plutonium
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Plutonium, Pu, 94 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Actinoids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | Ac , 7 , f | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery metal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-07-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-117-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.288 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 2 · 10 −15 ppm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 244.0642 u | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 151 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 187 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Rn ] 5 f 6 7 s 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 6th.02576 (25) eV ≈ 581.4 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 11.5 (4) eV ≈ 1 110 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 21st.1 (4) eV ≈ 2 040 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 35.0 (4) eV ≈ 3 380 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 49.0 (1.9) eV ≈ 4 730 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modifications | 6th | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | monoclinic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 19.816 g cm −3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 6.2 · 10 −4 ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 912.5 K (639.4 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 3509 K (3230 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 12.29 · 10 −6 m 3 · mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 325 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 11.48 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 2260 m s −1 at 293.15 K. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 130 J kg −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 6.8 · 10 5 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 6.74 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +3, +4 , +5, +6, (+7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −2.031 V (Pu 3+ + 3 e - → Pu) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.28 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard and safety information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Radioactive |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Plutonium is a chemical element with the element symbol Pu and the atomic number 94. It therefore has the highest atomic number of all naturally occurring elements. In the periodic table it is in the group of actinides ( 7th period , f-block ) and is one of the transuranic elements . It was named after the dwarf planet Pluto .
Plutonium is a toxic and radioactive heavy metal . However, it is only found in the smallest traces in very old rocks. The amount that is artificially generated in nuclear power plants is greater .
As one of the few fissile elements, it plays an important role in the construction of nuclear weapons . It was the fissile material of the atomic bomb that was dropped on Nagasaki on August 9, 1945 ( Fat Man ). During the operation of nuclear reactors , the uranium in the fuel elements turns into plutonium.
history

Plutonium was discovered by the Americans Glenn T. Seaborg , J. W. Kennedy , E. M. McMillan , Michael Cefola and Arthur Wahl . On December 14, 1940, they produced the isotope 238 Pu by bombarding uranium 238 U with deuterons in a cyclotron . For this purpose, samples of the isotope 238 U in the form of the oxide U 3 O 8 were first applied in a thin layer to a copper plate. In the nuclear reaction with deuterons, two neutrons are emitted; the neptunium that is initially formed decays to 238 Pu. Arthur Wahl provided the clear evidence for the element 94 on 23/24. February 1941.
The second isotope was generated by bombardment with fast neutrons:
- The times given are half-lives .
In March 1942 they named it after Pluto , which was then considered the outermost planet , which in turn is named after the god of the underworld of the same name : “[...] named after the planet Pluto on the other side of Neptune Derivation of the name of Pluto, the god of the underworld more justified! ”This is how the three heaviest elements known at the time, uranium, neptunium and plutonium, were named after the planets Uranus , Neptune and Pluto.
The first weighable amount of about 4 µg was isolated in August / September 1942 by Burris B. Cunningham , M. Cefola and Louis B. Werner .
The discovery was kept secret during World War II . Plutonium was first produced on a larger scale as part of the US Manhattan Project . The atomic bomb, with which the Trinity test caused the first nuclear weapon explosion in mankind, and Fat Man , the bomb with which the Japanese city of Nagasaki was destroyed, contained plutonium 239 Pu as a fissile material. Joseph Hamilton carried out plutonium distribution studies on test persons, which are controversial today due to the extremely toxic effects of plutonium.
In Germany, even before the discovery of plutonium, Carl Friedrich von Weizsäcker had pointed out that a new fissile element 239 Eka Re (Eka-Rhenium) would have to be created in nuclear reactors . Even Fritz Houtermans said in 1942 the existence of transuranic elements in a secret report theoretically expected. However , as far as we know today, no significant quantities of plutonium were produced in the German uranium project until the end of the war.
Occurrence
Natural occurrence
Plutonium is the last, but extremely rare, previously known naturally occurring element of the periodic table . With a content of 2 · 10 −19 % by weight, it is one of the rarest elements in the earth's crust. In uranium deposits it can be produced in tiny amounts through the absorption of naturally released neutrons from uranium. There should be one plutonium atom for every 140 billion uranium atoms. In 1951, the American chemist DF Peppard extracted 239 Pu from a Congolese pitchblende concentrate . 100 tons of pitchblende were required for each microgram.
From the natural reactors of Oklo in Gabon and from a neighboring uranium deposit it is known that nuclear fission occurred there as a chain reaction in a natural environment about 1.5 to 2 billion years ago over several millennia . The accumulation of fission neutrons at 238 U resulted in about 2 to 4 tons of 239 Pu. In some parts of the Oklo deposit, the direct fission of 239 Pu contributed significantly to the total core fission . About a third of the total split 235 U is said to have come from the alpha decay of 239 Pu. Any remains of the generated plutonium have now completely decayed.
With sophisticated trace analysis succeeded in mineral Bastnäsit that after the site Bastnäs in Sweden , was named the smallest traces of long-lived plutonium isotope 244 prove Pu. This plutonium comes from the time the solar system was formed , so it is a primordial nuclide . The quantities found are so small that they were only discovered in 1971, long after the artificial production of plutonium in nuclear reactors.
Artificial occurrence
Plutonium is produced in nuclear weapons and in nuclear reactors through the transmutation of uranium . In the meantime (2016) the nuclear powers and other states that operate nuclear power plants have a total inventory of hundreds of tons of plutonium artificially produced in this way, including Russia 180 tons and the USA 90 tons of separated plutonium.
Release due to anthropogenic causes
Between 1945 and 1980, plutonium was anthropogenically released in an amount of three to five tons through above-ground nuclear weapon tests, traces of which are detectable worldwide. Additional quantities were released through various unintended events and accidents.
- Releases in nuclear weapons accidents and accidents in nuclear weapons laboratories
- Failed space missions and re-entry of satellites with radionuclide batteries , such as through Transit 5BN-3 , Kosmos 954, and Apollo 13
- Fire in the reactor at the Sellafield plutonium factory (then Windscale) in 1957
- Accidents involving nuclear submarines
- in waste water from nuclear research facilities and from processing plants
- In the past there has been legal and illegal dumping of radioactive waste into the oceans
- Most of the plutonium that escaped during the Chernobyl reactor disaster remained within a 100-kilometer radius of the reactor. Also in 1957 during the Kyschtym accident in the Russian Mayak plant, considerable amounts of plutonium escaped, which were mainly deposited locally and regionally.
Extraction and presentation
Plutonium is inevitably produced in nuclear power plants operated with 238 U-rich isotope mixtures. Part of the 238 U used is converted into 239 Pu by capturing a neutron and subsequent beta decay .
- (HWZ: 24110 a)
- The times given are half-lives .
In most cases, another neutron leads to nuclear fission, but the isotope 240 Pu (HWZ: 6560 a) is sometimes formed . Since this isotope is difficult to split, further neutron capture leads to the formation of 241 Pu (HWZ: 14 a), which in turn is easy to split. However, not all atoms are split, so that in some of them the breeding process to 242 Pu (HWZ: 373000 a) and even heavier isotopes can be continued. However, because the fissile 243 Pu has a very short half-life (5 h), further neutron capture, which usually leads to fission or - in rarer cases - to the production of plutonium 244 Pu, is unlikely. The plutonium breeding process is therefore practically over with the 243 Pu and leads to the americium isotope 243 Am (HWZ: 7370 a) via the beta decay of 243 Pu .
Since each stage of these successive nuclear reactions takes a certain amount of time, the relative amounts of isotopes in the reactor core change over time. The rates at which the nuclear reactions take place depend on the velocity distribution of the neutrons. However, because a large part of the easily cleavable isotopes is split and not converted into other isotopes, the possible yield (efficiency) of the breeding process decreases with the generation of each additional easily split isotope.
The lighter isotope 238 Pu is produced specifically if required. It is created by capturing several neutrons from the uranium isotope 235 U. First, a 236 U nucleus is formed in an excited state, which has a half-life of 120 nanoseconds and is very likely to split. However, excited 236 U-nuclei can also pass into the long-lived ground state through emission of gamma radiation . Further neutron capture and β-decay results in neptunium 237 Np. After a certain irradiation time, the neptunium, which consists almost exclusively of 237 Np, is extracted from the fuel rods . The neptunium is then reintroduced into a reactor in the form of pure neptunium fuel rods and irradiated with neutrons. It is converted by neutron capture into 238 Np, which decays to 238 Pu when beta radiation is emitted.
- The times given are half-lives .
The fuel rods so treated also contain heavier isotopes of plutonium. In addition, some of the neptunium atoms are also hit by neutrons with more than 6.27 MeV energy, which results in a small amount of 236 Pu. This decays over the thorium series , in which the strong gamma emitter thallium 208 Tl occurs.
If 239 Pu is split by fast neutrons that are not slowed down, the average number of newly released neutrons per split atomic nucleus is particularly high. In such a reactor, therefore, theoretically more 238 U can be converted into new 239 Pu than is simultaneously consumed by cleavage. It is therefore called a breeder reactor or “fast breeder”. In practice, however, a maximum conversion rate of 0.7 has been achieved so far, so the functioning of a breeder reactor economy has not yet been demonstrated on a large scale.
After production, the plutonium is found in the spent fuel elements together with the fission products and unused residual nuclear fuel. Through the PUREX process , the plutonium produced and the uranium that is also desired can be extracted from them in reprocessing plants. To do this, the material is first dissolved in nitric acid and the plutonium and uranium extracted with tri-n-butyl phosphate . The fission products and other components remain behind. Around 20 tons of plutonium are produced each year, mainly in the form of the isotope 239 Pu.
The transfer of fissile material (such as 239 Pu and 241 Pu) as well as materials that are suitable for their production to states that do not have nuclear weapons is subject to the control of the International Atomic Energy Agency (IAEA) according to Section III of the Nuclear Non-Proliferation Treaty . In Germany, the Atomic Energy Act regulates the handling of fissile material. It determines who is allowed to transport and own plutonium in Germany and under what conditions.
properties
Physical Properties
Under normal conditions, plutonium is a shiny silver heavy metal with a high density (19.86 g / cm 3 ). As with all actinides , only radioactive isotopes exist of plutonium . It is self-heating; for every 100 g of plutonium there is around 0.2 kW of heat output (based on 239 Pu). Compared to other metals, plutonium is a poor conductor of heat and electricity . The metal crystallizes in a total of six allotropic modifications, depending on the temperature . Some of these differ significantly in their density. The modification α-Pu, which is stable at room temperature, is monoclinic . In plutonium there is the rare case of a density anomaly at higher temperatures ; the density increases again during the phase transition to the δ 'and ε modification. As with water , the density also increases when it melts . Liquid plutonium has the highest viscosity of all elements in the liquid state. Despite an abnormally high magnetic susceptibility for metals and the tendency to order at low temperatures, plutonium shows no order over large areas and must therefore be described as paramagnetic . However, the constant heating caused by the decay of the plutonium 239 Pu interferes with the measurement . This means that temperatures close to absolute zero cannot be achieved.
Modifications at atmospheric pressure Name of
the phaseStable in the
temperature rangeDensity (temperature) Crystal system Bravais grid Space group α-Pu 0 K - 395 K 19.77 g / cm 3 (293 K) monoclinic primitive P 2 1 / m (No. 11) β-Pu 395K - 479K 17.7 g / cm 3 (395 K) monoclinic body-centered I 2 / m (No. 12, position 3) γ-Pu 479K - 592K 17.14 g / cm 3 (479 K) orthorhombic face-centered Fddd (No. 70) δ-Pu 592 K - 730 K 15.9 g / cm 3 (592 K) monoclinic base-centered Cm (No. 8) δ'-Pu 730K - 749K 16.0 g / cm 3 (730 K) tetragonal body-centered I 4 / mmm (No. 139) ε-Pu 749K - 914K 16.5 g / cm 3 (749 K) cubic body-centered In 3 m (No. 229) liquid 914K - 3503K 16.63 g / cm 3 (914 K) - - -
Furthermore, a high-pressure modification is known which was obtained from α-Pu at a pressure above 40 GPa and which crystallizes in space group P 6 3 (space group no. 173) .
Chemical properties
Plutonium is a base and very reactive metal. In the air it reacts quickly with oxygen and humidity. The metal initially becomes matt and is covered with a dark blue-black oxide skin; when standing in the air for a long time, a thick, gray-green, powdery, abrasive oxide layer forms. The metal reacts with most non-metals and water when heated . At room temperature, however, it is not attacked by water or alkaline solutions . It is not soluble in concentrated nitric acid because of its passivation . Plutonium is soluble in hydrochloric acid and fluoride-containing nitric acid. The fluoride ions suppress the passivation of the metal that would otherwise occur. The chemical properties of plutonium are similar to those of other actinides. Similar to many other of these elements, the strong radioactivity determines the chemical properties of plutonium, since bonds can be broken by the heat generated. The radiation released can also break bonds.
Plutonium has a number of compounds in which it can exist in the oxidation states +3 to +7. This means that plutonium, together with neptunium, forms the highest oxidation level of all actinides. The most stable level is +4. In aqueous solution, the plutonium ions have characteristic colors, the Pu 3+ ion is violet, Pu 4+ brown, Pu V O 2 + purple, Pu VI O 2 2+ orange and Pu VII O 2 3+ green.
Biological aspects
A biological function of the plutonium is not known. Further research and investigation focused on microbial interactions with plutonium in order to remediate contaminated landfills and environments. Enterobacteria of the genus Citrobacter can precipitate Pu (IV) from aqueous solution due to the phosphatase activity in their cell wall and bind it as a lanthanum phosphate complex.
Isotopes
20 isotopes and 15 nuclear isomers with mass numbers from 228 to 247 were measured for plutonium . The half-lives are between 37 · 10 −12 s for the isomer 236 m 1 Pu and 80 million years for 244 Pu. The longest-lived isotopes with half-lives greater than 11 days have mass numbers between 236 and 244. The isotope 243 Pu is an exception with a half-life of less than 5 hours. Some of the plutonium isotopes are seen as starting points for radioactive decay series .
- 236 Pu is divided into the thorium series . It comes with a half-life of 2.858 years through α-decay to the intermediate level 232 U, whichdecayswith a half-life of 68.9 years to 228 Th, which is on the main strand of the series. This isotope is only incubated in tiny amounts in nuclear reactors that run on uranium.
- 237 Pu converts with a half-life of 45.2 days to 99.9958% by electron capture into the neptunium isotope 237 Np, which is the official starting point of the neptunium series . The remaining 0.0042% decays through α-decay to uranium 233 U, which also decays via the neptunium series.
- 238 Pu is an α-emitter with a half-life of 87.7 years. It first decays into 234 U and continues through the decay chain of the uranium-radium series .
- 239 Pu is the most commonly produced isotope of plutonium. It has a half-life of 24,110 years and mainly decays with emission of α-radiation in 235 U. Further decay follows the uranium-actinium series for natural radioactivity, whichbeginsat 235 U. Spontaneous splitting occurs ina proportion of 3 · 10 −10 %.
- 240 Pu decays with a half-life of 6564 years through α-radiation in 236 U. This uranium isotope decays with a half-life of 23.4 million years to the natural 232 Th. The further decay follows the thorium series.
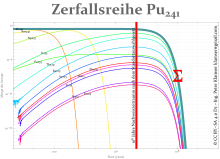
- 241 Pu is often referred to as the beginning of the Neptunium series because it (when the series is extended) comes before the Neptunium. It decays with a half-life of 14.35 years and a probability of 99.9975% with a β-decay to 241 Am, and with a 0.0025% probability under α-decay to 237 U. 241 Am decays under α-decay and 237 U through β-decay to the same long-lived neptunium isotope 237 Np, which is an alpha emitter and has a half-life of 2.14 million years. This very long half-life is the problem for the safety evidence of repositories if 241 Pu is not transmuted to isotopes in mixed oxide fuel elements in a nuclear fission, in whose decay series there is no 237 Np.
- 242 Pu decays through the same chain of decay as 238 Pu. However, while 238 Pucomes to the decay chainas a side arm of 234 U, 242 Pu is still before 238 U. Plutonium 242 Pu decays through α-decay into 238 U, the beginning of the natural uranium-radium series. With a half-life of 375,000 years, it isthe longest-lived isotopeafter 244 Pu.
- 243 Pu is short-lived with a half-life of 4.956 h. It is first converted into americium 243 Amby β-radiation, whichconverts intoneptunium 239 Np and furtherdecaysto 239 Pu. It is an extension of the uranium-actinium series.
- 244 Pu is the only naturally occurring plutonium isotope because of its long half-life of 80 million years. It is the starting point of the thorium series, which is why it is sometimes also called the plutonium-thorium series. 244 Pu decays through α-decay to 240 U, this by two β-decays over 240 Np to 240 Pu, this then again by two further α-decays over 236 U to 232 Th. This is followed by the decay of the thorium series.
Cleavage
All plutonium isotopes with an odd number of neutrons are among the few nuclides that are easily, i. H. are easily fissionable even by thermal neutrons . The corresponding cross-section for the 239 Pu is 752 Barn (b) and for the 241 Pu 1010 b, for the even-numbered 238 Pu, 240 Pu and 242 Pu, however, it is only 17 b, 0.4 b and <0.2 b, respectively. The very short-lived even 236 Pu (half-life 2.9 years) has a medium-sized fissure cross-section of 169 b.
All long-lived isotopes of plutonium also split spontaneously . The spontaneous fission rate is lowest at 239 Pu and increases sharply towards both the lighter and heavier isotopes. Both isotopes with an odd and an even number of neutrons are affected by spontaneous fission. In particular, 240 Pu has a spontaneous cleavage rate that is approximately 70,000 times higher than 239 Pu. Since the neutrons released during the spontaneous fission of an atomic bomb can lead to pre-ignition and greatly reduced explosive effects, 240 Pu is undesirable for nuclear weapons. Gun plutonium contains as little as possible of 240 Pu, but is never completely free of it.
All plutonium isotopes, including those with an even number of neutrons, can be split by fast neutrons and are therefore in principle suitable for the construction of nuclear weapons. The fissionability of plutonium by fast neutrons decreases with an increasing number of neutrons.
The critical mass relevant for atomic bomb construction is determined by the fissionability with slow as well as with fast neutrons, because due to the comparatively small gap cross-sections with fast neutrons (1 to 3 barns) in an atom bomb some of the neutrons after numerous collisions with plutonium nuclei but is slowed down to thermal energies before it can trigger another nuclear fission. At 236 Pu the critical mass is 8.04–8.42 kg, at 237 Pu, which can be split very well by both fast and slow neutrons, only 3.1 kg. However, both of the above isotopes are not used for nuclear weapons because of their high spontaneous fission rate, short half-life, high heat production and complicated extraction. According to calculations , the 238 Pu used for nuclear batteries has a critical mass of approx. 9.04-10.31 kg. For the most important isotope for nuclear weapons, 239 Pu, the critical mass (as well as for all other information without moderator and / or reflector) is 10 kg. With 241 Pu it is already 12.27–13.04 kg.
Section 2, Paragraph 1 of the Atomic Energy Act (Germany) assigns the plutonium isotopes 239 Pu and 241 Pu as "special fissile substances" to nuclear fuels.
Two of the many possibilities for neutron-induced fission of 239 Pu:
use
Only 238 Pu and 239 Pu are used in larger quantities. 238 Pu, when hatched from Neptunium, is contaminated with other isotopes of plutonium. Only 238 Pu, which is brooded via the detour of Curium 242 cm, is free from 236 Pu.
239 Pu is always contaminated with 240 Pu and even smaller amounts of 241 Pu and 242 Pu.
Use in nuclear power plants
During the operation of nuclear reactors, the uranium in the fuel elements turns into plutonium. After separation in a reprocessing plant , this is processed together with enriched uranium to make MOX fuel elements for light water reactors . There, the use of MOX instead of pure uranium fuel increases certain operational risks slightly: the proportion of delayed neutrons (see criticality ) decreases by a few percent and the fast neutron flow - which causes radiation damage to the reactor pressure vessel - increases by a few percent.
A MOX fuel with about ten times the enrichment of the fissile isotopes is used in breeder reactors .
Military use
For nuclear weapons suitable weapons plutonium (English weapons grade plutonium ) has as much 239 Pu and a minimum of 240 included Pu. From a content of about 92% 239 Pu, plutonium is considered weaponized. According to the IAEA, however, any plutonium is in principle suitable for military purposes. Plutonium is designated as reactor plutonium, which occurs during normal operation of nuclear power plants; it can contain up to 31% 240 Pu.
240 Pu cannot be fissioned by thermal neutrons, but it decays by spontaneous fission . This releases neutrons, which can cause the plutonium bomb to pre-ignite undesirably and make the calculation of the explosive force inaccurate. Exact ignition and precise prediction of the explosive force is desirable for military purposes. The decay heat of the alpha emitter 238 Pu also has a disruptive effect.
For the production of weapons plutonium in nuclear reactors, the shortest possible irradiation time is required: the longer this is, the more 240 Pu is created from 239 Pu (see also nuclear reactor breeding reactions ). For this reason, weapons plutonium can only sensibly be obtained from a nuclear reactor with ongoing power generation if it is a pressure tube reactor , because only with this reactor can individual fuel elements be replaced during operation (reactor types e.g. CANDU , RBMK ). In contrast, in German nuclear power plants, all fuel elements are located together in the reactor pressure vessel , and fuel element removal requires a complex shutdown ("shutdown") of the plant, which should not be kept secret (e.g. no more water vapor clouds over the cooling towers).
Russia produced its weapons plutonium in purpose-built ADE reactors ; the last of them was shut down in 2010 after 46 years of operation.
In the Plutonium Management and Disposition Agreement in 2010, the USA and Russia agreed to reduce their stocks of weapons plutonium by 34 tons each. Secretary of State Hillary Clinton and Sergei Lavrov signed a supplementary protocol in Washington. This costs Russia 2.5 billion dollars; the USA will take over $ 400 million of this. The plutonium can be disposed of after mixing with other nuclear waste or by converting it into MOX elements.
Radionuclide batteries for space travel
238 Pu heats up through its own radioactive decay to the point of incandescence, and emits only very small amounts of gamma radiation , so that one gets by with the thinnest shield compared to five other potentially suitable nuclides. It is therefore used in oxidized form as chemically inert plutonium dioxide to generate electrical energy in radionuclide batteries .
Because of their longevity, radionuclide batteries are used in interplanetary space travel , especially for space probes that are supposed to reach the outer solar system. Because solar cells no longer supply enough energy at a great distance from the sun. Such nuclear batteries were built into the Voyager probes , Cassini-Huygens (1997–2005 for Saturn) or New Horizons (2006–2015 for Pluto), for example . In the past, radionuclide batteries with plutonium 238 Pu were also used in orbiting satellites.
In 1964, the US Transit 5BN-3 satellite with a radionuclide battery on board burned up in a false start about 50 kilometers above the Pacific. The satellite contained almost one kilogram of plutonium, which was then measurably distributed over the entire northern hemisphere.
In 1996 the Russian Mars 96 probe , in which Germany was involved, crashed with 270 grams of plutonium on board - in the Pacific or on the South American mainland.
236 Pu-free 238 Pu was used in cardiac pacemakers in the 1970s , and was produced via the not very productive and therefore expensive incubation of Curium 242 Cm. This is created by capturing neutrons from americium 241 Am, which in turn is obtained from 241 Pu.
- The times given are half-lives .
Neutron source
Furthermore, 238 Pu is used together with beryllium as a neutron source, whereby an α-particle from the decay of the plutonium hits the beryllium nucleus and is incorporated into it, emitting a neutron.
toxicity
Like many other heavy metals, plutonium is poisonous and particularly harmful to the kidneys. It also binds to proteins in the blood plasma and is deposited in the bones and liver, among other things. The lethal dose for a human is probably in the double-digit milligram range, for dogs the LD 50 dose is 0.32 mg / kg body weight. However, the chemical toxicity of plutonium is exceeded by many other substances.
Much more dangerous than the chemical effect is - because of the various physical properties isotope-dependent - its high level of radioactivity, the genetic damage and thus u. a. Can cause cancer , but also symptoms like normal heavy metal poisoning. Even the inhalation of 40 nanograms 239 Pu is sufficient to reach the limit value of the annual activity intake for inhalation and ingestion . The α-radiation emitted by plutonium 239 Pu is already shielded outside the body by the top layer of skin made of dead cells, but this protection does not exist in the case of incorporation , for example through inhalation of dust containing plutonium, or through contaminated food.
According to investigations by Arnulf Seidel from the Institute for Radiation Biology at the Karlsruhe Nuclear Research Center , small doses of 239 Pu lead to bone cancer in dogs in long-term experiments only after ten years at the earliest, and it is five times more dangerous than radium. The reason for this can be an uneven distribution of the plutonium in the skeleton, which leads to highly irradiated areas.
Like 240 Pu, which is always co-incubated in nuclear reactors, 241 Pu decays with a half-life of about 14 years into americium 241 Am, which emits large amounts of relatively soft gamma radiation . In stored plutonium, the concentration of 241 Am peaks after about 70 years. Because the plutonium isotopes themselves hardly emit gamma radiation, this radiation (and thus the thickness of the required shielding) initially increases significantly due to the americium formed, and then decreases again after around 70 years of storage. Because of the longer half-life of 241 Am (432 years), this decrease occurs much more slowly than the increase.
safety instructions
Classifications according to the CLP regulation are not available, although the chemical toxicity is known. Extreme caution is required when handling plutonium, especially because of its high level of radioactivity . Since the α radiation from plutonium only has a short range, special care must be taken to ensure that the metal does not get into the body. Since heat is generated during the decay , it must be dissipated. The best way to do this is to keep plutonium under dry, circulating air. Finely divided plutonium is pyrophoric .
Furthermore, it is imperative to prevent the creation of a critical mass that leads to a nuclear chain reaction and thus to an uncontrolled release of energy and radiation. Sub-criticality can be achieved either by sufficiently small masses or a safe geometry. In this case, the surface is large enough that more neutrons are lost than are produced in neutron-induced fission. Another possibility is the use of neutron-absorbing materials such as boron , which intercept them before possible new fission reactions. In principle, it should be noted that the critical mass can also be greatly reduced by the presence of certain substances, especially water, due to their neutron moderating or reflecting effect.
links
→ Category plutonium compound
Oxides
The most stable and most important oxygen compound is plutonium dioxide (PuO 2 ). This compound is a solid with a high melting temperature. It is stable towards water and not soluble in it. Plutonium is therefore used in radionuclide batteries and nuclear power plants in the form of this oxide. In addition to plutonium dioxide, plutonium (III) oxide Pu 2 O 3 and plutonium (II) oxide PuO are also known.
Halides
Plutonium forms numerous compounds with the halogens fluorine , chlorine , bromine and iodine . A corresponding plutonium compound in the +3 oxidation state is known of all halogens. There are also plutonium (IV) fluoride , plutonium (IV) chloride and plutonium (VI) fluoride .
| Oxidation number | F. | Cl | Br | I. |
| +6 |
Plutonium (VI) fluoride PuF 6 red-brown |
|||
| +4 |
Plutonium (IV) fluoride PuF 4 red-brown |
Plutonium (IV) chloride PuCl 4 |
||
| +3 |
Plutonium (III) fluoride PuF 3 violet |
Plutonium (III) chloride PuCl 3 green |
Plutonium (III) bromide PuBr 3 green |
Plutonium (III) iodide PuI 3 green |
Boride
There are four known plutonium borides . They are used to reduce the radioactivity of plutonium.
Organometallic compounds
Analogous to uranocene , an organometallic compound in which uranium is complexed by two cyclooctatetraene ligands, the corresponding complexes of thorium , protactinium , neptunium, americium and also of plutonium, (η 8 -C 8 H 8 ) 2 Pu, were prepared.
Analytics
Because of its rarity, there are no classical, wet-chemical detection methods for plutonium. Therefore only instrumental procedures are used.
Instrumental quantitative analysis of plutonium
α spectrometry
Plutonium is often detected via the α radiation of the isotopes 239 (40) Pu and 238 Pu. A direct analysis is often not possible, so that previous separation techniques have to be carried out. Ion exchange chromatography is often used here. With the help of α-spectrometry 239 (40) Pu could be determined in maritime sediments with a detection limit of 1 mBq / g.
Elemental Mass Spectrometry (MS)
For the determination of plutonium are used in mass spectrometry ICP-ionization (ICP, inductively-coupled plasma mass spectrometry with inductively coupled plasma ) and AMS ( Accelerator Mass Spectrometry ) is used. Compared to ICP-MS, AMS is more sensitive, but complex and costly in terms of equipment, since a particle accelerator has to be used for ionization. With the AMS, a detection limit of around 10 6 atoms of the 239 Pu isotope was achieved on the VERA system in Vienna . With the help of the ICP technique, a detection limit of 10 8 atoms 239 Pu could be achieved, which corresponds to an activity of 0.1 mBq.
Optical emission spectrometry (OES)
Plutonium can also be detected with a laser-based variant of optical emission spectrometry (OES). In the Laser Induced Breakdown Spectroscopy (LIBS) to use laser pulses to vaporize and emission stimulation of the sample. A wide range of lines is available for emission measurement, whereby the lines at 295.16 nm, 300.06 nm and 363.22 nm are mostly used due to the best intensity values. With this technique, a detection limit of 10 −8 g / mL could be achieved. The same detection limit could be achieved with optical emission spectrometry using inductively coupled plasma (ICP-OES).
Laser Induced Photoacoustic Spectrometry (LIPAS)
With the LIPAS technology, a high-energy laser pulse is sent into the sample solution, which induces a photoacoustic wave. The amplitude of this wave is determined with the aid of a piezoelectric detector. With this technique, hexavalent plutonium could be detected with a detection limit of 0.5 µg / mL.
Proof of production outside the reactor
Researchers at Sandia National Laboratories want to use the antineutrinos emitted during the beta decay of fission products to measure the production of plutonium in nuclear reactors so that the IAEA is no longer dependent on estimates and no plutonium can be diverted unnoticed for the construction of nuclear weapons . Because of the extremely high production rate of antineutrinos in nuclear reactors, a detector with 1 m 3 of detector liquid in front of the nuclear power plant would be sufficient.
Plutonium inventory
At the end of 2009, Germany reported a plutonium inventory of 5.4 t of separated, unirradiated plutonium in fresh MOX fuel elements or other manufactured products to the IAEA. In addition, there were 86.9 t of plutonium in irradiated fuel elements, which were stored at the German reactors, and a further 5.9 t in irradiated fuel that was stored at other locations.
At the end of 2010, Switzerland reported to the IAEA that it had a plutonium inventory of less than 50 kg of separated plutonium. In addition, there was 13 t of plutonium in irradiated fuel elements, which were stored at the reactor sites, and a further 4 t in irradiated fuel, which was stored at other sites.
The global inventory of plutonium is given as of 1999. The information is based on estimates by the Department of Energy . The numbers in brackets indicate the amount of plutonium extracted from the spent fuel. For Kazakhstan, according to the Bulletin of the Atomic Scientist, plutonium quality has been misclassified by the Department of Energy and should be commercial. Weapons grade plutonium contains less than 7% of the isotope 240 Pu. Commercial grade plutonium is made up of fuel grade plutonium with 7 to 18% 240 Pu and reactor grade plutonium with more than 19% 240 Pu.
State
(as of 1999)weapon capable (in t) commercial
quality (in t)Argentina 0 6th Belgium 0 23-31 Brazil 0 0.6 Great Britain 7.6 98.4 (51) People's Republic of China 1.7-2.8 1.2 France 6-7 151-205 (70) Germany 0 75-105 (17) India 0.15-0.25 6th Israel 0.3-0.5 0 Japan 0 119–262 (21) Kazakhstan 2–3 * 0 North Korea 0.025-0.035 0 Russia 140-162 65 (30) United States 85 257.2 (14.5) total 242.3-267.4 802.4-1037.4 (~ 203.5)
In 2000, the USA and Russia concluded an agreement to dispose of or defuse 34 t of plutonium each. Against the background of political tensions, the Kremlin declared in October 2016 that “Russia is no longer able to implement this agreement on its own”.
literature
- David L. Clark, Siegfried S. Hecker, Gordon D. Jarvinen, Mary P. New: Plutonium. In: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Eds.): The Chemistry of the Actinide and Transactinide Elements. Springer, Dordrecht 2006, ISBN 1-4020-3555-1 , pp. 813-1264 ( doi: 10.1007 / 1-4020-3598-5_7 ).
Web links
- Entry on plutonium. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2015.
- Shenda M. Baker: Plutonium , Chemical & Engineering News, 2003
- Plutonium: Human Health Fact Sheet (English, PDF, 57 kB)
- Plutonium Manufacture and Fabrication (English)
- Institute for Energy and Environmental Research (English)
- Contaminated Plutonium , Deutschlandfunk
Individual evidence
- ↑ https://www.seilnacht.com/Lexikon/94Pluton.html Thomas Seilnacht on Plutonium, accessed on Nov. 9, 2019
- ↑ The values of the atomic and physical properties (info box) are taken from www.webelements.com (plutonium) , unless otherwise stated .
- ↑ a b c d e Entry on plutonium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 13, 2020.
- ↑ a b c d e Entry on plutonium at WebElements, https://www.webelements.com , accessed on June 13, 2020.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Magnetic Susceptibility of the Elements and Inorganic Compounds, pp. 4-145. The values there are related to g · mol −1 and are given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b c Harry H. Binder: Lexicon of chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 , pp. 469-476.
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this element has either not yet been classified or a reliable and citable source has not yet been found.
- ^ GT Seaborg, E. McMillan, JW Kennedy, AC Wahl: Radioactive Element 94 from Deuterons on Uranium. In: Physical Review . 69 (7-8), 1946, pp. 366-367 ( doi: 10.1103 / PhysRev.69.367 ).
- ^ JW Kennedy, GT Seaborg, E. Segrè, AC Wahl: Properties of Element 94. In: Physical Review . 70 (7-8), 1946, pp. 555-556 ( doi: 10.1103 / PhysRev.70.555 ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1948.
- ↑ Marco Fontani: The Lost element. Oxford University Press, 2014, ISBN 978-0-19-938336-8 ( limited preview in Google Book Search).
- ↑ BB Cunningham, LB Werner: The First Isolation Of Plutonium. In: Journal of the American Chemical Society . 71 (5), 1949, pp. 1521-1528 ( doi: 10.1021 / ja01173a001 ).
- ^ Carl Friedrich von Weizsäcker: A possibility of generating energy from uranium 238, July 17, 1940. In: Secret documents on the German atomic program 1938–1945. Deutsches Museum , accessed March 8, 2010 .
- ↑ Markus Becker: Nuclear Forensics: "Heisenberg Cube" reveals details about Hitler's nuclear program. In: Spiegel Online . March 19, 2009. Retrieved May 7, 2009 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1949.
- ↑ DF Peppard, MH Studier, MV Gergel, GW Mason, JC Sullivan, JF Mech: Isolation of Microgram Quantities of Naturally-occurring Plutonium and Examination of its Isotopic Composition. In: J. Am. Chem. Soc. 73 (6), 1951, pp. 2529-2531 ( doi: 10.1021 / ja01150a034 ).
- ^ A b D. C. Hoffman, FO Lawrence, JL Mewherter, FM Rourke: Detection of Plutonium-244 in Nature. In: Nature . 234, 1971, pp. 132-134 ( doi: 10.1038 / 234132a0 ).
- ↑ a b kernenergie-wissen.de: What is plutonium? ( Memento from December 25, 2013 in the Internet Archive )
- ↑ Pavel Podvig: Can the US-Russia plutonium disposition agreement be saved? Bulletin of the Atomic Scientists, April 28, 2016.
- ↑ Plutonium in the depths of the oceans ; Plutonium in the environment en.wo, accessed May 2, 2012.
- ^ Karlsruhe (Germany) Nuclear Research Center: KFK. . 1993 ( limited preview in Google Book Search).
- ^ Johannes Friedrich Diehl: Radioactivity in food. John Wiley & Sons, 2008, ISBN 978-3-527-62374-7 , p. 82 ( limited preview in Google book search).
- ↑ Rudolf Stagl: Effects of the disclosure obligation of the plutonium processing plant Rocky Flats on perception and land market in the Denver / Boulder area (Colorado, USA) . Reimer, 1986, ISBN 978-3-496-00881-1 ( limited preview in Google book search).
- ↑ Lasse Ringius: Radioactive waste disposal at sea - public ideas, transnational policy entrepreneurs, and environmental regimes. MIT Press, Cambridge 2001, ISBN 0-262-18202-5 , p. 23 ( limited preview in Google Book Search), accessed May 2, 2012.
- ↑ IAEA press release on Chernobyl (1995) p. 9 ( Memento of April 13, 2006 in the Internet Archive ) (PDF; 180 kB).
- ↑ dtv atlas on chemistry. Volume 1, dtv, 2000.
- ^ German translation of the Nuclear Non-Proliferation Treaty of the German Federal Foreign Ministry .
- ↑ Law on the peaceful use of nuclear energy and protection against its dangers (Atomic Energy Act) .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 2149.
- ^ A b c Norman N. Greenwood, Alan Earnshaw: Chemistry of the elements. 1st edition. VCH Verlagsgesellschaft, Weinheim 1988, ISBN 3-527-26169-9 .
- ↑ a b c Plutonium: An Element at odds with itself. In: Los Alamos Science. 26, 2000. (PDF; 881 kB)
- ↑ www.kernchemie.de (Plutonium - element with many facets) .
- ^ WH Zachariasen, FH Ellinger: The Crystal Structure of alpha Plutonium Metal. In: Acta Cryst. 16, 1963, pp. 777-783 ( doi: 10.1107 / S0365110X63002012 ).
- ^ WH Zachariasen, FH Ellinger: The Crystal Structure of beta Plutonium Metal. In: Acta Cryst. 16, 1963, pp. 369-375 ( doi: 10.1107 / S0365110X63000992 ).
- ^ WH Zachariasen: Crystal Chemical Studies of the 5f-Series of Elements. XXIV. The Crystal Structure and Thermal Expansion of γ-Plutonium. In: Acta Cryst. 8, 1955, pp. 431-433 ( doi: 10.1107 / S0365110X55001357 ).
- ↑ KT Moore, P. Söderlind, AJ Schwartz, DE Laughlin: Symmetry and Stability of δ Plutonium: The Influence of Electronic Structure. In: Physical Review Letters . 96 (20), 2006, pp. 206402 / 1-206402 / 4 ( doi: 10.1103 / PhysRevLett.96.206402 ).
- ↑ FH Ellinger: Crystal structure of delta 'plutonium and the thermal expansion characteristics of delta, delta' and epsilon plutonium. In: Journal of Metals . 8, 1956, pp. 1256-1259.
- ↑ JB Ball, JA Lee, PG Mardon, JAL Robertson: Determination de quelques proprietes physiques du plutonium metal. In: Memoires Scientifiques de la Revue de Metallurgie . 57, 1960, pp. 49-56.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Density of Molten Elements and Representative Salts, pp. 4-141.
- ↑ S. Dabos-Seignon, JP Dancausse, R. Low, S. Heathman, U. Benedict: Pressure induced phase transition in α-Pu. In: Journal of Alloys and Compounds . 190, 1993, pp. 237-242 ( doi: 10.1016 / 0925-8388 (93) 90404-B ).
- ↑ Georg Brauer (Ed.): Handbook of Preparative Inorganic Chemistry . 3., reworked. Edition. tape II . Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 1293 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1956.
- ^ The Biochemical Periodic Tables - Plutonium .
- ↑ P. Yong, LE Macaskie: Bioaccumulation of Lanthanum, Uranium and Thorium, and Use of a Model System to develop a Method for the Biologically-mediated Removal of Plutonium from Solution . In: Journal of Chemical Technology & Biotechnology . tape 71 , no. 1 , 1998, p. 15-26 , doi : 10.1002 / (SICI) 1097-4660 (199801) 71: 1 <15 :: AID-JCTB773> 3.0.CO; 2-8 .
- ↑ a b c d e f g h i j k G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties. In: Nuclear Physics. Volume A 729, 2003, pp. 3-128. doi : 10.1016 / j.nuclphysa.2003.11.001 . ( PDF ; 1.0 MB).
- ↑ G. Pfennig, H. Klewe-Nebenius, W. Seelmann-Eggebert (eds.): Karlsruher Nuklidkarte . 6th edition. correct. Edition 1998.
- ↑ Isotope data for plutonium-236 on atom.kaeri.re.kr , accessed on August 12, 2012.
- ↑ isotope data on plutonium atom.kaeri.re.kr , accessed on 12 August 2012; For details, first select the isotope, then click on "n-XS Summary".
- ↑ a b Institut de Radioprotection et de Sûreté Nucléaire : Evaluation of nuclear criticality safety data and limits for actinides in transport ( Memento of November 18, 2014 in the Internet Archive ) (PDF, pp. 15-16).
- ↑ Updated Critical Mass Estimates for Plutonium-238 .
- ↑ Information Circle on Nuclear Energy (Ed.): Basic Knowledge of Nuclear Energy ( Memento from June 17, 2012 in the Internet Archive ) (PDF; 8.9 MB).
- ↑ Cassini-Huygens: Spacecraft (see Table 2–3) ( Memento from January 19, 2012 in the Internet Archive ) (PDF; 625 kB).
- ^ Institute for Energy and Environmental Research .
- ↑ Erich Übelacker: Atomic energy. (= What is what. Volume 3). Tessloff Verlag, Nuremberg 1995, ISBN 3-7886-0243-0 , p. 29.
- ↑ World Nuclear Association: plutonium (English) ( Memento of 29 December 2013, Internet Archive ).
- ^ Reactor plutonium and weapon plutonium (as of 2005).
- ↑ Weapons plutonium reactor shut down after 46 years. In: Russia News. April 15, 2010.
- ↑ Nuclides for RTGs (PDF; 297 kB) last page.
- ↑ Cassini-Huygens: Spacecraft (see Table 2–2) ( Memento from January 19, 2012 in the Internet Archive ) (PDF; 625 kB).
- ↑ Quotation: "Its radioactivity is measurable" on all continents and at any altitude, "stated a report by the OECD in 1989." In: Die Zeit. 39/1997.
- ^ Dispute over the plutonium drive of the Saturn probe Cassini. In: The time. 39/1997.
- ↑ Pacemaker with plutonium (Eng.)
- ↑ Plutonium pacemaker: atomic battery in the chest. In: Spiegel Online . November 22, 2009, accessed April 5, 2015 .
- ↑ Basic knowledge about nuclear energy: Plutonium batteries ( Memento from December 26, 2013 in the Internet Archive ).
- ↑ University of Oldenburg: Dangerousness of uranium-238 and plutonium-239 in comparison .
- ↑ Franz Frisch: Clip and clear, 100 × energy. Bibliographisches Institut AG, Mannheim 1977, ISBN 3-411-01704-X , p. 184.
- ↑ BREDL Southern Anti-plutonium Campaign ( Memento of 29 April 2015, Internet Archive ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1972.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1968–1971.
- ↑ Christoph Elschenbroich : Organometallchemie. 6th edition. Wiesbaden 2008, ISBN 978-3-8351-0167-8 , p. 589.
- ↑ T. Miura, S. Oikawa, T. Kishimoto, S. Banba, T. Morimoto: Rapid separation of plutonium in environmental samples using an anion exchange resin disk. In: Journal of Radioanalytical and Nuclear Chemistry . 250, 2001, pp. 449-452 ( doi: 10.1023 / A: 1017936703216 ).
- ↑ E. Hrnecek, P. Steier, A. Wallner: Determination of plutonium in environmental samples by AMS and alpha spectrometry. In: Applied Radiation and Isotopes . 63 (5-6), 2005, pp. 633-638 ( doi: 10.1016 / j.apradiso.2005.05.012 . PMID 15982894 ).
- ↑ L. Fifield, R. Cresswell, M. di Tada, T. Ophel, J. Day, A. Clacher, S. King, N. Priest: Accelerator mass spectrometry of plutonium isotopes. In: Nuclear Instruments and Methods in Physics Research B . 117 (3), 1996, pp. 295-303 ( doi: 10.1016 / 0168-583X (96) 00287-X ).
- ^ A b X. Claudon, J. Birolleau, M. Lavergne, B. Miche, C. Bergey: Simultaneous determination of americium and plutonium by inductively coupled plasma-atomic emission spectrometry. In: Spectrochimica Acta . 42B (1-2), 1987, pp. 407-411 ( doi: 10.1016 / 0584-8547 (87) 80080-0 ).
- ↑ C. Pasquini, J. Cortez, L. Silva, F. Gonzaga: Laser Induced Breakdown Spectroscopy. In: Journal of the Brazilian Chemical Society . 18 (3), 2007 ( doi: 10.1590 / S0103-50532007000300002 ).
- ↑ N. Surugaya, S. Sato, S. Jitsukata, M. Watahiki: Application of Laser-induced Photoacoustic Spectroscopy for Determination of Plutonium Concentration in Nuclear Waste Solutions. In: Analytical Sciences . 24, 2008, pp. 527-530. PMID 18403847 .
- ↑ K. Adelhelm, W. Faubel, H. Ache: Laser induced photoacoustic spectroscopy in liquid samples: temperature and solvent effects. In: Fresenius Journal of Analytical Chemistry . 338, 1990, pp. 259-264 ( doi: 10.1007 / BF00323020 ).
- ↑ Antineutrinos monitor plutonium production .
- ↑ Communication Received from Germany. Concerning its Policies regarding the Management of Plutonium. ( Memento from October 22, 2013 in the Internet Archive ) PDF at www.iaea.org
- ↑ Communication Received from Switzerland. Concerning its Policies Regarding the Management of Plutonium. ( Memento from October 22, 2013 in the Internet Archive ) PDF at www.iaea.org
- ↑ a b World Plutonium Inventories. In: The Bulletin of the Atomic Scientist. September / October 1999, p. 71.
- ↑ Russia stops the destruction of weapons-grade plutonium orf.at, October 3, 2016.
![{\ mathrm {^ {{238}} _ {{\ 92}} U \ + \ _ {{1}} ^ {{2}} D \ \ longrightarrow \ _ {{\ 93}} ^ {{238} } Np \ + \ 2 \ _ {{0}} ^ {{1}} n \ quad; \ quad _ {{\ 93}} ^ {{238}} Np \ {\ xrightarrow [{2,117 \ d}] {\ beta ^ {-}}} \ _ {{\ 94}} ^ {{238}} Pu}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e1cea0855d09c23ed65d8571e341ca5b6e018ea6)
![{\ mathrm {^ {{238}} _ {{\ 92}} U \ + \ _ {{0}} ^ {{1}} n \ \ longrightarrow \ _ {{\ 92}} ^ {{239} } U \ {\ xrightarrow [{23.5 \ min}] {\ beta ^ {-}}} \ _ {{\ 93}} ^ {{239}} Np \ {\ xrightarrow [{2.3565 \ d }] {\ beta ^ {-}}} \ _ {{\ 94}} ^ {{239}} Pu}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/12492af52719f167cf2db01c018abf105d690225)




![\ mathrm {^ {235} _ {\ 92} U \ + \ ^ {1} _ {0} n \ \ longrightarrow \ ^ {236m} _ {\ 92} U \ \ xrightarrow [120 \ ns] {} \ ^ {236} _ {\ 92} U \ + \ \ gamma}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8c21496787df33e6024968eb4a98ac23d3035b9a)
![{\ mathrm {^ {{236}} _ {{\ 92}} U \ + \ _ {{0}} ^ {{1}} n \ \ longrightarrow \ _ {{\ 92}} ^ {{237} } U \ {\ xrightarrow [{6.75 \ d}] {\ beta ^ {-}}} \ _ {{\ 93}} ^ {{237}} Np}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0ddd879b76c59f77664f22db1fa2a50b2e029905)
![{\ mathrm {^ {{237}} _ {{\ 93}} Np \ + \ _ {{0}} ^ {{1}} n \ \ longrightarrow \ _ {{\ 93}} ^ {{238} } Np \ {\ xrightarrow [{2,117 \ d}] {\ beta ^ {-}}} \ _ {{\ 94}} ^ {{238}} Pu}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/88e4b7fc6cd5d7f51d47d58061f3abe4e147765c)





![{\ mathrm {^ {{241}} _ {{\ 94}} Pu \ {\ xrightarrow [{14.35 \ a}] {\ beta ^ {-}}} \ _ {{\ 95}} ^ { {241}} On \ {\ xrightarrow {(n, \ gamma)}} \ _ {{\ 95}} ^ {{242}} on \ {\ xrightarrow [{16.02 \ h}] {\ beta ^ {-}}} \ _ {{\ 96}} ^ {{242}} cm \ \ left ({\ xrightarrow [{162.8 \ d}] {\ alpha}} \ _ {{\ 94}} ^ {{238}} Pu \ right)}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d6e05aaf59bc4d437fa8fa526b3d79bd33d12568)
