manganese
| properties | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Manganese, Mn, 25 | |||||||||||||||||||||||||||||||||||||||
| Element category | Transition metals | |||||||||||||||||||||||||||||||||||||||
| Group , period , block | 7 , 4 , d | |||||||||||||||||||||||||||||||||||||||
| Appearance | silvery metallic (steel white) | |||||||||||||||||||||||||||||||||||||||
| CAS number | 7439-96-5 | |||||||||||||||||||||||||||||||||||||||
| EC number | 231-105-1 | |||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.277 | |||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.085% | |||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 54.938044 (3) and | |||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 140 (161) pm | |||||||||||||||||||||||||||||||||||||||
| Covalent radius | low-spin: 139 pm, high-spin: 161 pm | |||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Ar ] 3 d 5 4 s 2 | |||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 7th.434 037 9 (12) eV ≈ 717.28 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 15th.63999 (7) eV ≈ 1 509.03 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 33.668 (12) eV ≈ 3 248.5 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 51.21 (12) eV ≈ 4 941 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 72.41 (10) eV ≈ 6 987 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||||||||
| Modifications | four | |||||||||||||||||||||||||||||||||||||||
| density | 7.43 g / cm 3 (25 ° C ) | |||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.0 | |||||||||||||||||||||||||||||||||||||||
| magnetism |
paramagnetic ( : Χ m = 9.0 x 10 -4 ; : = 8.2 x 10 -4 )
|
|||||||||||||||||||||||||||||||||||||||
| Melting point | 1519 K (1246 ° C) | |||||||||||||||||||||||||||||||||||||||
| boiling point | 2373 K (2100 ° C) | |||||||||||||||||||||||||||||||||||||||
| Molar volume | 7.35 × 10 −6 m 3 mol −1 | |||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 225 kJ / mol | |||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 13.2 kJ mol −1 | |||||||||||||||||||||||||||||||||||||||
| Speed of sound | 5150 m s −1 at 293.15 K. | |||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 479.5 J kg −1 K −1 | |||||||||||||||||||||||||||||||||||||||
| Work function | 4.1 eV | |||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 6.94 × 10 5 A · V −1 · m −1 | |||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 7.8 W m −1 K −1 | |||||||||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 1, 2 , 3, 4, (5), 6, 7 | |||||||||||||||||||||||||||||||||||||||
| Normal potential | −1.18 V (Mn 2+ + 2 e - → Mn) | |||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.55 ( Pauling scale ) | |||||||||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||||||||
| NMR properties | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| MAK |
Switzerland: 0.5 mg m −3 (measured as inhalable dust ) |
|||||||||||||||||||||||||||||||||||||||
| Toxicological data |
|
|||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||||||||
Manganese [ maŋˈɡaːn ] is a chemical element with the element symbol Mn and the atomic number 25. In the periodic table it is in the 7th subgroup (7th IUPAC group ), the manganese group . Manganese is a silvery-white, hard, very brittle transition metal , which in some properties is similar to iron .
Manganese occurs naturally as brownstone and is broken down in large quantities. 90% of the mined manganese is used in the steel industry in the form of ferromanganese as an alloy component of steel . It removes oxygen and sulfur from the steel and improves the hardening process at the same time. Manganese (IV) oxide , which is used as a cathode in alkaline manganese batteries , is also economically important .
The element is of great biological importance as a component of various enzymes . It works at a central point in the photosynthesis cycle, where a manganese-calcium cluster is responsible for the oxidation of water to oxygen.
history
Naturally occurring manganese oxides such as manganese dioxide have long been known and used as natural pigments. Black manganese oxide pigments, for example, were found in the 17,000 year old cave paintings in the Ekain and Lascaux caves . In the glass manufacturing manganese compounds are from the fourth century AD in the Roman Empire used. Manganese has two different functions. If brownstone is used, it colors the glass intensely brown-violet. If, on the other hand, trivalent manganese oxide is added to ferrous glasses, it discolors them by oxidizing the green-colored divalent iron to the pale yellow trivalent iron, which together with the violet of the manganese gives a gray "discolored" appearance.
The first extraction of the element was probably achieved in 1770 by Ignatius Gottfried Kaim (1746–1778), who reduced manganese dioxide with carbon and thereby obtained impure manganese, which he called the brownstone king. However, this discovery has not become very well known. In 1774, Carl Wilhelm Scheele recognized that brownstone must contain an unknown element, in the same year Johan Gottlieb Gahn, at Scheele's suggestion, produced manganese by reducing brownstone with carbon. Manganesium was initially chosen as the name after the Latin name for brownstone manganesia nigra , but after the discovery of magnesium it was abbreviated to manganese (ium) due to possible confusion . Braunstein was named magnes feminei sexus (since brownstone is not magnetic) by Pliny because of its similarity to magnetic iron (or magnes masculini sexus ) , which became manganesia in the Middle Ages .
In 1839 it was recognized that manganese improved the malleability of iron . When in 1856 Robert Forester Mushet (1811-1891) showed that the addition of manganese would allow the mass production of steel in the Bessemer process , manganese was used in large quantities for steel production in a short time . Braunstein also gained technical importance from 1866 when Walter Weldon developed the Weldon process for producing chlorine , in which hydrochloric acid is oxidized to chlorine with the help of manganese dioxide.
Occurrence
Manganese is a common element on earth , in the continental crust it occurs with a content of 0.095% as frequently as phosphorus or fluorine . It is the third most common transition metal after iron and titanium . It does not occur elementarily, but always in connections. In addition to manganese silicates and manganese carbonate, it is mainly bound in oxides. Common minerals are the mineral group of the brown stones , manganite , hausmannite , braunite , rhodochrosite and rhodonite . The manganese occurs in different oxidation states as bivalent, trivalent and tetravalent manganese, which sometimes also occur in the same mineral as in hausmannite.
While many compounds of divalent manganese are easily soluble in water, compounds in higher oxidation states are usually sparingly soluble and also physically and chemically stable. This is why manganese ores are mainly formed under oxidative conditions. Although iron behaves in a similar way to manganese and also oxidizes from easily soluble bivalent to poorly soluble trivalent iron under oxidative conditions, there are only a few iron-manganese mixed ores. This is due to the fact that manganese requires much higher oxygen concentrations for oxidation than iron.
Minable manganese ores can be geologically divided into three groups. The first type is rhodochrosite - braunite ores, which are trapped in Precambrian volcanic rocks. These ores are mainly found around the southern Atlantic , for example in Brazil , Guyana , the Ivory Coast , Ghana , Burkina Faso or in the Congo . Ores of the second type are found in heavily oxidized, iron and silicate-rich sedimentary rocks from the Proterozoic . The deposits of this type at Hotazel in South Africa and Corumbá in Brazil are among the largest manganese deposits in the world. The third type includes manganese shale ores that are formed by sedimentation in shallow shelf seas . This type includes occurrences in Gabon , Ukraine and other countries around the Black Sea .
Around 75% of the known manganese resources are in the Kalahari of South Africa. There are also larger manganese deposits in Ukraine, Brazil, Australia , India , Gabon and China . The largest manganese producing countries are South Africa, Australia, Gabon, China and Brazil, with total world production in 2018 at 18 million tons.
Manganese occurs in larger quantities in so-called manganese nodules , nodular, up to 20 centimeters large, porous concretions of heavy metal oxides in the deep sea , which can consist of up to 50% manganese. Particularly high concentrations of manganese nodules are found in the Pacific south of Hawaii and in the Indian Ocean . The mining of manganese nodules, especially for the extraction of copper, cobalt and nickel, has been intensively investigated at times, but has so far failed due to high technical requirements and high mining costs with comparatively low prices for metals mined on land.
Extraction and presentation
Minable manganese ores contain at least 35% manganese. Depending on the content and other elements contained, the ores are preferred for various applications. Manganese ore used for metallurgical purposes contains between 38 and 55% manganese and is mined underground using opencast or chamber mining methods. There is also battery-grade ore, which contains at least 44% manganese and may only contain a small amount of copper , nickel and cobalt , so that it is suitable for the production of alkaline-manganese batteries , as well as chemical-grade ore, which is used for the production of pure manganese and manganese compounds.
Pure manganese is not required for most of the applications. Instead, ferromanganese , an iron-manganese alloy with 78% manganese, is extracted. This is produced by reducing oxidic manganese and iron ores with coke in an electric furnace. Another alloy, which is produced in this way, the manganese-iron-silicon alloy silicomanganese . Here, quartz is also introduced into the furnace as a silicon source.
Pure manganese cannot technically be obtained by reduction with carbon, since in addition to manganese, stable carbides , in particular Mn 7 C 3 , are also formed. Pure manganese is only produced at temperatures above 1600 ° C, but at this temperature some of the manganese evaporates, so that this route is not economical. Instead, manganese is obtained through hydrometallurgy . Here manganese ore is oxidized, leached and subjected to electrolysis . For the latter, a manganese sulfate solution that is as pure as possible is used, which is electrolyzed with stainless steel electrodes at 5–7 V. Pure manganese is produced at the cathode and oxygen at the anode , which then reacts with manganese ions to form manganese dioxide.
Smaller amounts of sulfur dioxide or selenium dioxide are added to the electrolyte to reduce energy consumption.
In addition, manganese can also be extracted by reducing manganese oxides with aluminum ( aluminothermics ) or silicon.
| rank | country | advancement | Reserves |
|---|---|---|---|
| 1 | South Africa | 5500 | 230,000 |
| 2 | Australia | 3100 | 99,000 |
| 3 | Gabon | 2300 | 65,000 |
| 4th | People's Republic of China | 1800 | 54,000 |
| 5 | Brazil | 1200 | 110,000 |
| 6th | Ghana | 850 | 13,000 |
| 7th | India | 770 | 33,000 |
| 8th | Ukraine | 740 | 140,000 |
| 9 | Malaysia | 510 | k. A. |
| 10 | Mexico | 220 | 5000 |
properties
Physical Properties
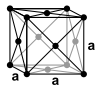
Manganese is a silver-white, hard, very brittle heavy metal . It melts at 1246 ° C and boils at 2100 ° C. In contrast to most other metals, manganese does not crystallize in the closest packing of spheres or in the body-centered cubic crystal structure at room temperature, but in the unusual α-manganese structure. A total of four different modifications are known that are stable at different temperatures. Manganese is paramagnetic at room temperature , the α-modification becomes antiferromagnetic below a Néel temperature of 100 K , while β-manganese shows no such behavior.
The α-manganese structure is thermodynamically stable up to a temperature of 727 ° C. It is a distorted, cubic structure with 58 atoms in the unit cell . The manganese atoms of the structure can be divided into four groups with different environments and coordination numbers between 12 and 16. Above 727 ° C to 1095 ° C, another unusual structure, the cubic β-manganese structure, with 20 formula units per unit cell and coordination numbers of 12 and 14 for the manganese atoms, is thermodynamically more favorable. Only above 1095 ° C does the metal crystallize in a tight packing of spheres, the face-centered cubic crystal structure (γ-manganese, copper type). At 1133 ° C, this finally changes into a body-centered cubic structure (δ-manganese, tungsten type).
Chemical properties
| Oxidation states of manganese | |
|---|---|
| 0 | Mn 2 (CO) 10 |
| +1 | MnC 5 H 4 CH 3 (CO) 3 |
| +2 | MnCl 2 , MnO , MnCO 3 , manganese (II) acetate |
| +3 | MnF 3 , Mn 2 O 3 , manganese (III) acetate |
| +4 | MnO 2 |
| +5 | K 3 MnO 4 |
| +6 | K 2 MnO 4 |
| +7 | KMnO 4 , Mn 2 O 7 |
As a base metal, manganese reacts with many non-metals . Compact manganese reacts slowly and superficially with oxygen , whereas finely divided manganese is pyrophoric in the air and reacts quickly to manganese (II, III) oxide . Manganese also reacts with fluorine , chlorine , boron , carbon , silicon , phosphorus , arsenic and sulfur , whereby the reactions take place only slowly at room temperature and are only faster at higher temperatures. The element only reacts with nitrogen at temperatures above 1200 ° C to form manganese nitride Mn 3 N 2 ; it does not react with hydrogen .
Like other base elements, manganese dissolves in dilute acids with the evolution of hydrogen ; in contrast to chromium , it is not passivated by a dense oxide layer . This reaction takes place slowly in water too. If it is dissolved in concentrated sulfuric acid, sulfur dioxide is formed. In aqueous solution, Mn 2+ ions, which are colored pink in the complex [Mn (H 2 O) 6 ] 2+ , are particularly stable to oxidation or reduction. This is due to the formation of an energetically favored half-filled d-shell (d5). Manganese ions in other oxidation states also have characteristic colors, so trivalent manganese ions are red, tetravalent brown, pentavalent ( hypomanganate , MnO 4 3− ) blue, hexavalent ( manganate , MnO 4 2− ) green and seven valued ( permanganate , MnO 4 - ) violet.
Isotopes
A total of 28 isotopes and eight other core isomers of manganese between 44 Mn and 72 Mn are known. Only one of these, 55 Mn, is stable, so manganese is a pure element . Furthermore, 53 Mn has a long half-life of 3.74 million years . All other isotopes have short half-lives, of which 54 Mn with 312.3 days are the longest.
The longest-lived radioactive manganese isotope 53 Mn occurs in traces in nature. It forms through spallation reactions in ferrous rocks. 54 Fe reacts with 3 He from cosmic rays and the short-lived 53 Fe is formed, which decays to 53 Mn.
use
Pure manganese is only used to a very limited extent. 90% of the manganese extracted is used as ferromanganese , mirror iron or silicon manganese in the steel industry. Since manganese forms very stable manganese-oxygen compounds, it has a deoxidizing effect like aluminum and silicon and intensifies the effect of these elements. It also prevents the formation of the easily melting iron sulphide and thus has a desulphurising effect. At the same time, the solubility of nitrogen in steel is increased, which promotes austenite formation. This is important for many stainless steels. Another important property of manganese in steel is that it increases the hardenability of the steel.
Also in alloys with non-ferrous metals , in particular copper alloys and aluminum-manganese alloys , manganese is used. It increases the strength , corrosion resistance and ductility of the metal. The alloy Manganin (83% copper, 12% manganese and 5% nickel) has, similar to constantan or - even better - Isaohm , a low electrical temperature coefficient , i. H. the electrical resistance is only slightly dependent on the temperature. These materials are therefore widely used in electrical measuring devices .
Manganese is also used as an activator in phosphors . According to current knowledge, the wavelength of the emitted light is between 450 and 750 nm (Mn 2+ ) or 620 and 730 nm (Mn 4+ ), depending on the oxidation level . BaMgAl 10 O 17 : Eu 2+ , Mn 2+ (green emitter) and Mg 14 Ge 5 O 24 : Mn 4+ (red emitter) are of particular practical importance as phosphors in white LEDs .
YInMn blue is a mixed oxide of yttrium, indium and manganese oxides, which shows a very pure and brilliant blue.
Pure manganese is produced in the order of about 140,000 tons per year. It is used to a large extent for the production of special steels and aluminum alloys. Zinc-manganese ferrites for electronic components are also produced from it.
Biological importance
Manganese is an essential element for all living things and a component of various enzymes . There it acts in various ways, including as a Lewis acid , to form the enzyme structure and in redox reactions . In some bacteria it is also used to generate energy. Shewanella putrefaciens , a bacterium found in the sea , operates anaerobic respiration with Mn 4+ as the terminal electron acceptor, which is reduced to Mn 2+ in the process.
Manganese plays an essential role in photosynthesis , namely in the oxidation of water to oxygen in photosystem II . The central component of the photosensor system is a complex of four manganese atoms and one atom of calcium , which are connected together by oxygen bridges, the oxygen-producing complex ( oxygen-evolving complex , OEC). Here, in a multi-stage cycle, the Kok cycle, in which the manganese changes between the trivalent and tetravalent oxidation state, water is split by sunlight and oxygen, electrons and protons are released.
In manganese-containing superoxide dismutases , which are found in mitochondria and peroxisomes , the reaction of superoxide to form oxygen and hydrogen peroxide is catalyzed by redox reactions with divalent and trivalent manganese ions.
Dioxygenases , through which molecular oxygen is incorporated into special organic molecules, usually contain iron, but several manganese-containing dioxygenases are known from the bacteria Arthrobacter globoformis and Bacillus brevis , among others . Manganese peroxidase , an enzyme discovered in the fungus Phanerochaete chrysosporium , is one of the few known enzymes that break down lignin . Manganese is also involved in the reaction of arginases , hydrolases , kinases , decarboxylases and transferases such as pyruvate carboxylase , mevalonate kinase and glycosyl transferase , as well as certain ribonucleotide reductases and catalases .
Manganese is absorbed by humans through the small intestine and is mainly stored in the liver , bones , kidneys and the pancreas . Inside the cell, the element is mainly found in mitochondria , lysosomes and in the cell nucleus . In the brain, manganese is bound to special proteins, mainly to the glutamate ammonium ligase in astrocytes . The total amount of manganese in the human body is around 10 to 40 mg, the daily requirement is around 1 mg and the average manganese intake in Germany is around 2.5 mg.
Manganese deficiency is rare; in animals with a low manganese diet, skeletal changes, neurological disorders , defects in the carbohydrate metabolism as well as growth and fertility disorders occurred. Foods particularly rich in manganese are black tea , wheat germ , hazelnuts , oat flakes , soybeans , flax seeds , blueberries , aronia berries and whole-grain rye bread .
Safety and toxicity
Like many other metals, manganese is flammable in a finely divided state and reacts with water. Therefore only metal fire extinguishers (class D) or sand can be used to extinguish . Compact manganese, on the other hand, is not flammable.
If dust containing manganese is inhaled in high doses, it has a toxic effect. This initially results in damage to the lungs with symptoms such as cough, bronchitis and pneumonitis . Manganese also has a neurotoxic effect and damages the central nervous system . This manifests itself in manganism , a disease with Parkinson's- like symptoms such as motor disorders. For manganese dusts therefore a MAC value exists of 0.02 mg / m 3 for especially fine dust, which in the pulmonary alveoli can penetrate and 0.2 mg / m 3 for respirable dusts.
Diseases caused by manganese or its compounds are included in Germany as No. 1105 in the list of occupational diseases . Exposure can arise during the extraction, transport, processing and use of manganese or its compounds, provided that these substances are inhaled as dust or smoke. This also applies to electric welding with coated electrodes containing manganese.
proof
The qualitative chemical detection of manganese ions can be provided by the formation of violet permanganate after a reaction with lead (IV) oxide , ammonium peroxodisulfate (with silver ions as a catalyst) or hypobromite in an alkaline solution.
- Reaction of manganese with lead (IV) oxide in acidic solution
The so-called alkaline fall can be used for separation in the context of the cation separation process, in which manganese is oxidized to solid manganese (IV) oxide hydroxide by a mixture of hydrogen peroxide and sodium hydroxide solution and then precipitates.
- Reaction of manganese with hydrogen peroxide and sodium hydroxide solution to form manganese (IV) oxide hydroxide
Further possible detection reactions , which can also be used as a preliminary sample , are the phosphorus salt pearl , which turns violet due to the formation of manganese (III) ions, and the oxidation melt , in which a green melt of manganate (VI) is produced by the reaction with nitrate ions. (MnO 4 2− ), blue manganate (V) (MnO 4 3− ) is also formed with low oxygen supply If an acid is added, purple permanganate forms.
Quantitatively, manganese can be determined by atomic absorption spectroscopy (at 279.5 nm), by photometric determination of permanganate, the absorption maximum being at 525 nm, or by titration . In this case, Mn 2+ ions are titrated with permanganate using the Vollhard-Wolff manganometric method , with the formation of manganese dioxide. The end point can be recognized by the pink coloration caused by remaining permanganate.
By adding formaldoxime reagent to a solution of manganese (II) salts, an orange to red-brown colored metal complex is formed.
links
Manganese compounds in the oxidation states between −3 and +7 are known. The most stable are divalent, trivalent and tetravalent manganese compounds, the lower levels are mainly found in complexes , the higher levels in compounds with oxygen .
Oxygen compounds
With oxygen manganese forming compounds in the oxidation states +2 to +7, and at higher levels +5, +6 and +7 especially anionic manganate and manganese halogen oxides, but also the green, oily, explosive liquid manganese (VII) oxide known are. The seven-valent, violet permanganates (MnO 4 - ) are predominantly of importance , with potassium permanganate in particular being of economic importance. This is used, among other things, as a strong oxidizing agent in organic reactions , detection reactions in the context of manganometry and medicinally as an astringent and disinfectant . The pentavalent blue hypomanganates (MnO 4 3− ) and hexavalent green manganates (MnO 4 2− ) are more unstable and intermediate products in permanganate production. There are also complex permanganates such as hexamanganato (VII) manganese (IV) acid, (H 3 O) 2 [Mn (MnO 4 ) 6 ] · 11H 2 O, a deep purple compound that is only stable at low temperatures . Manganese (IV) oxide is mainly used as a cathode material in alkaline manganese batteries . When the battery is discharged, manganese oxide hydroxide and manganese (II) hydroxide are produced . Furthermore, the divalent manganese (II) oxide , the trivalent manganese (III) oxide and manganese (II, III) oxide are also known.
As manganese hydroxides are manganese (II) hydroxide , manganese (III) oxide hydroxide and manganese (IV) oxide hydroxide known. White manganese (II) hydroxide precipitated from manganese (II) salts with sodium hydroxide solution is, however, unstable and is easily oxidized to manganese (III, IV) oxide hydroxide by atmospheric oxygen. Because of its easy oxidizability, manganese (II) hydroxide is used to fix oxygen in the Winkler method .
Halogen compounds
With the halogens fluorine , chlorine , bromine and iodine , the divalent compounds as well as manganese (III) and manganese (IV) fluoride and manganese (III) chloride are known. Corresponding bromine and iodine compounds do not exist, since Br - and I - ions reduce Mn (III) to Mn (II). Technically important Manganhalogenid is obtained by reaction of manganese (IV) oxide with hydrochloric acid recoverable manganese (II) chloride , inter alia, for the production of dry batteries , corrosion-resistant and hard magnesium alloys as well as the synthesis of the anti-knock agent (methylcyclopentadienyl) manganese tricarbonyl used (MMT) becomes.
More manganese compounds

Manganese does not form a binary compound with hydrogen that is stable at room temperature , only manganese (II) hydride could be represented in an argon matrix at low temperatures .
Many complexes of manganese, mainly in the +2 oxidation state , are known. These are mainly in the form of high-spin complexes with five unpaired electrons and a correspondingly strong magnetic moment . The crystal field and ligand field theory does not predict any preferred geometry here . Accordingly, depending on the ligand tetrahedral , octahedral , square -planare or dodecahedral geometry of Mn 2+ - complexes known. The complexes show (by quantum-mechanically forbidden ) d-d transitions weak staining, with octahedral Mn 2+ - complexes usually slightly pink, tetrahedral yellow-green colored. With very strong ligands such as cyanide, there are also low-spin complexes with only one unpaired electron and a strong ligand field splitting . The complexes in lower oxidation states include Dimangandecacarbonyl Mn 2 (CO) 10 with the oxidation state 0 of manganese and a manganese-manganese single bond. Other similar complexes such as Mn (NO) 3 CO with the lowest known oxidation state −3 in manganese are known.
Mangafodipir is a liver-specific paramagnetic contrast agent that is approved for magnetic resonance imaging (MRI) . The contrast enhancing effect is based on the paramagnetic properties of Mn 2+ - ion represented by the five unpaired electrons are caused. The toxic effect of the Mn 2+ ions is suppressed in Mangafodipir by complexing with the ligand dipyridoxyl diphosphate (DPDP or Fodipir). It is superior to standard MRI contrast media based on gadolinium for imaging the liver .
The metallocene in manganese is manganocene . This, in comparison with ferrocene one electron less and thus contrary to the 18-electron rule only 17 electrons. Nevertheless, it may because of the favorable high-spin d 5 - Configuration not to Mn + reduced be and is in solid state in a polymer prior structure.
The category: Manganese compounds provides an overview of manganese compounds .
literature
- David B. Wellbeloved, Peter M. Craven, John W. Waudby: Manganese and Manganese Alloys. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2005 ( doi: 10.1002 / 14356007.a16_077 ).
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1607-1620.
- Heinz Cassebaum : The position of the brown stone investigations by JH Pott (1692–1777) in the history of manganese. In: Sudhoff's archive. 63, 1979, No. 2, pp. 136-153.
Web links
- Entry to manganese. In: Römpp Online . Georg Thieme Verlag, accessed on March 29, 2011.
- Mineral Atlas: Manganese (Wiki)
Individual evidence
- ↑ a b Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (manganese) unless otherwise stated .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e entry on manganese in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e entry on manganese at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1339.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b c Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics . Volume 6: Solids. 2nd Edition. Walter de Gruyter, 2005, ISBN 3-11-017485-5 , p. 361.
- ↑ a b c d e f Entry on manganese, powder in the GESTIS substance database of the IFA , accessed on April 30, 2017(JavaScript required) .
- ↑ Schweizerische Unfallversicherungsanstalt (Suva): Limit values - current MAK and BAT values (search for 7439-96-5 or manganese and its inorganic compounds ), accessed on November 2, 2015.
- ↑ Emilie Chalmin, Michel Menu, Colette Vignaud: Analysis of rock art painting and technology of Palaeolithic painters. In: Measurement Science and Technology . 14, 2003, pp. 1590-1597, doi: 10.1088 / 0957-0233 / 14/9/310 .
- ↑ E. Chalmin, C. Vignaud, H. Salomon, F. Farges, J. Susini, M. Menu: Minerals discovered in paleolithic black pigments by transmission electron microscopy and micro-X-ray absorption near-edge structure. In: Applied Physics A . 83, 2006, pp. 213-218, doi: 10.1007 / s00339-006-3510-7 .
- ^ EV Sayre, RW Smith: Compositional Categories of Ancient Glass. In: Science . 133, 1961, pp. 1824-1826, doi: 10.1126 / science.133.3467.1824 .
- ↑ W. Patrick McCray: Glass Making in renaissance Italy: The innovation of venetian cristallo. In: JOM - Journal of the Minerals, Metals and Materials Society . 50, 1998, pp. 14-19, doi: 10.1007 / s11837-998-0024-0 .
- ^ E. Rancke-Madsen: The Discovery of an Element. In: Centaurus . 19, 1975, pp. 299-313, doi: 10.1111 / j.1600-0498.1975.tb00329.x .
- ^ Justus von Liebig, Johann C. Poggendorff, Friedrich Wöhler, Hermann Kolbe: Concise dictionary of pure and applied chemistry. Volume 5, 1851, pp. 594-595 ( limited preview in Google book search)
- ↑ a b c L. A. Corathers, JF Machamer: Manganese. In: Society for Mining, Metallurgy and Exploration (US): Industrial minerals & rocks: commodities, markets, and uses. 7th edition. SME, 2006, ISBN 0-87335-233-5 , pp. 631–636 ( limited preview in Google book search).
- ^ William H. Brock: Viewegs Geschichte der Chemie . Vieweg, Braunschweig 1997, ISBN 3-540-67033-5 , p. 182.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 85th edition. CRC Press, Boca Raton, Florida, 2005. Section 14, Geophysics, Astronomy, and Acoustics; Abundance of Elements in the Earth's Crust and in the Sea.
- ↑ https://prd-wret.s3-us-west-2.amazonaws.com/assets/palladium/production/atoms/files/mcs-2019-manga.pdf
- ↑ a b c d e f g David B. Wellbeloved, Peter M. Craven, John W. Waudby: Manganese and Manganese Alloys. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2005 ( doi: 10.1002 / 14356007.a16_077 ).
- ^ R. Elliott, K. Coley, S. Mostaghel, M. Barati: Review of Manganese Processing for Production of TRIP / TWIP Steels, Part 1: Current Practice and Processing Fundamentals. In: JOM. 70, 2018, p. 680, doi : 10.1007 / s11837-018-2769-4 .
- ↑ Selenium and Tellurium. ( Memento of November 13, 2011 in the Internet Archive ) (PDF; 51 kB) In: 2010 Minerals Yearbook. Retrieved May 29, 2013.
- ↑ United States Geological Survey: World Mine Production and Reserves
- ↑ JS Kasper, BW Roberts: Antiferromagnetic Structure of α-Manganese and a Magnetic Structure Study of β-Manganese. In: Physical Review . 101, 1956, pp. 537-544, doi: 10.1103 / PhysRev.101.537 .
- ↑ a b K. Schubert: A model for the crystal structures of the chemical elements. In: Acta Crystallographica . 1974, B30, pp. 193-204, doi: 10.1107 / S0567740874002469 .
- ^ A b J. A. Oberteuffer, JA Ibers: A refinement of the atomic and thermal parameters of α-manganese from a single crystal. In: Acta Crystallographica . 1970, B26, pp. 1499-1504, doi: 10.1107 / S0567740870004399 .
- ↑ a b C. B. Shoemaker, DP Shoemaker, TE Hopkins, S. Yindepit: Refinement of the structure of β-manganese and of a related phase in the Mn-Ni-Si system. In: Acta Crystallographica . B34, 1978, pp. 3573-3576, doi: 10.1107 / S0567740878011620 .
- ↑ a b R. GW Wykhoff: Crystal structures. 1963, 1, pp. 7-83.
- ^ Max Schmidt: VII. Subgroup . In: Inorganic Chemistry II. . Wissenschaftsverlag, 1968, pp. 100-109.
- ↑ a b c d e entry on manganese. In: Römpp Online . Georg Thieme Verlag, accessed on March 29, 2011.
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1608-1609.
- ↑ G. Audi, FG Kondev, Meng Wang, WJ Huang, S. Naimi: The NUBASE2016 evaluation of nuclear properties. In: Chinese Physics C. 41, 2017, S. 030001, doi : 10.1088 / 1674-1137 / 41/3/030001 ( full text ).
- ↑ J. Schaefer, T. Faestermann, G. Herzog, K. Knie, G. Korschinek, J. Masarik, A. Meier, M. Poutivtsev, G. Rugel, C. Schlüchter: Terrestrial manganese-53 - A new monitor of Earth surface processes. In: Earth and Planetary Science Letters . 251, 2006, pp. 334-345, doi: 10.1016 / j.epsl.2006.09.016 .
- ↑ Shigeo Shionoya, William M. Yen, Hajime Yamamoto (Eds.): Phosphor Handbook. 2nd Edition. CRC Press, Boca Raton, FL 2006, ISBN 0-8493-3564-7 , pp. 153ff.
- ↑ Michael T. Madigan, John M. Martinko, Thomas Lazar (translator) and Freya Thomm-Reitz (translator): Brock Mikrobiologie . 11th, updated edition. Pearson Studium, 2009, ISBN 978-3-8273-7358-8 , p. 644.
- ↑ J. Yano et al. a .: Where Water Is Oxidized to Dioxygen: Structure of the Photosynthetic Mn 4 Ca Cluster. In: Science . 314, 2006, pp. 821-825, doi: 10.1126 / science.1128186 .
- ^ RG Alscher: Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. In: Journal of Experimental Botany . 53, 2002, pp. 1331-1341, doi: 10.1093 / jexbot / 53.372.1331 .
- ^ A b Neil A. Law, M. Tyler Caudle, Vincent L. Pecoraro: Manganese Redox Enzymes and Model Systems: Properties, Structures, and Reactivity. In: Advances in Inorganic Chemistry . 46, 1998, pp. 305-440, doi: 10.1016 / S0898-8838 (08) 60152-X .
- ^ A. Takeda: Manganese action in brain function. In: Brain Research Reviews . 41, 2003, pp. 79-87, doi: 10.1016 / S0165-0173 (02) 00234-5 .
- ↑ Cem Ekmekcioglu, Wolfgang Marktl: Essential trace elements: Clinic and nutritional medicine. Springer, 2006, ISBN 3-211-20859-3 , p. 148 ( limited preview in the Google book search).
- ↑ Annette Santa Maria, Sandra Sulsky: Risk Assessment of an Essential Element: Manganese. In: Journal of Toxicology and Environmental Health, Part A . 73, 2010, pp. 128-155, doi: 10.1080 / 15287390903337118 .
- ↑ Leaflet on BK No. 1105 Announcement of the BMA v. May 19, 1964, BArbBl Specialist Part Occupational Safety and Health 1964, 128f.
- ^ J. Strähle, E. Schweda: Jander · Blasius - Introduction to the inorganic-chemical practical course. 14th edition. S. Hirzel Verlag, Stuttgart 1995, ISBN 3-7776-0672-3 , pp. 186-192.
- ^ J. Strähle, E. Schweda: Jander · Blasius - Introduction to the inorganic-chemical practical course. 14th edition. S. Hirzel Verlag, Stuttgart 1995, ISBN 3-7776-0672-3 , p. 460.
- ^ J. Strähle, E. Schweda: Jander · Blasius - Introduction to the inorganic-chemical practical course. 14th edition. S. Hirzel Verlag, Stuttgart 1995, ISBN 3-7776-0672-3 , pp. 378-379.
- ^ OG Koch: Analytical Chemistry of Manganese. Springer-Verlag, 2013, ISBN 978-3-642-69853-8 , pp. 95ff.
- ↑ Bernt Krebs, Klaus-Dieter Hasse: Hexamanganato (VII) manganese (IV) acid: a "pseudopermanganic acid" . In: Angewandte Chemie . tape 86 , no. 17 , 1974, p. 647-648 , doi : 10.1002 / anie.19740861708 .
- ↑ Heinrich Remy : Textbook of Inorganic Chemistry. Volume II, Akademische Verlagsgesellschaft Geest & Portig, Leipzig 1961, pp. 255-258.
- ↑ Erwin Riedel, Christoph Janiak: Inorganic Chemistry . Walter de Gruyter, 2011, ISBN 978-3-11-022566-2 , p. 831 ( limited preview in Google Book search).
- ↑ Arno H. Reidies: Manganese compounds. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2005 ( doi: 10.1002 / 14356007.a16_123 ).
- ^ MF Bellin: MR contrast agents, the old and the new. In: European Journal of Radiology Volume 60, Number 3, December 2006, pp. 314-323, doi: 10.1016 / j.ejrad.2006.06.021 . PMID 17005349 .
- ↑ Christoph Elschenbroich: Organometallchemie. 6th edition. Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , pp. 460-468.