phosphorus
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Phosphorus, P, 15 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Non-metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 15 , 3 , p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | white-beige (W) dark red (R) black (S) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7723-14-0 (red) 12185-10-3 (white) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-115-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.286 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.09% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 30.973761998 (5) et al | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 100 (98) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 107 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 180 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Ne ] 3 s 2 3 p 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 10.486 686 (15) eV ≈ 1 011.81 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 19th.76949 (4) eV ≈ 1 907.47 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 30th.20264 (9) eV ≈ 2 914.11 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 51.44387 (12) eV ≈ 4 963.58 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 65.02511 (12) eV ≈ 6 273.97 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modifications | 6th | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | orthorhombic (black) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | white: 1.83 g / cm 3 red: 2.0 ... 2.4 g / cm 3 black: 2.69 g / cm 3 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism |
diamagnetic (red: Χ m = −1.9 10 −5 black: = −2.9 10 −5 ) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | white: 317.3 K (44.2 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | white: 553.2 K (280 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 17.02 · 10 −6 m 3 · mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 51.9 kJ / mol (P 4 ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 0.64 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | red: 3900 Pa white: 3300 Pa at 293 K |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | red: 685.6 J kg −1 K −1 at 298 K. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 0.236 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | ± 3, 4, 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.19 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MAK |
Switzerland: 0.02 mg m −3 (white / yellow phosphorus, inhalable dust ) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicological data |
White phosphorus |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
Phosphorus (from ancient Greek φωσφόρος phōsphóros , German 'light-bearing' , from the glow of white phosphorus when reacting with oxygen ) is a chemical element with the symbol P and the atomic number 15. In the periodic table it is in the fifth main group , or 15. IUPAC -Group or nitrogen group .
Phosphorus occurs in several, very different modifications . The white phosphorus , made up of P 4 molecules, is the easiest of all to produce, but thermodynamically not the most stable modification, and it is also toxic and easily flammable.
Phosphorus compounds are essential for all living things and are involved in the structure and function of organisms in central areas such as DNA and the cellular energy supply ( ADP / ATP ). The biogeochemical conversion of phosphorus takes place within the framework of the phosphorus cycle .
history




Phosphorus was discovered in 1669 by Hennig Brand , a German pharmacist and alchemist , when he was evaporating urine to dryness in search of the “ Philosopher's Stone ” . When the dry urine residue, which - as is now known - also contains phosphates (salts of phosphoric acid), glowed in the absence of air - i.e. in the absence of an oxidizing agent - the reduction of the phosphates with the organic compounds also contained in the urine resulted in whiter whiter as a reducing agent Phosphorus that glowed in the dark due to its chemiluminescence .
Although phosphorus was not used except as a night lamp at that time, it was weighed against gold . Hennig Brand did not get rich from this discovery and sold the recipe to the alchemist Johann Daniel Kraft , who in turn made a fortune with it. He demonstrated the production of phosphorus in 1677 before Robert Boyle . It is remarkable that Hennig Brand also presented his Phosphorus in Hanover to the scientist and philosopher Gottfried Wilhelm Leibniz , who wrote a poem out of enthusiasm about the mysterious light carrier “Phosphorus Mirabilis”.
White phosphorus was initially used as a remedy due to its fascinating property - chemiluminescence . Later it was of great importance in making matches . However, because white phosphorus is highly toxic, severe poisoning has often occurred among workers who came into contact with it. The phosphorus fumes mainly penetrated the body through damaged teeth and led to phosphorus necrosis, especially of the jaw . The phosphorus jaw , English phossy jaw , was one of the first occupational diseases to be diagnosed as such.
White phosphorus played a role as a weapon in military history . It was used as a filling material for incendiary bombs , the so-called phosphor bombs . The British Air Force used a mixture of white phosphorus and rubber during World War II . The viscous mass sticks due to the rubber and is therefore difficult to strip off. It caused poorly healing wounds on the skin.
Chemists discovered early on that white phosphorus turned red when exposed to light, even in a vacuum. Jöns Jakob Berzelius suspected that this was a modification of white phosphorus, which was only proven in 1847 by Anton Schrötter von Kristelli (Anton Schrötter) in Vienna, who isolated and analyzed the substance. Violet phosphorus was discovered by Johann Wilhelm Hittorf in 1865 . and the letterpress variant black phosphor Percy Williams Bridgman 1914.
Origin of phosphorus
Phosphorus finds its origin in neutrino sources in the cosmos, which are supernovae . In the center of which had been hydrogen to helium , this to carbon and oxygen , and this to silicon , phosphorus and sulfur , and finally to iron , cobalt and nickel fused what finally within milliseconds to the collapse of the star led ( Nukleosynthese ). However, the proven amount of phosphorus in our galaxy lags behind the calculations.
Occurrence
On earth, especially in the earth's crust, phosphorus occurs exclusively in bound form, i.e. not native, mostly in the form of phosphates (content in the earth's crust: ~ 0.09%, about 1.2 kilograms per ton). Typical minerals are the apatites Ca 5 (PO 4 ) 3 (F, Cl, OH). Fluorapatite in particular and phosphorite interspersed with calcium carbonate are the most important phosphates from an economic point of view. There are also other phosphorus-containing minerals, such as the Wavellite Al 3 (PO 4 ) (F, OH) · 5 H 2 O, Vivianite Fe 3 ( PO 4 ) 2 · 8 H 2 O and the turquoise CuAl 6 [(PO 4 ) (OH 2 )] 4 · 4 H 2 O.
The largest deposits of phosphate minerals are found in Africa, China and the USA ( Florida ). Four countries have around 80% of the world's phosphate rock reserves that can be economically mined using current technology : Morocco (together with Western Sahara 36.5%), China (23.7%), Jordan and South Africa (9.6% each) . The continental deposits are only sufficient for a few decades; Estimates from the 2000s vary between 50 (2008) and 130 years (2006). However, as a result of the newly found deposits mainly in North Africa and Iraq, an estimate by the German government from 2012 assumes that no shortage is imminent and that the stocks known to date will last until around 2400. Furthermore, there are large deposits under water, which at the moment cannot be mined economically.
In addition to minerals, phosphorus is also found in deposits of bird droppings from sea birds , the so-called guano (contains 7–8%, rarely up to 60% Chile nitrate and a maximum of around 40% phosphates). This is mainly found on some islands in the Pacific Ocean, such as Nauru or Kiribati and in South America ( Peru / Chile ). Phosphorus stocks on Nauru have been falling continuously since the mid-1970s and are now almost completely exhausted.
Sewage sludge also contains large amounts of phosphates. By urban mining the phosphoric acid can be recovered from the sewage sludge.
Of the approximately 180 million tonnes of rock phosphates extracted annually worldwide (as of 2010), around 90% are used in the production of fertilizers . Phosphorus cannot be replaced by any other substance in fertilizers.
Phosphorus also has an important meaning in the organic world (as bound or particulate bound organic phosphate in organisms and in detritus ) and occurs in various areas of fauna and flora : for example as hydroxylapatite Ca 5 (PO 4 ) 3 OH, which is one of the The main components of the structural substance found in bones and teeth. Furthermore, phosphorus compounds play an important role in living organisms as components of nucleic acids and as a component of the energy carrier ATP .
Phosphorus is formed in massive stars when burning oxygen from oxygen at temperatures above 1.5 · 10 9 Kelvin and densities of at least 10 10 kg / m 3 .
It is postulated that the phosphorus usable for early living things was only available through meteorites that came to earth during the Hadaic era . The phosphate, which already occurs on earth, is inert and difficult to dissolve and would therefore have only been of limited use for the first living beings. In contrast, the scribes brought along by the meteorites react with water to form reduced phosphides . These would be plausible starting materials for a prebiotic synthesis of phosphorylated biomolecules (such as ribonucleic acid ).
Extraction and presentation
Phosphorus is extracted from phosphate minerals such as phosphorite or apatite , in which these are heated to 1500 ° C in an electric smelting reduction furnace together with quartz gravel and thus converted into white phosphorus. The furnace is designed as a closed low-shaft furnace, the heat is supplied via Söderberg electrodes .
The carbon mass contained in the electrode acts as a reducing agent and the silicon dioxide in the quartz acts as a slag former. The gaseous phosphorus produced during the process is condensed and collected under water.
Manufacturer
After the bankruptcy of Thermphos , the last European manufacturer, the supply of white phosphorus is based almost exclusively on the Kazphosphate company , which operates a plant in Tschimkent . Other manufacturers are Monsanto with a plant in Soda Springs ( Idaho ) and various Chinese companies.
Modifications and properties
Phosphorus occurs in four allotropic modifications as white, red, black, and purple phosphorus. Each of these basic types forms different crystal structures . This leads to very large differences in physical properties and reactivity.
Since the other modifications are difficult to obtain directly, white phosphorus is always produced first and this is then converted into other modifications. These can be converted into one another by high pressure and high temperature. The black phosphorus is actually the most stable modification at room temperature, but the others are metastable due to the slow conversion rate . White phosphorus can be produced in the laboratory by heating red phosphorus in the absence of oxygen. Conversely, red phosphorus can also be produced by heating white phosphorus to around 180-350 ° C for several hours.
Gaseous state
In the phosphorus vapor, CP 4 tetrahedra predominate as the smallest molecular units below 1200 ° .
The PP distance in the tetrahedral P 4 molecules is r g = 2.1994 (3) Å, the PPP angle is 60 °. The structure was determined by gas electron diffraction .
The degree of dissociation is ~ 1% at 800 ° C. Between 1200 and 2000 ° C, P 2 molecules with a nitrogen-analogous valence electron structure predominate ; above 2000 ° C, these slowly dissociate to atomic phosphorus with increasing temperatures.
White phosphorus
White phosphorus is the most volatile and reactive modification of phosphorus. It has a density of 1.82 g / cm 3 , a melting point of 44.25 ° C and a boiling point of 280 ° C and is translucent and waxy. When contaminated, the white phosphorus is also known as yellow phosphorus . The cubic white phosphorus is very easily soluble in phosphorus trichloride and carbon disulfide CS 2 ; 100 g carbon disulphide dissolve more than 1 kg phosphorus. Phosphorus is slightly soluble in carbon tetrachloride , benzene or ether . It is practically insoluble in water.
At −76.9 ° C, the cubic shape (α-shape) changes into a hexagonal shape (β-shape) (rotation of the free outer electrons "frozen"). In any form (α-, β-, in solution) white phosphorus forms P 4 tetrahedra with a bond angle of 60 °.
In a finely divided state, white phosphorus ignites by itself in the air; from around 50 ° C even compact pieces ignite and burn to form phosphorus (V) oxide . Therefore, white phosphorus must be kept underwater. Burning phosphorus must not be extinguished with water as there is a risk that the phosphorus dust will be washed into fine cracks and will ignite again after the water has evaporated. Burning phosphorus is best extinguished with sand.
White phosphorus can show a bluish chemiluminescence in air . This arises from the gaseous P 4 present in the environment due to the high vapor pressure of white phosphorus , which reacts to P 4 O 10 by gas phase oxidation via P 4 O 6 . Usually in a violent exothermic reaction, phosphorus combines with halogens, metals or sulfur. The resulting compounds are phosphorus sulfides , phosphorus (III) or phosphorus (V) compounds and phosphides . Under the action of strong alkalis at high temperatures, phosphorus disproportionates to phosphine and hypophosphite. Phosphorus has a high affinity for oxygen , so it has a strong reducing effect . So z. B. sulfuric acid reduced to sulfur dioxide when heated with white phosphorus .
The phosphorus pentoxide produced when phosphorus is burned is highly hygroscopic and, together with the humidity, forms a thick mist of phosphoric acid. White phosphorus is therefore used in smoke grenades.
White phosphorus is highly toxic; even about 50 mg can be fatal to an adult human. Death only occurs after five to ten days. White phosphorus is also only slowly excreted. The slow toxicity makes (e) phosphorus suitable as a rat poison . So-called "Phosphorlatwergen" were used for this. Because of the general dangers and because more suitable agents are available, white phosphorus is obsolete as a rat poison.
The toxicity of white phosphorus is mainly attributed to its high reducing capacity, which disrupts intracellular oxidative metabolic processes such as protein and carbohydrate synthesis. This mainly concerns enzymatically controlled metabolic processes in the liver. The highly poisonous phosphines formed by reaction with water , which are strong metabolic poisons and have a special affinity for the central nervous system , represent a further danger . White phosphorus can be rendered harmless with a copper (II) sulfate solution. In the process, poorly soluble copper (I) phosphide is formed .
Since 1845 were among workers, mainly in the industrial production of matchsticks heavy with white phosphorus, jaw - necrosis observed (Engl. Phossy jaw ). In the 19th century, workers handling phosphorus baths without protection (often children and young people who were busy packing the matches) quickly became incapacitated. There was no effective therapy. The patients were severely disfigured, often unemployed, the mortality rate was 20%. The occupational health problem of phosphorus necrosis , especially massive jaw necrosis, led to the first occupational medical consequences in the history of modern medicine. In 1906, the Bern Convention led to the ban on white phosphorus in the manufacture of matches. Similar jaw necrosis can be seen today with bisphosphonate therapy ( bisphosphonate-associated bone necrosis ).
Black phosphorus
The most stable modification at room temperature exists in one amorphous and three crystalline forms. Due to its polymeric form, black phosphorus is insoluble, significantly less flammable, very inert and has a density of 2.69 g / cm 3 . Therefore, like red phosphorus, black phosphorus is non-toxic. The crystal lattice on which the black phosphorus is based consists of corrugated double layers in which the phosphorus atoms are pyramidal connected to three other phosphorus atoms in the vicinity at a bond angle of 100 °. In this configuration, phosphorus has semiconductor properties . In humid air, black phosphorus is oxidized a little faster than red phosphorus, but it is covered with a colorless, viscous liquid skin made of phosphoric acids, so that further oxygen access is prevented and inflammation is made more difficult. The normal black phosphorus crystallizes orthorhombically ; at 80,000 bar this changes reversibly into a rhombohedral and at 110,000 bar into a cubic metallic modification.
Black phosphorus is formed from white phosphorus under high pressure (12,000 bar) and increased temperature (200 ° C) and is very different from the aforementioned modification in terms of its color. It looks gray-black, shiny and fibrous like wood. A low pressure modification has also recently been made.
Red phosphorus
A number of amorphous and crystalline forms with density variations between 2.0 and 2.4 g / cm 3 and melting points between 585 ° C and 610 ° C are grouped together under the name red phosphorus . Red phosphorus is generally amorphous, but can be converted into monoclinic Hittorfian (violet) phosphorus by recrystallization from molten lead , which forms a three-dimensionally cross-linked polymeric form.
Red phosphorus is obtained by heating white phosphorus for several hours to around 260 ° C in the absence of air. A slow conversion also happens when exposed to light. Iodine catalyzes the conversion of white to red phosphorus.
The differences between the crystalline parts in red phosphorus determine the different forms of the same. The grain size , the type of lattice , impurities and the various saturations of the edge groups with halogens , oxygen and hydroxyl groups have an influence here .
Although red phosphorus is not self-igniting, it can be caused to ignite suddenly or even to explode with strong oxidizing agents through low energy input (friction, impact). An example of this is Armstrong's mixture , which is used to strike safety matches. In terms of reactivity, violet phosphorus is more like black phosphorus, while Schenck's phosphorus is much more reactive than “normal” red phosphorus.
In contrast to white phosphorus, red phosphorus is not toxic. His first description is attributed to Anton Schrötter von Kristelli .
Bright red phosphorus
Light red or Schenck's phosphorus ( Rudolf Schenck , 1902) is produced by boiling white phosphorus in phosphorus tribromide (PBr 3 ). The product is a mixed compound of phosphorus with 10 to 30% bromine, the density of which is 1.88 g / cm 3 .
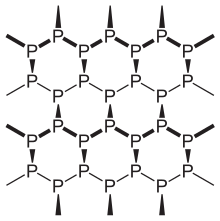
Violet / Hittorf's phosphorus
Violet phosphor is formed when white phosphorus is heated to approx. 550 ° C for one to two weeks.
He was discovered by Johann Wilhelm Hittorf . It is a non-conductive polymer that is insoluble in CS 2 . The structure elucidation succeeded in the late 1960s at the University of Stuttgart by Herbert Thurn . The purple phosphor is also non-toxic.
Phosphorus nanorods
In August 2004, German researchers succeeded in isolating and structurally characterizing two further modifications of the multi-faceted element: Phosphorus nanorods . In these two modifications, the phosphorus atoms are in the form of chain molecules (polymers). The discoverers of the new modifications are Arno Pfitzner from the University of Regensburg and Hellmut Eckert from the Westfälische Wilhelms-Universität Münster . The red-brown fibers, which differ significantly from the red phosphorus modification, are stable in the dry state for weeks in the air. Electron microscopy showed this red-brown phosphorus form as long, parallel nanorods with diameters of approximately 0.34 nm (nanometers) to 0.47 nm.
Isotopes
Phosphorus has only one stable isotope , 31 P; this is the only naturally occurring isotope of phosphorus. It is therefore a pure element (anisotopic).
Phosphorus has several other isotopes, all of which are radioactive. The phosphorus isotope 33 P has the longest half-life of 25.3 days . 32 P has a half-life of 14.3 days and is used in medicine. It is also used as a tracer in molecular biology . It can be used, for example, to radioactively mark gene probes and to detect them by means of autoradiography .
use
The majority (80%) of the white phosphorus produced is burned to form phosphorus (V) oxide (P 2 O 5 ), which is used as a starting material for the production of phosphoric acid and for the preparation of various phosphates . The majority of all phosphates, on the other hand, are used as fertilizers . Phosphorus (V) oxide is also one of the most effective dehydrating substances ( drying agents ).
Another part is to phosphorus trichloride (PCl 3 ) and phosphorus (V) sulfide (P 4 S 10 processed), in turn, as base materials for the production of flame retardants , additives , plasticizers and pesticides are used.
Red phosphorus is used to make matches . Paradoxically, finely divided red phosphorus is also added to plastics (e.g. polyamide ) as a flame retardant: the reaction products of phosphorus, atmospheric oxygen and water (air humidity, residual moisture in the plastic) form a charred protective layer against the flames. In addition, formed phosphorus suboxides act, e.g. B. PO, as a radical scavenger and in this way interrupt the combustion in the gas phase.
Furthermore, the phosphates, which are important as fertilizers, are obtained directly from calcium phosphate by breaking it down with sulfuric acid . The so-called superphosphate is produced . Around 60% of the world's sulfuric acid production is required for this.
Red and white phosphorus are also used for military purposes. The very poisonous and self-igniting white phosphorus is used in incendiary ammunition such as phosphor bombs and was previously also used in smoke ammunition. In modern smoke ammunition, however, the non-toxic red phosphorus is used in mixtures with oxidizing agents and metallic fuels. If fog is the goal, one tries to obtain the finest possible dust from P 2 O 5 , of which every particle acts hygroscopically as a condensation nucleus for air humidity. The mist then consists of small droplets of dilute phosphoric acid, a rather weak acid.
Until the 1980s, experiments were also carried out with white phosphorus in schools, which was forbidden for health reasons. Now only red phosphorus may be used in experiments.
The radioactive phosphorus isotope 32 P is used in medicine and research as a tracer to observe metabolic processes (for example in 32P post labeling ) or to treat diseases, such as in the nuclear medicine therapy of polycythemia vera . An outdated method of diagnosing choroidal melanoma was the radiophosphorus test .
In the ground
Phosphorus enters the soil naturally through apatite weathering or from decomposed organic matter. The atmospheric deposition plays only a subordinate role with phosphorus. Phosphorus is an important core nutrient . Using artificial fertilizers , humans increase the phosphorus content in the soil. Erosion can be regarded as the main factor behind the losses. The direct leaching into the groundwater is very low and apart from negligible amounts of phosphine gas there is no outgassing from the soil. Phosphate has a very poor solubility . For example, adsorption on Fe and Al hydroxides in acidic soils leads to phosphate fixation. Calcium precipitates in basic soils .
In general, phosphate fractions in the soil can be classified in various ways. Two frequently used classifications are the classification according to solubility and the classification according to phosphate types.
Classification of three different phosphate fractions in the soil according to solubility:
- Phosphate available in the soil solution is directly available to plants. However, this is the smallest fraction with 1–2 kg / ha.
- Unstable phosphate is loosely bound to iron and aluminum oxides or clay minerals through specific sorption. 450–900 kg / ha can thus be present in the soil. Plant-available phosphate can be formed from this fraction by absorption.
- Stable phosphate is of practically no importance for plant nutrition, although with 3000–6000 kg / ha it is the largest of the three fractions. The most important representatives here are apatites and calcium phosphates.
These fractions are in a dynamic equilibrium with one another and can sometimes merge into one another over very long periods of time.
Classification of three different phosphate fractions in the soil according to the phosphate types:
- Inorganic phosphate is the fraction that is present in primary phosphorus minerals (e.g. apatite ), in secondary phosphorus minerals ( Fe , Al or Ca minerals ) or adsorbed on Fe and Al hydroxides .
- Dissolved phosphate is the proportion that is present in dissolved form in the soil solution as H 2 PO 4 - or as HPO 4 2− . Dissolved phosphate is directly available to plants.
- Organic phosphate is a collective term for all other organic compounds that are present in the soil. This includes phosphorus in humus, phosphorus adsorbed on organic molecules, microbial phosphorus and phosphorus in plant debris.
The point in time at which the maximum global phosphate production is reached is known as Peak Phosphorus .
Biological importance
physiology
Phosphorus is essential for all biological organisms. Phosphorus compounds are part of the DNA and RNA molecules, the carrier substance for the genetic information of all living things. The high phosphorus compound adenosine triphosphate plays a crucial role in the energy metabolism (activated sugar) of the cells. Phosphorus is also contained in sugar phosphates, phospholipids and coenzymes . The phosphorylation is a key regulatory mechanisms in organisms. Phosphates are also an integral part of the pH buffer system in the blood .
The dry matter of terrestrial plants contains 0.15% to 0.50% phosphorus, that of mammals such as humans about 4%. The framework of bones and teeth consists mainly of hydroxyapatite (Ca 5 (PO 4 ) 3 OH). The body of a person weighing 70 kg contains about 700 grams of phosphorus, of which 600 g are firmly bound in the bone system.
The daily requirement of an adult is about 0.75 grams of phosphorus; It is particularly abundant in dairy products, meat, fish and bread. The availability of phosphate often acts as a limiting growth factor for plants, which is why large amounts of fertilizer containing phosphate have to be applied in agriculture.
White phosphorus and phosphorus compounds such as phosphane and numerous phosphoric acid esters are very toxic.
plants
Phosphorus fulfills various essential functions in plants. It is part of lipids and therefore a structural element. In DNA and RNA, it is the bridge between two riboses . Covalently bound to adenosine , it serves as a universal form of energy transfer in cells. Furthermore, phosphorus has an influence on the carbohydrate balance , photosynthesis and the water balance of plants.
In order to make the phosphorus found in the soil available for metabolism, plants have to release organically or inorganically bound phosphorus, which they can absorb as H 2 PO 4 - . The same goes for microorganisms and fungi that live in the soil. Many microorganisms, fungi and plants release enzymes into the soil which hydrolyse the organic phosphoric acid esters and thus release inorganic phosphate, which can be absorbed and metabolized by the organisms. These enzymes are called phosphatases . Depending on the optimal pH range of the phosphatase, a distinction is made between acidic (pH 4–5) and basic phosphatases. If the plant absorbs more phosphate than it can use in lipids, nucleic acids and bound to adenosine, it stores the excess as organically bound form in the vacuoles . Phosphatases also help at this point to convert the phosphate back into the inorganic free form.
If plants suffer from phosphate deficiency, they show various symptoms. The leaf areas are reduced and the habitus is reduced overall; anthocyanin discoloration may occur, necrosis may develop. Due to the accumulation of starch in the chloroplasts, the leaves become “rigid”. The development of flowers, seeds and fruits is reduced or delayed. Since the chlorophyll synthesis is not reduced as much by phosphate deficiency as the ratio of the leaf area decreases, hyperchlorophyllation occurs in the leaves, which is expressed by a deep green color.
Since the phosphate concentration is greatly reduced in the rhizosphere , especially in the area of one to two mm around the roots, some plants react to phosphate deficiency with increased root growth.
ecology
The phosphorus cycle or phosphorus cycle is the constant migration and biogeochemical conversion of the bio-element phosphorus in water, in soils and in biomass.
Because of the central ecological importance of phosphate, quantitative phosphorus analysis also plays an important role in the practice of chemical water and soil monitoring, for example in the determination of phosphate contamination (increased phosphate concentration) in water from over-fertilized agricultural areas in the area.
proof
Spectroscopically
The method of choice for the detection of phosphorus compounds is 31 P- NMR spectroscopy. 31 P is the only naturally occurring phosphorus isotope and has a nuclear spin quantum number of 1/2. Compared to hydrogen, the relative sensitivity is only 6.6%. The resonance range is approx. 700 ppm (P 4 , for example, has a shift of −520 ppm). 85% phosphoric acid can be used as a standard. Since phosphorus is a spin 1/2 nucleus, the spectra can be evaluated very well. If the hydrogen is also decoupled, a sharp signal usually results. The phosphorus shift is highly dependent on its binding partner, so it is very well suited for the identification of known compounds. In the case of unknown compounds, the informative value is often limited because only rarely can a compound class be exclusively assigned to a spectral range.
| Derivatives with | Chemical shift (δ in ppm) |
|---|---|
| 3-string P | −180 to +200 |
| 4-string P | −120 to +130 |
| 5-string P | −100 to −20 |
| 6-string P | −220 to −130 |
Wet chemical
The quantitative and qualitative determination of phosphorus is carried out via the phosphate ( more precisely orthophosphate PO 4 3− ). For this purpose, bound phosphorus is converted into phosphate by oxidizing digestion, if necessary .
Qualitative evidence
In the detection reaction with sodium molybdate , a yellow solution of sodium molybdophosphate is obtained in acidic solution ; a color reagent made from dissolved ascorbic acid is added to the solution and heated in a water bath. This results in molybdenum blue , which can be quantitatively and photometrically determined.
In the detection reaction with ammonium heptamolybdate , a yellow precipitate of ammonium molybdophosphate is obtained in acidic solution . Taking into account that the heptamolybdate enters into an equilibrium in aqueous solution:
the following reaction equation results:
In alkaline solution of ammonia phosphate falls in the presence of magnesium - ions as magnesium ammonium phosphate from:
The proof can also be carried out in the form of zirconium hydrogen phosphate :
As stated in many textbooks, the form Zr 3 (PO 4 ) 4 is incorrect; this compound does not form in aqueous solutions!
Historically, the Mitscherlich sample is interesting for the detection of white phosphorus, which was primarily used in suspected phosphorus poisoning. The contents of the stomach are heated with water, the white phosphorus, which is volatile with the water vapor, then condenses and when it comes into contact with atmospheric oxygen, it lights up ( chemiluminescence ).
For structural investigations of compounds that contain phosphorus, 31 P nuclear magnetic resonance spectroscopy is suitable .
Quantitative evidence
Gravimetry
For gravimetric determination, a yellow molybdophosphate ion can be formed from phosphate and molybdate ions in a strong hydrochloric acid solution:
With 8-hydroxyquinoline ( called HOx or oxine for short ) a sparingly soluble precipitate of oxine-12-molybdo-1-phosphate is formed, which is then dried at 160 ° C and weighed in anhydrous form.
- Precipitation form:
- Weighing form:
- (Color: dark orange)
The coarsely crystalline precipitate contains only 1.37% phosphorus. This means that smaller amounts of phosphate in particular can be easily determined (see micromole method ).
Volumetry
Volumetric phosphate determinations are precipitation with La 3+ or Bi 3+ - standard solutions and subsequent back titration with EDTA performed.
Colorimetry / photometry
For the determination of low concentrations of phosphate in freshwater samples, a deep blue antimony-phosphorus molybdate complex is formed, which is only given its intense color by a reducing agent, usually ascorbic acid . This enables sensitive proofs down to the range of approx. 0.6 mg PO 4 / l (approx. 0.2 mg P / l).
The procedure is standardized in EN ISO 6878 (formerly EN 1189 or DIN 38405 D11).
safety instructions
White phosphorus
White phosphorus can ignite in air if it is finely divided. The auto-ignition temperature is around 34 ° C, which is relatively close to room temperature. The reaction to phosphorus pentoxide is strongly exothermic . Toxic phosphines can be formed with water and alkali hydroxides . Strong oxidizing agents usually react explosively.
The disposal of ammunition containing phosphorus in shallow sections of the Baltic Sea after the Second World War repeatedly leads to serious injuries and deaths. The amber-colored lumps of phosphorus are washed up and thus endanger fishermen and tourists in particular. According to statistics, 168 people died from ammunition residues in the Baltic Sea after the Second World War and 250 people were injured, some seriously. Experts are now even assuming that the numbers are significantly higher.
Acute poisoning with white phosphorus (phosphorus poisoning ) manifests itself in gastrointestinal disorders, liver damage with severe metabolic disorders and damage to the heart and kidneys. Chronic poisoning leads to disturbances of the general condition and damage to blood and bones ( osteoporosis ), especially of the jaw , even in small amounts .
Doses of 15 mg or more of white phosphorus can cause severe toxic effects. Amounts from 50 mg (~ 1 mg / kg body weight) can be lethal .
School experiments with white phosphorus, such as the phosphorus bell experiment , are not permitted under more recent regulations; white phosphorus may not be stored in schools due to its dangerousness.
Other modifications
The other known modifications of phosphorus are non-toxic in their pure form because of their insolubility in water and lower reactivity. They also only ignite at a higher temperature (red phosphorus only at 260 ° C).
links
Phosphorus is very reactive and forms covalent bonds with many non-metals . It occurs in all oxidation states between -3 and +5 and coordination numbers 1 to 6, mostly 3 to 4. The oxidation numbers -3 and +5 are preferred.
Hydrogen compounds
Phosphanes (the old name phosphine is no longer IUPAC- compliant, but is almost exclusively used in chemical literature, especially in Anglo-Saxon literature) denote compounds of trivalent phosphorus with hydrogen or the replacement of one or more hydrogen atoms with organic groups as binding partners. The organic group must be linked directly to the phosphorus atom via the carbon atoms of the basic structure. If the organic group is bound to the phosphorus atom by an oxygen atom (i.e. unit POC, e.g. in P (OPh) 3 ), one speaks of phosphorous acid esters or phosphites .
Oxides
With oxygen , phosphorus forms various compounds of the general formula P 4 O n (n = 6–10, 18), since phosphorus can be present in several oxidation states. Both phosphorus-oxygen single bonds and double bonds as well as bridging phosphorus-oxygen-phosphorus bonds are possible.
Phosphorus oxides form structures similar to adamantane :
- Phosphorus trioxide P 4 O 6 is a white, soft compound that is very toxic and reactive and reacts quickly to form phosphorus pentoxide.
- Phosphorus tetraoxide P 2 O 4 is a mixed oxide. It can be obtained by oxidizing phosphorus trioxide into carbon tetrachloride .
- Phosphorus pentoxide P 4 O 10 is the most important phosphorus oxide. It is very hygroscopic and is used as a drying agent.
There are also other phosphorus oxides that cannot be isolated under normal conditions. Of these, the phosphorus monoxide PO is probably the most common phosphorus-containing molecule in interstellar clouds .
Oxo acids and salts
A large number of phosphorus-oxygen acids and their corresponding salts with one or more phosphorus atoms can be derived from these oxides :
| Oxidation level of phosphorus |
Structural formula | Name of the acid | Name of the salts | |
|---|---|---|---|---|
| Monophosphoric acids | ||||
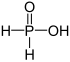
| + I | Phosphinic acid | Phosphinates | ||
| + III | Phosphonic acid | Phosphonates | ||
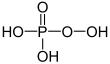
| + V | phosphoric acid | Phosphates | ||
| + V | Peroxomonophosphoric acid | Peroxomonophosphates | ||
| Diphosphoric acids | ||||
| + II | Hypodiphosphonic acid | Hypodiphosphonates | ||
| + III | Diphosphonic acid | Diphosphonates | ||
| + IV | Hypodiphosphoric acid | Hypodiphosphates | ||
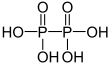
| + V | Diphosphoric acid | Diphosphates | ||
| + V | Peroxodiphosphoric acid | Peroxodiphosphates | ||
There are also sulfur-containing derivatives of these acids, e.g. B. thiophosphoric acid , the salts of which are called thiophosphates .
There are also a number of phosphorous minerals , the most important of which are hydroxyapatite and fluoroapatite . Further phosphorus minerals can be found in the category: phosphorus mineral .
Halogen compounds
Phosphorus forms a large number of compounds with the halides fluorine , chlorine , bromine and iodine . The most important connection types are PX 3 , P 2 X 4 and PX 5 . The fluorine compounds are gaseous, the chlorine compounds are mostly liquid, bromine and iodine compounds are solid. Many phosphorus halogen compounds are poisonous. All compounds are sensitive to hydrolysis and must be protected from humidity during storage.
Examples of this class of compounds are phosphorus trichloride , phosphorus pentachloride , diphosphorus tetrafluoride and phosphorus triiodide . Oxygen and sulfur halogen compounds of the type POX 3 (e.g. phosphorus oxychloride ) and PSX 3 , as well as polymeric oxide halides of the type (POX) n are also known . The halides of phosphorus are among the first non-metallic halides to be investigated by researchers such as Joseph Louis Gay-Lussac , Humphry Davy and Pierre Louis Dulong at the beginning of the 19th century .
Other inorganic compounds
Phosphorus forms a number of phosphorus sulfides with sulfur , which in their structure z. T. resemble the phosphorus- oxygen compounds. The structure is based on a P 4 tetrahedron, the edges and tips of the tetrahedron are occupied differently with sulfur atoms. They have the general formula P 4 S x (x = 3–10). They are made by heating red phosphorus and sulfur in the appropriate proportions. Phosphorus pentasulphide (P 4 S 10 ) is the most important of these. Tetraphosphorus trisulfide (P 4 S 3 ) is still used in part for the ignition material of matches . Compounds with selenium are also known.
Nitrogen and phosphorus form nitrides of the composition PN and P 3 N 5 . Phosphorus nitride chlorides (phosphorus nitrile dichlorides) are not known in the monomeric state. They have the general formula (PNCl 2 ) x with a ring or chain structure. They arise from the reaction of ammonium chloride with phosphorus pentachloride and belong to the group of phosphazenes, compounds of the general formula (PNH 2 ) x . Polydichlorophosphazene has properties like synthetic rubber , but it is unstable. By replacing the chlorine atoms with alkoxy groups or perfluoroalkoxy groups, however, chemically and thermally stable polymers with elastomeric properties are obtained.
Organic compounds

Among the organic phosphorus compounds one can distinguish between those with a phosphorus-carbon bond and those without a phosphorus-carbon bond. The first include derivatives of phosphines in which hydrogen atoms are replaced by one or more organic radicals. This group also includes phosphine oxides (R 3 PO), alkylphosphinic acids (R 2 PO (OH)) and alkylphosphonic acids (R-PO (OH) 2 ) or their salts. Examples of the second group are esters of phosphinic acid , phosphonic acid or phosphoric acid , which are referred to as phosphites , phosphonates or phosphates . Organic phosphorus compounds - for example triphenylphosphine , phenyldichlorophosphine or phosphorylides - play a role in many organic reactions, e.g. B. the Wittig reaction plays an important role.
In biochemistry, the phosphoric acid esters are particularly relevant. They are a vital part of many metabolic processes and part of DNA . Important molecules are:
literature
- John Emsley: Phosphorus - an element of life and death . Weinheim 2001, ISBN 3-527-30421-5 .
- Harry H. Binder: Lexicon of the chemical elements - the periodic table in facts, figures and data . Hirzel, Stuttgart 1999, ISBN 3-7776-0736-3 .
- F. Krafft: Phosphorus. From light matter to chemical element. In: Angewandte Chemie . 81 (17/18), 1969, pp. 634-645; doi: 10.1002 / anie.19690811703 .
- Ludwig Maier: Phosphorus compounds and their technical importance. In: Chemistry in Our Time . 9th Year, No. 4, 1975, pp. 109-116; doi: 10.1002 / ciuz.19750090403 .
- Jochen Metzger: Phosphorus time bomb. If this raw material runs out, the apocalypse threatens. In: PM world of knowledge. No. 8, 2010, ISSN 1863-9313 , pp. 58-64.
Web links
- CEEP Phosphates Website of the TU-Darmstadt and the CEEP for phosphorus recovery
- The Phosphorus Crisis - The End of Mankind? TV documentary by arte , May 2013, directed by Christiane Schwarz and Marcel Weingärtner
- Phosphate reserves Lecture on global reserves, mining, consumption and price development (PDF; 3.3 MB)
Individual evidence
- ↑ a b Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (phosphor) , unless otherwise stated .
- ^ IUPAC, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e Entry on phosphorus in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e Entry on phosphorus at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ↑ a b c d e f Entry on tetraphosphorus (white phosphorus) in the GESTIS substance database of the IFA , accessed on August 9, 2016(JavaScript required) .
- ↑ a b c d Entry on phosphorus, red in the GESTIS substance database of the IFA , accessed on August 9, 2016(JavaScript required) .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 747.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-142-4-147. The values there are based on g / mol and are given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data. 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ Entry on red / white phosphorus in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 14, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 12185-10-3 or Phosphor white / yellow ), accessed on November 2, 2015.
- ^ A b National Technical Information Service. Vol. AD-B011-150.
- ↑ a b c d Entry on phosphorus in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Pesticide Chemicals Official Compendium. Association of the American Pesticide Control Officials, Inc., 1966, p. 901.
- ↑ American Heart Journal . Vol. 84, 1972, p. 139.
- ^ Archives of Internal Medicine . Vol. 83, 1949, p. 164.
- ^ Alphons Oppenheim: Brand, Hennig . In: Allgemeine Deutsche Biographie (ADB). Volume 3, Duncker & Humblot, Leipzig 1876, p. 236.
- ↑ Alexander P. Hardt: Pyrotechnics , Pyrotechnica Publications, Post Falls Idaho USA 2001, ISBN 0-929388-06-2 , pp. 74 ff.
- ^ Mary Weeks, Discovery of the elements, Journal of Chemical Education 1956, p. 135.
- ^ Schrötter, New Modification of Phosphorus , Liebigs Annalen der Chemie, Volume 68, 1848, pp. 247-253
- ↑ Anton Schrötter: About a new allotropic state of phosphorus . In: JC Poggendorff (Ed.): Annals of Physics and Chemistry, Third Series . 157 (Pogg. Ann. 81), no. 10 . Johann Ambrosius Barth, 1850, ISSN 1521-3889 , p. 276–298 , doi : 10.1002 / andp.18501571009 ( online at Gallica Bibliothèque nationale de France ).
- ^ W. Hittorf, On the knowledge of phosphorus , Annalen der Physik, Volume 202, 1865, pp. 193-228
- ↑ Bridgman, Two new modifications of phosphor , Journal of the American Chemical Society, Volume 36, 1914, pp. 1344-1363.
- ↑ Martina Davids: Neutrino sources in the cosmos: Supernovae . Neutrino Seminar, RWTH Aachen University, WS2003 / 2004, November 24, 2003, (PDF) , viewed February 16, 2020.
- ↑ G. Cescutti, F. Matteucci, E. Caffau, P. François: Chemical evolution of the Milky Way: the origin of phosphorus . In: Astrophysics of Galaxies , doi: 10.1051 / 0004-6361 / 201118188 .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 , pp. 928-931.
- ↑ "Phosphorus Depletion: Bacterial Acid Generators - Rulers of Life and Death?" (Schattenblick, April 15, 2008).
- ↑ “ The earth's available reserves of phosphate, which is the primary ingredient in fertilizers, could be exhausted within the next 50 to 130 years ”, in: A blooming waste, Website University of Technology, Sydney , November 6, 2006; alternatively: “ Depletion of current economically exploitable reserves are estimated at somewhere from 60 to 130 years. Using the median reserves estimates and under reasonable predictions, it appears that phosphate reserves would last for at least 100+ years ”; in Arne Haarr, EUREAU (European Union of National Associations of Water Suppliers and Waste Water Services): The Reuse of Phosphorus .
- ↑ Answer of the Federal Government to the minor question of the members Cornelia Behm, Friedrich Ostendorff, Dorothea Steiner, other members and the parliamentary group BÜNDNIS 90 / DIE GRÜNEN. German Bundestag, December 15, 2012, accessed on August 27, 2019.
- ↑ Phosphate at USGS Mineral Resources .
- ↑ M. Okrusch, S. Matthes: Mineralogie: An introduction to special mineralogy, petrology and deposit science. 7th edition. Springer, 2005, ISBN 3-540-23812-3 .
- ↑ Media release from the building department of the Canton of Zurich: Converting sewage sludge into raw material: New process suitable for industrial production. June 3, 2019, accessed October 14, 2019 .
- ↑ Odenwald's universe: Does the shortage of phosphorus mean the end of mankind? on: focus.de , May 9, 2008.
- ↑ Dietmar Kunath: Phosophor. In: Claus Schaefer, Torsten Schröer (Hrsg.): The large lexicon of aquaristics. Eugen Ulmer, Stuttgart 2004, ISBN 3-8001-7497-9 , p. 772.
- ^ A. Matthew et al .: Evidence for reactive reduced phosphorus species in the early Archean ocean. In: PNAS. 110 (25), 2013, pp. 10089-10094. PMID 23733935 ; pdf (free full text access, English)
- ↑ Entry on phosphorus. In: Römpp Online . Georg Thieme Verlag, accessed on January 13, 2015.
- ^ Soda Springs. ( Memento of July 24, 2016 in the Internet Archive ) at: monsanto.com
- ↑ Federal contract site reveals US reliance on Monsanto and Israeli firm for White Phosphorus supply , February 7, 2013.
- ↑ Horst Briehl: Chemistry of materials . Springer-Verlag, 2014, ISBN 978-3-658-06225-5 , pp. 24 ( limited preview in Google Book search).
- ↑ Ralf Steudel : Chemistry of Non-Metals From Structure and Bond to Application . Walter de Gruyter, 2008, ISBN 978-3-11-021128-3 , p. 360 ( limited preview in Google Book Search).
- ↑ Brandi M. Cossairt, Christopher C. Cummins, Ashley R. Head, Dennis L. Lichtenberger, Raphael JF Berger, SA Hayes, NW Mitzel, G. Wu ,: On the Molecular and Electronic Structures of AsP 3 and P 4 . In: Journal of the American Chemical Society . tape 132 , no. 24 , June 23, 2010, ISSN 0002-7863 , p. 8459-8465 , doi : 10.1021 / ja102580d ( acs.org [accessed July 3, 2020]).
- ^ Alfons Klemenc: Inorganic chemistry on a physical-chemical basis . Springer-Verlag, 2013, ISBN 978-3-7091-7793-8 , pp. 202 ( limited preview in Google Book search).
- ↑ Stefanie Ortanderl, Ulf Ritgen: Chemistry for Dummies. The textbook . John Wiley & Sons, 2014, ISBN 978-3-527-70924-3 , pp. 541 ( limited preview in Google Book search).
- ^ Dietrich Wertz: Luminescence . diplom.de, 2004, ISBN 3-8324-8284-9 , p. 29 ( limited preview in Google Book search).
- ↑ retro; bib - Page from the manual of the druggist practice : vermin remedies.
- ↑ J. Sedlmeyer: About phosphorus poisoning . In: German journal for all forensic medicine. Volume 19, No. 1, December 1932, pp. 365-383; ( doi: 10.1007 / BF01750213 ).
- ↑ Leaflet on BK No. 1109: Diseases caused by phosphorus or its inorganic compounds , notice of the BMA of February 25, 1981, BArbBl Heft 4/1981.
- ↑ How copper sulfate solution works in detoxifying white phosphorus. on: www.chemieunterricht.de .
- ↑ Stefan Lange, Peer Schmidt, Tom Nilges: Au 3 SnP 7 @Black Phosphorus: An Easy Access to Black Phosphorus. In: Inorg. Chem. 46 (10), 2007, pp. 4028-4035; doi: 10.1021 / ic062192q .
- ↑ G. Brauer (Ed.): Handbook of Preparative Inorganic Chemistry. 2nd Edition. Vol. 1, Academic Press 1963, pp. 518-525.
- ↑ Basics and main group elements Volume 1: Basics and main group elements . Walter de Gruyter GmbH & Co KG, 2016, ISBN 3-11-049585-6 , p. 852 ( limited preview in Google Book search).
- ↑ H. Thurn, H. Krebs: About structure and properties of semi-metals. XXII. The crystal structure of Hittorf's phosphorus. In: Acta Cryst. B25, 1969, pp. 125-135.
- ↑ H. Thurn: The crystal structure of Hittorf's phosphorus. Dissertation . Technical University of Stuttgart, 1967.
- ↑ Arno Pfitzner, Michael F. Bräu, Josef Zweck, Gunther Brunklaus, Hellmut Eckert: Phosphorus Nanorods - Two Allotropic Modifications of a Long-Known Element. In: Angew. Chem. Int. Ed. 43, 2004, pp. 4228-4231; doi: 10.1002 / anie.200460244 .
- ↑ E.-C. Koch: Specials Materials in Pyrotechnics: IV. The Chemistry of Phosphorus and its Compounds. In: J. Pyrotech. 21, 2005, p. 39; Abstract .
- ↑ E.-C. Koch: Specials Materials in Pyrotechnics: V. Military Applications of Phosphorus and its Compounds. In: Propellants Explos. Pyrotech. 33, 2008, p. 165; doi: 10.1002 / prep.200700212 .
- ↑ Dietmar Kunath: Phosphorus. In: Claus Schaefer, Torsten Schröer (Hrsg.): The large lexicon of aquaristics. 2 volumes. Eugen Ulmer, Stuttgart 2004, ISBN 3-8001-7497-9 , 772.
- ↑ Emanuel Epstein: The Anomaly of Silicon in Plant Biology. In: Proc. Natl. Acad. Sci. USA 91, 1994, p. 11; doi: 10.1073 / pnas.91.1.11 .
- ^ Lincoln Taiz, Eduardo Zeiger: Physiology of plants. Spektrum, Akad. Verlag, Heidelberg / Berlin 1998, ISBN 3-8274-0537-8 .
- ↑ K. Mengel: Nutrition and metabolism of the plant. Gustav Fischer Verlag, Jena 1991, pp. 324–334.
- ↑ Ammunition Remnants : Grenades in the Baltic Sea. ( Memento of March 14, 2008 in the Internet Archive ) on: sueddeutsche.de , January 9, 2008.
- ↑ Remnants of ammunition in the Baltic Sea. “The authorities show no interest”. on: sueddeutsche.de , January 8, 2008.
- ↑ Stefan Nehring: Ammunition accidents - and no end. on: travemuende-aktuell.de , 2015.
- ↑ DGUV.de: BGR / GUV-SR 2003 Teaching in schools with hazardous substances (online version; PDF file; 9.52 MB), p. 24, accessed on April 25, 2011.
- ^ A b Nils Wiberg, Egon Wiberg, Arnold Frederik Hollemann: Inorganische Chemie . Volume 1: Basics and main group elements. 103rd edition. De Gruyter, Berlin, Boston 2017, ISBN 978-3-11-051854-2 , pp. 783 ff . (accessed via De Gruyter Online)




















![{\ displaystyle {\ ce {PO4 ^ 3- + 12MoO4 ^ 2- + 24H3O + + 3NH4 + <=> (NH4) 3 [P (Mo3O10) 4] + 36 H2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cb26219c3cab9f7c0612f33ef53af1422e1214ff)


![{\ displaystyle {\ ce {H2PO4- + 12 [MoO2Cl3 (H2O)] + 26 H2O -> [P (Mo3O10) 4] ^ 3- + 26 H3O + + 36Cl-}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6363079e9a055be6d511005c74fb931ca9f8abd5)
![{\ displaystyle {\ ce {[P (Mo3O10) 4] ^ 3- + 3HOx + 3H3O + -> (H2Ox) 3 [P (Mo3O10) 4] + 3H2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3f23c5ca64a72c9d6438fce291f757851636270c)
![\ mathrm {(H_2Ox) _3 [P (Mo_3O_ {10}) _ 4] \ cdot x \ H_2O}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b4a4ac6f7c69607fc6cb08462cc8acb0da733f77)
![\ mathrm {(H_2Ox) _3 [P (Mo_3O_ {10}) _ 4]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4015e1b4d1a83dc0b2c857e26fa6cdef99a7a9da)