: Experience with epoetin alfa and acquired immunodeficiency syndrome anemia . In: Semin Oncol , 1998, 25, pp. 64-68. PMID 9671334
- FR Dunphry et al .: Erythropoietin reduces anemia and transfusions: a randomized trial with or without erythropoietin during chemotherapy . In: Cancer , 1999, 86, pp. 1362-1367. PMID 10506726
- H. Feldmann et al .: Blood flow and oxygenation status of human tumors. Clinical investigations . In: Strahlenther Onkol , 1999, 175, pp. 1-6. PMID 9951511
- C. Gasche et al .: Sequential treatment of anemia in ulcerative colitis with intravenous iron and erythropoietin . In: Digestion , 1999, 60, pp. 262-267. PMID 10343140
- M. Cazzola: Haematopoietic growth factors in the treatment of myelodysplastic syndromes . In: Forum (Genova) , 1999, 9, pp. 49-57. PMID 10101210
- M. Milano, R. Collomp: Erythropoietin and neuroprotection: a therapeutic perspective . In: J Oncol Pharm Pract , 2005, 11, pp. 145-149. PMID 16595066
- H. Ehrenreich et al .: Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin . In: Mol Psychiatry , 2007, 12 (2), pp. 206-220. PMID 17033631
- M. Krebs et al .: Neuroprotective agents in schizophrenia and affective disorders . In: Expert Opin Pharmacother , 2006, 7, pp. 837-848. PMID 16634707
First generation EPO compounds
- L. Ehrlich: Use of EPOGEN for treatment of anemia associated with chronic renal failure . In: Crit Care Nurs Clin North , 1990, Am 2, pp. 101-113. PMID 2357306
- Patent US5441868 : Production of recombinant erythropoietin. Inventor: FK Lin.
- PL Storring et al .: Epoetin alfa and beta differ in their erythropoietin isoform composition and biological properties . In: Br J Haematol , 1998, 100, pp. 79-89. PMID 9450795
- V. Skibeli et al .: Sugar profiling proves that human serum erythropoietin differs from recombinant human erythropoietin . In: Blood , 2001, 98, pp. 3626-3634. PMID 11739166
- A. Bren et al .: A comparison between epoetin omega and epoetin alfa in the correction of anemia in hemodialysis patients: a prospective, controlled crossover study . In: Artif Organs , 2002, 26, pp. 91-97. PMID 11879235
- R. Deicher, WH Horl: Differentiating factors between erythropoiesis-stimulating agents: a guide to selection for anemia of chronic kidney disease . In: Drugs , 2004, 64, pp. 499-509. PMID 14977387
- J. Glaspy, Y. Beguin: Anemia management strategies: optimizing treatment using epoetin beta (NeoRecormon) . In: Oncology , 2005, 69 Suppl. 2, pp. 8-16. PMID 16244505
- T. Littlewood: Epoetin alfa (Eprex) and quality of life . In: Curr Med Res Opin. , 2005, 21 Suppl 2: S1-S2. PMID 15969857
Next generation EPO compounds
- FC Wrighton et al .: Small peptides as potent mimetics of the protein hormone erythropoietin . In: Science , 1996, 273, pp. 458-464. PMID 8662529
- A. Battaglia et al .: The fusion protein MEN 11303 (granolocyte-macrophage colony stimulating factor / erythropoietin) acts as a potent inducer of erythropoiesis . In: Exp Hematol , 2000, 28, pp. 490-498. PMID 10812238
- JQ Hudson, RM Sameri: Darbepoetin alfa, a new therapy for the management of anemia of chronic kidney disease . In: Pharmacotherapy , 2002, 22, pp. 141S-149S. PMID 12222584
- GG Kochendoerfer et al .: Design and chemical synthesis of homogeneous polymer-modified erythropoiesis protein . In: Science , 2003, 299, pp. 884-887. PMID 12574628
- AJ Bitonti et al .: Delivery of an Erythropoietin-Fc Fusion Protein by Inhalation in Humans through an Immunoglobulin Transport Pathway . In: J Aerosol Med , 2004, 18, pp. 294-303. PMID 16181004
- IC Macdougall: CERA (Continuous Erythropoietin Receptor Activator): a new erythropoiesis-stimulating agent for the treatment of anemia . In: Curr Hematol Rep , 2005, 4, pp. 436-440. PMID 16232379
- SY Chen et al .: Synthetic erythropoietic proteins: tuning biological performance by site-specific polymer attachment . In: Chem Biol , 2005, 12, pp. 371-383. PMID 15797221
- F. Fiordaliso et al. (2005), A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury Proc Natl Acad Sci, 102, pp. 2046-2051. PMID 15671158
- SR Hamilton et al .: Humanization of yeast to produce complex terminally sialylated glycoproteins . In: Science , 2006, 313, pp. 1441-1443. PMID 16960007
- Österborg AC et al .: A novel erythropoiesis-stimulating agent (AMG114) with 131-hour half-life effectively treats chemotherapy-induced anemia when administered as 200 mcg every 3 weeks . In: J Clin Oncol , 2006, 24, No. 18S, p. 8626. PMID 16982323
- Q. Fan et al .: Preclinical evaluation of Hematide, a novel erythropoiesis stimulating agent, for the treatment of anemia . In: Exp Hematol , 2006, 34, pp. 1303-1311. PMID 16982323
- TR Coleman et al .: Cytoprotective doses of erythropoietin or carbamylated erythropoietin have markedly different procoagulant and vasoactive activities . In: Proc Natl Acad Sci USA , 2006, 103, pp. 5965-5970. PMID 16585502
Imitation drugs (biosimilars) / The "Eprex" case
- S. Louët: Lessons from Eprex for biogeneric firms . In: Nature Biotechnology , 2003, 21 (9), pp. 956-957. PMID 12949539
- H. Schellekens: Biosimilar epoetins: how similar are they? In: EJHP Practice , 2004, 3, pp. 243–247. PMID 16006274
- K. Boven et al .: The increased incidence of pure red cell aplasia with an Eprex formulation in uncoated rubber stopper syringes . In: Kidney International , 2005, 67, pp. 2346-2353. PMID 15882278
- SK Niazi: Handbook of Biogeneric Therapeutic Proteins . 1st edition. CRC Press, 2005, ISBN 0-8493-2991-4
- V. Zylka-Menhorn, ME Tippmann: Biopharmaceuticals are “inimitable” . In: Deutsches Ärzteblatt , 2006, Volume 103, Issue 6: A311-A314.
Dosage forms
- KF Pistel et al .: Biodegradable recombinant human erythropoietin loaded microspheres prepared from linear and star-branched block copolymers: (...) . In: J Control Release , 1999, 59, pp. 309-325. PMID 10332063
- Patent US5674534 : Composition for sustained release of non-aggregated erythropoietin. Inventor: SE Zale.
EPO doping
- J. Scott, GC Phillips: Erythropoietin in sports: a new look at an old problem . In: Curr Sports Med Rep , 2005, 4, pp. 224-226. PMID 16004833
- Diamanti-Kandarakis E. et al .: Erythropoietin abuse and erythropoietin gene doping: detection strategies in the genomic era . In: Sports Medicine , 2005, 35, pp. 831-840. PMID 16180943
- W. Jelkmann: Novel erythropoietic agents: A threat to sportsmanship . (PDF) In: Medicina Sportiva , 2007, 11, pp. 32–42.
- K. Sharpe et al .: A third generation approach to detect erythropoietin abuse in athletes . In: Haematologica , 2006, 91, pp. 356-363. PMID 16503554
Verification procedure
- F. Lasne, J. de Ceaurriz: Recombinant erythropoietin in urine . In: Nature , 2000, 405, p. 635. PMID 10864311
- F. Lasne: Double-blotting: a solution to the problem of nonspecific binding of secondary antibodies in immunoblotting procedures . In: J Immunol Methods , 2003, 276, pp. 223-226. PMID 12738375
- M. Beullens et al .: False-positive detection of recombinant human erythropoietin in urine following strict physical exercise . In: Blood , 2006, 107, pp. 4711-4713. PMID 16493001
- F. Lasne: No doubt about the validity of the urine test for detection of recombinant human erythropoietin . In: Blood , 2006, 108, pp. 1778-1779. PMID 16926299
- W. Jelkmann: Erythropoiesis stimulating agents and techniques: a challenge for doping analysts . In: Curr Med Chem , 2009, 16 (10), pp. 1236-1247. PMID 19355882
- W. Jelkmann, C Lundby: Blood doping and its detection . In: Blood , 2011, 118 (9), pp. 2395-404. doi: 10.1182 / blood-2011-02-303271 , PMID 21652677 .
Web links
- EPO doping in cycling on cycling4fans.de
- EPO - Risks and Side Effects ; Independent information offer from the National Anti Doping Agency (NADA)
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) on: Epoetin
Individual evidence
- ↑ Ulrich Kuhlmann, Joachim Böhler, Friedrich C. Luft, Mark Dominik Alscher, Ulrich Kunzendorf: Nephrology - Pathophysiology - Clinic - Renal Replacement Procedures , Thieme Verlag , Stuttgart, 2015, ISBN 978-3-13-700206-2 , p. 409
- ↑ Bodó E. et al. 2007, Human hair follicles are an extrarenal source and a nonhematopoietic target of erythropoietin. FASEB J . 21: 3346-54. PMID 17540710 .
- ↑ Foley RN (2008), Erythropoietin: Physiology and molecular mechanisms. Heart Fail Rev. 13: 405-414. PMID 18236154 .
- ↑ Adamcio B. et al. (2008), Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol. 2008 Sep 9; 6 (1): 37. PMID 18782446 .
- ↑ D. Burger, F. Xiang, L. Hammoud, X. Lu, Q. Feng: Role of heme oxygenase-1 in the cardioprotective effects of erythropoietin during myocardial ischemia and reperfusion. In: Am. J. Physiol. Heart Circ. Physiol. 296, 2009, pp. H84-H93 PMID 18996987 .
- ↑ UniProt P01588
- ↑ B. Agoram, K. Aoki, S. Doshi, C. Gegg, G. Jang, G. Molineux, L. Narhi, S. Elliott: Investigation of the effects of altered receptor binding activity on the clearance of erythropoiesis-stimulating proteins : Nonerythropoietin receptor-mediated pathways may play a major role. In: J Pharm Sci 98, 2009, pp. 2198-2211 PMID 18837016 .
- ↑ Narhi LO et al. (1991), The effect of carbohydrate on the structure and stability of erythropoietin. J. Biol. Chem. 266: 23022-23026 PMID 1744097 .
- ↑ Brines M. et al. (2008), Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl. Acad. Sci. USA 105: pp. 10925-10930. PMID 18676614 .
- ↑ Fu-Kuen Lin (1985), DNA sequences encoding erythropoietin. U.S. Patent 4,703,008.
- ↑ Lee-Huang S. (1984), Cloning and expression of human erythropoietin cDNA in Escherichia coli. Proc Natl Acad Sci U S A 81: 2708-2712. PMID 6371819 .
- ↑ Jacobs K. et al. (1985), Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature 313: 806-810. PMID 3838366 .
- ↑ E. Lacson, J. Rogus, M. Teng, JM Lazarus, RM Hakim: The association of race with erythropoietin dose in patients on long-term hemodialysis. In: Am. J. Kidney Dis. 52, 2008, pp. 1104-1114 PMID 18824287 .
- ^ WC Winkelmayer, J. Liu, MA Brookhart: Altitude and all-cause mortality in incident dialysis patients. In: JAMA 301, 2009, pp. 508-512 PMID 19190315 .
- ^ M. Gallieni: Iron in the treatment of anemia in dialysis patients: an important support to erythropoietin. In: Int J Artif Organs 21, 1998, pp. 681-686 PMID 9894741 .
- ↑ G. Amendola, R. Di Concilio, G. D'Urzo, P. Danise, G. Parisi, F. della Ragione, F. Rossi, B. Nobili, S. Perrotta: Erythropoietin treatment can prevent blood transfusion in infantile pyknocytosis . In: Br. J. Haematol. 143, 2008, pp. 593-595 PMID 18783402 .
- ↑ H. Ehrenreich, K. Weissenborn, H. Prange, D. Schneider, C. Weimar, K. Wartenberg, PD Schellinger, M. Bohn, H. Becker, M. Wegrzyn, P. Jähnig, M. Herrmann, M. Knauth, M. Bähr, W. Heide, A. Wagner, S. Schwab, H. Reichmann, G. Schwendemann, R. Dengler, A. Kastrup, C. Bartels,.: Recombinant human erythropoietin in the treatment of acute ischemic stroke . In: Stroke 40, 2009, pp. E647 – e656 PMID 19834012 .
- ↑ Ehrenreich H. et al. (2007), Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain 2007; 130 (Pt 10): 2577-88. PMID 17728357 .
- ↑ S. Boesch, B. Sturm, S. Hering, H. Goldenberg, W. Poewe, B. Scheiber-Mojdehkar: Friedreich's ataxia: clinical pilot trial with recombinant human erythropoietin. In: Ann. Neurol. 62, 2007, pp. 521-524 PMID 17702040 .
- ↑ Grunfeld JF et al. (2007), Erythropoietin delays disease onset in an amyotrophic lateral sclerosis model. Experimental Neurology 204: 260-263 PMID 17174305 .
- ↑ Lykissas MG et al. (2007), Axonal regeneration stimulated by erythropoietin: An experimental study in rats. J Neurosci Methods 164: 107-115. PMID 17532473 .
- ↑ Ehrenreich H. et al. (2006), Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry 12: 206-220 PMID 17033631 .
- ↑ K. Miskowiak, B. Inkster, S. Selvaraj, R. Wise, GM Goodwin, CJ Harmer: Erythropoietin improves mood and modulates the cognitive and neural processing of emotion 3 days post administration. In: Neuropsychopharmacology 33, 2008, pp. 611-618 PMID 17473836 .
- ↑ M. Joyeux-Faure: Cellular protection by erythropoietin: new therapeutic implications? In: J. Pharmacol. Exp. Ther. 323, 2007, pp. 759-762 PMID 17717190 (Review).
- ↑ Bogoyevitch MA (2004), An update on the cardiac effects of erythropoietin cardioprotection by erythropoietin and the lessons learned from studies in neuroprotection. Cardiovasc. Res. 63: 208-216. PMID 15249178 .
- ↑ Mihov D. et al. (2009), Erythropoietin protects from reperfusion-induced myocardial injury by enhancing coronary endothelial nitric oxide production. Eur J Cardiothorac Surg. 35 (5): 839-846. PMID 19237290 .
- ^ "EPO fails to help heart attack victims in study" .
- ↑ Darbepoetin-alfa without benefit in heart failure , Deutsches Ärzteblatt from January 18, 2013 (accessed January 19, 2013).
- ^ H. Sorg, C. Krueger, T. Schulz, MD Menger, F. Schmitz, B. Vollmar: Effects of erythropoietin in skin wound healing are dose related. In: FASEB J. 23, 2009, pp. 3049-3058 PMID 19403513 .
- ↑ Hannover Medical School (MHH), EPO in wound therapy: MHH tests innovative healing concept without side effects . Press release from March 24, 2010.
- ^ Achim-Peter Neubauer, Wolfgang Voss, Michael Wachtendorf, Tanja Jungmann: Erythropoietin Improves Neurodevelopmental Outcome of Extremely Preterm Infants . In: Annals of Neurology . tape 67 , no. 5 , May 2010, p. 657-666 , doi : 10.1002 / ana.21977 .
- ↑ mdr.de : Study in Göttingen: Doping against Covid-19. Retrieved July 7, 2020 .
- ↑ International nonproprietary names (INN) for biologicals and biotechnological substances (PDF; 179 KB).
- ↑ Jelkmann W. (2009), Efficacy of recombinant erythropoietins: is there unity of international units? In: Nephrol Dial Transplant . 24: 1366-1368. PMID 19225013 .
- ^ Cotes PM, Bangham DR (1966), The international reference preparation of erythropoietin. Bull World Health Organ. 35: 751-760. PMID 5297809 .
- ↑ Annable L. et al. (1972), The second international reference preparation of erythropoietin, human, urinary, for bioassay. Bull World Health Organ 47: 99-112. PMID 4538911 .
- ↑ Storring PL et al. (1992), The international standard for recombinant DNA-derived erythropoietin: collaborative study of four recombinant DNA-derived erythropoietins and two highly purified human urinary erythropoietins. J endocrinol. 134: 459-484. PMID 1402553 .
- ↑ Behr-Gross ME (2007), Collaborative study for the establishment of erythropoietin BRP batch 3. Pharmeuropa Bio. 2007 (1): 49-66. PMID 18413137 .
- ↑ CNNMoney.com: "Missing the $ 20 billion biogeneric boom," August 15, 2006 .
- ^ "Kirin, Sankyo to terminate joint marketing of renal anemia drug ESPO (...) by next march" ( Memento of September 28, 2007 in the Internet Archive ).
- ↑ Macdougall IC (2008), Novel erythropoiesis-stimulating agents: a new era in anemia management. Clin J Am Soc Nephrol. 3 (1): 200-207. PMID 18077782 .
- ↑ "CERA: Different interaction with the erythropoietin receptor than epoetin" .
- ↑ European public assessment report for Mircera .
- ↑ Specialist information Mircera (PDF) June 2006.
- ↑ "Cepo instead Epo" FAZnet of 8 July 2004 .
- ↑ Fiordaliso F. et al. (2005), A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury , Proc Natl Acad Sci 102: 2046-2051. PMID 15671158 .
- ↑ Fahres F. et al. (2007), Development of a long-acting erythropoietin by fusing the carboxyl-terminal peptide of human chorionic gonadotropin beta-subunit to the coding sequence of human erythropoietin. Endocrinology 148: 5081-5087 PMID 17641000 .
- ↑ Acquisition of Shire Pharmaceuticals on April 21, 2005 ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. .
- ^ "New treatment a success for anemia associated with chronic kidney disease" ( Memento from September 28, 2007 in the Internet Archive ).
- ^ "New treatment for renal anemia produced by human cells ..." Press release of March 15, 2007 ( Memento of September 27, 2007 in the Internet Archive ).
- ↑ Quarterly report from Shire and announcement from CEO Angus Russel from July 31, 2008 ( Memento from January 5, 2011 in the Internet Archive ).
- ↑ D. Kodama, D. Nishimiya, K. Iwata, K. Yamaguchi, K. Yoshida, Y. Kawabe, M. Motono, H. Watanabe, T. Yamashita, K. Nishijima, M. Kamihira, S. Iijima: Production of human erythropoietin by chimeric chickens. In: Biochem. Biophys. Res. Commun. 367, 2008, pp. 834-839 PMID 18201556 .
- ^ "Roche licenses Synthetic Erythropoiesis Protein from Gryphon Sciences" ( Memento from March 20, 2006 in the Internet Archive ).
- ↑ Affymax and Takeda Announce a Nationwide Voluntary Recall of All Lots of OMONTYS® (peginesatide) Injection ( Memento from April 18, 2015 in the Internet Archive ), notification on the Affymax Inc. homepage of February 23, 2013 (accessed on February 27 2013).
- ↑ posters of ProMetic Life Sciences Inc. for preparation PBI-1402 .
- ↑ Aachener Nachrichten: Baesweiler: Biotech: Aplagen in insolvency proceedings. In: aachener-nachrichten.de. April 8, 2010, accessed November 2, 2018 .
- ↑ Z. Liu, VS Stoll, PJ Devries, CG Jakob, N. Xie, RL Simmer, SE Lacy, DA Egan, JE Harlan, RR Lesniewski, EB Reilly: A potent erythropoietin-mimicking human antibody interacts through a novel binding site. In: Blood 110, 2007, pp. 2408-2413 PMID 17620453 .
- ↑ Qureshi SA et al. (1999), Mimicry of erythropoietin by a nonpeptide molecule. Proc Natl Acad Sci U.S.A. 96: 12156-12161. PMID 10518592 .
- ↑ Bouman-Thio E. et al. (2008), A phase I, single and fractionated, ascending-dose study evaluating the safety, pharmacokinetics, pharmacodynamics, and immunogenicity of an erythropoietin mimetic antibody fusion protein (CNTO 528) in healthy male subjects. J Clin Pharmacol. 48 (10): 1197-207. PMID 18812609 .
- ↑ "We have repoxygen in the cool compartment" FAZ from January 31, 2006 .
- ↑ The "Biopumpe" of Medgenics .
- ↑ Akagi S. et al. (2004), The critical role of SRC homology domain 2-containing tyrosine phosphatase-1 in recombinant human erythropoietin hyporesponsive anemia in chronic hemodialysis patients. J Am Soc Nephrol. 15: 3215-3224. PMID 15579525 .
- ↑ Rinna A., Forman HJ (2008), SHP-1 inhibition by 4-hydroxynonenal activates Jun N-terminal kinase and glutamate cysteine ligase. , Am J Respir Cell Mol Biol. 39: 97-104. PMID 18276794 .
- ^ "Astellas Acquires the Rights to FG-2216 and FG-4592 (...) for Europe and Other Regions (...)" press release of April 28, 2006 ( Memento of August 7, 2008 in the Internet Archive ).
- ↑ "Akebia Announces Initiation of Phase 1 Clinical Study of AKB-6548" press release from September 15, 2009.
- ↑ "CrystalGenomics had Entered into oral hypoxia therapeutics development", press release of 19 September 2006 ( Memento of 8 October 2007 at the Internet Archive ).
- ↑ Nakano Y. et al. 2004, Oral administration of K-11706 inhibits GATA binding activity, enhances hypoxia-inducible factor 1 binding activity, and restores indicators in an in vivo mouse model of anemia of chronic disease. Blood 104: 4300-4307 PMID 15328158 .
- ↑ A. Angelillo-Scherrer, L. Burnier, D. Lambrechts, RJ Fish, M. Tjwa, S. Plaisance, R. Sugamele, M. DeMol, E. Martinez-Soria, PH Maxwell, G. Lemke, SP Goff, GK Matsushima, HS Earp, M. Chanson, D. Collen, S. Izui, M. Schapira, EM Conway, P. Carmeliet: Role of Gas6 in erythropoiesis and anemia in mice. In: J. Clin. Invest. 118, 2008, pp. 583-596 PMID 18188450 PMC 217618 (free full text).
- ↑ Guidelines for similar biological-medical products ( Memento of September 21, 2008 in the Internet Archive ) (PDF; 105 KB).
- ↑ Guidelines for similar biological-medical products that contain recombinant erythropoietin ( Memento of July 18, 2006 in the Internet Archive ).
- ↑ PlasmaSelect ( Memento from February 22, 2014 in the Internet Archive ).
- ^ "Biggest Pharmaceutical Plant to Open Soon" ( Memento of February 17, 2007 in the Internet Archive ).
- ↑ generic recombinant EPO from Cristália / Blausigel ( Memento of March 4, 2005 in the Internet Archive ).
- ↑ Pliva press release of February 22, 2006 ( Memento of December 15, 2007 in the Internet Archive ).
- ↑ Nicholas Buhay: WARNING LETTER 320-06-02. Public Health Service, Food and Drug Administration, April 28, 2006, accessed July 10, 2009 .
- ↑ Stada press release of March 30, 2006 ( Memento of May 24, 2006 in the Internet Archive ).
- ↑ "STADA: Registration documents for erythropoietin biosimilar submitted to EMEA" ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. .
- ↑ "Bibitec completes first active ingredient development for a biosimilar." Press release from NewLab BioQuality AG ( Memento of October 4, 2009 in the Internet Archive ).
- ↑ "STADA is reorganizing biosimilar projects - Epo-zeta distribution rights to Hospira" Press release of November 20, 2006 ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. .
- ^ Committee for Medicinal Products for Human Use Summary of Positive Opinion for Silapo .
- ↑ STADA receives approval for erythropoietin-zeta - introduction in the 1st quarter of 2008 ( Memento of July 17, 2011 in the Internet Archive ) (PDF; 39 KB).
- ^ Seth E. Cockrum: FDA Approves Retacrit as a Biosimilar to Epogen®. Announcement on lexicology.com dated May 15, 2018 (accessed May 15, 2018).
- ↑ Press release by Therapeutic Proteins of May 12, 2006 ( Memento of October 4, 2009 in the Internet Archive ).
- ↑ Public assessment report (EPAR) of the European Medicines Agency (EMA) on: Binocrit .
- ↑ Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Epoetin Alfa Hexal .
- ^ Public assessment report (EPAR) of the European Medicines Agency (EMA) on: Abseamed .
- ↑ Briefly Informed , Dtsch Arztebl 2010; 107 (17)
- ↑ SS Park, J. Park, J. Ko, L. Chen, D. Meriage, J. Crouse-Zeineddini, W. Wong, BA Kerwin: Biochemical assessment of erythropoietin products from Asia versus US Epoetin alfa manufactured by Amgen. In: J Pharm Sci 98, 2009, pp. 1688-1699 PMID 18781649 .
- ↑ Generics and Biosimilars Initiative: Biosimilar EPOs show the same or better quality. dated November 12, 2010
- ↑ Pistel KF et al. (1999) Biodegradable recombinant human erythropoietin loaded microspheres prepared from linear and star-branched block copolymers: (...) , J Control Release 59: 309-325. PMID 10332063 .
- ↑ Zale SE (1999), Composition for sustained release of non-aggregated erythropoietin , US Patent 5,674,534.
- ↑ Longhu L. et al. (2009), Sustained release of erythropoietin using biodegradable gelatin hydrogel microspheres persistently improves lower leg ischemia. J Am Coll Cardiol 53: 2378-2388.
- ↑ “NanoVentures Australia Reaches Important Milestone in Development of Pulmonary Drug Delivery Technology” ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. dated May 31, 2009.
- ↑ Henke M. et al. (2003), Erythropoietin to treat head and neck cancer patients with anemia undergoing radiotherapy: randomized, double-blind, placebo-controlled trial. The Lancet 362: 1255-1260. PMID 14575968 .
- ↑ Leyland-Jones B. et al. (2005), Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. Journal of Clinical Oncology 23: 5960-5972. PMID 16087945 .
- ↑ Baz R. et al. (2007), Recombinant human erythropoietin is associated with increased overall survival in patients with multiple myeloma. Acta Haematologica 117: 162-167. PMID 17148935 .
- ↑ San Miguel JF, García-Sanz R. (1998), Recombinant human erythropoietin in the anemia of multiple myeloma and non-Hodgkin's lymphoma. Medical Oncology 15 Suppl 1: S29-S34. PMID 9785334 .
- ↑ Wright JR et al. (2007), Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. Journal of Clinical Oncology 25: 1027-1032. PMID 17312332 .
- ^ "Amgen Comments on Phase 3 Study in Cancer Patients with Anemia Not Due to Concurrent Chemotherapy" ( Memento from May 16, 2008 in the Internet Archive ).
- ↑ "Study of the importance of Novel Erythropoiesis Stimulating Protein (Aranesp) for the effect of radiotherapy in patients with primary squamous cell carcinoma of the head and neck." .
- ^ "Roche Halts Enrollment in Study of Biologic Drug Cera Due to Safety Concerns" ( Memento of July 7, 2007 in the Internet Archive ).
- ^ FDA-Alert on Erythropoiesis Stimulating Agents (ESA) ( Memento of November 13, 2007 in the Internet Archive ).
- ↑ Aapro M. et al. (2008), Effect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and / or taxane-based chemotherapy: results of the Breast Cancer-Anemia and the Value of Erythropoietin (BRAVE) study. Journal of Clinical Oncology 26: 592-598. PMID 18235117 .
- ↑ Sina Vogt: side effect risk of death: drug for blood formation in the criticism. Cologne University Hospital, press release from May 11, 2009 at Informationsdienst Wissenschaft (idw-online.de), accessed on September 15, 2015.
- ↑ Bohlius J. et al. (2009), Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomized trials. Lancet 373 (9674): 1532-1542. PMID 19410717 .
- ↑ Bennet CL et al. (2008), Venous Thromboembolism and Mortality Associated With Recombinant Erythropoietin and Darbepoetin Administration for the Treatment of Cancer-Associated Anemia. JAMA 299: 914-924.
- ↑ Treatment with hematopoietic growth factors (PDF) Status: November 2008, German Society for Hematology and Oncology
- ↑ G-BA therapy information: prescribe erythropoiesis-stimulating agents in the case of renal insufficiency , press release Joint Federal Committee of September 22, 2011
- ↑ Ritter S. (2005), Erythropoietin in Chemical & Engineering News No. 83 (25).
- ↑ Fox JL (2007), FDA likely to further restrict erythropoietin use for cancer patients. Nature Biotechnology 25: 607-608. PMID 17557084 .
- ^ "Erythropoietin: Pick-me-ups and lifesavers" ( Memento of September 7, 2005 in the Internet Archive ).
- ^ "A Comprehensive Report of Erythropoietins" ( Memento of February 22, 2014 in the Internet Archive ).
- ↑ Häussler B (2008), IGES Institute.
- ^ Arnd Krüger : EPO games in Sydney too? Archived copy ( memento of the original dated December 31, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Alessandro Donati: World Traffic in Doping Substances (PDF) WADA, February 2007, (PDF, 542 KB).
- ↑ Red List online, as of September 2009.


