copper
| properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Copper, Cu, 29 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Transition metals | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 11 , 4 , d | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | salmon pink, metallic | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-50-8 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-159-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.326 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.01% | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 63,546 (3) u | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 135 (145) pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 132 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 140 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Ar ] 3 d 10 4 s 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 7th.726 380 (4) eV ≈ 745.48 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 20th.29239 (6) eV ≈ 1 957.92 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 36.841 (12) eV ≈ 3 554.6 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 57.38 (5) eV ≈ 5 536 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 79.8 (7) eV ≈ 7 700 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | Cubic area-centered | |||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 8.92 g / cm³ (20 ° C ) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 3.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | diamagnetic ( Χ m = −9.6 10 −6 ) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1357.77 K (1084.62 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 2868 K (2595 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 7.11 · 10 −6 m 3 · mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 305 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 13.3 kJ mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 3570 m · s −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 385 J kg −1 K −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Work function | 4.65 eV | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 58.1 · 10 6 A · V −1 · m −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 400 W m −1 K −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanically | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modulus of elasticity | 100… 130 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson's number | 0.34 ... 0.35 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 1, 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | 0.340 V (Cu 2+ + 2 e - → Cu) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.9 ( Pauling scale ) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| MAK |
Switzerland: 0.1 mg m −3 (measured as inhalable dust ) |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||||||||||||||||||||
Copper ( Latin Cuprum ) is a chemical element with the element symbol Cu and the atomic number 29. It is a transition metal , in the periodic table it is in the 4th period and the 1st subgroup (group 11 according to the new count) or copper group . The Latin name cuprum is derived from (aes) cyprium " ore from the Greek island of Cyprus ", where copper was mined in ancient times.
As a relatively soft metal, copper is easily malleable and tough. It is used in many ways as an excellent conductor of heat and electricity . It also belongs to the group of coin metals .
As a weakly reactive heavy metal , copper is a semi-precious metal .
history

Copper, gold , silver and tin were the first metals that mankind got to know in their development. Because copper is easy to work with, it was used by the oldest known cultures around 10,000 years ago. The time of its widespread use from the 5th millennium BC. Until the 3rd millennium BC BC is also called Copper Age depending on the region . In Hujayrat al-Ghuzlan in Jordan there was already around 4,000 BC. A mass production site of copper. In alchemy , copper was associated with Venus / femininity ♀ ( planetary metals ) and viewed as a compound of sulfur and mercury. The first mirrors were made from this metal. During the late East Mediterranean Bronze Age , copper was mined mainly in Cyprus and exported from there in mostly approx. 30 kg heavy copper bars in the form of cattle hides (so-called ox skin bars ). Fragments of Cypriot oxhide bars from the 16th to the 11th centuries BC Chr. Can be found in large parts of the Mediterranean, as far as Sardinia, in the Balkans and even north of the Alps ( deposit found in Oberwilflingen ). The largest pre-industrial copper producer was the Roman Empire with an estimated annual production of 15,000 t.
Later copper was alloyed with tin and lead to form bronze . This harder and technically more resistant alloy gave its name to the Bronze Age . The distinction between lead and tin was only introduced with increasing knowledge of metals, so that from today's perspective the term bronze is only correctly applied to tin-copper alloys with a high copper content.
The golden yellow copper-zinc alloy " brass " was already known in ancient Greece . It was smelted by processing the respective ores together, but it was only the Romans who made increasing use of this process. In ancient Colombia , the gold-copper alloy Tumbaga was widely used.
Copper as a mineral
Natural occurrences of native copper, i.e. in its elemental form, were known long before the International Mineralogical Association (IMA) was founded. Copper is therefore recognized as a so-called grandfathered mineral as an independent type of mineral.
According to the systematics of minerals according to Strunz (9th edition) , copper is classified under system no. "1.AA.05" (elements - metals and intermetallic compounds - copper cupalite family - copper group) or in the outdated 8th edition classified under I / A.01 ( copper series ). The systematics of minerals according to Dana , which is mainly used in English-speaking countries , lists the element mineral under the system no. 01/01/01/03 ( gold group ).
In nature, copper usually forms in basaltic lavas either in the form of “copper-red”, metallic shining nuggets (solidified from the melt) or in branched structures, so-called dendrites . Occasionally, crystalline training can also be found. Copper occurs in paragenesis with various, mostly secondary copper minerals such as bornite , chalcosine , cornwallite , cuprite , azurite and malachite as well as tenorite , but can also be associated with many other minerals such as calcite , clinoclase , prehnite , pumpellyite , quartz and silver .
Pseudomorphism from copper to aragonite
Copper ores are common. Copper is made from chalcopyrite ( copper pyrites , CuFeS 2 ), chalcosine ( copper luster , Cu 2 S), more rarely also from bornite ( colored copper pebbles , Cu 5 FeS 4 ), atacamite (CuCl 2 Cu (OH) 2 ), malachite (Cu 2 [(OH) 2 | CO 3 ]) and other ores. In 2019, 636 copper minerals were known. The minerals with the highest copper concentration in the compound are cuprite (up to 88.8%) and algodonite (up to 83.6%) as well as paramelaconite , tenorite and chalcosine (up to 79.9%).
Occurrence and extraction
According to the German Copper Institute, copper occurs in the earth with a content of around 0.006% and is in 23rd place in terms of the frequency of the elements in the earth's crust . Often copper occurs in solid form , i.e. in elemental form. Worldwide (as of 2017) there are currently over 3000 sites known for solid copper, including in Afghanistan , Argentina , Australia , Belgium , Bolivia , Brazil , Bulgaria , Chile , China , the Democratic Republic of the Congo , Germany , Finland , France , Greece , India , Iran , Ireland , Italy , Japan , Canada , Kazakhstan , Morocco , Mexico , Mongolia , Namibia , New Zealand , Norway , Austria , Peru , the Philippines , Poland , Portugal , Romania , Russia , Zambia , Sweden , Switzerland , Zimbabwe , Slovakia , Spain , South Africa , the Czech Republic , Turkey , Ukraine , Hungary , the United States of America (USA) and the United Kingdom (UK).
Solid copper could also be detected in several rock samples from the Mid-Atlantic Ridge and from the moon , which the probe of the Luna 24 mission brought from the Mare Crisium .
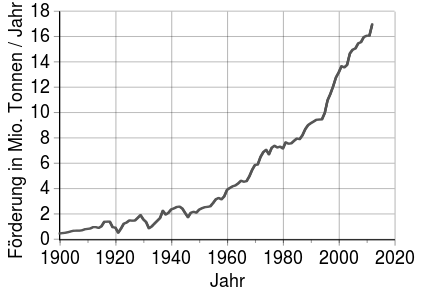
Promotion and reserves
The most important copper producer is Chile, followed a long way by Peru and China. In Europe, Poland , Portugal and Sweden are noteworthy. The most important export countries were organized in the CIPEC from 1967 to 1988 . CIPEC included Chile, Peru and Papua New Guinea , on whose island of Bougainville one of the world's largest copper mines led to a civil war in 1988.
The copper mines on the Keweenaw Peninsula in Lake Superior (USA) were historically significant . There was the world's largest deposit of native copper there. Mining took place there in pre-Columbian times. In Germany, copper shale was mined in the Mansfeld region until 1990 . In Cornwall (England) there was significant copper mining, especially in the 18th and 19th centuries.
| rank | country | advancement | Reserves |
|---|---|---|---|
| 1 | Chile | 5800 | 170,000 |
| 2 | Peru | 2400 | 83,000 |
| 3 | People's Republic of China | 1600 | 26,000 |
| 4th | United States | 1200 | 48,000 |
| 5 | Democratic Republic of Congo | 1200 | 20,000 |
| 6th | Australia | 950 | 88,000 |
| 7th | Zambia | 870 | 19,000 |
| 8th | Indonesia | 780 | 51,000 |
| 9 | Mexico | 760 | 50,000 |
| 10 | Russia | 710 | 61,000 |
Extraction
Raw copper
The most important furnaces for copper extraction are the flame furnace and, since 1980, the flash smelter .
For the production of copper, so-called copper stone (Cu 2 S with varying contents of FeS and a Cu content of approx. 70%) is first extracted from copper pebbles (CuFeS 2 ) . For this purpose, the starting material is roasted with the addition of coke and the iron oxides contained are slagged with silica-containing additives . This iron silicate slag floats on the copper stone and can therefore be easily poured off.
- Roasting work:
- Melting work:
The copper stone obtained in this way is processed into raw copper (also known as black copper ). To do this, it is poured into a converter and air is blown into this melt. In a first stage (slag bubbles) the iron sulfide contained therein is roasted to iron oxide and this is bound by slagged quartz to form slag, which can be poured off. In a second step (cooking bubbles) , two thirds of the remaining Cu 2 S is oxidized to Cu 2 O. The oxide then reacts with the remaining sulphide to form raw copper.
- Slag bubbles:
- Cooking bubbles:
The raw copper has a copper content of 98%. In addition to base metals such as iron and zinc, the remaining 2% also contains precious metals such as silver and gold.
Refining
The electrolytic refining of copper is carried out in a copper (II) sulfate solution containing sulfuric acid with a raw copper anode and a pure copper cathode . During electrolysis, all metals that are less noble than copper are oxidized and go into solution as cations , while the more noble metals sink as anode sludge .
Reaction equation of electrolytic refining:
- anode
- cathode
While the anode slowly dissolves with the formation of cations, only copper, the electrolytic copper, with a mass fraction of w (Cu) = 99.99% , is deposited on the cathode due to the reduction of copper ions .
The anode sludge that is created as a by-product is later recycled and serves as the starting material for the extraction of the precious metals.
Copper is extracted in refineries . In Europe, Aurubis AG (formerly Norddeutsche Affinerie) with its headquarters in Hamburg is known for this, and it used to be the Duisburger Kupferhütte (today DK Recycling).
Copper can also be obtained as so-called cement copper by precipitation from copper sulphate solution with iron . The process of precipitation is called cementation . The copper obtained is often contaminated. The precipitation of copper on iron from naturally occurring metal salt solutions has been practiced in China since 1086 AD.
Copper can also be represented by an aluminothermic reaction . A mixture of copper (II) oxide and aluminum powder is used as thermite . By using a superplasticizer (e.g. calcium fluoride ), the yield can be increased because the elemental metals, in contrast to the slag produced, cannot dissolve in the superplasticizer. The aluminothermic extraction is not economical because of the aluminum required for it.
properties
Physical Properties

With a density of 8920 kg / m³, copper belongs to the heavy metals , which crystallizes in a face-centered cubic manner and thus has a cubic closest packing of spheres with the space group Fm 3 m (space group no. 225) . The lattice parameter for pure copper is 0.3615 nm (corresponds to 3.615 Å ) with 4 formula units per unit cell .
Copper is a very good conductor of heat . Its melting point is 1083.4 ° C. Copper is also a very good electrical conductor with an electrical conductivity of 58 · 10 6 S / m. Its conductivity is only slightly worse than silver and significantly better than gold . Since all admixtures dissolved in copper, especially impurities such as phosphorus and iron, greatly reduce the conductivity, the highest levels of purity are often sought for conductor materials .
The Mohs hardness of copper is 2.5 to 3, which corresponds to a Vickers hardness (VHN) of 77-99 with a test force of 100 g. By cold forming which is strength of 150 to 200 MPa (as cast state) is increased to values of about 450 MPa. The elongation at break is 4.5% with hardness values around 100 HB . Deformed and then soft-annealed copper with a strength of 200 to 240 MPa has an elongation at break greater than 38% and hardness values around 50 HB.
The softness of copper partly explains its high electrical conductivity and high thermal conductivity , which is the second highest among pure metals at room temperature after silver . This is because the resistivity for electron transport in metals at room temperature is primarily based on the scattering of electrons during thermal vibrations of the lattice , which are relatively weak in a soft metal .
Forging is very possible at temperatures of 700 to 800 ° C. Cold deformations can easily be carried out without intermediate annealing.
As a bare metal , copper has a light red color, the line color is pink-red. The red color is due to the fact that it absorbs the complementary green and blue light a little more at normal temperature . It tarnishes when exposed to air and initially turns red-brown. With further weathering and corrosion , the smooth surface is very slowly (often over centuries) lost and the color changes from red-brown to blue-green due to the formation of a patina .
Copper is one of the few metallic elements with a natural color other than gray or silver. Pure copper is orange-red and turns reddish in air . The characteristic color of copper results from the electronic transitions between the filled 3d and the half empty 4s atomic shell - the energy difference between these shells corresponds to orange light .
As with other metals , galvanic corrosion occurs when copper is brought into contact with another metal .
Chemical properties
| Oxidation states of copper | |
|---|---|
| +1 | CuCl , Cu 2 O , CuH , Cu 2 C 2 |
| +2 | CuCl 2 , CuO , CuSO 4 , copper (II) acetate |
| +3 | KCuO 2 , K 3 CuF 6 |
| +4 | Cs 2 CuF 6 |
Copper occurs in the oxidation states 0, +1, +2, +3 and +4, +1 and +2 are the most common, with +2 being the most stable oxidation state in aqueous solutions ; Level +4 is extremely rare (for example in Cs 2 CuF 6 ). Copper (II) salts (e.g. copper sulfate ) are usually blue or green in color. In chemical terms, copper has in some cases similar properties to the elements silver and gold, which are in the same group . A layer of metallic copper is deposited on an iron nail that is dipped in a solution of copper sulphate , for which iron goes into solution as iron sulphate because iron is less noble than copper (see also voltage series ). Copper is normally not attacked by hydrochloric acid , but is strongly attacked in the presence of oxygen ; it is dissolved by hot sulfuric acid . It also dissolves in nitric acid and aqua regia . A mixture of hydrochloric acid or sulfuric acid with hydrogen peroxide dissolves copper very quickly. The metal is also attacked by organic acids . Against alkalis it is stable. In red heat it reacts with oxygen and forms a thick layer of copper oxide . Copper is passivated by fluorine and its compounds . Depending on the grain size , copper powder is ignitable or combustible . The metal in compact form is not combustible and, after forming a thin oxide layer of air and water not attacked, so it is against clean air and water resistant .
In liquid copper dissolve oxygen and hydrogen , which in the solidification of the melt to water vapor be able to implement and thus the cause of gas porosity in the casting form.
Cracks and cavities can occur in oxygen-containing copper grades on contact with hydrogen-containing gases , which leads to what is known as hydrogen embrittlement in copper .
Biological properties
Compared to many other heavy metals, copper is only relatively weakly toxic to higher organisms. A person can consume 0.04 grams of copper per day without harming their health. In a free form that is not bound to proteins , copper has an antibacterial effect; here, as with silver, one speaks of the oligodynamic effect , which is why z. B. also flower water, which is kept in copper vessels or in which a copper coin is placed, does not rot so quickly.
Bactericidal properties
Copper is toxic to many microorganisms even in low concentrations. Therefore (but also because it is easy to lay) water end lines often contain copper. Due to the bactericidal properties of copper, large-scale trials are being carried out to determine whether it makes economic sense to equip hospital rooms with copper-coated door handles. A clinical study from 2008/2009 shows that in the Asklepios Clinic Wandsbek , Hamburg, after replacing 50 door handles / plates and light switches, the MRSA germs were reduced to 63%. A study from Chile found a reduction in the number of germs on objects made of copper alloys by up to 92% at a humidity of 7.2 to 19.7%. A multi-center study from 2010/2011 from the USA shows that the infection rate in "copper rooms" drops by almost 60%, on the copper objects themselves by over 80%. In 2013 the Clinic for Pediatric and Adolescent Medicine in the Niederberg Clinic in North Rhine-Westphalia converted to copper alloys.
The toxic effect arises from the fact that copper ions bind to thiol groups of proteins and peroxidize lipids in the cell membrane , which leads to the formation of free radicals which damage the DNA and cell membranes. In humans, for example, in the case of Wilson's disease (copper storage disease), this leads to organ damage with a high copper excess.
Copper alloys with a copper content of at least 60% also show a toxic effect against noroviruses .
Effect against snails
The copper in the copper wire or copper foil, which acts as a barrier to endangered plants, is oxidized by the slime. This creates an irritant substance that prevents the snail from crawling any further.
Biological copper demand
In most multicellular cells, copper is a component of many enzymes ( metalloenzymes ) and is therefore a vital trace element . Copper is a component of blue hemocyanin , which is used as a blood pigment in molluscs and arthropods to transport oxygen.
The daily requirement of an adult person is 1.0–1.5 milligrams. In the human body, copper is mainly stored in the liver.
Copper is found mainly in chocolate, liver, grains, vegetables and nuts. Copper deficiency rarely occurs in humans. A deficiency is mainly possible with long-lasting diarrhea, precocious children, after long-lasting malnutrition or malabsorption due to diseases such as B. sprue , Crohn's disease or cystic fibrosis . Ingesting high doses of zinc , iron, or molybdate can also lead to decreased levels of copper in the body. The Menkes disease is a rare congenital disorder of copper metabolism.
Excess copper and poisoning
Excess copper is released into the digestive system with the bile for excretion.
Copper sulfate (copper vitriol) is a strong emetic and has therefore been used to treat many poisonings , for example from white phosphorus , which in this special case also has the advantage that the phosphorus is bound as poorly soluble copper phosphide at the same time .
In the rare hereditary disease Wilson's disease , the excretion of copper is impaired and there is an increased accumulation of copper, first in the liver, then, when this excretes the copper into the bloodstream, also in other organs. Another equally rare disease of the copper metabolism is Menkes syndrome . The copper can be absorbed by the cells, but then no longer transported in an orderly manner, so that some organs have an increased copper content, while others have a reduced copper content.
Copper and Alzheimer's disease
The connection between copper and the development of Alzheimer's disease has been discussed again and again . As early as 2003, researchers suspected that copper slows down the production of amyloid A and that a lack of copper promotes Alzheimer's disease. A subsequent pilot study with 70 Alzheimer's patients, however, could not show any protective effect from an increased copper intake, even if there was a stabilization in the decrease in Abeta42 in the CSF , a disease marker of Alzheimer's disease.
Other studies showed that copper could be harmful to the brain . A study with the ionophore PBT2 as an active ingredient against Alzheimer's disease showed good results in a phase II study. The active ingredient binds not only zinc , but also copper and thus reduces the concentration of copper in the brain.
A new study shows that copper is deposited in the brain capillaries with long-term high intake and can damage the blood-brain barrier there . This hinders the removal of beta-amyloid , and the accumulation of the substance then causes Alzheimer's disease .
use


Copper is used pure or as an alloy in electrical installations , for pipelines (heating, water, gases), for precision parts, coins , cutlery , works of art, musical instruments and much more.
If it is used in contact with other metals, it leads to contact corrosion when exposed to moisture .
After silver, copper has the second highest electrical conductivity of all substances, ahead of gold. a. used for:
- electrical lines , jumper wires and power cables with small cross sections, overhead lines
- Conductor tracks on circuit boards and partly in integrated circuits
- Electrical machines: wire windings in transformers , chokes / coils and electric motors
- Components: anode bodies of magnetrons , clamps , component connection legs, contact carriers, compression sleeves
It is true that aluminum is cheaper and, in terms of mass per length, a better electrical conductor than copper. But it is more voluminous. I.a. Because of this and also because copper can be contacted better and it has a higher flexural fatigue strength, it is usually preferred as a conductor over aluminum, except when weight or price is important.
Wires and strands made of so-called Oxygen Free Copper ( OFC , English for oxygen-free copper with a purity of> 99.99%) have a very fine-grained crystal structure and a particularly high fatigue strength. They are used for cables and wires that are subject to high mechanical stress.
Alloys of copper and magnesium are used for overhead lines. A compromise has to be found between increasing tensile strength and decreasing conductivity.
Copper has a high reflectivity in the infrared range and is therefore used as a mirror for carbon dioxide laser beams and for coating glass ( insulating glass ).
Because of its high thermal conductivity and corrosion resistance, it is well suited as a material for heat exchangers , heat sinks and mounting plates for power semiconductors .
In arts and crafts , sheet copper is driven , that is, deformed by hammering, which is easily possible due to its softness. In the fine arts, copper is still used today to manufacture printing plates for copperplate engravings and etchings .
Roofs are also covered with copper sheet, which then forms a permanent greenish patina , which consists of various basic copper hydroxides or copper carbonates . This patina, which is often incorrectly referred to as “verdigris” (see copper acetate ), protects the underlying metal well against further corrosion , so that copper roofs can have a service life of several centuries. Copper nails are used in traditional slate roofs .
Alloys
Copper is also a component of many alloys such as B. brass (with zinc), bronze (with tin) and nickel silver (with zinc and nickel). These copper alloys are widely used because of their good properties, such as color, corrosion resistance and processability. A distinction is made between wrought alloys (brass and nickel silver) and cast materials ( gunmetal , bronzes): Wrought alloys are brought into the desired shape by plastic forming (hot forming: rolling, forging, etc. or cold forming: wire drawing, hammering, cold rolling, deep drawing, etc.), while cast materials are usually difficult or impossible to form plastically.
Depending on the addition of nickel , the copper's own color disappears and yellowish to white corrosion-resistant alloys ( copper-nickel ) are formed.
Many coin materials are made on a copper basis, so the metal called " Nordic gold " of the gold-colored parts of the euro coins is a copper-zinc-aluminum-tin alloy. The coin metals of the 1 DM coins valid until 2001 and the light-colored parts of the euro coins consist of cupronickel alloys.
Copper compounds are used in color pigments , as toners , in medical preparations and galvanic surface coatings.
proof

Copper colors the borax pearl in the oxidizing flame zone from blue to blue-green, in the reducing flame zone no discoloration is noticeable or the pearl is colored red to red-brown. In the classic cation separation process , copper is precipitated in the hydrogen sulfide group and detected there as a blue complex in the copper group. The latter color is based on the fact that solutions of copper (II) ions with ammonia form a deep blue copper tetrammine complex , [Cu (NH 3 ) 4 ] 2+ (see also complex formation reaction ).
A potassium hexacyanoferrate (II) solution precipitates copper (II) ions as copper (II) hexacyanoferrate (II), Cu 2 [Fe (CN) 6 ]. This detection reaction is very sensitive; i.e., it also indicates low levels of copper.
Copper salts color the flame (Bunsen burner flame) green to blue ( flame color , spectral analysis ).
The quantitative determination can be carried out by electrogravimetry on a platinum mesh cathode from a solution containing sulfuric acid, copper (II). Copper can be determined dimensionally by iodometry or complexometry ( titration with Titriplex / complexon III with indicator Murexid ). In the track area which is Differenzpulspolarographie available (half-wave potential -0.62 V vs. SCE in 1 M thiocyanate solution). Ultra traces of copper are determined by means of inverse voltammetry , graphite tube AAS or ICP-MS .
Copper (II) ions form a blue complex with cuprizone (oxalic acid biscyclohexylidene hydrazide ) in a weakly alkaline solution.
links
Oxides and hydroxides
Copper (I) oxide is reddish and has a cubic crystal structure with the space group Pn 3 m (space group no. 224) . It is used as a pigment in glass , ceramics , enamel , porcelain glaze and as an optical glass polish , insecticide , catalyst for ammonia production , solvent for chrome iron ores , in galvanic electrodes , in pyrotechnics , cloud formation , corrosion inhibitors , galvanizing processes , electronics , textiles , as a flame retardant , fuel additive , Catalyst used in pollution control, printing and photocopying and as a wood preservative .
Copper (II) oxide is a black, amorphous or crystalline solid and forms a monoclinic crystal structure with the space group C 2 / c (space group no. 15) . It is used in the ceramics industry to color glasses , glazes and enamels blue, green or red. It is occasionally used to incorporate mineral additives to protect against copper deficiency in animal nutrition. Its other applications include the preparation of solutions for the pulp industry.
Copper (II) hydroxide is blue and is used for production of pulp , battery electrodes used and other copper salts. It is used as a dressing agent in dyeing , as a pigment and feed additive , in the treatment of storage rot in cranberries and as a fungicide against bacterial weak points in lettuce, peaches, cranberries and walnuts.
Halides
Copper (II) chloride is a brown, highly hygroscopic powder. It is used as a catalyst for organic and inorganic reactions , mordant for dyeing and printing of textiles , pigment for glass and ceramics , wood preservative , disinfectant , insecticide , fungicide and herbicide , as well as catalyst in the production of chlorine from hydrogen chloride used. Copper (II) chloride dihydrate (CuCl 2 · 2 H 2 O) is a blue-green solid .
Copper (I) chloride is white and has a crystal structure of the zinc blende type with space group F 4 3 m (space group no. 216) . It is used as a catalyst for many organic reactions . Ammonia solutions of copper (I) chloride are used to purify gases from carbon monoxide .
Other inorganic compounds
Copper sulfate occurs naturally as chalcanthite (copper sulfate pentahydrate, Cu [SO 4 ] · 5H 2 O) and as boothite (copper sulfate heptahydrate, Cu [SO 4 ] · 7H 2 O). It is used for preservation of hides for tanning of leather , for the production of copper salts, for preserving pulpwood and ground wood pulp , to combat the growth of algae in stagnant water . It is also used in electroplating solutions , washing and metal marking paints, petroleum refineries , pyrotechnics and many other industrial applications.
Organic compounds

Copper (II) acetate (verdigris) forms dark green crystals . It is used as a fungicide , catalyst for organic reactions , pigment for ceramics , insecticide , mold inhibitor , preservative for cellulosic materials , stabilizer for polyurethanes and nylons , corrosion inhibitor and fuel additive.
Price development
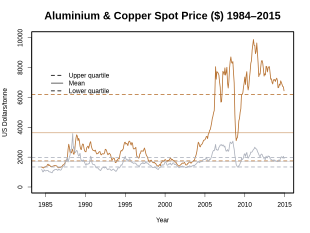
Copper is a relatively expensive metal. Its price is largely based on the world's major commodity exchanges and futures exchanges . The London Metal Exchange (LME) is the leader in copper trading.
The world market price for copper is subject to strong fluctuations : It experienced one of the greatest fluctuations in 2008, when the price for copper was traded on the LME on July 2nd at the interim high of USD 8,940 / t and up until December 23, 2008 at its 10-year low of $ 2,825 fell. The copper price then recovered in less than 4 months by April 15, 2009 to 4,860 USD / t. The copper price reached its 10-year high on February 14, 2011 at 10,180 USD / t.
From March 2012 to March 2013 the copper price peaked on April 2, 2012 to USD 8,619.75 and on August 2, 2012 to USD 7,288.25. A similar range was also found from October 2012 to March 2013 between 8,350 USD / t and 7,577 USD / t.
In August 2014, the world market price for copper was around 7,000 USD / t. At the exchange rate at the time, this was EUR 5,875 / t.
The high price of copper is also causing an increase in thefts of objects containing copper. Earthing cables from railways are particularly affected. For example, Deutsche Bahn AG suffered around 14 million euros in damage in 2015.
One of the biggest financial scandals in recent history is the Sumitomo affair . It was based on the trade in copper. As a result of the discovery, the copper price fell by 27% within a day in 1996.
literature
- Harry H. Binder: Lexicon of the chemical elements - the periodic table in facts, figures and data. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- JW Howard: The story of copper . In: Journal of Chemical Education . tape 6 , no. 3 , 1929, pp. 413-431 , doi : 10.1021 / ed006p413 ( PDF ).
Web links
- Mineral Atlas: Copper (data), Mineral Atlas: Mineral Portrait / Copper (history, smelting etc.)
- deutschlandfunk.de , Das Feature , July 18, 2017, Michael Faulmüller: Copper - Element of Discord: A Story of Eternal Struggle
- kupfer-institut.de (German Copper Institute DKI)
- London Metal Exchange , lme.com: Copper Price
Remarks
- ↑ The values for the properties (info box) are taken from www.webelements.com (copper) , unless otherwise stated .
Individual evidence
- ↑ a b Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e Entry on copper in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e Entry on copper at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1509.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data. 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ^ A. Lossin: Copper. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag, Weinheim 2005, doi : 10.1002 / 14356007.a07_471 .
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics. Volume 6: Solids. 2nd Edition. Walter de Gruyter, 2005, ISBN 3-11-017485-5 , p. 361.
- ^ Buildingmaterials.de: Copper ( Memento from November 15, 2009 in the Internet Archive )
- ^ Building material collection of the Faculty of Architecture at the Technical University of Munich: Metals - Copper .
- ↑ Glyconet ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. .
- ↑ a b c Entry on copper in the GESTIS material database of the IFA , accessed on April 25, 2017 (JavaScript required)
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for copper and its inorganic compounds ), accessed on March 4, 2020.
- ^ Wilhelm Hassenstein: The fireworks book from 1420. 600 years of German powder weapons and gunsmithing. Reprint of the first print from 1529 with translation into Standard German and explanations, Munich 1941, p. 104.
- ↑ For the oxhide bars , their distribution and the Bronze Age copper trade, see: Serena Sabatini: Revisiting Late Bronze Age oxhide ingots. Meanings, questions and perspectives. In: Ole Christian Aslaksen (Ed.): Local and global perspectives on mobility in the Eastern Mediterranaean (= Papers and Monographs from the Norwegian Institute at Athens, Volume 5). The Norwegian Institute at Athens, Athens 2016, ISBN 978-960-85145-5-3 , pp. 15-62.
- ↑ Sungmin Hong, Jean-Pierre Candelone, Clair C. Patterson, Claude F. Boutron: History of Ancient Copper Smelting Pollution During Roman and Medieval Times Recorded in Greenland Ice. In: Science . Volume 272, No. 5259, 1996, pp. 246-249 (247, Fig. 1 & 2; 248, Tab. 1)
- ^ IMA / CNMNC List of Mineral Names; July 2019 (PDF 1.67 MB; copper see p. 44)
- ↑ IMA / CNMNC List of Mineral Names - Copper (English, PDF 1.8 MB, p. 64)
- ↑ Webmineral - Minerals Arranged by the New Dana classification. 01/01/01 Gold group
- ^ Copper . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 58 kB ; accessed on January 22, 2018]).
- ^ Mineral Atlas: Copper
- ↑ Webmineral - Mineral Species sorted by the element Cu (Copper ).
- ^ Deutsches Kupferinstitut - Availability of copper
- ↑ a b List of localities for solid copper in the Mineralienatlas and Mindat
- ↑ World Heritage Cornish Mining ( Memento of the original from February 17, 2011 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ United States Geological Survey: World Mine Production and Reserves
- ↑ Page no longer available , search in web archives: copper and goods from it .
- ↑ TN Lung: The history of copper cementation on iron - The world's first hydrometallurgical process from medieval China. In: Hydrometallurgy . Volume 17, Issue 1, November 1986, pp. 113-129; doi: 10.1016 / 0304-386X (86) 90025-3 .
- ^ Ralph WG Wyckoff: Crystal Structures . 2nd Edition. tape 1 . John Wiley & Sons, New York, London, Sydney 1963, pp. 3 (in the appendix ).
- ^ Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 34 .
- ↑ H. Keller, K. Eickhoff: Kuper and Copper Alloys, Springer-Verlag 2013, 54 pages, page 7
- ↑ George L. Trigg, Edmund H. Immergut: Encyclopedia of applied physics , Volume 4: Combustion to Diamagnetism. VCH Publishers, 1992, ISBN 978-3-527-28126-8 , pp. 267-272 (accessed May 2, 2011).
- ^ William Chambers, Robert Chambers: Chambers's Information for the People , 5th. Edition, Volume L, W. & R. Chambers, 1884, ISBN 978-0-665-46912-1 , p. 312.
- ^ Galvanic Corrosion . In: Corrosion Doctors . Retrieved April 29, 2011.
- ^ University of Siegen: Reaction of metals with hydrochloric acid .
- ↑ Facts on the subject - sulfuric acid ( Memento of March 8, 2001 in the Internet Archive )
- ^ University of Siegen: Reaction of metals with nitric acid .
- ↑ eLexicon chemistry: copper chloride .
- ↑ Daily intake of 0.5 mg / kg harmlessly according to: AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1434.
- ↑ Jens Oliver Bonnet: Copper against germs: expectations were exceeded. Asklepios Kliniken Hamburg GmbH, press release from June 16, 2009 from Informationsdienst Wissenschaft (idw-online.de), accessed on September 15, 2015.
- ↑ Germkiller copper - topic to "know up to date: the power of cell pirates" .
- ↑ Has it arrived in everyday clinical practice? Antimicrobial construction materials based on solid copper , on krankenhaushygiene.de
- ↑ A. Ala, AP Walker, K. Ashkan, JS Dooley, ML Schilsky: Wilson's disease. In: The Lancet . Volume 369, Number 9559, February 2007, pp. 397-408, doi: 10.1016 / S0140-6736 (07) 60196-2 . PMID 17276780 .
- ^ SL Warnes, CW Keevil: Inactivation of norovirus on dry copper alloy surfaces. In: PLoS One. 8 (9), 2013, e75017. PMID 24040380 , PMC 3767632 (free full text, PDF).
- ↑ ratschlag24.com: Copper wire against snail plague . ( Memento from April 11, 2013 in the web archive archive.today ) March 17, 2008.
- ↑ sat1.de: broadcast 24: clever! - Knowledge book ( Memento from May 26, 2011 in the Internet Archive ).
- ↑ med.de: Entry on copper , accessed on February 23, 2013.
- ↑ a b Merck Manual: Copper .
- ↑ JF Mercer: Menkes syndrome and animal models. In: The American journal of clinical nutrition. Volume 67, Number 5 Suppl, May 1998, pp. 1022S-1028S. PMID 9587146 . (Review).
- ↑ S. Lutsenko, NL Barnes et al. a .: Function and regulation of human copper-transporting ATPases. In: Physiological reviews . Volume 87, number 3, July 2007, pp. 1011-1046, doi: 10.1152 / physrev.00004.2006 . PMID 17615395 . (Review).
- ↑ TA Bayer: Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Aβ production in APP23 transgenic mice. In: Proceedings of the National Academy of Sciences. 100, 2003, pp. 14187-14192, doi: 10.1073 / pnas.2332818100 .
- ↑ Holger Kessler, Frank-Gerald Pajonk, Daniela Bach, Thomas Schneider-Axmann, Peter Falkai, Wolfgang Herrmann, Gerd Multhaup, Jens Wiltfang, Stephanie Schäfer, Oliver Wirths, Thomas A. Bayer: Effect of copper intake on CSF parameters in patients with mild Alzheimer's disease: a pilot phase, 2 clinical trial. In: Journal of Neural Transmission. 115, 2008, pp. 1651-1659, doi: 10.1007 / s00702-008-0136-2 .
- Jump up ↑ NG Faux, CW Ritchie, A. Gunn, A. Rembach, A. Tsatsanis, J. Bedo, J. Harrison, L. Lannfelt, K. Blennow, H. Zetterberg, M. Ingelsson, CL Masters, RE Tanzi, JL Cummings, CM Herd, AI Bush: PBT2 rapidly improves cognition in Alzheimer's Disease: additional phase II analyzes. In: Journal of Alzheimer's disease: JAD. Volume 20, Number 2, 2010, pp. 509-516, doi: 10.3233 / JAD-2010-1390 . PMID 20164561 .
- ↑ I. Singh, AP Sagare, M. Coma, D. Perlmutter, R. Gelein, RD Bell, RJ Deane, E. Zhong, M. Parisi, J. Ciszewski, RT Kasper, R. Deane: Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance. In: Proceedings of the National Academy of Sciences. S., doi: 10.1073 / pnas.1302212110 .
- ↑ Deutsches Kupferinstitut: Copper and its Applications ( Memento of the original from July 16, 2012 in the web archive archive.today ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ^ R. Neeb: Inverse polarography and voltammetry. Akademie-Verlag, Berlin 1969, pp. 185–188.
- ↑ a b Georg Brauer (Ed.), With the collaboration of Marianne Baudler u a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 979.
- ↑ a b c d National Pollutant Inventory: Copper and compounds
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 973.
- ↑ Leader in trading copper: London Metal Exchange - LME Copper . Retrieved March 15, 2013.
- ↑ a b c Development of the copper price at the London Metal Exchange in the period from July 2, 2008 to April 15, 2009 ( memento of the original from August 12, 2014 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Source: Handelsblatt database. Retrieved March 15, 2013.
- ↑ Highest price for copper on the London Metal Exchange in the last 10 years on February 14, 2011 Source: Handelsblatt. Retrieved March 15, 2013.
- ↑ Development of the copper price on the London Metal Exchange in the last 12 months Source: Handelsblatt. Retrieved March 15, 2013.
- ↑ Development of the price for copper on the London Metal Exchange in the last 6 months Source: Handelsblatt. Retrieved March 15, 2013.
- ↑ Current price for copper at the London Metal Exchange ( Memento of the original from August 12, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Source: Handelsblatt.
- ↑ This figure was determined with the template: Exchange rate.
- ↑ Theft of non-ferrous metal at Deutsche Bahn. (No longer available online.) Deutsche Bahn press office, archived from the original on December 23, 2016 ; accessed on December 22, 2016 .