Vanadium
| properties | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Vanadium, V, 23 | |||||||||||||||||||||||||||||||||
| Element category | Transition metals | |||||||||||||||||||||||||||||||||
| Group , period , block | 5 , 4 , d | |||||||||||||||||||||||||||||||||
| Appearance | steel gray metallic, shimmering bluish | |||||||||||||||||||||||||||||||||
| CAS number | 7440-62-2 | |||||||||||||||||||||||||||||||||
| EC number | 231-171-1 | |||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.337 | |||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.041% | |||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||
| Atomic mass | 50.9415 (1) and | |||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 135 (171) pm | |||||||||||||||||||||||||||||||||
| Covalent radius | 153 pm | |||||||||||||||||||||||||||||||||
| Electron configuration | [ Ar ] 3 d 3 4 s 2 | |||||||||||||||||||||||||||||||||
| 1. Ionization energy | 6th.746 187 (21) eV ≈ 650.91 kJ / mol | |||||||||||||||||||||||||||||||||
| 2. Ionization energy | 14th.634 (7) eV ≈ 1 412 kJ / mol | |||||||||||||||||||||||||||||||||
| 3. Ionization energy | 29.3111 (25) eV ≈ 2 828.09 kJ / mol | |||||||||||||||||||||||||||||||||
| 4. Ionization energy | 46.709 (5) eV ≈ 4 506.7 kJ / mol | |||||||||||||||||||||||||||||||||
| 5. Ionization energy | 65.28165 (17) eV ≈ 6 298.72 kJ / mol | |||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic | |||||||||||||||||||||||||||||||||
| density | 6.11 g / cm 3 (20 ° C ) | |||||||||||||||||||||||||||||||||
| Mohs hardness | 7.0 | |||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 3.8 10 −4 ) | |||||||||||||||||||||||||||||||||
| Melting point | 2183 K (1910 ° C) | |||||||||||||||||||||||||||||||||
| boiling point | 3680 K (3407 ° C) | |||||||||||||||||||||||||||||||||
| Molar volume | 8.32 · 10 −6 m 3 · mol −1 | |||||||||||||||||||||||||||||||||
| Heat of evaporation | 444 kJ / mol | |||||||||||||||||||||||||||||||||
| Heat of fusion | 21.5 kJ mol −1 | |||||||||||||||||||||||||||||||||
| Speed of sound | 4560 m s −1 at 293.15 K. | |||||||||||||||||||||||||||||||||
| Specific heat capacity | 489 J kg −1 K −1 | |||||||||||||||||||||||||||||||||
| Electric conductivity | 5 · 10 6 A · V −1 · m −1 | |||||||||||||||||||||||||||||||||
| Thermal conductivity | 31 W m −1 K −1 | |||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||
| Oxidation states | +5 , +4, + 3, + 2 | |||||||||||||||||||||||||||||||||
| Electronegativity | 1.63 ( Pauling scale ) | |||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||
| NMR properties | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||
Vanadium , also outdated vanadium , is a chemical element with the symbol V and the atomic number 23. It is a steel-gray, bluish shimmering transition metal that is very soft in its pure state . In the periodic table, the metal, together with the heavier niobium , tantalum and dubnium, forms the 5th IUPAC group or vanadium group . Most of the vanadium is used as so-called ferrovanadium in steel production. The addition of vanadium to chrome - vanadium steels increases the toughness and thus increases the resistance of the steel.
The element has different biological meanings and is essential for many living things . So it plays a role in the control of enzymes of phosphorylation and is used by bacteria to nitrogen fixation used. On the other hand, it or its compounds are suspected of causing chromosome aberrations as a mutagenic clastogen and thus acting as a poison and carcinogen .
The best-known compound of vanadium is vanadium (V) oxide , which is used as a catalyst for the production of sulfuric acid .
history
The later vanadium was discovered for the first time in 1801 by the Spanish mineralogist Andrés Manuel del Río in a Mexican lead ore, later vanadinite . He called the new element Panchromium first because of the multicolored nature of the compounds , later Erythronium , because the salts turned red when acidified. However, del Rio revoked the discovery a short time later, when first Alexander von Humboldt and later the French chemist HV Collett-Desotils claimed that the new element was contaminated chromium because of its similarity to chromium compounds .
The rediscovery of the element succeeded in 1830 by the Swedish chemist Nils Gabriel Sefström . He examined iron from the Swedish iron ore mine Taberg by dissolving it in hydrochloric acid . In the process, he discovered, among other known substances, an unknown element that resembled chromium in some properties and uranium in others , but was not one of these elements after further research. He named the new element after Vanadis , an epithet of the Nordic deity Freyja . A short time later, Friedrich Wöhler , who had already dealt with the task at Berzelius, provided evidence of the identity of vanadium with erythronium.
Metallic vanadium was first in 1867 by Henry Enfield Roscoe by reduction of vanadium (II) chloride with hydrogen produced. John Wesley Marden and Malcolm Rich were able to obtain 99.7% pure vanadium for the first time in 1925 by reducing vanadium (V) oxide with calcium.
Vanadium was first used in 1903 when the first vanadium-containing steel was produced in England . The element's increased use in the steel industry began in 1905 when Henry Ford started using vanadium steels in the construction of automobiles .
Occurrence
Vanadium is a common element on earth, its share in the continental crust is about 120 ppm . Zirconium , chlorine and chromium have a similar abundance of elements . The element is mainly found bound in various minerals . Despite the abundance of vanadium, deposits with high concentrations of the element are rare, and many vanadium minerals are not common. Compared to the earth's crust, the content in seawater is significantly lower, it is around 1.3 μg / l.
The most important vanadium minerals include, in particular, vanadates such as vanadinite [Pb 5 (VO 4 ) 3 Cl], Descloizit Pb (Zn, Cu) [OH | VO 4 ] and carnotite [K 2 (UO 2 ) 2 (VO 4 ) 2 · 3H 2 O], as well as the vanadium sulfide Patrónit VS 4 . Most of the vanadium is found in traces in other minerals, especially iron ores such as magnetite . The vanadium content of titanium magnetite ores is usually between 0.3 and 0.8%, but can reach up to 1.7% in some South African ores.
Animals and plants contain vanadium, so humans contain about 0.3 mg / kg of the element. This is mostly located in cell nuclei or mitochondria . Some living things, especially some species of sea squirt and the fly agaric , are able to enrich vanadium. The vanadium content in sea squirts is up to 10 7 times higher than in the surrounding sea water. Due to the vanadium content of living beings, coal and crude oil that arise from them also contain vanadium. The content is up to 0.1%. Particularly high levels of vanadium are found in petroleum from Venezuela and Canada.
In 2015, a total of 79,400 tons of vanadium ore were mined (calculated as vanadium metal). The most important producing countries are South Africa , China and Russia . With known reserves totaling 15 million tons (as of 2015), a delivery bottleneck for vanadium is not expected in the foreseeable future.
Vanadium as a mineral
Vanadium has been recognized as a mineral by the International Mineralogical Association (IMA) since 2012 . It was first discovered by Mikhail Ostrooumov as a resublimation product in the high-temperature fumaroles of the Mexican volcano Colima . Potassium vanadium sulfide Colimait (K 3 VS 4 ) and the vanadium oxide Shcherbinait (V 5+ 2 O 5 ) , which were also discovered there for the first time, appeared as accompanying minerals .
The first description was published by Ostrooumov and Yuri Taran in 2015, initially in the Macla. Revista de la Sociedad Española de Mineralogía and 2016 in Mineralogical Magazine .
Besides its type locality at the volcano Colima vanadium was dignified yet only at a unspecified Hibonit -Fundstätte in Argentina's Sierra de los Comechingones be discovered. Another find in the Rhovan opencast vanadium mine near Rustenburg in the South African province of Northwest has not yet been confirmed.
According to the systematics of minerals according to Strunz (9th edition) , vanadium is classified under system no. 1.AF.05 (elements - metals and intermetallic compounds - iron-chromium family - iron group).
Extraction and presentation
Vanadium is represented in several steps. First of all, vanadium (V) oxide must be obtained from various starting materials . This can then be reduced to elementary metal and cleaned if necessary.
Possible starting materials from which vanadium can be extracted are vanadium ores such as carnotite or patronite, vanadium-containing titanium-magnetite ores and petroleum . Vanadium ores were important for production in the past, but no longer play an important role and have mainly been replaced by titanium-magnetite ores.
If iron ores containing vanadium are reduced to iron in the blast furnace process , the vanadium initially remains in the pig iron . In order to process the pig iron further into steel , oxygen is blown in during the refining process . The vanadium goes into the slag . This contains up to 25% vanadium (V) oxide and is the most important source for the extraction of the metal. In order to obtain the pure vanadium (V) oxide, the finely ground slag is roasted in an oxidizing manner with sodium salts such as sodium chloride or sodium carbonate . In the process, water-soluble sodium metavanadate is formed , which is separated from the remaining slag by leaching. The resulting insoluble ammonium polyvanadate precipitates out of the solution by adding acid and ammonium salts . This can be converted to vanadium (V) oxide by roasting . The oxide can also be obtained from other vanadium-containing ores in an identical way. The vanadium can be extracted from petroleum by forming an emulsion with the addition of water and magnesium nitrate . The further processing takes place as with the extraction from iron ores.
The actual vanadium extraction takes place by reducing the vanadium (V) oxide with other metals. Aluminum , calcium , ferrosilicon or carbon can be used as reducing agents ; with the latter, however, carbides are formed in the reaction , which are difficult to separate from the metal.
- Reduction with calcium
In order to obtain pure vanadium, expensive calcium or aluminum is used as a reducing agent, since the cheaper ferrosilicon cannot achieve high purity. While pure vanadium is obtained directly with calcium, a vanadium-aluminum alloy is initially formed with aluminum, from which pure vanadium is obtained by sublimation in a vacuum .
A large part of the vanadium is not used as a pure metal, but in the form of the iron-vanadium alloy ferrovanadium , which contains at least 50% vanadium. In order to produce this, it is not necessary to extract the pure vanadium beforehand. Instead, the vanadium and iron-containing slag is reduced to ferrovanadium with ferro-silicon and lime . This alloy is sufficient for most technical applications.
The purest vanadium can be produced either electrochemically or according to the Van-Arkel-de-Boer method . For this purpose, the pure vanadium is melted together with iodine in an empty glass ampoule. The vanadium (III) iodide formed in the heated ampoule decomposes on a hot tungsten wire to form highly pure vanadium and iodine.
- Reaction in the Van Arkel de Boer process
properties
Physical Properties
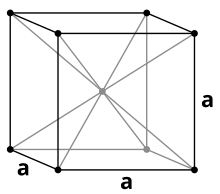
Vanadium is a non-magnetic, tough, malleable and clearly steel-blue heavy metal with a density of 6.11 g / cm 3 . Pure vanadium is relatively soft, but becomes harder when other elements are added and then has high mechanical strength. In most of its properties it is similar to its neighbor in the periodic table, titanium . The melting point of pure vanadium is 1910 ° C, but this is significantly increased by impurities such as carbon . With a carbon content of 10% it is around 2700 ° C. Vanadium crystallizes like chromium or niobium in a body-centered cubic crystal structure with the space group Im 3 m (space group no. 229) and the lattice parameter a = 302.4 pm as well as two formula units per unit cell .
Below a critical temperature of 5.13 K vanadium becomes a superconductor . Just like pure vanadium, alloys of vanadium with gallium , niobium and zirconium are superconducting. At temperatures below 5.13 K, vanadium, like the vanadium group metals niobium and tantalum , shows in tiny lumps of up to 200 atoms a hitherto unexplained, spontaneous electrical polarization that is otherwise only exhibited by non-metallic substances .
Chemical properties
Vanadium is a base metal and is able to react with many non-metals . In the air it remains shiny metallic for weeks. When viewed over longer periods of time, clearly visible green rust is perceived. If vanadium is to be preserved, it must be kept under argon. In the heat it is attacked by oxygen and oxidized to vanadium (V) oxide . While carbon and nitrogen only react with vanadium when it is incandescent , the reaction with fluorine and chlorine takes place in the cold.
Compared with acids and bases is vanadium usually stable at room temperature because of a thin passivating oxide layer; In this state it is only attacked by hydrofluoric acid and strongly oxidizing acids such as hot nitric acid , concentrated sulfuric acid and aqua regia .
Up to a temperature of 500 ° C is vanadium capable of hydrogen to absorb . The metal becomes brittle and can be easily powdered. The hydrogen can be removed at 700 ° C in a vacuum.
Isotopes
A total of 27 isotopes and a further 6 core isomers are known of vanadium . Of these, two occur naturally. These are the isotopes 50 V with a natural frequency of 0.25% and 51 V with a frequency of 99.75%. 50 V is weakly radioactive , with a half-life of 1.5 · 10 17 years it decays 83% with electron capture to 50 Ti, 17% with β - decay to 50 Cr. Both cores can be used for investigations with NMR spectroscopy .
The most stable artificial isotopes are 48 V with a half-life of 16 days and 49 V with a half-life of 330 days. These are used as tracers . All other isotopes and core isomers are very unstable and disintegrate in minutes or seconds.
use
Only a small percentage of pure vanadium is used as a cladding material for nuclear fuels due to its small neutron capture cross- section . However, more resistant vanadium alloys can also be used. Over 90% of production is used in a variety of alloys , mostly with the metals iron, titanium, nickel, chromium, aluminum or manganese. Only a small part is used in compounds, mostly as vanadium (V) oxide.
With 85% of the vanadium produced, by far the largest part is consumed in the steel industry . Since this does not require high purities, ferrovanadium is used as a raw material. Even in small quantities, vanadium increases the strength and toughness of steels and thus their wear resistance significantly. This is caused by the formation of hard vanadium carbide . Depending on the application, different amounts of vanadium are added; structural steels and tool steels contain only small amounts (0.2 to 0.5%) of vanadium, high-speed steel up to 5%. Vanadium-containing steels are mainly used for tools and springs that are subject to mechanical stress. Steels that contain cobalt in addition to iron and vanadium are magnetic.
Titanium alloys, which contain vanadium and mostly aluminum, are particularly stable and heat-resistant and are used in aircraft construction for load-bearing parts and turbine blades of aircraft engines.
Vanadium compounds can be used for electrochemical energy storage in redox flow cells , see Vanadium redox accumulator . In this application, the vanadium salts are used in acidic aqueous solutions that can be stored in tanks.
proof
A preliminary test is provided by the phosphorus salt bead , in which vanadium appears characteristic green in the reduction flame. The oxidation flame is pale yellow and therefore too unspecific.
Qualitative evidence for vanadium is based on the formation of peroxovanadium ions. To do this, an acidic solution containing vanadium in the +5 oxidation state is mixed with a little hydrogen peroxide . The reddish-brown [V (O 2 )] 3+ cation is formed. This reacts with larger amounts of hydrogen peroxide to form the pale yellow peroxovanadic acid H 3 [VO 2 (O 2 ) 2 ].
Vanadium can be quantitatively determined by titration . For this purpose, a vanadium - containing sulfuric acid solution is oxidized with potassium permanganate to pentavalent vanadium and then back-titrated with an iron (II) sulfate solution and diphenylamine as an indicator . A reduction of existing pentavalent vanadium with iron (II) sulfate to the tetravalent oxidation state and subsequent potentiometric titration with potassium permanganate solution is also possible.
In modern analytics , vanadium can be detected using several methods. These are, for example, atomic absorption spectrometry at 318.5 nm and spectrophotometry with N-benzoyl-N-phenylhydroxylamine as the color reagent at 546 nm.
Biological importance

Vanadium compounds have various biological meanings. It is characteristic of vanadium that it occurs both anionically as vanadate and cationically as VO 2 + , VO 2+ or V 3+ . Vanadates are very similar to phosphates and accordingly have similar effects. Since vanadate binds more strongly to suitable enzymes than phosphate, it is able to block and thus control enzymes of phosphorylation . This concerns, for example, the sodium-potassium-ATPase , which controls the transport of sodium and potassium into cells. This blockage can be quickly removed with desferrioxamine B , which forms a stable complex with vanadate. Vanadium also affects glucose uptake. It is able to stimulate glycolysis in the liver and inhibit the competitive process of gluconeogenesis . This leads to a lowering of the glucose level in the blood. It is therefore being investigated whether vanadium compounds are suitable for the treatment of type 2 diabetes mellitus . However, no clear results have yet been found. Vanadium also stimulates the oxidation of phospholipids and suppresses the synthesis of cholesterol by inhibiting squalene synthase , a microsomal enzyme system in the liver. Consequently, a deficiency causes increased levels of cholesterol and triglycerides in the blood plasma .
Vanadium plays a role in photosynthesis in plants . It is able to catalyze the reaction to form 5-aminolevulinic acid without enzyme. This is an important precursor to the formation of chlorophyll .
In some organisms there are vanadium-containing enzymes, so some types of bacteria have vanadium-containing nitrogenases for nitrogen fixation . These are, for example, species of the genus Azotobacter and the cyanobacterium Anabaena variabilis . However, these nitrogenases are not as efficient as the more common molybdenum nitrogenases and are therefore only activated when there is a molybdenum deficiency. Other vanadium-containing enzymes can be found in brown algae and lichens . These have vanadium-containing haloperoxidases with which they build up organochlorine, bromine or iodine-organic compounds.
The function of vanadium, which is present in large quantities in sea squirts as the metalloprotein vanabine , is not yet known. It was originally assumed that the vanadium, similar to the hemoglobin, serves as an oxygen transporter; however, this has been found to be wrong.
Hazards
Like other metal dusts, vanadium dust is flammable. Vanadium and its inorganic compounds have been shown to be carcinogenic in animal experiments . They are therefore classified in carcinogen category 2. If vanadium dust is inhaled by workers in metal smelting for a long time, it can lead to so-called vanadism . This recognized occupational disease can manifest itself in irritation of the mucous membranes , green-black discoloration of the tongue and chronic bronchial, lung and intestinal diseases.
links
Vanadium can be present in compounds in various oxidation states . Often levels are +5, +4, +3 and +2, more rarely are +1, 0, −1 and −3. The most important and most stable oxidation states are +5 and +4.
Aqueous solution
Vanadium can easily be converted into different oxidation states in aqueous solution. Since the different vanadium ions have characteristic colors, color changes occur.
In acidic solution pentavalent vanadium forms colorless VO 2 + ions, which upon reduction to blue first tetravalent VO 2+ are ions. The trivalent level with V 3+ ions is green, the lowest level that can be achieved in aqueous solution, the divalent V 2+ ion is gray-violet.
Oxygen compounds
The most important and most stable vanadium-oxygen compound is vanadium (V) oxide V 2 O 5 . This orange-colored compound is used in large quantities as a catalyst for the production of sulfuric acid. There it acts as an oxygen carrier and is reduced to another vanadium oxide, vanadium (IV) oxide VO 2 during the reaction . Further known vanadium oxides are vanadium (III) oxide V 2 O 3 and vanadium (II) oxide VO.
In an alkaline solution, vanadium (V) oxide forms vanadates , salts with the anion VO 4 3− . In contrast to the analogous phosphates , however, the vanadate ion is the most stable form; Hydrogen and dihydrogen vanadates as well as free vanadium acid are unstable and only known in dilute aqueous solutions. If basic vanadate solutions are acidified, polyvanadates are formed instead of hydrogen vanadates, in which up to ten vanadate units accumulate. Vanadates can be found in various minerals, examples are vanadinite , descloicite and carnotite .
Halogen compounds
Vanadium forms a large number of compounds with the halogens fluorine , chlorine , bromine and iodine . In the oxidation states +4, +3 and +2 there are compounds with all halogens, only with iodine only compounds in the stages +2 and +3 are known. Of these halides, however, only the chlorides vanadium (IV) chloride and vanadium (III) chloride are technically relevant. Among other things, they serve as a catalyst for the production of ethylene-propylene-diene rubber .
Vanadium oxide chlorides
Vanadium also forms mixed salts with oxygen and chlorine, the so-called vanadium oxide chlorides . Vanadium (III) oxychloride, VOCl, is a yellow-brown, water-soluble powder. The vanadium (IV) oxychloride, VOCl 2 , used in photography and as a textile stain consists of green, hygroscopic crystal tablets which dissolve in water with a blue color. Finally, vanadium (V) oxychloride, VOCl 3 , is a yellow liquid that is very easily hydrolyzed by water . VOCl 3 is used as a catalyst component in low-pressure ethylene polymerization .
More vanadium compounds
In organic vanadium compounds, vanadium reaches its lowest oxidation states 0, −I and −III. The metallocenes , the so-called vanadocenes , are particularly important here. These are used as catalysts for the polymerization of alkynes .
Vanadium carbide VC is used in powder form for plasma spraying and plasma powder build-up welding . Furthermore, vanadium carbide is added to hard metals in order to reduce grain growth .
literature
- Günter Bauer among others: Vanadium and Vanadium Compounds. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2000, doi : 10.1002 / 14356007.a27_367 .
- Norman N. Greenwood, Alan Earnshaw: Chemistry of the Elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 .
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1542–1552.
Web links
- Entry to vanadium. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
Individual evidence
- ↑ a b Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (vanadium) unless otherwise stated .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e Entry on vanadium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e entry on vanadium at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1260.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data. 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet Version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Fluid Properties; Enthalpy of Fusion, pp. 6-135.
- ↑ Entry on vanadium, powder in the GESTIS substance database of the IFA , accessed on April 30, 2017 (JavaScript required)
- ↑ Juan J. Rodríguez-Mercado, Rodrigo A. Mateos-Nava, Mario A. Altamirano-Lozano: DNA damage induction in human cells exposed to vanadium oxides in vitro. In: Toxicology in Vitro. 25, No. 8, 2011, pp. 1996-2002, doi: 10.1016 / j.tiv.2011.07.009 .
- ^ LR Caswell: Andres del Rio, Alexander von Humboldt, and the Twice-Discovered Element. (PDF; 124 kB). In: Bull. Hist. Chem. 28 (1), 2003, pp. 35-41.
- ↑ NG Sefstöm: About the vanadium, a new metal found in the rod iron from Eckersholm, an ironworks that gets its ore from Taberg in Småland. In: Annal. d. Physics . 97 (1), 1831, pp. 1-4.
- ↑ “In ancient times, the goddess Vanadis lived in the far north, beautiful and lovable. One day there was a knock on the door. The goddess remained seated comfortably and thought: it could be knocked again, but there was no more knocking, instead the knocking went down the stairs. The goddess was curious [...] Oh! [...] that's Schalk Wöhler. [...] After a few days there was another knock on the door; but there was knocking over and over again. The goddess finally came herself and opened the door. Sefström entered, and from this meeting Vanadin was born. ”( Letter from Berzelius to Wöhler of January 22, 1831. In: O. Wallach (Ed.): Correspondence between J. Berzelius and F. Wöhler . Leipzig 1901. )
- ↑ Vanadium. In: Encyclopædia Britannica. 2008. Encyclopædia Britannica Online, accessed October 6, 2008 (online)
- ↑ a b c d e f g h i j Günter Bauer et al: Vanadium and Vanadium Compounds. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2000, doi : 10.1002 / 14356007.a27_367 .
- ↑ a b c d Dieter Rehder: Bioinorganic chemistry of vanadium. In: Angew. Chem. 103, 1991, pp. 152-172.
- ↑ a b c d e f Entry on vanadium. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1542.
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1543.
- ^ US Geological Survey: Vanadium. In: Mineral Commodity Summaries. January 2008 (PDF; 84 kB).
- ^ PA Williams, f. Hatert, M. Pasero, SJ Mills: IMA / CNMNC Newsletter 14: New minerals and nomenclature modifications approved in 2012 and 2013 . In: Mineralogical Magazine . tape 77 , no. 1 , 2013, p. 1–12 ( main.jp [PDF; 125 kB ; accessed on January 1, 2018]).
- ↑ Mikhail Ostrooumov, Yuri Taran: Discovery of Native Vanadium, a New Mineral from the Colima Volcano, State of Colima (Mexico) . In: Macla. Revista de la Sociedad Española de Mineralogía . tape July 20 , 2015, p. 109–110 ( ehu.eus [PDF; 134 kB ; accessed on January 1, 2018]).
- ↑ Mikhail Ostrooumov, Yuri Taran: Vanadium, V - a new native element mineral from the Colima volcano, State of Colima, Mexico, and implications for fumarole gas composition . In: Mineralogical Magazine . tape 80 , no. 2 , April 2016, p. 371–382 , doi : 10.1180 / minmag.2016.080.006 (accessed via De Gruyter Online).
- ↑ Mindat - Vanadium (English)
- ^ Mineralienatlas : Strunz 9 systematics: iron-chromium family
- ↑ K. Schubert: A model for the crystal structures of the chemical elements. In: Acta Crystallographica . 30, 1974, pp. 193-204, doi: 10.1107 / S0567740874002469 .
- ^ Aaron Waxler, William S. Corack: Superconductivity of Vanadium. In: Physical Review. 85, (1), 1952, pp. 85-90, doi: 10.1103 / PhysRev . 85.85 .
- ↑ T. Krome: Metals on the wrong track. About the unusual low-temperature behavior of tiny lumps of metal. In: Spektrumdirekt.de. May 22, 2003; Abstract .
- ↑ Ramiro Moro, Xiaoshan Xu, Shuangye Yin, Walt A. de Heer: Ferroelectricity in Free Niobium Clusters. In: Science . Vol. 300, No. 5623, 2003, pp. 1265-1269, doi: 10.1126 / science.1083247 .
- ↑ a b G. Audi, FG Kondev, Meng Wang, WJ Huang, S. Naimi: The NUBASE2016 evaluation of nuclear properties. In: Chinese Physics C. 41, 2017, S. 030001, doi : 10.1088 / 1674-1137 / 41/3/030001 ( full text ).
- ↑ Vanadium. In: Lexicon of Physics. Retrieved July 9, 2008.
- ^ A b G. Jander, E. Blasius, J. Strähle: Introduction to the inorganic-chemical practical course. 14th edition. S. Hirzel Verlag, Stuttgart 1995, ISBN 3-7776-0672-3 , pp. 218-219.
- ^ DM Smith, RM Pickering, GT Lewith: A systematic review of vanadium oral supplements for glycaemic control in type 2 diabetes mellitus. In: QJM: An International Journal of Medicine . 101, (5), 2008, pp. 351-358, doi: 10.1093 / qjmed / hcn003 .
- ↑ Vanadium. In: Lexicon of Biology. Retrieved July 9, 2008.
- ^ A b Wolfgang Kaim, Brigitte Schwederski: Bioinorganische Chemie. 4th edition. Teubner, Wiesbaden 2005, ISBN 3-519-33505-0 , pp. 241-243.
- ↑ DFG press release on changes in the list of MAK and BET values ( memento of August 13, 2007 in the Internet Archive ), July 19, 2005.
- ↑ Vanadium Oxide Chloride. In: Lexicon of Chemistry. Retrieved July 9, 2008.
- ↑ Entry on organic vanadium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ Use of vanadium carbide from the HC Starck Group.